Abstract
Background
Older patients with Hodgkin lymphoma (HL) often have comorbid cardiovascular disease; however, the impact of pre-existing heart failure (HF) on the management and outcomes of HL is unknown.
Objectives
The aim of this study was to assess the prevalence of pre-existing HF in older patients with HL and its impact on treatment and outcomes.
Methods
Linked Surveillance, Epidemiology, and End Results (SEER) and Medicare data from 1999 to 2016 were used to identify patients 65 years and older with newly diagnosed HL. Pre-existing HF, comorbidities, and cancer treatment were ascertained from billing codes and cause-specific mortality from SEER. The associations between pre-existing HF and cancer treatment were estimated using multivariable logistic regression. Cause-specific Cox proportional hazards models adjusted for comorbidities and cancer treatment were used to estimate the association between pre-existing HF and cause-specific mortality.
Results
Among 3,348 patients (mean age 76 ± 7 years, 48.6% women) with newly diagnosed HL, pre-existing HF was present in 437 (13.1%). Pre-existing HF was associated with a lower likelihood of using anthracycline-based chemotherapy regimens (OR: 0.42; 95% CI: 0.29-0.60) and a higher likelihood of lymphoma mortality (HR: 1.25; 95% CI: 1.06-1.46) and cardiovascular mortality (HR: 2.57; 95% CI: 1.96-3.36) in models adjusted for comorbidities. One-year lymphoma mortality cumulative incidence was 37.4% (95% CI: 35.5%-39.5%) with pre-existing HF and 26.3% (95% CI: 25.0%-27.6%) without pre-existing HF. The cardioprotective medications dexrazoxane and liposomal doxorubicin were used in only 4.2% of patients.
Conclusions
Pre-existing HF in older patients with newly diagnosed HL is common and associated with higher 1-year mortality. Strategies are needed to improve lymphoma and cardiovascular outcomes in this high-risk population.
Key Words: anthracycline cardiotoxicity, cardio-oncology, cardioprotection, geriatric oncology
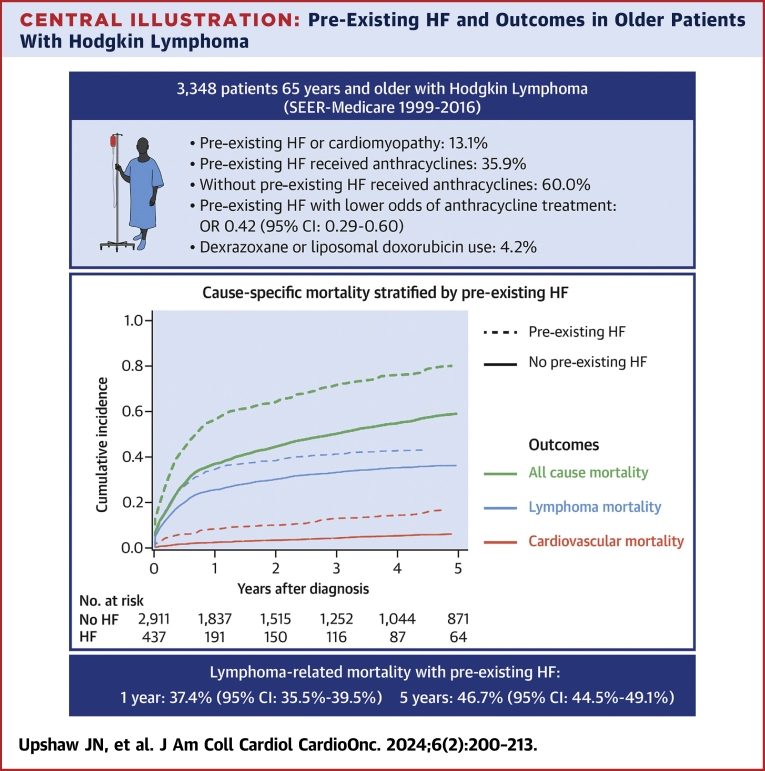
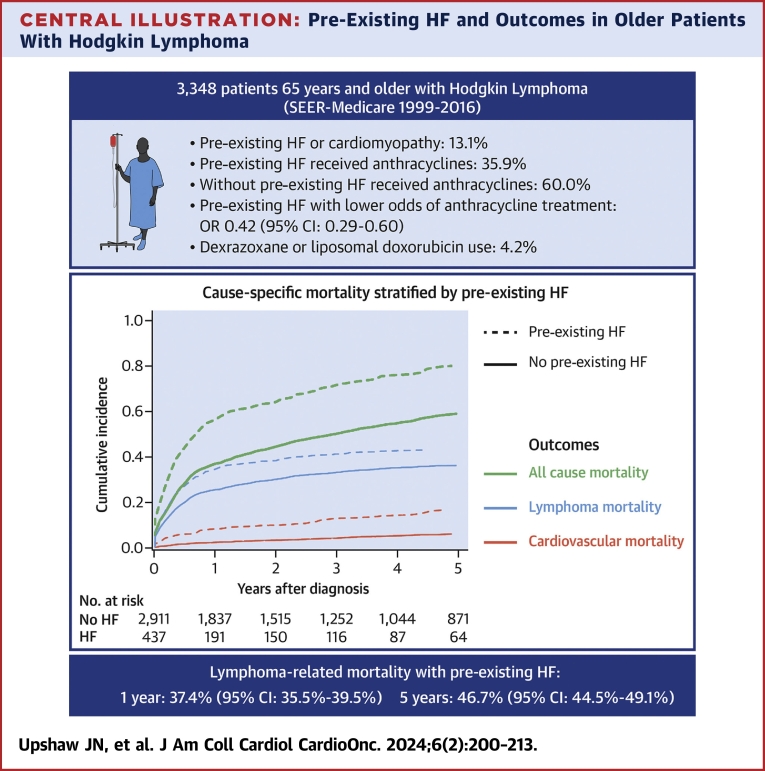
Central Illustration
Anthracycline-based chemotherapy regimens remain the preferred first-line therapy for patients with Hodgkin lymphoma (HL).1 Although the risk for heart failure (HF) after HL treatment is well described, few studies have evaluated the impact of pre-existing HF or cardiomyopathy on outcomes in patients with HL.2, 3, 4 Approximately 20% of patients with HL are 65 years or older at the time of diagnosis; however, this age group disproportionally accounts for more than 60% of HL deaths, reflecting the excellent outcomes among younger patients and the worse progression-free survival among older patients with HL.5 Cardiac and noncardiac comorbidities increase with age, and standard HL regimens may be limited by treatment-related toxicity, especially among patients with multiple comorbidities or impaired functionality.6,7 Although total comorbidity burden has been associated with worse outcomes and higher treatment-related toxicity,6,7 the extent to which pre-existing HF affects HL treatment and outcomes has not been well studied.
In published studies, clinical HF events were reduced with the addition of dexrazoxane to doxorubicin8, 9, 10 or the substitution of conventional doxorubicin with liposomal formulations11,12 with preserved oncologic efficacy. However, the majority of the studies in adults have enrolled patients with metastatic breast cancer, and data on HL are limited to single-arm phase 2 studies.13,14 The American Society of Clinical Oncology clinical practice guidelines on the prevention and monitoring of cardiac dysfunction in survivors of adult cancer recommend cardioprotective strategies in patients planning to receive high cumulative doses of anthracyclines or with multiple cardiac risk factors, with the caveat that much of the evidence comes from patients with advanced breast cancer.15 Our group recently reported that pre-existing HF was associated with less anthracycline use, higher lymphoma mortality, and low use of cardioprotective agents in older patients with diffuse large B-cell lymphoma (DLBCL), another aggressive lymphoma that is commonly treated with anthracyclines.16
The goal of this study was to extend these findings through the detailed assessment of prevalent HF at the time of HL diagnosis and the associations of pre-existing HF with anthracycline-based chemotherapy, cardioprotective medications, and lymphoma and cardiac-specific mortality in a national population-based sample of older patients with newly diagnosed HL.
Methods
Data sources and study population
We used linked Surveillance, Epidemiology, and End Results (SEER) and Medicare data from 1999 to 2016. The National Cancer Institute’s (NCI) SEER program is a system of population-based cancer registries that capture more than 25% of the U.S. population diagnosed with cancer and include patient demographics, date of cancer diagnosis, cancer characteristics, initial cancer treatments and follow-up of vital status and cause of death. Linkage to Medicare offers additional information on outpatient therapies, diagnostic tests, procedures, and hospitalizations ascertained from billing claims by hospitals, outpatient facilities, and physicians, with 94% of those 65 years and older in SEER registries matched to Medicare enrollment records (Supplemental Methods). For this study, we included individuals 65 years and older with newly diagnosed HL from 2000 to 2015 with fee-for-service Medicare Parts A and B continuously in the year prior to lymphoma diagnosis and in whom the lymphoma diagnosis did not first appear on a death certificate. For analyses that included neurohormonal antagonist and statin therapy, the population was additionally restricted to those with Medicare Part D (2007-2016). The Tufts Health Sciences Institutional Review Board determined that the present study was exempt from review (Code of Federal Regulations 46.104[4]), and the requirement to obtain informed consent was waived. The Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines for cohort studies were followed.17
Covariate definitions
HF or cardiomyopathy required at least 1 of the following: 1) 1 primary inpatient discharge diagnosis; 2) 2 outpatient diagnoses; 3) 3 secondary inpatient discharge diagnoses; 4) 3 emergency department diagnoses; or 5) 2 secondary inpatient discharge diagnoses plus 1 outpatient diagnosis as previously described.18 Individual sociodemographic variables such as age, sex, race, ethnicity, marital status, and Medicaid dual eligibility and census tract–level information such as income and educational status were derived from the SEER registry. Other baseline cardiac and noncardiac comorbidities were defined on the basis of International Classification of Diseases-9th Revision (ICD-9) and International Classification of Diseases-10th Revision (ICD-10) diagnostic codes in the 365 days prior to HL diagnosis from Medicare inpatient (Medicare Provider Analysis and Review), Medicare outpatient (outpatient claims), and physician visit (carrier claims) data requiring at least 2 codes appearing on separate days. A full list of ICD-9 and ICD-10 codes is available in Supplemental Table 1. Of note, this database does not contain echocardiographic data such as left ventricular ejection fraction (LVEF), and thus we were unable to categorize HF according to LVEF. In addition, the claims-based diagnostic codes may include some patients with cardiomyopathy but without the clinical syndrome of HF. Frailty was defined using the claims-based frailty index, which includes 21 claims and has been cross-validated with other frailty measures.19 We excluded comorbidity diagnoses made in the same month as the lymphoma diagnosis to reduce misclassification biases, as cancer diagnoses in SEER include the month and year of diagnosis only. Hospital-level variables were determined from SEER and included number of beds, medical school affiliation, teaching status, NCI cancer center designation, Commission on Cancer accreditation, and cooperative group membership.
Cancer treatment
Cancer treatment was determined using Healthcare Common Procedure Coding Systems codes, ICD-9 and ICD-10 codes, diagnosis-related group codes, and revenue center codes (Supplemental Methods, Supplemental Table 2). Chemotherapy was categorized as anthracycline-containing if the patient received at least 1 infusion of doxorubicin or liposomal doxorubicin, non-anthracycline-containing if the patient received at least 1 intravenous chemotherapy or targeted therapy but no doxorubicin, and no chemotherapy if no systemic chemotherapy or targeted therapy was given. In addition, patients treated with anthracycline were further subdivided into those receiving the first anthracycline dose in the first 3 months after HL diagnosis (early anthracycline group) and those receiving their first anthracycline dose 3 months or more after HL diagnosis (late anthracycline group). Radiation therapy in the year after diagnosis and hematopoietic cell transplantation in the 3 years after diagnosis were determined.
Cardioprotective medications
Dexrazoxane and liposomal doxorubicin use was determined using Healthcare Common Procedure Coding System codes (Supplemental Table 2). For the subset of patients with Medicare Part D, prescriptions for beta-blockers, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), or beta-hydroxy beta-methylglutaryl reductase inhibitors (statins) were identified using the National Drug Code directory. Prevalent users were defined by at least 1 prescription filled in the 4 months prior to HL diagnosis, and new users were defined if a prescription was filled in the 6 months after HL diagnosis (not including the month of HL diagnosis) among those without prescriptions in the 4 months prior to HL diagnosis.
Outcomes
Cause of death was defined using the SEER cause-of-death recode and grouped into lymphoma mortality, cardiovascular mortality, nonlymphoma cancer mortality, and noncardiovascular and noncancer mortality (Supplemental Methods). HF hospitalizations were defined as an inpatient admission with a primary discharge diagnosis code of HF (Supplemental Methods).
Missing data
Missing values for patient- and hospital-level covariates were imputed using multiple imputation to create 10 imputed data sets. The imputation model included all patient- and hospital-level characteristics, along with the outcome of interest. Variables were imputed using a fully conditional specification method. Logistic regression was used for dichotomous variables (derived from categorical characteristics), and predictive mean matching was used for continuous variables. Multivariable regression models that include covariates with missing data were estimated in each of the imputed data sets and results pooled using Rubin’s rules.
Statistical analysis
Baseline characteristics are summarized as mean ± SD for normally distributed continuous variables, median (Q1-Q3) for skewed continuous variables, and frequencies and percentages for categorical variables. Cell counts with values <11 were suppressed to avoid reidentification of patients according to SEER-Medicare policy.
All analyses were conducted using SAS version 9.4 (SAS Institute).
Associations between pre-existing HF and cancer treatment
The associations between pre-existing HF (exposure) and the outcome of receiving anthracycline chemotherapy compared with nonanthracycline chemotherapy were modeled with multivariable logistic regression using 2 sequential models, with results presented as ORs with 95% CIs. The first model included patient-level covariates (age, sex, race, ethnicity, cancer stage, hypertension, diabetes, hyperlipidemia, coronary artery disease, atrial fibrillation, peripheral vascular disease, ischemic stroke, valvular heart disease, chronic obstructive pulmonary disease, dementia, moderate or severe renal dysfunction, frailty, and any prior cancer diagnosis), and the second model additionally included geographic characteristics, social determinants of health (SDOH), and hospital-level variables (SEER region; metropolitan, nonurban metropolitan, or rural; Medicaid dual eligibility; marital status; census tract poverty indicator; household income; NCI cancer center designation; Commission on Cancer accreditation; hospital cooperative group status; hospital classified as referral center; teaching hospital; medical school affiliation; and number of beds). We repeated these sequential models with the outcomes of: 1) any chemotherapy vs no chemotherapy; 2) early anthracycline chemotherapy (in the first 3 months after diagnosis) vs nonanthracycline chemotherapy in the first 3 months; and 3) early vs late anthracycline chemotherapy. We also used the same sequential models to understand the associations between pre-existing HF and the use of cardioprotective medications (either dexrazoxane or liposomal doxorubicin) in the subcohort of patients who received early anthracycline therapy.
Association between pre-existing HF and cause-specific mortality
The cumulative incidence of cause-specific mortality was estimated using Gray’s competing risk method. The association between pre-existing HF and cause-specific mortality was estimated using a cause-specific Cox proportional hazards model with adjustment for baseline comorbidities and time-varying treatment covariates in sequential models, with results presented as HRs with 95% CIs.20,21 The cause-specific proportional hazards model censors for other causes of death. The unadjusted model included pre-existing HF (exposure) and the outcome cause-specific mortality. The first model included patient-level covariates (age, sex, race, ethnicity, cancer stage, diabetes, chronic obstructive pulmonary disease, dementia, chronic kidney disease, and any prior cancer diagnosis), the second model additionally included SDOH and hospital-level variables (SEER region; metropolitan, nonurban metropolitan, or rural; Medicaid dual eligibility; census tract poverty indicator; NCI cancer center designation; medical school affiliation; and number of beds), and the third model additionally included time-varying treatment information (anthracycline treatment including number of claims, radiation therapy, and cardioprotective medications liposomal formulations and dexrazoxane). Given the potential for effect modification by cancer stage, we repeated these analyses stratified by early stage (I or II) or advanced stage (III or IV).
Association between anthracycline use and cause-specific mortality among patients with pre-existing HF
The association between anthracycline use in the first 90 days (vs nonanthracycline chemotherapy in the first 90 days) and cause-specific mortality among patients with pre-existing HF was estimated using cause-specific Cox proportional hazards model with adjustment for baseline comorbidities, with results presented as HRs with 95% CIs. Cancer treatment was modeled as a time-varying covariate. In these models, patients without pre-existing HF and patients who did not receive any chemotherapy treatment in the first 90 days were excluded. Analyses were repeated stratified by early or advanced cancer stage.
Association between anthracycline use and HF hospitalizations among patients with pre-existing HF
The association between anthracycline use in the first 90 days (vs nonanthracycline chemotherapy in the first 90 days) and time to HF hospitalization among patients with pre-existing HF was estimated using Cox proportional hazards models that accounted for the competing risk for death and adjusted for baseline comorbidities. In these models, patients without pre-existing HF and patients who did not receive any chemotherapy treatment in the first 90 days were excluded, and cancer treatment was modeled as a time-varying covariate. Analyses were repeated stratified by early or advanced cancer stage and results presented as HRs with 95% CIs.
For all the Cox proportional hazards models, the linearity assumption was evaluated using Martingale residuals. The proportional hazards assumption was evaluated using weighted Schoenfeld residuals. There were no violations of proportional hazards for the main independent covariate of interest (pre-existing HF) in any of the models. However, for violations of proportional hazards for the other covariates in the models, these were addressed with stratification (categorical variables) or the addition of a time interaction term (continuous variables).
Poisson regression models were used to assess temporal trends in pre-existing HF, anthracycline, dexrazoxane, and liposomal doxorubicin by year from 2000 to 2016, with an offset for the total number of people per year.
Results
Study population
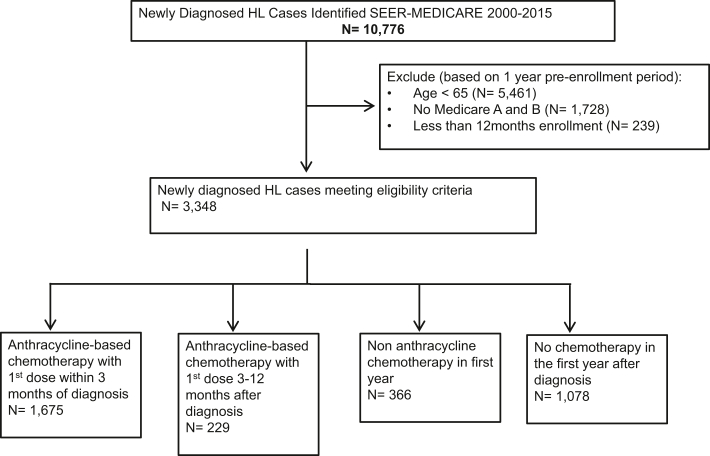
Among 10,776 patients with newly diagnosed HL identified in the SEER-Medicare database from 2000 to 2015, 5,461 were excluded because of age <65 years and 1,967 because of lack of continuous enrollment in Medicare Parts A and B for the past 12 months, resulting in a final study cohort of 3,348 patients (Figure 1). Baseline patient-level, census tract–level, and hospital-level variables are shown stratified by treatment strategy (Table 1, Supplemental Table 3). Of 3,348 included patients, 1628 (48.6%) were women, and the mean age was 76.1 ± 6.9 years. Cardiovascular and noncardiovascular comorbidities were prevalent in the cohort, including HF (13.1%), coronary artery disease (29.0%), atrial fibrillation (13.4%), peripheral vascular disease (15.8%), hypertension (67.2%), diabetes (31.2%), and hyperlipidemia (59.1%). In the first year after HL diagnosis, 56.9% of patients received anthracycline-based chemotherapy, 10.9% received nonanthracycline chemotherapy, and 32.2% received no chemotherapy. Radiation therapy was used in 23.4% of patients, and fewer than 1% of the patients were treated with hematopoietic cell transplantation.
Figure 1.
Consolidated Standards of Reporting Trials Diagram
A flow diagram detailing the study cohort. HL = Hodgkin lymphoma; SEER = Surveillance, Epidemiology, and End Results.
Table 1.
Baseline Characteristics of the Cohort Stratified by Cancer Treatment
| Total Cohort (N = 3,348) | Early Anthracycline Treatmenta (n = 1,675, 50.0%) | Late Anthracycline Treatmentb (n = 229, 6.8%) | Nonanthracycline Chemotherapy (n = 366, 10.9%) | No Chemotherapy (n = 1,078, 32.2%) | |
|---|---|---|---|---|---|
| Age, y | 76.1 ± 6.9 | 74.3 ± 5.9 | 74.3 ± 6.3 | 78.0 ± 7.1 | 78.6 ± 7.4 |
| Female | 1,628 (48.6) | 812 (48.5) | 120 (52.4) | 177 (48.4) | 519 (48.1) |
| Race | |||||
| Black | 181 (5.4) | 71 (4.2) | <11c | 21 (5.7) | 79 (7.3) |
| White | 3,058 (91.3) | 1,547 (92.4) | 212 (92.6) | >339 (>92.6)c | 957 (88.8) |
| Otherd | 109 (3.3) | 57 (3.4) | <11c | <11c | 42 (3.9) |
| Hispanice | 289 (8.6) | 143 (8.5) | 18 (7.9) | 28 (7.7) | 100 (9.3) |
| Stage | |||||
| I | 713 (21.3) | 311 (18.6) | 51 (22.3) | 71 (19.4) | 280 (26.0) |
| II | 755 (22.6) | 387 (23.1) | 62 (27.1) | 82 (22.4) | 224 (20.8) |
| III | 871 (26.0) | 493 (29.4) | 56 (24.5) | 106 (29.0) | 216 (20.0) |
| IV | 817 (24.4) | 402 (24.0) | 47 (20.5) | 90 (24.6) | 278 (25.8) |
| Unknown | 192 (5.7) | 82 (4.9) | 13 (5.7) | 17 (4.6) | 80 (7.4) |
| B symptoms | |||||
| Present | 1,197 (35.8) | 628 (37.5) | 114 (49.8) | 137 (37.4) | 411 (38.1) |
| Absent | 1,334 (39.8) | 672 (40.1) | 65 (28.4) | 139 (38.0) | 365 (33.9) |
| Unknown | 817 (24.4) | 375 (22.4) | 50 (21.8) | 90 (24.6) | 302 (28.0) |
| Heart failure/cardiomyopathyf | 437 (13.1) | 132 (7.9) | 25 (10.9) | 92 (25.1) | 188 (17.4) |
| Hypertension | 2,250 (67.2) | 1,106 (66.0) | 141 (61.6) | 276 (75.4) | 727 (67.4) |
| Diabetes | 1,045 (31.2) | 499 (29.8) | 65 (28.4) | 123 (33.6) | 358 (33.2) |
| Hyperlipidemia | 1,977 (59.1) | 1,062 (63.4) | 138 (60.3) | 239 (65.3) | 538 (49.9) |
| Coronary artery disease | 970 (29.0) | 415 (24.8) | 52 (22.7) | 159 (43.4) | 344 (31.9) |
| Prior myocardial infarction | 168 (5.0) | 62 (3.7) | <11c | 37 (10.1) | 61 (5.7) |
| Atrial fibrillation/flutter | 448 (13.4) | 163 (9.7) | 25 (10.9) | 79 (21.6) | 181 (16.8) |
| Valvular heart disease | 502 (15.0) | 226 (13.5) | 23 (10.0) | 79 (21.6) | 174 (16.1) |
| Peripheral vascular disease and carotid artery disease | 529 (15.8) | 210 (12.5) | 37 (16.2) | 78 (21.3) | 204 (18.9) |
| Ischemic stroke | 226 (6.8) | 80 (4.8) | 13 (5.7) | 26 (7.1) | 107 (9.9) |
| Chronic bronchitis/emphysema | 705 (21.1) | 335 (20.0) | 43 (18.8) | 90 (24.6) | 237 (22.0) |
| Dementia | 80 (2.4) | 23 (1.4) | <11c | <11c | 44 (4.1) |
| Moderate or severe renal disease | 272 (8.1) | 114 (6.8) | 17 (7.4) | 29 (7.9) | 112 (10.4) |
| Any prior cancer diagnosis | 529 (15.8) | 243 (14.5) | 38 (16.6) | 73 (19.9) | 175 (16.2) |
| Frailty (CFI19) | 0.18 ± 0.15 | 0.14 ± 0.11 | 0.14 ± 0.10 | 0.22 ± 0.14 | 0.24 ± 0.18 |
Values are mean ± SD or n (%).
CFI = claims-based frailty index; SEER = Surveillance, Epidemiology, and End Results.
Early anthracycline refers to those receiving their first anthracycline dose in the first 3 months after lymphoma diagnosis.
Late anthracycline refers to those receiving their first anthracycline dose 3 months or more after lymphoma diagnosis.
Cell counts with values <11 were suppressed to avoid reidentification of patients according to SEER-Medicare policy.
Other race in the SEER race recode includes: American Indian or Alaska Native and Asian or Pacific Islander.
Hispanic ethnicity defined by SEER. Hispanic ethnicity coding is independent of race coding.
Heart failure or cardiomyopathy was defined from International Classification of Diseases-9th Revision or International Classification of Diseases-10th Revision diagnostic codes; see “Methods” and Supplemental Appendix for details. The claims-based diagnostic codes may include some patients with cardiomyopathy but without the clinical syndrome of HF.
Associations between pre-existing HF and cancer treatment
Pre-existing HF was associated with lower odds of treatment with anthracycline chemotherapy in the first year compared with nonanthracycline chemotherapy (OR: 0.42; 95% CI: 0.29-0.60) (Table 2). Among those with pre-existing HF, only 35.9% of patients received anthracyclines in the first year, compared with 60.0% of patients without pre-existing HF (Table 3). Dexrazoxane or liposomal doxorubicin formulations were used in 4.1% of anthracycline-treated patients without pre-existing HF and <7% of anthracycline-treated patients with pre-existing HF (Table 3). Among patients treated with anthracyclines in the first year, pre-existing HF was not associated with higher odds of cardioprotective medication use with liposomal formulation or dexrazoxane (OR: 0.83; 95% CI: 0.34-2.07) (Table 2). Among patients with HF at the time of HL diagnosis, only one-half were on beta-blockers, and slightly more than one-half were on ACEIs or ARBs at the time of lymphoma diagnosis (Table 4).
Table 2.
Association Between Pre-Existing HF (Compared With No Pre-Existing HF) and Cancer Therapy
| Cancer Treatment Choice: OR (95% CI) |
|||||
|---|---|---|---|---|---|
| Anthracycline vs Nonanthracycline Chemotherapy (First Year) | Any Chemotherapy vs No Chemotherapy | Early vs Late Anthracycline | Early Anthracycline vs Nonanthracycline Chemotherapy | Cardioprotective Therapy (Dexrazoxane or Liposomal Doxorubicin) Among Anthracycline-Treated Patients | |
| Model A (adjusted for clinical variables)a | 0.42 (0.30-0.60) | 0.85 (0.66-1.09) | 0.56 (0.33-0.94) | 0.39 (0.27-0.55) | 0.89 (0.36-2.16) |
| Model B (adjusted for clinical variables, SDOH, and hospital variables)b | 0.42 (0.29-0.60) | 0.87 (0.67-1.11) | 0.54 (0.32-0.92) | 0.38 (0.26-0.55) | 0.83 (0.34-2.07) |
Comparison is between patients with pre-existing HF and those without pre-existing HF.
HF = heart failure; SDOH = social determinants of health.
Adjusted for age, sex, race, Hispanic ethnicity, advanced stage (III or IV vs I or II), hypertension, diabetes, hyperlipidemia, coronary artery disease, atrial fibrillation, peripheral vascular disease, valvular heart disease, prior ischemic stroke, any prior cancer diagnosis, chronic bronchitis or emphysema, dementia, moderate or severe renal dysfunction, dementia, and frailty.
Adjusted for model A variables as well as Surveillance, Epidemiology, and End Results region; metropolitan, nonurban metropolitan, or rural; Medicaid dual eligibility; marital status; census tract poverty indicator; household income; percentage without a high school diploma; National Cancer Institute cancer center designation; Commission on Cancer accreditation; hospital cooperative group membership (as of 2002); hospital classification as a referral center; teaching hospital; hospital medical school affiliation; and number of beds
Table 3.
Liposomal Anthracyclines and Dexrazoxane Use in the First Year Stratified by Pre-Existing HF
| HL All Patients (n = 3,348) | HL, No Pre-Existing HF (n = 2,911) | HL, Pre-Existing HF (n = 437) | P Value | |
|---|---|---|---|---|
| Doxorubicin (nonliposomal) | 1,886/3,348 (56.3) | 1,730/2,911 (59.4) | 156/437 (35.7) | <0.001a |
| Either doxorubicin or liposomal doxorubicin | 1,903/3,348 (56.8) | 1,746/2,911 (60.0) | 157/437 (35.9) | <0.001a |
| Liposomal doxorubicinb | 48/1,903 (2.5) | 44/1,746 (2.5) | <11/157 (<7)c | 1.00d |
| Dexrazoxaneb | 32/1,903 (1.7) | 28/1,746 (1.6) | <11/157 (<7)c | 0.33d |
| Either liposomal anthracycline or dexrazoxaneb | 79/1,903 (4.2) | 72/1,746 (4.1) | <11/157 (<7)c | 0.84a |
Values are n/N (%).
HF = heart failure; HL = Hodgkin lymphoma.
P values were estimated using the chi-square test.
Among patients treated with any anthracycline or liposomal anthracycline in the first year. The denominator includes doxorubicin and liposomal doxorubicin.
Cell counts with values <11 were suppressed to avoid reidentification of patients according to Surveillance, Epidemiology, and End Results and Medicare policy.
P values were estimated using the Fisher exact test.
Table 4.
Neurohormonal Antagonist and Statin Prescriptions in the Subset of Patients With Medicare Part D
| Prevalent Usersa |
New Usersb |
|||||
|---|---|---|---|---|---|---|
| All Patients (n = 980) |
Patients With Prevalent HF (n = 128) |
P Value | All Patients | Patients With Prevalent HF | P Value | |
| Beta-blocker | 215 (21.9) | 59 (46.1) | <0.001c | 77 (10.1) | 17 (24.6) | <0.001c |
| ACEI or ARB | 439 (44.8) | 75 (58.6) | 0.001c | 39 (7.2) | <11d | 0.009e |
| Statin | 426 (43.5) | 65 (50.8) | 0.07c | 31 (5.6) | <11d | 0.15e |
Values are n (%).
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; HF = heart failure.
Prevalent users were defined by at least 1 prescription filled in the 4 months prior to lymphoma diagnosis.
New users were defined if a prescription was filled in the 6 months after lymphoma diagnosis (not including the month of lymphoma diagnosis) among those without prescriptions in the 4 months prior to lymphoma diagnosis.
P values were estimated using the chi-square test.
Cell counts with values <11 were suppressed to avoid reidentification of patients according to Surveillance, Epidemiology, and End Results and Medicare policy.
P values were estimated using the Fisher exact test.
Pre-existing HF and risk for cardiovascular and lymphoma mortality
In those with pre-existing HF, the cumulative incidence of lymphoma-specific mortality was 37.4% (95% CI: 35.5%-39.5%) at 1 year and 46.7% (95% CI: 44.5%-49.1%) at 5 years (Central Illustration, Table 5). The cumulative incidence of cardiovascular mortality was 7.9% (95% CI: 7.0%-8.9%) at 1 year and 14.5% (95% CI: 12.9%-16.2%) at 5 years. Pre-existing HF was associated with higher risk for lymphoma mortality (HR: 1.50; 95% CI: 1.29-1.75) in unadjusted models (Table 6). This association was attenuated but remained significant after adjusting for clinical, SDOH, and hospital variables (HR: 1.25; 95% CI: 1.06-1.46) and further attenuated and no longer statistically significant after adjusting for cancer treatment variables (HR: 1.12; 95% CI: 0.95-1.31). Pre-existing HF was associated with higher risk for cardiovascular mortality (HR: 3.36; 95% CI: 2.61-4.31) in unadjusted models. This association was attenuated but remained significant in models adjusted for clinical, SDOH, and hospital-level variables (HR: 2.57; 95% CI: 1.96-3.36). Results were consistent when stratified by early (Supplemental Table 4) or advanced stage (Supplemental Table 5) HL.
Central Illustration.
Pre-Existing HF and Outcomes in Older Patients With Hodgkin Lymphoma
Cumulative incidence of lymphoma mortality (blue), cardiovascular mortality (red), and all-cause mortality (black) stratified by the presence or absence of pre-existing heart failure (HF). The dashed line represents patients with HF at the time of lymphoma diagnosis, and solid lines represent patient without HF at the time of lymphoma diagnosis. In addition to the cause-specific mortality results, the figure also summarizes the other key findings of the study, including the prevalence of pre-existing HF, associations with anthracycline treatment, and low use of the cardioprotective agents dexrazoxane and liposomal doxorubicin. SEER = Surveillance, Epidemiology, and End Results.
Table 5.
Cumulative Incidence of Cause-Specific Mortality Stratified by Presence or Absence of Pre-Existing HF
| Cumulative Incidence (95% CI) |
||||
|---|---|---|---|---|
| Lymphoma Mortality | Cardiovascular Mortality | Nonlymphoma Cancer Mortality | Non-CV, Noncancer Mortality | |
| Pre-existing HF 1-y outcomes | 37.4 (35.5-39.5) | 7.9 (7.0-8.9) | 4.4 (3.8-5.1) | 8.4 (7.4-9.4) |
| No pre-existing HF 1-y outcomes | 26.3 (25.0-27.6) | 2.9 (2.6-3.3) | 3.2 (2.8-3.6) | 4.5 (4.1-5.0) |
| Pre-existing HF 5-year outcomes | 46.7 (44.5-49.1) | 14.5 (12.9-16.2) | 7.0 (6.0-8.1) | 14.0 (12.6-15.6) |
| No pre-existing HF 5-y outcomes | 35.9 (34.4-37.6) | 6.8 (6.1-7.5) | 6.1 (5.4-6.9) | 9.4 (8.6-10.3) |
Unadjusted cumulative incidence estimates using competing risks for 4 different cause-specific mortalities.
CV = cardiovascular; HF = heart failure.
Table 6.
Associations Between Pre-Existing HF and Cause-Specific Mortality
| HR (95% CI) |
||||
|---|---|---|---|---|
| Lymphoma Mortality | Cardiovascular Mortality | Nonlymphoma Cancer Mortality | Non-CV, Noncancer Mortality | |
| Cohort sample size | 3,331 | 3,331 | 3,331 | 3,331 |
| Number of events | 1,300 | 368 | 257 | 464 |
| Unadjusteda | 1.50 (1.29-1.75) | 3.36 (2.61-4.31) | 1.42 (0.97-2.07) | 1.97 (1.53-2.53) |
| Model A (adjusted for clinical variables)b | 1.23 (1.05-1.44) | 2.61 (2.00-3.39) | 1.22 (0.82-1.81) | 1.45 (1.11-1.89) |
| Model B (adjusted for clinical variables, SDOH, and hospital variables)c | 1.25 (1.06-1.46) | 2.57 (1.96-3.36) | 1.21 (0.82-1.80) | 1.42 (1.09-1.86) |
| Model C (clinical and treatment variables)d | 1.12 (0.95-1.31) | 2.40 (1.83-3.16) | 1.12 (0.74-1.69) | 1.33 (1.02-1.73) |
Cox proportional hazards model using competing risks for 4 different cause-specific mortalities. Comparison is between patients with pre-existing HF and those without pre-existing HF.
Unadjusted includes pre-existing HF as only independent variable.
Adjusted for age (including an age-time interaction term), sex, race, Hispanic ethnicity, diabetes, any prior cancer diagnosis, chronic bronchitis or emphysema, dementia, and moderate or severe renal dysfunction and stratified by advanced cancer stage (III or IV vs I or II).
Adjusted for model A variables plus Surveillance, Epidemiology, and End Results region; metropolitan, nonurban metropolitan, or rural; Medicaid dual eligibility; National Cancer Institute cancer center designation; hospital medical school affiliation; number of beds; and stratification for census tract poverty indicator.
Adjusted for model B variables plus number of anthracycline claims (time varying), radiation (time varying), and cardioprotective medications (dexrazoxane or liposomal formulations, time varying).
Association between anthracycline vs nonanthracycline chemotherapy and lymphoma mortality and HF hospitalization among patients with pre-existing HF
Among patients with pre-existing HF who were treated with any chemotherapy in the first 90 days after diagnosis, anthracycline use compared with nonanthracycline chemotherapy was associated with lower risk for lymphoma mortality (HR: 0.44; 95% CI: 0.28-0.71) in models adjusted for baseline clinical variables (Table 7). Results were similar when stratified by early stage HL (Supplemental Table 6) or advanced stage HL (Supplemental Table 6), although the number of events was small and thus CIs were wide. No association was seen between anthracycline use compared with nonanthracycline chemotherapy and cardiovascular mortality (HR: 0.62; 95% CI: 0.33-1.15) or time to first HF hospitalization (HR: 1.07; 95% CI: 0.76-1.51) in models adjusting for baseline clinical variables (Tables 7 and 8).
Table 7.
Association Between Anthracycline Chemotherapy vs Nonanthracycline Chemotherapy and Cause-Specific Mortality Among Patients With Pre-Existing HF
| HR (95% CI) |
||||
|---|---|---|---|---|
| Lymphoma Mortality | Cardiovascular Mortality | Nonlymphoma Cancer Mortality | Non-CV, Noncancer Mortality | |
| Cohort sample size | 245 | 245 | 245 | 245 |
| Number of events | 88 | 49 | 15 | 40 |
| Unadjusteda | 0.51 (0.32-0.80) | 0.60 (0.35-1.04) | 0.47 (0.17-1.32) | 0.47 (0.24-0.91) |
| Model A (adjusted for clinical variables)b | 0.44 (0.28-0.71) | 0.62 (0.33-1.15) | 0.44 (0.15-1.32) | 0.36 (0.18-0.74) |
Cox proportional hazards model using competing risks for 4 different cause-specific mortalities. Patients without pre-existing HF were excluded. Patients not receiving any chemotherapy in the first 90 days were excluded. Cancer treatment was modeled as a time-varying covariate in the first 90 days.
Unadjusted includes anthracycline use within the first 90 days (time varying) as the only independent variable.
Adjusted for age, sex, Hispanic ethnicity, advanced stage (III or IV vs I or II), diabetes, any prior cancer diagnosis, chronic bronchitis/emphysema, dementia, and moderate or severe renal dysfunction and stratified by race.
Table 8.
Association Between Anthracycline Chemotherapy vs Nonanthracycline Chemotherapy and HF Hospitalization Among Patients With Pre-Existing HF
| Anthracycline Use vs Nonanthracycline | HF Admissions |
||
|---|---|---|---|
| All Patients | Early Stage | Advanced Stage | |
| Cohort sample size | 245 | 87 | 150 |
| Number of events | 135 | 46 | 84 |
| Unadjusteda | 0.99 (0.71-1.39) | 0.93 (0.53-1.63) | 0.95 (0.62-1.46) |
| Model A (adjusted for clinical variables)b | 1.07 (0.76-1.51) | 0.73 (0.39-1.38) | 0.98 (0.62-1.55) |
Values are HR (95% CI). Cox proportional hazards model of time to first HF admission accounting for the competing risk of death.
HF = heart failure.
Unadjusted includes anthracycline use within the first 90 days (time varying) as the only independent variable.
Adjusted for age, sex, race, Hispanic ethnicity, diabetes, any prior cancer diagnosis, chronic bronchitis or emphysema, dementia, and moderate or severe renal dysfunction. The model including all stages was additionally adjusted for advanced stage (III or IV vs I or II).
Longitudinal trends in pre-existing HF and cancer treatment
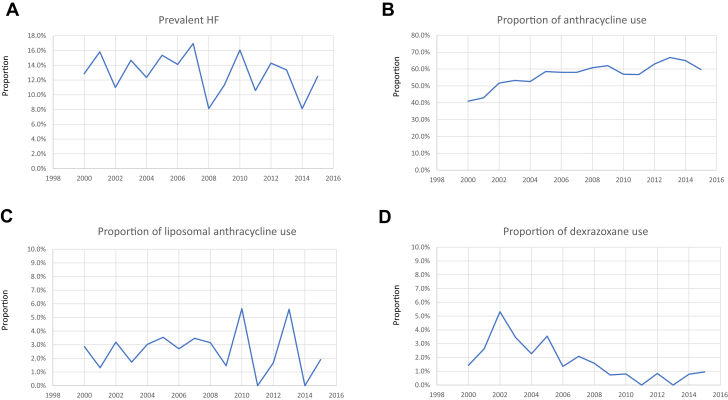
There was no significant change in the percentage of patients with pre-existing HF at the time of lymphoma diagnosis when assessed by year from 2000 to 2016 (Figure 2). There was a modest increase in anthracycline use from 2000 to 2016 (P < 0.001 for linear trend). There was no significant change in the use of liposomal doxorubicin over the study period, but there was a significant decrease in the use of dexrazoxane (P < 0.001 for linear trend).
Figure 2.
Temporal Trends
Temporal trends in the proportion of patients with new lymphoma diagnoses with pre-existing heart failure (HF) or cardiomyopathy (A), proportion of anthracycline use in the first year after diagnosis (B), and proportion of patients treated with anthracyclines in the first year who received liposomal doxorubicin (C) or dexrazoxane (D). Trends over time were estimated using Poisson regression.
Discussion
In a population-based analysis of older patients with HL, our main findings are as follows: 1) pre-existing HF was present in 13.1% of patients with HL; 2) pre-existing HF was associated with lower use of anthracyclines; 3) the cardioprotective agents dexrazoxane and liposomal doxorubicin were used infrequently (4.2%), even in patients with pre-existing HF; and 4) pre-existing HF was associated with an increased risk for lymphoma mortality in models adjusted for baseline comorbidities.
The prognosis for patients with HL <60 years of age is excellent, with 5-year survival and sustained cures in >85% of the patients. However, older patients continue to have a poor prognosis but with heterogeneity in outcomes by cancer stage, histology, Epstein-Barr virus positivity, treatment intensity, comorbidities, functionality, and frailty.6,7,22 Although competing causes of death from non-lymphoma-related comorbidities are higher among older patients, our findings suggest that even among patients with pre-existing HF, the risk for lymphoma-related mortality is 3- to 4-fold higher than that for cardiovascular-related mortality in the first 5 years after diagnosis. Of note, the association between pre-existing HF and lymphoma mortality was no longer significant after additionally adjusting for cancer treatment as a time-varying covariate. We hypothesize that the higher lymphoma mortality in patients with pre-existing HF may be mediated in part by the lower use of anthracyclines. In support of this, in exploratory analysis, anthracycline-based chemotherapy was associated with lower lymphoma mortality compared with non-anthracycline-based therapy among patients with pre-existing HF, although we recognize that residual confounding and selection bias are potential concerns in this observational analysis. Our findings motivate additional studies to understand whether select patients with pre-existing HF can safely receive anthracycline-based chemotherapy regimens with cardioprotection.
Our group recently reported a similar SEER-Medicare analysis of older patients with DLBCL, another aggressive lymphoma for which the first-line chemotherapy regimen includes anthracyclines, with higher disease prevalence and thus a larger sample size for the analysis.16 Interestingly, in the DLBCL cohort, we found a similar prevalence of pre-existing HF (13.9%) and similar associations between pre-existing HF and lower anthracycline use (OR: 0.55; 95% CI: 0.49-0.61) and higher risk for lymphoma mortality (adjusted HR: 1.24; 95% CI: 1.18-1.31) as we found in this study of patients with HL.16 In both studies, 1-year lymphoma mortality was high in patients with pre-existing HF (41.8% [95% CI: 40.5%-43.2%] for DLBCL and 37.4% [95% CI: 35.5%-39.5%] for HL), with lymphoma mortality exceeding cardiovascular mortality by more than 4-fold at 1 year. These analyses together highlight the poor 1-year outcomes in older patients with either DLBCL or HL who have pre-existing HF, driven largely by high lymphoma-related mortality. HL and DLBCL are common aggressive lymphomas, and anthracycline-containing regimens remain the standard of care for these malignancies. Although frontline anthracycline-based regimens are different for DLBCL (rituximab, cyclophosphamide, doxorubicin, vincristine sulfate, and prednisone) and HL (doxorubicin, bleomycin, vinblastine sulfate, and dacarbazine or doxorubicin, vinblastine sulfate, and dacarbazine), with higher dose intensity for HL regimens and different treatment-related toxicities, such as risk for lung toxicity with bleomycin, the cumulative anthracycline doses and risks for cardiotoxicity are similar for both DLBCL and HL. In addition, observational studies suggest that anthracycline-free regimens are associated with worse lymphoma outcomes for both lymphoma types.7,23, 24, 25, 26
In randomized trials of patients with breast cancer, dexrazoxane given with doxorubicin8, 9, 10 or the substitution of doxorubicin with liposomal doxorubicin11,12 was associated with a decrease in clinical HF events with preserved oncologic efficacy.27 However, neither dexrazoxane nor liposomal doxorubicin is approved by the U.S. Food and Drug Administration for the prevention of anthracycline-associated HF in adults newly diagnosed with HL or for use in patients with reduced LVEFs. Studies in adults with lymphoma have included 1 small randomized trial of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone vs rituximab, cyclophosphamide, liposomal doxorubicin, vincristine, and prednisone in patients with DLBCL, with less subclinical cardiotoxicity and similar lymphoma outcomes.28 In a multicenter, single-arm study of patients with HL at increased risk for HF because of age ≥70 years (n = 41) or established cardiac disease (n = 6), the substitution of a liposomal formulation of doxorubicin in combination with bleomycin, vinblastine, and dacarbazine was associated with progression-free survival of 70% and overall survival of 43% with grade 3 or higher cardiac events in 2 patients (4%).13 Gemcitabine, vinorelbine, and liposomal doxorubicin have been studied in the setting of relapsed HL after an initial course of doxorubicin-containing regimens and is included in the National Comprehensive Cancer Network guidelines as an option for second-line therapy.1 To our knowledge, this is the first study to explore the prevalence of “off label” use of these agents in patients with HL, and we found low use of dexrazoxane (1.7%) or liposomal doxorubicin (2.5%) across all patients and no signal for any increase in the use of these agents over the study period from 2000 to 2016. “Permissive cardiotoxicity” refers to the continuation of effective cancer therapies despite cardiac risk or emerging cardiotoxicity while also optimizing cardiac medications, especially in cases in which alternative cancer therapies are inferior to the cardiotoxic regimen.29 Future studies are needed to explore the safety of permissive cardiotoxicity in patients with aggressive lymphomas in the context of optimized HF guideline-directed medical therapy, infusional cardioprotective strategies, and close cardiac monitoring.
Nonanthracycline regimens such as brentuximab vedotin and dacarbazine have shown activity in frontline therapy for HL in older patients who were not candidates or declined anthracycline-based chemotherapy.30 Immune checkpoint inhibitor therapy is effective for relapsed or refractory HL31 and is being evaluated in combination with standard anthracycline-based chemotherapy in the frontline setting.32 A phase 2 study of nivolumab and brentuximab vedotin for frontline therapy for older patients with HL showed activity but did not meet the primary response rate threshold of 68%.33 Although novel agents may eventually eliminate the need for anthracyclines, at the present time, anthracycline-based regimens are associated with better lymphoma outcomes even in older patients with comorbidities, although further studies are needed to assess optimal regimens for patients with pre-existing HF, multiple comorbidities, or documented geriatric syndrome.7,23,24
Although lymphoma mortality was the most common cause of death in this cohort, cardiovascular mortality was also high among patients with pre-existing HF, occurring in 7.9% of patients at 1 year and 14.5% at 5 years. In the subset of our cohort with Medicare Part D in whom prescription medication information was available, only 46.1% with pre-existing HF were treated with beta-blockers, and 58.6% with pre-existing HF were treated with ACEIs or ARBs. Although we do not have access to ejection fraction, vital signs, or laboratory values and thus cannot determine if these therapies were indicated, our findings suggest possible opportunities to improve optimal guideline-directed medical therapy for all cardiac comorbidities and cardiac risk factors to improve lymphoma and cardiovascular outcomes.34, 35, 36, 37 Cardio-oncology programs have been established at many hospitals with the goal of improving the cardiovascular care of patients with cancer through multidisciplinary collaboration.15,38,39
Study limitations
First, this was an observational study, and residual confounding and selection bias were likely. Selection bias is especially relevant when interpreting the association between anthracycline chemotherapy and outcomes, as more fit or healthier patients are more likely to receive anthracyclines and also may have lower cardiovascular risk because of factors incompletely adjusted for in our analysis.
Second, HF, comorbidities, and cancer treatments were ascertained using claims data, and therefore we do not have access to clinical data such as LVEF, symptom burden, and biomarkers such as natriuretic peptides. We were therefore unable to categorize HF as HF with reduced ejection fraction, HF with mildly reduced ejection fraction, or HF with preserved ejection fraction.40
Third, claims for doxorubicin allow the determination of the number of cycles of doxorubicin; however, we were unable to determine if there were dose reductions of chemotherapy or if doxorubicin was given as a continuous infusion.
Fourth, additional medications have been shown to reduce morbidity and mortality in patients with HF since the study period, including the angiotensin receptor neprilysin inhibitor sacubitril-valsartan, as well as sodium glucose cotransporter 2 inhibitors. There are now 4 foundational medications recommended for the treatment of patients with HF with reduced ejection fraction,34 and future studies are needed to assess whether these medications are being routinely used in the population of patients with comorbid HF and HL and if they improve cardiovascular and oncologic outcomes in patient with established HF and lymphoma.
Conclusions
Pre-existing HF was present in 13.1% of older patients with HL and was associated with lower use of anthracyclines and higher risk for lymphoma and cardiovascular mortality in adjusted analyses. The cardioprotective agents dexrazoxane and liposomal doxorubicin were used infrequently (4.2%), and use was similar in patients with and those without pre-existing HF. Among patients with pre-existing HF, anthracycline use was associated with lower lymphoma mortality and no signal for increased cardiovascular mortality of HF hospitalizations, although these findings are hypothesis generating only given the observational study design and concerns for residual confounding bias. Randomized trials of strategies to reduce lymphoma and cardiovascular mortality in this high-risk patient population are needed. Future studies could evaluate close collaboration between oncology and cardiology, interventions to optimize guideline-directed medical therapy for HF, the selective use of cardioprotective medications with anthracycline-based regimens, or novel nonanthracycline regimens.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Older patients with HL and pre-existing HF or cardiomyopathy are less likely to be treated with conventional anthracycline-based chemotherapy and have high 1-year mortality. Dexrazoxane and liposomal doxorubicin were used infrequently.
TRANSLATIONAL OUTLOOK: Close collaboration between oncology and cardiology, as well as clinical trials and prospective registries, are needed to evaluate strategies to reduce lymphoma and cardiovascular mortality in patients with pre-existing HF and newly diagnosed aggressive lymphomas, such as HL. Additional studies are needed to assess whether anthracycline-based chemotherapy with cardioprotective strategies can be safely given and are associated with improved outcomes in patients with Hodgkin lymphoma and established cardiomyopathy or HF.
Funding Support and Author Disclosures
This study is supported by National Institute of Health grant K08HL146959 (to Dr Upshaw). The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code § 103885; the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under cooperative agreement 1NU58DP007156; the NCI’s SEER program under contract HHSN261201800032I awarded to the University of California-San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, the Department of Public Health, the NCI, and the Centers for Disease Control and Prevention or their contractors and subcontractors. The funders had no role in the design and conduct of the trial, analysis of data, or writing of the manuscript. Mr Nelson is employed by OM1 (but was employed by Tufts Medical Center while conducting the present research). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors acknowledge the efforts of the NCI; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Husam Abdel-Qadir, MD, PhD, served as the Guest Associate Editor for this paper.
Paaladinesh Thavendiranathan, MD, MSc, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, results, and references, please see the online version of this paper.
Appendix
References
- 1.Hodgkin lymphoma (version 2.2022). National Comprehensive Cancer Network. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1439
- 2.van Nimwegen F.A., Schaapveld M., Janus C.P., et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175(6):1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 3.de Vries S., Haaksma M.L., Jozwiak K., et al. Development and validation of risk prediction models for coronary heart disease and heart failure after treatment for Hodgkin lymphoma. J Clin Oncol. 2023;41(1):86–95. doi: 10.1200/JCO.21.02613. [DOI] [PubMed] [Google Scholar]
- 4.Dores G.M., Curtis R.E., Dalal N.H., Linet M.S., Morton L.M. Cause-specific mortality following initial chemotherapy in a population-based cohort of patients with classical Hodgkin lymphoma, 2000-2016. J Clin Oncol. 2020;38(35):4149–4162. doi: 10.1200/JCO.20.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute . National Cancer Institute; Bethesda, MD: 2022. Surveillance, Epidemiology, and End Results. Cancer statistics. [Google Scholar]
- 6.Evens A.M., Advani R.H., Helenowski I.B., et al. Multicenter phase II study of sequential brentuximab vedotin and doxorubicin, vinblastine, and dacarbazine chemotherapy for older patients with untreated classical Hodgkin lymphoma. J Clin Oncol. 2018;36(30):3015–3022. doi: 10.1200/JCO.2018.79.0139. [DOI] [PubMed] [Google Scholar]
- 7.Orellana-Noia V.M., Isaac K., Malecek M.K., et al. Multicenter analysis of geriatric fitness and real-world outcomes in older patients with classical Hodgkin lymphoma. Blood Adv. 2021;5(18):3623–3632. doi: 10.1182/bloodadvances.2021004645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Baat E.C., Mulder R.L., Armenian S., et al. Dexrazoxane for preventing or reducing cardiotoxicity in adults and children with cancer receiving anthracyclines. Cochrane Database Syst Rev. 2022;9:CD014638. doi: 10.1002/14651858.CD014638.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swain S.M., Whaley F.S., Gerber M.C., et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15(4):1318–1332. doi: 10.1200/JCO.1997.15.4.1318. [DOI] [PubMed] [Google Scholar]
- 10.Marty M., Espie M., Llombart A., et al. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline-based chemotherapy. Ann Oncol. 2006;17(4):614–622. doi: 10.1093/annonc/mdj134. [DOI] [PubMed] [Google Scholar]
- 11.Batist G., Harris L., Azarnia N., Lee L.W., Daza-Ramirez P. Improved anti-tumor response rate with decreased cardiotoxicity of non-pegylated liposomal doxorubicin compared with conventional doxorubicin in first-line treatment of metastatic breast cancer in patients who had received prior adjuvant doxorubicin: results of a retrospective analysis. Anticancer Drugs. 2006;17(5):587–595. doi: 10.1097/00001813-200606000-00014. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien M.E., Wigler N., Inbar M., et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 13.Salvi F., Luminari S., Tucci A., et al. Bleomycin, vinblastine and dacarbazine combined with nonpegylated liposomal doxorubicin (MBVD) in elderly (>/=70 years) or cardiopathic patients with Hodgkin lymphoma: a phase-II study from Fondazione Italiana Linfomi (FIL) Leuk Lymphoma. 2019;60(12):2890–2898. doi: 10.1080/10428194.2019.1608529. [DOI] [PubMed] [Google Scholar]
- 14.Picardi M., Giordano C., Pugliese N., et al. Liposomal doxorubicin supercharge-containing front-line treatment in patients with advanced-stage diffuse large B-cell lymphoma or classical Hodgkin lymphoma: Preliminary results of a single-centre phase II study. Br J Haematol. 2022;198(5):847–860. doi: 10.1111/bjh.18348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armenian S.H., Lacchetti C., Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline summary. J Oncol Pract. 2017;13(4):270–275. doi: 10.1200/JOP.2016.018770. [DOI] [PubMed] [Google Scholar]
- 16.Upshaw J.N., Nelson J., Rodday A.M., et al. Association of preexisting heart failure with outcomes in older patients with diffuse large B-cell lymphoma. JAMA Cardiol. 2023;8(5):453–461. doi: 10.1001/jamacardio.2023.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E., Altman D.G., Egger M., et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 18.Go A.S., Lee W.Y., Yang J., Lo J.C., Gurwitz J.H. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296(17):2105–2111. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 19.Segal J.B., Huang J., Roth D.L., Varadhan R. External validation of the claims-based frailty index in the national health and aging trends study cohort. Am J Epidemiol. 2017;186(6):745–747. doi: 10.1093/aje/kwx257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putter H., Fiocco M., Geskus R.B. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 22.Rodday A.M., Hahn T., Kumar A.J., et al. Association of treatment intensity with survival in older patients with Hodgkin lymphoma. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng P.T.M., Villa D., Gerrie A.S., et al. The outcome of older adults with classic Hodgkin lymphoma in British Columbia. Blood Adv. 2022;6(22):5924–5932. doi: 10.1182/bloodadvances.2022008258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goh Z., Latimer M., Lewis K.L., et al. Characteristics and outcomes of older patients with classical Hodgkin lymphoma: an Australasian Lymphoma Alliance, and Lymphoma and Related Diseases Registry study. Clin Lymphoma Myeloma Leuk. 2023;23(5):370–378. doi: 10.1016/j.clml.2023.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Lin R.J., Behera M., Diefenbach C.S., Flowers C.R. Role of anthracycline and comprehensive geriatric assessment for elderly patients with diffuse large B-cell lymphoma. Blood. 2017;130(20):2180–2185. doi: 10.1182/blood-2017-05-736975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida M., Nakao T., Horiuchi M., et al. Analysis of elderly patients with diffuse large B-cell lymphoma: aggressive therapy is a reasonable approach for “unfit” patients classified by comprehensive geriatric assessment. Eur J Haematol. 2016;96(4):409–416. doi: 10.1111/ejh.12608. [DOI] [PubMed] [Google Scholar]
- 27.Henriksen P.A., Rankin S., Lang N.N. Cardioprotection in patients at high risk of anthracycline-induced cardiotoxicity: JACC: CardioOncology primer. J Am Coll Cardiol CardioOnc. 2023;5(3):292–297. doi: 10.1016/j.jaccao.2023.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fridrik M.A., Jaeger U., Petzer A., et al. Cardiotoxicity with rituximab, cyclophosphamide, non-pegylated liposomal doxorubicin, vincristine and prednisolone compared to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in frontline treatment of patients with diffuse large B-cell lymphoma: a randomised phase-III study from the Austrian Cancer Drug Therapy Working Group [Arbeitsgemeinschaft Medikamentose Tumortherapie AGMT] (NHL-14) Eur J Cancer. 2016;58:112–121. doi: 10.1016/j.ejca.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Porter C., Azam T.U., Mohananey D., et al. Permissive cardiotoxicity: the clinical crucible of cardio-oncology. J Am Coll Cardiol CardioOnc. 2022;4(3):302–312. doi: 10.1016/j.jaccao.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedberg J.W., Forero-Torres A., Bordoni R.E., et al. Frontline brentuximab vedotin in combination with dacarbazine or bendamustine in patients aged >/=60 years with HL. Blood. 2017;130(26):2829–2837. doi: 10.1182/blood-2017-06-787200. [DOI] [PubMed] [Google Scholar]
- 31.Armand P., Engert A., Younes A., et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018;36(14):1428–1439. doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brockelmann P.J., Goergen H., Keller U., et al. Efficacy of nivolumab and AVD in early-stage unfavorable classic Hodgkin lymphoma: the randomized phase 2 German Hodgkin Study Group NIVAHL trial. JAMA Oncol. 2020;6(6):872–880. doi: 10.1001/jamaoncol.2020.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheson B.D., Bartlett N.L., LaPlant B., et al. Brentuximab vedotin plus nivolumab as first-line therapy in older or chemotherapy-ineligible patients with Hodgkin lymphoma (ACCRU): a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2020;7(11):e808–e815. doi: 10.1016/S2352-3026(20)30275-1. [DOI] [PubMed] [Google Scholar]
- 34.Heidenreich P.A., Bozkurt B., Aguilar D., et al. 2022 American College of Cardiology/American Heart Association/Heart Failure Society of America guideline for the management of heart failure: executive summary. J Card Fail. 2022;28(5):810–830. doi: 10.1016/j.cardfail.2022.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.January C.T., Wann L.S., Calkins H., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Fihn S.D., Blankenship J.C., Alexander K.P., et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Thorac Cardiovasc Surg. 2015;149(3):e5–e23. doi: 10.1016/j.jtcvs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Herrmann J., Lenihan D., Armenian S., et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J. 2022;43(4):280–299. doi: 10.1093/eurheartj/ehab674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyon A.R., Dent S., Stanway S., et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 2021;27(4):387-413.https://doi.org/10.1016/j.cardfail.2021.01.022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.