Abstract
Background
The relationship between genotype and phenotypical vascular and cardiac properties in paediatric Loeys-Dietz syndrome (LDS) patients are not well characterized. This study explores the phenotypical differences in aortic properties and cardiac structural and functional parameters between paediatric LDS patients with TGFBR1 and TGFBR2 mutations.
Methods
We included 32 LDS patients with either TGFBR1 (n = 17) or TGFBR2 (n = 15) mutations. Echocardiographic data included aortic dimensions, distensibility, strain, and stiffness at the level of the annulus, sinuses of Valsalva, sinotubular junction, ascending aorta, and descending aorta. Parameters for left ventricular size and function were also recorded.
Results
Demographics were similar between the groups. Patients with TGFBR2 were more likely to have undergone aortic surgery (47% vs 12%, P = 0.057) and use angiotensin receptor blockers (93% vs 47%, P = 0.015). Aortic z scores were significantly larger in the TGFBR2 group at the level of the aortic valve annulus (P = 0.007), sinuses of Valsalva (P = 0.001), sinotubular junction (P = 0.001), and ascending aorta (P = 0.054). Patients with TGFBR2 also had significantly lower aortic distensibility and strain coupled with higher stiffness index at the level of the annulus, sinotubular junction, and ascending aorta. Parameters for the descending aorta, cardiac morphology, and cardiac function were similar between the groups.
Conclusions
Paediatric LDS patients with TGFBR2 present with more severe cardiovascular phenotypes than patients with TGFBR1 with larger aortic dimensions and increased aortic stiffness. Our findings suggest that genotypes should be taken into consideration in the clinical management of paediatric LDS patients.
Résumé
Contexte
Les liens entre le génotype des enfants atteints du syndrome de Loeys-Dietz (SLD) et les particularités phénotypiques vasculaires et cardiaques n’ont pas encore été bien caractérisés. La présente étude vise à explorer les différences phénotypiques entre les propriétés de l’aorte et les paramètres cardiaques structuraux et fonctionnels des enfants atteints du SLD qui présentent une mutation du gène TGFBR1 et ceux qui présentent une mutation du gène TGFBR2.
Méthodologie
Nous avons inclus dans notre analyse 32 patients atteints du SLD présentant une mutation de TGFBR1 (n = 17) ou de TGFBR2 (n = 15). Les données échocardiographiques colligées incluaient les dimensions de l’aorte, sa distensibilité, sa déformation (strain) et sa rigidité au niveau de l’anneau aortique, des sinus de Valsalva, de la jonction sinotubulaire, de l’aorte ascendante et de l’aorte descendante. Les paramètres ayant trait à la taille et à la fonction du ventricule gauche ont également été consignés.
Résultats
Les caractéristiques démographiques étaient comparables dans les deux groupes. Les patients présentant une mutation du gène TGFBR2 étaient plus susceptibles d’avoir subi une intervention chirurgicale de l’aorte (47 % vs 12 %, p = 0,057) et de prendre un antagoniste des récepteurs de l’angiotensine (93 % vs 47 %, p = 0,015). Les scores z aortiques étaient significativement plus élevés chez les patients présentant une mutation de TGFBR2 pour les dimensions de l’anneau de la valve aortique (p = 0,007), des sinus of Valsalva (p = 0,001), de la jonction sinotubulaire (p = 0,001) et de l’aorte ascendante (p = 0,054). Les patients avec une mutation de TGFBR2 présentaient aussi une élasticité et une déformation aortiques significativement plus faibles ainsi qu’une rigidité accrue au niveau de l’anneau aortique, de la jonction sinotubulaire et de l’aorte ascendante. Les paramètres de l’aorte descendante, les caractéristiques morphologiques cardiaques et la fonction cardiaque étaient comparables pour les deux groupes.
Conclusions
Chez les enfants atteints du SLD, une mutation du gène TGFBR2 se traduisait par des phénotypes plus défavorables que dans le cas d’une mutation du gène TGFBR1 et se caractérisait par des dimensions et une rigidité aortiques accrues. Nos observations indiquent qu’il convient de prendre le génotype des patients en considération lors de la prise en charge clinique des enfants atteints du SLD.
Loeys-Dietz syndrome (LDS) is an autosomal dominant connective tissue disorder caused by mutations in any of the following 6 genes: TGFBR1, TGFBR2, TGFB2, TGFB3, SMAD2, or SMAD3.1 One of the major contributors to morbidity and mortality in LDS is aortic pathology including aortic aneurysms, dissection, and rupture.2 Clinical management is complicated by the significant phenotypic heterogeneity between patients. LDS-causing mutations have been associated with phenotypes ranging from normal to severe cardiovascular involvement in childhood requiring multiple surgical interventions.3
Recent studies highlighted important phenotypic differences between LDS genotypes, especially between the 2 most common LDS genotypes (TGFBR1 and TGFBR2). Aortic features differ between the 2 populations, with more aggressive aortic disease identified in patients with TGFBR2 mutations.4, 5, 6 These data are mainly based on adult LDS cohorts and suggest that there may be gene-dependent differences in the impact on aortic wall architecture and biophysical properties. LDS-associated mutations can also alter the cardiac extracellular matrix composition, which plays an important role in cardiac structure and function, but less is known about the impact of genotypical variation on cardiac structure and function.7
In this study, we aimed to further explore the relationship between genotype and cardiovascular phenotype in a paediatric cohort of LDS patients with TGFBR1 and TGFBR2 mutations.
Methods
Study population
Paediatric patients followed by our institution with a genetically confirmed diagnosis of LDS between January 2006 and June 2020 were included. Inclusion criteria were a clinically confirmed pathogenic or likely pathogenic variant of an LDS-associated mutation (TGFBR1 or TGFBR2) and at least 1 echocardiographic assessment with blood pressure, height, and weight measurements on the day of imaging. Current medications, family history, and the presence of other phenotypic presentations at the time of echocardiography were also collected. In applicable cases, echocardiographic assessment performed after surgical aortic repair or replacement was excluded. Children were excluded from the study if they had clinical features of LDS without genetic confirmation or had LDS with non-TGFBR1 or non-TGFBR2 mutations.
Echocardiographic assessments
Echocardiograms were performed according to a standardized clinical protocol based on the American Society of Echocardiography guidelines, including standard measurements of aortic dimensions, cardiac chamber dimensions, and systolic and diastolic function.8 The most recent echocardiographic measurements (presurgical, when applicable) were abstracted from clinical reports or measured from digitally stored images (SyngoDynamics; Siemens, Munich, Germany). Echocardiograms that had missing measurements in the reports were measured offline by a trained observer who was blinded to the participants’ clinical and genetic information. If multiple measurements of the same parameter were collected from the same echocardiogram, the average of these measurements was used in the analysis. Using standardized and validated methods,9 aortic measurements were collected from the parasternal short-axis view. Measurements of the descending aorta were collected from the suprasternal long-axis view. Aortic measurements and associated calculations of biophysical properties were obtained based on inner edge-to-inner edge measurements for the aortic root, sinuses of Valsalva, sinotubular junction, and ascending aorta. Left ventricular structural and functional measurements were collected from M-mode echocardiograms. Aortic biophysical properties were assessed by the calculating aortic distensibility, strain, and stiffness index based on the following formulae:9,10
where AOs is the systolic aortic dimension, AOd is the diastolic aortic dimension, ln is the natural logarithm, and SBP and DBP are the systolic and diastolic blood pressure, respectively.
Measurements of left ventricular dimensions included left ventricular end-diastolic dimension (LVEDD), left ventricular posterior wall end-diastole (LVPWD), and interventricular septum end-diastole (IVSD) were taken in M-mode. Left ventricular mass (LVM) was calculated using the following American Society of Echocardiography–recommended formula:11
Parameters of systolic function included ejection fraction (M-mode), fractional shortening, and mitral annulus tissue Doppler S′-velocity. Parameters of diastolic function included mitral valve E-wave velocity, A-wave velocity, A-wave time, and E-wave deceleration time. Isovolumetric relaxation time and septal and lateral mitral annulus tissue Doppler velocities (E′ and A′) were measured. The mitral E/A ratio and lateral and septal E/E′ ratio were calculated. z scores were calculated based on the formulas and values published by the Pediatric Heart Network (PHN).12
Statistical analyses
Continuous data were reported as means (±standard deviation) or medians (interquartile range [IQR]), depending on the distribution. Categorical data are presented as proportions (percentages) and were compared using Pearson’s χ2 test. Aortic measurements and left ventricular structural and functional measurements were compared using the unpaired Student t test or the Mann-Whitney Wilcoxon test, depending on the distribution. A P value of <0.05 was considered statistically significant. All statistical analyses were conducted using RStudio version 2023.03.1+446.
Results
Study population
In total, 32 patients met the eligibility criteria. According to the available genetic testing, patients were divided into either TGFBR1 (n = 17) or TGFBR2 (n = 15). Characteristics of each group are shown in Table 1. The median age at the time of echocardiographic imaging was 11.0 (IQR: 4.7-16.2) and 10.0 (IQR: 6.6-14.3) in the TGFBR1 and TGFBR2, respectively (P = 0.478). Both groups comprised more males (65% and 53%, respectively). Body mass index was not significantly different between the groups (P = 0.315).
Table 1.
Demographic, pharmacologic, and phenotypic parameters in 32 paediatric Loeys-Dietz syndrome (LDS) patients
| General characteristics | TGFBR1 (n = 17) | TGFBR2 (n = 15) | P |
|---|---|---|---|
| Demographics | |||
| Age at echo (y) | 11.0 (4.7-16.2) | 10.0 (6.6-14.3) | 0.478 |
| Sex, male | 11 (65) | 8 (53) | 0.769 |
| Height (cm) | 159 (111.5-174.0) | 143 (116.2-167.5) | 0.427 |
| Weight (kg) | 45.3 ± 30.2 | 35.7 ± 24.9 | 0.332 |
| BSA (m2) | 1.33 ± 0.57 | 1.15 ± 0.54 | 0.362 |
| Aortic surgery | |||
| History of aortic surgery | 2 (12) | 7 (47) | 0.057 |
| Aortic dissection | 0 (0) | 1 (14) | – |
| Age at aortic surgery (y) | 13.5 (12.3-14.7) | 6.0 (2.3-12.0) | 0.222 |
| ARB | 8 (47) | 14 (93) | 0.015 |
| β-Blocker | 7 (41) | 12 (80) | 0.061 |
| ACEi | 0 (0) | 1 (7) | 0.949 |
| None | 6 (35) | 1 (7) | 0.127 |
| Family history | |||
| Positive family history for LDS | 9 (53) | 4 (27) | 0.250 |
| Presence of other phenotypic characteristics | |||
| Arterial tortuosity | 12 (71) | 13 (87) | 0.272 |
| Craniofacial features | 3 (18) | 7 (47) | 0.077 |
| Pectus deformities | 3 (18) | 7 (47) | 0.077 |
| Dural ectasia | 4 (24) | 5 (33) | 0.538 |
| Scoliosis | 3 (18) | 5 (33) | 0.307 |
Data are presented as n (%), mean ± standard deviation, or median (interquartile range).
Bold values indicate P < 0.05.
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BSA, body surface area.
Significantly more patients in the TGFBR2 group had a history of aortic surgery than those in the TGFBR1 group (47% vs 12%, P = 0.057). One patient in the TGFBR2 group experienced an aortic dissection. Patients with TGFBR1 were more likely to undergo aortic surgery in late childhood, whereas the age at aortic surgery in patients with TGFBR2 was more variable (IQR: 12.3-14.7 years vs 2.3-12.0 years, respectively). Significantly more patients in the TGFBR2 group were treated with angiotensin receptor blockers (93% vs 47%, P = 0.015).
The presence of arterial tortuosity, craniofacial features, pectus deformities, dural ectasia, and scoliosis was similar between the groups. The list of each patient’s individual genetic variants is shown in Supplemental Table S1.
Aortic dimensions
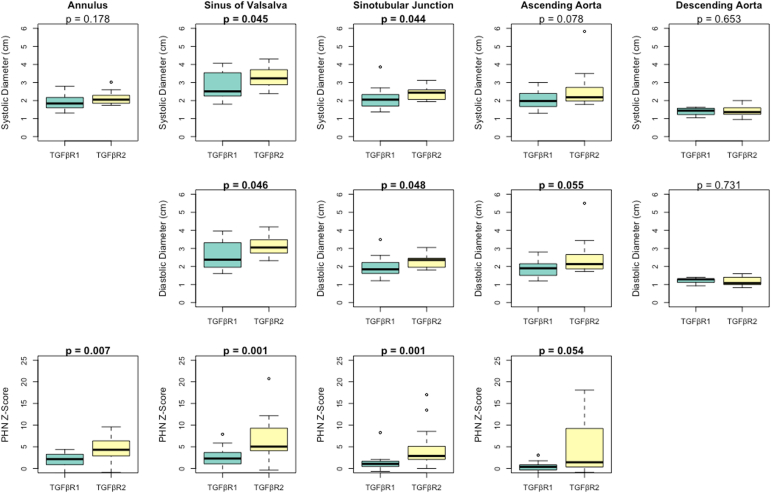
Aortic dimension results are summarized in Figure 1. Patients in the TGFBR2 group had significantly larger systolic diameters for the sinuses of Valsalva and sinotubular junction. The systolic dimensions were similar at the aortic valve annulus, ascending aorta, and descending aorta between the patients with TGFBR1 and TGFBR2.
Figure 1.
Aortic dimensions at the annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta in 32 paediatric Loeys-Dietz syndrome patients. PHN, Pediatric Heart Network.
Patients in the TGFBR2 group had significantly larger diastolic diameters for the sinuses of Valsalva and sinotubular junction. The diastolic diameter was similar for the ascending and descending aorta between patients with TGFBR1 and TGFBR2. The diastolic diameter of the aortic annulus was excluded as it is not clinically considered.
The PHN z score was significantly larger for patients with TGFBR2 at the level of the aortic annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta. There are no PHN z scores for the descending aorta to report.
Aortic biophysical properties
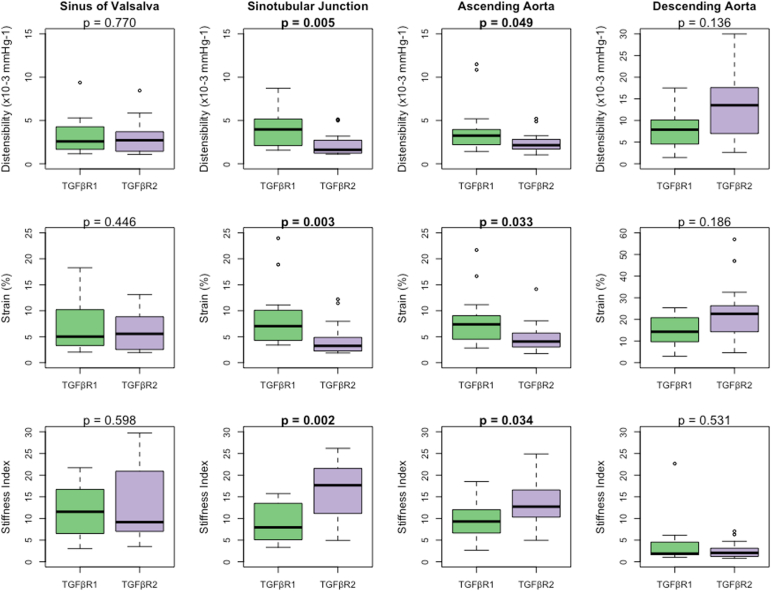
The results of aortic biophysical properties are summarized in Figure 2. Patients in the TGFBR2 group had significantly lower distensibility at the level of the sinotubular junction and ascending aorta. Distensibility was similar at the level of the sinuses of Valsalva and descending aorta between patients with TGFBR1 and TGFBR2.
Figure 2.
Aortic biophysical properties at the annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta in 32 paediatric Loeys-Dietz syndrome patients.
Patients in the TGFBR2 group had significantly lower strain in the sinotubular junction and ascending aorta. Strain was similar at the level of the sinuses of Valsalva and descending aorta between patients with TGFBR1 and TGFBR2.
Patients in the TGFBR2 group had significantly higher stiffness at the level of the sinotubular junction and ascending aorta. The stiffness index of the sinuses of Valsalva and descending aorta was similar between patients with TGFBR1 and TGFBR2.
Left ventricular structural and functional parameters
Our results did not show statistically significant differences for left ventricular dimensions between the TGFBR1 and TGFBR2 mutation groups (Table 2). The LVPWD z score was significantly higher for patients with TGFBR2 but was within a normal clinical range. Significantly more patients in the TGFBR2 group had aortic regurgitation when compared with the TGFBR1 group. There were no significant differences in left ventricular diastolic or systolic functional parameters between the 2 groups (Table 3).
Table 2.
Left ventricular structural parameters in 32 paediatric Loeys-Dietz syndrome patients
| Left ventricular structural parameters | TGFBR1 (n = 17) | TGFBR2 (n = 15) | P |
|---|---|---|---|
| Structural measurements | |||
| LVEDD (cm) | 4.32 ± 0.80 | 4.33 ± 0.78 | 0.958 |
| LVEDD, PHN z score | 0.08 ± 0.93 | 1.12 ± 1.92 | 0.071 |
| LVPWD (cm) | 0.58 ± 0.17 | 0.65 ± 0.12 | 0.204 |
| LVPWD, PHN z score | −0.26 (−1.36 to 0.62) | 0.52 (−0.05 to 1.01) | 0.011 |
| IVSD (cm) | 0.65 ± 0.14 | 0.63 ± 0.19 | 0.721 |
| IVSD, PHN z score | 0.20 ± 0.89 | 0.29 ± 1 | 0.806 |
| LVM (g) | 87.14 ± 46.76 | 89.24 ± 53.88 | 0.453 |
| LVM, PHN z score | −5.79 ± 0.03 | −5.79 ± 0.03 | 0.824 |
| LVM indexed to BSA (g/m2) | 63.41 (56.7-72.7) | 68.54 (60.9-87.7) | 0.278 |
| LVM indexed to height (g/mm2) | 0.56 ± 0.21 | 0.62 ± 0.28 | 0.515 |
| Valve function | |||
| Aortic regurgitation | 2 (12) | 8 (53) | 0.032 |
| Mitral valve regurgitation | 0 | 2 (13) | 0.410 |
| Mitral valve prolapse | 0 | 2 (13) | 0.410 |
Data are presented as n (%), mean ± standard deviation, or median (interquartile range).
Bold values indicate P < 0.05.
BSA, body surface area; IVSD, interventricular septum end-diastole; LVEDD, left ventricular end-diastolic diameter; LVM, left ventricular mass; LVPWD, left ventricular posterior wall end-diastole; PHN, Pediatric Heart Network.
Table 3.
Left ventricular diastolic and systolic parameters in 32 paediatric Loeys-Dietz syndrome patients
| Left ventricular functional parameters | TGFBR1 (n = 17) | TGFBR2 (n = 15) | P |
|---|---|---|---|
| Diastolic function | |||
| Mitral valve E-velocity (cm/s) | 83 (72.6-90.8) | 75 (72.0-89.5) | 0.850 |
| Mitral valve A-velocity (cm/s) | 43.8 ± 7.38 | 45.2 ± 12.7 | 0.733 |
| Mitral valve E/A ratio | 1.96 ± 0.56 | 1.97 ± 0.77 | 0.967 |
| Mitral valve E′-velocity (cm/s) | 16.3 ± 3.09 | 14.9 ± 3.52 | 0.279 |
| Mitral valve A′-velocity (cm/s) | 5.0 (4.2-7.0) | 6.5 (5.6-7.3) | 0.129 |
| Mitral valve E/E′ ratio | 4.81 (4.5-5.8) | 5.29 (4.8-5.5) | 0.451 |
| Isovolumetric relaxation time (ms) | 65.9 ± 15.4 | 57.9 ± 16.1 | 0.208 |
| Mitral valve deceleration time (ms) | 134 (116-169) | 143 (119.2-176.0) | 0.905 |
| Mitral valve A-wave time (ms) | 106.5 (96.5-148.5) | 106 (92.0-139.5) | 0.828 |
| Systolic function | |||
| Mitral valve S′-velocity (cm/s) | 10.0 (8.0-11.5) | 10.5 (8.1-12.1) | 0.380 |
| Ejection fraction (%) | 66.1 ± 5.41 | 67.7 ± 4.90 | 0.362 |
| Shortening fraction (%) | 35.1 (32.2-38.5) | 36.4 (33.6-39.4) | 0.484 |
Data are presented as mean ± standard deviation or median (interquartile range).
Discussion
In this study, we report the genotype-phenotype interactions in aortic dimensions and biophysical properties, left ventricular morphology, and left ventricle functional parameters in paediatric LDS patients with either TGFBR1 or TGFBR2 mutations. We demonstrate that at a young age, patients with TGFBR2 mutations have a more clinically unfavourable course requiring earlier surgical interventions and have significant differences in aortic dimensions and biophysical properties.
Genotype considerations in cardiac clinical management
Recent studies have highlighted important phenotypic differences between the 2 most commonly detected LDS genotypes (TGFBR1 and TGFBR2). In comparing the largest international cohort of adult LDS patients with either a TGFBR1 or TGFBR2 mutation, the Montalcino Aortic Consortium suggests that aortic features may differ between the 2 populations, with more aggressive aortic disease in patients with TGFBR2 mutations.4 Another group reported that aortic dissections occurred with minimal aortic root enlargement in patients with TGFBR2 mutations, whereas in patients with TGFBR1 mutation, dissections were only reported in association with significant aortic enlargement.6 When compared with patients with TGFBR1, adult LDS patients with the TGFBR2 mutation also have significantly higher cumulative risk of aortic events.5 These studies suggest that LDS patients harbouring TGFBR2 mutations manifest a distinct trajectory of disease progression, underscoring the imperative need for tailored and genotype-specific cardiac clinical management recommendations.
Our findings in a paediatric cohort are consistent with the existing literature. Our study illustrates that intrinsic variations in disease progression among LDS genotypes are extendable to paediatric populations, with a pronounced impact on the prevalence of aortic surgery, pharmacologic management, aortic dimensions, and aortic biophysical properties. This underscores the concept that distinct disease trajectories begin to manifest at an early age in individuals with LDS, emphasizing the significance of initiating genotype-specific clinical management strategies during the paediatric phase of care. Furthermore, our findings highlight the necessity for more close clinical monitoring of LDS patients with TGFBR2 mutations, even when their aortic measurements seemingly align with clinically accepted ranges. This approach to clinical management is warranted given our findings, coupled with previous research, which suggests that patients with TGFBR2 mutations may deviate from the conventional disease progression trends currently postulated for LDS patients.
Aortic dimensions and biophysical properties in prophylactic surgical planning
In this study, we delineated based on genotypes (TGFBR1 or TGFBR2 mutations) in LDS patients. First, considering only structural dimensions and using z scores to account for differences in body surface area, our findings show that PHN z scores were significantly higher in TGFBR2 patients compared with TGFBR1 at the level of the aortic annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta. These findings confirm a more unfavourable aortic disease progression as previously reported in LDS patients with TGFBR2 mutations.4, 5, 6
Aortic biophysical properties differed between the LDS genotypes. Previous studies comparing heterogeneous cohorts of Marfan syndrome and/or LDS patients with healthy controls have reported differences in aortic biophysical properties including lower aortic wall distensibility and increased aortic stiffness.13, 14, 15 Our data showed that these differences are applicable even when delineating between LDS genotypes. The differences in aortic distensibility, strain, and stiffness between the mutation types were observed at the level of the sinotubular junction and ascending aorta, but not at the level of the sinuses of Valsalva despite clinically significant increases in PHN z scores. This indicates that changes to aortic mechanical properties may precede aortic remodelling, suggesting that aortic biophysical properties may have independent predictive value for aortic disease progression. Indeed, lower aortic root strain and high aortic root stiffness are associated with a higher rate of aortic root dilation and surgical aortic root replacement. These associations are independent of the aortic root z score.16 It is important to note that our conclusions are speculative for 2 reasons. First, the biophysical properties are calculated using the same aortic dimensions and thus are innately correlated. Second, measuring larger and/or effaced aortic structures may lead to increased measurement variability. Longitudinal data are needed to evaluate the clinical applicability and significance of our observations.
Existing evidence suggests that the phenotypic manifestations of LDS in adults extend beyond the confines of the aortic root and sinuses of Valsalva, encompassing the sinotubular junction and ascending aorta as well.17,18 Our study demonstrates that the persistence of such manifestations extends to paediatric cohorts. This is consistent with previous paediatric surgical case reports.19,20 In the absence of paediatric-specific directives, the clinical management of paediatric LDS patients currently relies on adult guidelines, which focus predominantly on the surveillance of aortic root and sinus of Valsalva dimensions. Clinical guidelines by the American Heart Association and American College of Cardiology Joint Committee recommend prophylactic surgical interventions at aortic root diameters of ≥4.0 cm or 4.5 cm depending on the presence of other high-risk features.21 Although the risk for aortic dissection escalates at or beyond these dimensions in LDS, instances of aortic dissection have been documented in patients with aortic root dimensions ranging from 3.9 to 4.0 cm.22 Data from a single American centre on 11 adult LDS patients who experienced acute type A aortic dissections reported preoperative aortic root measurements ranging from 3.2 to 6.8 cm.23 Evidently, aortic dissections can occur in a broad range of aortic root diameters, and clinical guidelines for the risk stratification of LDS patients should not be primarily size dependent.
Although TGFBR1 and TGFBR2 mutations both impact the intracellular domain of their respective receptors and result in TGFβ oversignalling, nuanced variations in their downstream molecular pathogenesis result in distinct phenotypic presentations.24 Medial degeneration of the aorta, known as cystic medial necrosis, leads to pathological remodelling of aortic structure and is associated with a higher risk of aortic events.25 Indeed, pathohistologic analysis of aortic specimens from LDS patients demonstrated that patients with TGFBR2 mutations had significantly higher grades of cystic medial necrosis than those with TGFBR1 mutations.26 Considering these differential genetic mechanisms impacting aortic disease progression in LDS, it is likely that our patients in the TGFBR1 and TGFBR2 cohorts present distinct aortic structural arrangements that impact aortic biophysical properties and consequently the rate of dilatation. Our findings underscore the need for genotype-specific clinical management strategies of paediatric patients that extend beyond aortic root dimensions, encompassing a complete evaluation from the root to the ascending aorta and considering the inclusion of aortic biophysical properties. Such a comprehensive approach could be useful in the clinical management of aortopathy populations. Further research is required to determine whether aortic biophysical properties can improve long-term outcomes by accurately predicting and reducing the incidents of aortic events.
Impact of genotype on left ventricle morphologic and functional parameters
In the adult LDS population, there have been reports of impaired systolic function, cardiomyopathy, and/or heart failure.27,28 There is ongoing debate on whether primary cardiomyopathy exists in LDS patients, as cardiac remodelling can be attributed secondary to aortic dilation and/or valvular disease.29 Cardiac magnetic resonance imaging studies in LDS patients found increased extracellular volume, suggesting increased diffuse myocardial fibrosis in both paediatric and adult patients.27,30
Despite more frequent aortic regurgitation in patients with TGFBR2 compared with those with TGFBR1 mutations, we report no significant differences in left ventricular size and wall thickness between the groups. Furthermore, there were no echocardiographic differences in systolic and diastolic function between the groups. The occurrence of mitral valve prolapse was also similar between the groups. Further longitudinal studies will need to determine the impact of the 2 different genotypes on myocardial phenotype.
Limitations
The retrospective, single-centre design of the present study combined with the small sample size of the mutation groups is an inherent limitation. In addition, measuring larger and/or effaced aortic structures may lead to increased measurement variability and/or altered measurement accuracy. Finally, patients in this study were diagnosed and treated over a span of 14 years (2006-2020), during which standards of care and echocardiographic techniques were (and still are) evolving.
Conclusions
Our study demonstrates that during childhood, LDS patients with TGFBR2 mutations exhibit a more severe aortic phenotype than those with TGFBR1 mutations. This is evidenced by increased likelihood of aortic surgery, increased use of pharmacologic therapy, larger aortic dimensions, and differences in aortic biophysical properties. We did not observe differences in left ventricular morphology or function between the groups.
These findings underscore the importance of tailored LDS clinical management strategies based on genotypes, especially for patients with TGFBR2. Future research with larger paediatric cohorts and longitudinal follow-up is needed to better understand the role of aortic biophysical properties in the cardiovascular disease progression in paediatric LDS patients.
Acknowledgments
Ethics Statement
This study was approved by the institutional research ethics board (approval number: 1000071940).
Patient Consent
The authors confirm that patient consent is not applicable to this article. This is a retrospective study using de-identified data with a waiver of consent approved by the Research Ethics Board (REB).
Funding Sources
This study was partially funded by the SickKids Labatt Family Heart Center Innovation grant. NK received stipend support from the Loeys-Dietz SyndromeFoundation of Canada (LDSFC), Institute of Medical Science at the University of Toronto, and Queen Elizabeth II/Heart & Stroke Foundation of Ontario Graduate Scholarships in Science and Technology.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
To access the supplementary material accompanying this article, visit CJC Pediatric and Congenital Heart Disease at https://www.cjcpc.ca// and at https://doi.org/10.1016/j.cjcpc.2023.12.003
Supplementary Material
References
- 1.Schepers D., Tortora G., Morisaki H., et al. A mutation update on the LDS-associated genes TGFB2/3 and SMAD2/3. Hum Mutat. 2018;39:621–634. doi: 10.1002/humu.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeys B.L., Chen J., Neptune E.R., et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 3.Camerota L., Ritelli M., Wischmeijer A., et al. Genotypic categorization of Loeys-Dietz syndrome based on 24 novel families and literature data. Genes. 2019;10:764. doi: 10.3390/genes10100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jondeau G., Ropers J., Regalado E., et al. International registry of patients carrying TGFBR1 or TGFBR2 mutations: results of the MAC (Montalcino Aortic Consortium) Circ Cardiovasc Genet. 2016;9:548–558. doi: 10.1161/CIRCGENETICS.116.001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regalado E.S., Morris S.A., Braverman A.C., et al. Comparative risks of initial aortic events associated with genetic thoracic aortic disease. J Am Coll Cardiol. 2022;80:857–869. doi: 10.1016/j.jacc.2022.05.054. [DOI] [PubMed] [Google Scholar]
- 6.Tran-Fadulu V., Pannu H., Kim D.H., et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet. 2009;46:607–613. doi: 10.1136/jmg.2008.062844. [DOI] [PubMed] [Google Scholar]
- 7.Parikh M., Seidman M., Oudit G.Y. Critical role of extracellular matrix remodelling in patients with dilated cardiomyopathy: lessons from connective tissue disorders. Can J Cardiol. 2022;38:309–310. doi: 10.1016/j.cjca.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Lai W.W., Geva T., Shirali G.S., et al. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19:1413–1430. doi: 10.1016/j.echo.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Grattan M.J., Mertens L. Echocardiographic assessment of ventricular function in pediatric patients: a comprehensive guide. Future Cardiol. 2014;10:511–523. doi: 10.2217/fca.14.41. [DOI] [PubMed] [Google Scholar]
- 10.Akazawa Y., Motoki N., Tada A., et al. Decreased aortic elasticity in children with Marfan syndrome or Loeys-Dietz syndrome. Circ J. 2016;80:2369–2375. doi: 10.1253/circj.CJ-16-0739. [DOI] [PubMed] [Google Scholar]
- 11.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Lopez L., Colan S., Stylianou M., et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.117.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camarda J.A., Earing M.G., Dholakia R.J., et al. Biophysical properties of the aorta in patients with Marfan syndrome and related connective tissue disorders: evaluation with MRI and computational fluid dynamics modeling. J Cardiovasc Magn Reson. 2011;13:P219. [Google Scholar]
- 14.Munoz A.R., Guala A., Rodriguez Palomares J., et al. Aortic stiffness and hemodynamics in Loeys-Dietz syndrome by 4D flow CMR: a comparison with healthy volunteers and patients with Marfan syndrome. Eur Heart J. 2020;41:2336. [Google Scholar]
- 15.Cui J.Z., Harris K.C., Raedschelders K., et al. Aortic dimensions, biophysical properties, and plasma biomarkers in children and adults with Marfan or Loeys-Dietz syndrome. CJC Open. 2021;3:585–594. doi: 10.1016/j.cjco.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prakash A., Adlakha H., Rabideau N., et al. Segmental aortic stiffness in children and young adults with connective tissue disorders: relationships with age, aortic size, rate of dilation, and surgical root replacement. Circulation. 2015;132:595–602. doi: 10.1161/CIRCULATIONAHA.114.014934. [DOI] [PubMed] [Google Scholar]
- 17.Beckmann E. Is prophylactic aortic arch replacement justified in patients with Loeys-Dietz syndrome who present for ascending repair? J Card Surg. 2022;37:3693–3694. doi: 10.1111/jocs.16848. [DOI] [PubMed] [Google Scholar]
- 18.Weininger G., Zafar M., Ziganshin B.A., et al. Long-term risk of arch complications in Loeys Dietz syndrome patients undergoing proximal ascending aortic replacement. J Card Surg. 2022;37:3688–3692. doi: 10.1111/jocs.16855. [DOI] [PubMed] [Google Scholar]
- 19.Jaiswal P., Shetty V., Patel E., Shetty D. Repair of an aneurysm of the ascending aorta and arch in an infant with Loeys-Dietz syndrome. J Card Surg. 2018;33:286–288. doi: 10.1111/jocs.13605. [DOI] [PubMed] [Google Scholar]
- 20.Ozker E., Vuran C., Saritas B., Türköz R. Valve-sparing replacement of the ascending aorta and aortic arch in a child with Loeys-Dietz syndrome. Eur J Cardiothorac Surg. 2012;41:1184–1185. doi: 10.1093/ejcts/ezr235. [DOI] [PubMed] [Google Scholar]
- 21.Isselbacher E.M., Preventza O., Hamilton Black J., et al. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e334–e482. doi: 10.1161/CIR.0000000000001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacCarrick G., Black J.H., Bowdin S., et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16:576–587. doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams J.A., Hanna J.M., Shah A.A., et al. Adult surgical experience with Loeys-Dietz syndrome. Ann Thorac Surg. 2015;99:1275–1281. doi: 10.1016/j.athoracsur.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Loeys B.L., Dietz H.C. GeneReviews; 2018. Loeys-Dietz syndrome summary; pp. 1–32. [Google Scholar]
- 25.Fujiyoshi T., Minatoya K., Ikeda Y., et al. Impact of connective tissue disease on the surgical outcomes of aortic dissection in patients with cystic medial necrosis. J Cardiothorac Surg. 2017;12:97. doi: 10.1186/s13019-017-0663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seike Y., Matsuda H., Ishibashi-Ueda H., et al. Surgical outcome and histological differences between individuals with TGFBR1 and TGFBR2 mutations in Loeys-Dietz syndrome. Ann Thorac Cardiovasc Surg. 2021;27:56–63. doi: 10.5761/atcs.oa.20-00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokota T., Koiwa H., Matsushima S., et al. Loeys-Dietz cardiomyopathy? Long-term follow-up after onset of acute decompensated heart failure. Can J Cardiol. 2022;38:389–391. doi: 10.1016/j.cjca.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Eckman P.M., Hsich E., Rodriguez E.R., et al. Impaired systolic function in Loeys-Dietz syndrome. Circ Heart Fail. 2009;2:707–708. doi: 10.1161/CIRCHEARTFAILURE.109.888636. [DOI] [PubMed] [Google Scholar]
- 29.Alpendurada F., Wong J., Kiotsekoglou A., et al. Evidence for Marfan cardiomyopathy. Eur J Heart Fail. 2010;12:1085–1091. doi: 10.1093/eurjhf/hfq127. [DOI] [PubMed] [Google Scholar]
- 30.Karur G.R., Pagano J.J., Bradley T., et al. Diffuse myocardial fibrosis in children and adolescents with Marfan syndrome and Loeys-Dietz syndrome. J Am Coll Cardiol. 2018;72:2279–2281. doi: 10.1016/j.jacc.2018.07.095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.