Abstract
Sodium-glucose cotransporter-2 (SGLT2) inhibitors, originally approved for type 2 diabetes mellitus, have demonstrated efficacy in reducing cardiovascular events, particularly heart failure, in patients with and without diabetes. An intriguing research area involves exploring the potential application of SGLT2 inhibitors in cardio-oncology, aiming to mitigate the cardiovascular adverse events associated with anticancer treatments. These inhibitors present a unique dual nature, offering both cardioprotective effects and anticancer properties, conferring a double benefit for cardio-oncology patients. In this review, the authors first examine the established cardioprotective effects of SGLT2 inhibitors in heart failure and subsequently explore the existing body of evidence, including both preclinical and clinical studies, that supports the use of SGLT2 inhibitors in the context of cardio-oncology. The authors further discuss the mechanisms through which SGLT2 inhibitors protect against cardiovascular toxicity secondary to cancer treatment. Finally, they explore the potential anticancer effects of SGLT2 inhibitors along with their proposed mechanisms.
Key Words: anthracyclines, cancer, cardio-oncology, cardiotoxicity, SGLT2 inhibitors
Central Illustration
Highlights
-
•
Observational studies suggest sodium-glucose cotransporter-2 (SGLT2) inhibitors protect from cardiotoxic chemotherapy.
-
•
Preclinical studies show cardioprotective and anticancer effects of SGLT2 inhibitors.
-
•
Randomized clinical trials are warranted to test SGLT2 inhibitors in cardio-oncology.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors were originally developed to address type 2 diabetes mellitus (T2DM) by inhibiting glucose reabsorption in the renal proximal tubules.1 Beyond SGLT2 inhibitors’ glucose-lowering effects, extensive studies have highlighted their remarkable cardioprotective properties in patients, regardless of diabetes status.2, 3, 4, 5, 6 Currently, 2 SGLT2 inhibitors, empagliflozin and dapagliflozin, have gained approval for treating heart failure (HF) with reduced ejection fraction and HF with preserved ejection fraction.7, 8, 9, 10 Emerging evidence suggests that SGLT2 inhibitors exert direct protective effects on the cardiovascular system, independently of glycemic control.11 These effects encompass improved cardiac metabolism, reduced oxidative stress, modulation of neurohormonal pathways, attenuation of myocardial inflammation, and preservation of endothelial function.12, 13, 14 Such multifaceted mechanisms underscore their potential as therapeutic agents in various cardiac conditions.
One area of particular interest involves exploring the potential protective effects of SGLT2 inhibitors in mitigating cancer treatment–related cardiotoxicity and their role in cardio-oncology. Cardio-oncology is a clinical and scientific discipline that focuses on diagnosing, preventing, treating, and monitoring the cardiovascular consequences of cancer and its therapies.15 With the growing number of cancer survivors and the ongoing use of cardiotoxic cancer therapies, such as anthracyclines, proteasome inhibitors, and tyrosine kinase inhibitors, there is a pressing need for cardioprotective strategies. A plethora of preclinical studies, along with a growing number of observational clinical studies, have suggested promising cardioprotective roles for SGLT2 inhibitors in mitigating cancer treatment–related cardiotoxicity.16, 17, 18, 19, 20, 21
In the pursuit of improving the cardiovascular health of patients with cancer, it is crucial to develop cardioprotective agents that can effectively protect the heart without compromising the anticancer effects of cancer treatments. In this regard, several preclinical studies have demonstrated potential anticancer effects of SGLT2 inhibitors in various types of cancer, including breast, prostate, colorectal, and bladder cancer.22, 23, 24, 25 This growing preclinical evidence, highlighting the anticancer properties of SGLT2 inhibitors, further underscores their significance as promising cardioprotective agents against cancer therapy–related cardiotoxicity.
In this state-of-the-art review, we aim to underscore the potential role of SGLT2 inhibitors in cardio-oncology (Central Illustration). We begin with a discussion of the cardioprotective effects of SGLT2 inhibitors in HF. Next, we provide a comprehensive overview of the current preclinical research landscape and emerging clinical evidence pertaining to the use of SGLT2 inhibitors in cardio-oncology. We also discuss the mechanisms by which SGLT2 inhibitors may protect the cardiovascular system against the toxicity induced by cancer treatments. Finally, we explore the preclinical studies that demonstrate the potential anticancer effects of SGLT2 inhibitors, both as stand-alone therapies and in combination with other cancer treatments.
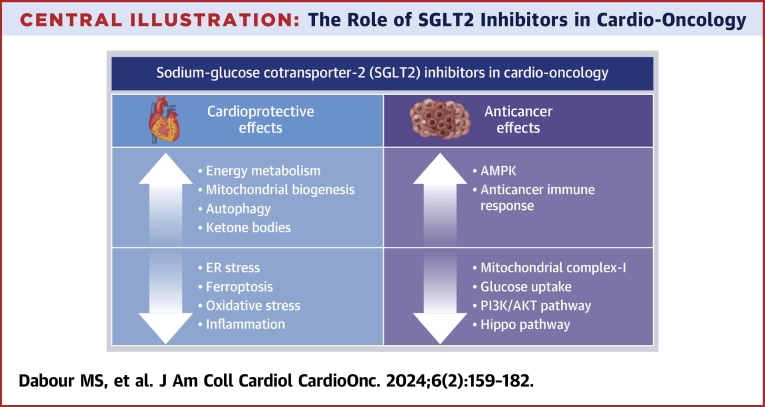
Central Illustration.
The Role of SGLT2 Inhibitors in Cardio-Oncology
This illustration summarizes both the cardioprotective and the anticancer mechanisms of sodium-glucose cotransporter-2 (SGLT2) inhibitors. AMPK = adenosine monophosphate–activated protein kinase; ER = endoplasmic reticulum; PI3K = phosphoinositide 3-kinase.
SGLT2 Inhibitors in HF
The serendipitous discovery of the cardioprotective effects of SGLT2 inhibitors originated from the EMPA-REG OUTCOME (BI 10773 [Empagliflozin] Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) trial. In this study, an unexpected 35% relative risk reduction in hospitalization for HF was observed in patients with T2DM and established cardiovascular disease treated with empagliflozin.2 Further insights into the cardiovascular benefits of 2 more SGLT2 inhibitors, canagliflozin and dapagliflozin, were gained from comprehensive cardiovascular outcome trials: the CANVAS (Canagliflozin Cardiovascular Assessment Study) program, which focused on patients with T2DM and established cardiovascular disease, and DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events–Thrombolysis In Myocardial Infarction 58), which focused on patients with T2DM at risk for cardiovascular disease.4,6 Both trials affirmed the benefits of SGLT2 inhibitors on hospitalization for HF, the composite of HF hospitalization or cardiovascular death, and renal composite outcomes. The clinical assessment of these agents extended to patients with HF as well as those with chronic kidney disease, regardless of their diabetic status. Both empagliflozin and dapagliflozin demonstrated efficacy in HF with reduced ejection fraction, as evidenced by reductions in the composite of HF hospitalization and cardiovascular death in the EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction) and DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) trials, respectively.3,5 Finally, the use of empagliflozin and dapagliflozin, supported by the findings of the EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction)26 and DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure)27 trials, respectively, was expanded to encompass the treatment of patients with HF with preserved ejection fraction. SGLT2 inhibitors also slowed the rate of kidney function deterioration while improving HF outcomes in patients with chronic kidney disease.28, 29, 30 These renal-protective effects may contribute to the overall effectiveness of SGLT2 inhibitors in patients with HF.
Highlights
-
•
SGLT2 inhibitors, initially developed for diabetes, have emerged as a therapy for HF and chronic kidney disease.
SGLT2 Inhibitors in Cardio-Oncology
Cardio-oncology
SGLT2 inhibitors, as previously discussed, have demonstrated improvement in cardiac outcomes in patients with HF, irrespective of diabetes. This suggests their potential role in cardio-oncology by improving cardiac outcomes in patients with cancer treated with cardiotoxic cancer therapies.
After recurrence of the primary cancer, cardiovascular disease emerges as the second leading cause of morbidity and mortality in cancer survivors.31 Anticancer treatments are associated with serious cardiovascular adverse events, including HF, hypertension, arrhythmias, and coronary artery disease. Among these treatments, anthracyclines, widely used in the treatment of patients with various cancer types, posed the highest risk for cardiotoxicity.32 Radiotherapy was associated with dose-dependent cardiac injury, potentially resulting in premature death because of ischemia, valvular disorders, or pericardial injury.33 Trastuzumab caused cardiotoxicity of varying severity, ranging from asymptomatic decline in left ventricular (LV) ejection fraction to symptomatic HF, an effect that can worsen when combined with anthracyclines.34 Patients undergoing cancer treatment with tyrosine kinase inhibitors such as sunitinib35 and immune checkpoint inhibitors such as nivolumab and ipilimumab also experienced cardiovascular adverse effects, including hypertension and HF.36 Similarly, carfilzomib was associated with hypertension, HF, and ischemia in more than 18% of patients.37 Therefore, it is critical to discover new therapeutic agents capable of preventing and/or reversing the cardiovascular complications arising from cancer treatments, all while preserving the essential therapeutic benefits against cancer. SGLT2 inhibitors have demonstrated promising potential in this regard; this is further detailed in subsequent discussion.
Clinical studies supporting the use of SGLT2 inhibitors in cardio-oncology
Emerging clinical evidence from retrospective observational studies supports the use of SGLT2 inhibitors in patients with cancer undergoing potentially cardiotoxic therapies, especially doxorubicin (DOX) (Table 1). A recent observational retrospective cohort study investigated the effectiveness and safety of SGLT2 inhibitors in patients with cancer and diabetes mellitus who were treated with anthracyclines.16 This study included 32 case patients on SGLT2 inhibitors during anthracycline treatment and 96 control patients treated with anthracyclines without SGLT2 inhibitors. The most common cancer type in both groups was lymphoma, followed by breast cancer. Over a median follow-up period of 1.5 years, SGLT2 inhibitors were associated with a lower rate of cardiac events, such as HF admissions and cardiomyopathy, compared with patients who did not receive SGLT2 inhibitors. Additionally, SGLT2 inhibitors appeared to be safe in this population, demonstrating improved overall mortality and lower rates of sepsis and neutropenic fever.
Table 1.
Clinical Studies Supporting the Use of SGLT2 Inhibitors in Cardio-Oncology
| First Author (Year) | Study Type | Population | Study Groups | Treatments | Key Findings |
|---|---|---|---|---|---|
| Gongora et al (2022)16 | Observational retrospective cohort study | Patients with DM and cancer treated with anthracyclines, aged >18 y, and patients with prior HF | Cases (n = 32): patients with DM and cancer on SGLT2 inhibitors during anthracycline treatment Control (n = 96): patients with DM cancer on anthracycline treatment without SGLT2 inhibitors |
CANA (34% [n = 11]), DAPA (16% [n = 5]), EMPA (50% [n = 16]) | Patients on SGLT2 inhibitors had ↓ cardiac events after anthracycline therapy, including ↓ HF admissions and a ↓ rate of cardiac dysfunction No new cases of anthracycline-induced cardiac dysfunction were observed in patients taking SGLT2 inhibitors |
| Abdel-Qadir et al (2023)18 | Observational population-based cohort study using medical records data sets | Patients aged ≥65 y with treated diabetes, no prior HF, receiving anthracycline-based chemotherapy for cancer | SGLT2 inhibitor–treated patients (n = 99) SGLT2 inhibitor–unexposed patients (n = 834) |
CANA, DAPA, EMPA | SGLT2 inhibitor exposure ↓ risk of HF hospitalization, but no significant difference in incident HF diagnosis SGLT2 inhibitor use was associated with a statistically nonsignificant ↓ rate of mortality |
| Avula et al (2024)38 | Retrospective cohort analysis of deidentified, aggregated patient data | Patients aged ≥18 y with histories of T2DM, cancer, and exposure to potentially cardiotoxic antineoplastic therapies, with subsequent diagnoses of cardiomyopathy or HF | Patients on SGLT2 inhibitors (n = 640) SGLT2 inhibitor–unexposed patients (n = 640), after propensity score matching |
CANA, DAPA, EMPA | Patients on SGLT2 inhibitors had ↓ risk of acute HF exacerbation and all-cause mortality Less frequent all-cause hospitalizations or emergency department visits, atrial fibrillation/flutter, acute kidney injury, and need for renal replacement therapy in patients on SGLT2 inhibitors |
CANA = canagliflozin; DAPA = dapagliflozin; DM = diabetes mellitus; EMPA = empagliflozin; HF = heart failure; SGLT2 = sodium-glucose cotransporter-2; T2DM = type 2 diabetes mellitus; ↑ = increase; ↓ = decrease.
Similarly, a retrospective cohort study based on population data used administrative data sets encompassing individuals ≥65 years of age, treated for diabetes, and undergoing anthracycline treatment between 2016 and 2019.18 During a median follow-up period of 1.6 years, the study included 933 patients, with 99 receiving SGLT2 inhibitors and 834 without SGLT2 inhibitors. The predominant cancer types in both groups were breast cancer and lymphoma. This study revealed that the use of SGLT2 inhibitors was associated with a lower rate of hospitalization for HF, but not incident HF, after anthracycline chemotherapy in older patients with cancer.
A recent retrospective study assessed the efficacy of SGLT2 inhibitors in patients experiencing cardiac dysfunction due to cancer therapy, including anthracyclines, alkylating agents, antimetabolites, monoclonal antibodies, tyrosine kinase inhibitors, proteasome inhibitors, and radiation therapy.38 The study focused on patients ≥18 years of age with histories of T2DM, cancer, and exposure to potentially cardiotoxic antineoplastic therapies, with subsequent diagnoses of cardiomyopathy or HF between 2013 and 2020. The study cohort included 1,280 patients experiencing cardiac dysfunction or HF related to cancer therapy (640 in each group, categorized on the basis of SGLT2 inhibitor use). Hematologic cancer was the most common, followed by gastrointestinal cancer, breast cancer, and lymphoma. Over a 2-year follow-up period, patients on SGLT2 inhibitors exhibited a lower risk for acute HF exacerbation and all-cause mortality, along with fewer all-cause hospitalizations or emergency department visits, incidences of atrial fibrillation or flutter, acute kidney injury, and the need for renal replacement therapy.
These findings suggest that SGLT2 inhibitors may be beneficial in reducing the risk for cardiotoxicity in patients with cancer. Nevertheless, randomized clinical trials are needed to determine the efficacy and risks of SGLT2 inhibitors in mitigating cardiotoxicity for patients undergoing cardiotoxic therapies. To this end, the EMPACT (Empagliflozin in the Prevention of Cardiotoxicity in Cancer Patients Undergoing Chemotherapy Based on Anthracyclines) trial, a randomized, multicenter, placebo-controlled, double-blind clinical trial (NCT05271162), is currently underway. This trial aims to determine the efficacy of empagliflozin in preventing LV systolic dysfunction in patients with cancer exposed to high cumulative doses of anthracyclines.
Highlights
-
•
Retrospective observational clinical studies suggest that SGLT2 inhibitors are associated with lower cardiac events and improved survival in patients with cancer receiving potentially cardiotoxic therapies.
-
•
There is an important need for prospective randomized clinical trials to determine the safety and efficacy of leveraging SGLT2 inhibitors in cardio-oncology.
Preclinical studies supporting the use of SGLT2 inhibitors in cardio-oncology
Subsequent to landmark studies demonstrating the cardioprotective effects of SGLT2 inhibitors in HF, numerous in vivo (Table 2) and in vitro (Table 3) studies, starting in 2019, have underscored the cardioprotective roles of SGLT2 inhibitors in mitigating cardiotoxicity induced by various cancer treatments, including DOX,17,39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 sunitinib,20 trastuzumab,21 cisplatin,19 ipilmumab,54,55 ponatinib,56 and carfilzomib.57 DOX has received particular attention because of its status as the most cardiotoxic cancer treatment and its widespread use.32 Researchers have investigated several models of DOX cardiotoxicity, including acute39,42,43,48,51 and chronic17,39,41,46, 47, 48 cardiotoxicity, cardiorenal syndrome,40 and conditions involving diabetic44 and nondiabetic rodents. Empagliflozin17,20,21,39, 40, 41, 42, 43,49,55,56 and dapagliflozin44, 45, 46,48,50, 51, 52, 53, 54,56 have emerged as the most commonly used SGLT2 inhibitors, followed by canagliflozin.19,57 Notably, no in vivo studies have reported the protective effects of canagliflozin against DOX, with only 1 in vivo study reporting the cardioprotective effects of canagliflozin against cisplatin19 and an in vitro study demonstrating its efficacy against carfilzomib.57 In vitro studies involved different types of cardiomyocytes,21,47 as well as human aortic56 and human umbilical vein endothelial cells.57
Table 2.
In Vivo Studies Demonstrating the Cardioprotective Effects of SGLT2 Inhibitors Against Cancer Treatment–Related Cardiotoxicity
| First Author (Year) | Species/Strain | Anticancer Treatment | SGLT2 Inhibitor Treatment | Key Findings | Proposed Mechanisms |
|---|---|---|---|---|---|
| Oh et al (2019)39 | Male C57BL/6J mice | DOX (15 mg/kg, single dose, i.p.) | EMPA (0.03% in NCD) | ↑ FS ↓ Hypertrophic pathologic changes Preserved LV mass ↓ Perivascular and interstitial fibrosis |
↑ βOHB levels |
| DOX (2.5 mg/kg every other day for 12 d, i.p.) | |||||
| Yang et al (2019)40 | Male Sprague-Dawley rats with subtotal nephrectomy | DOX, CRS (7 mg/kg, once every 5 d for 20 d, i.p.) | EMPA (20 mg/kg/d, i.p., from day 24 after CRS induction) | ↑ EF ↓ LV remodeling ↓ BNP |
↓ NOX-1, NOX-2, and oxidized protein ↓ TNF-α, NF-κB, IL-1β, and MMP-9 ↓ Mitochondrial-Bax, cleaved caspase-3, and cleaved PARP ↓ TGF-β and Smad3 ↓ γ-H2AX, cytosolic cytochrome c ↑ Mitochondrial cytochrome c |
| Wang et al (2020)41 | Male C57BL/6 mice | DOX (5 mg/kg, once per week for 4 wk, i.v. and i.p.) | EMPA (50 mg/kg/d in NCD for 5 wk, 1 wk before DOX) | ↑ FS ↓ cTnT ↓ BNP ↑ Contractility, ↓ DNA damage ↓ Cardiac fibrosis |
↑ Autophagic flux with ↑ in autophagosomes ↓ Accumulation of autolysosomes ↑ Beclin 1 in the heart ↑ TLR9 and SIRT3 in the heart ↑ Formation of beclin 1–TLR9–SIRT3 complex |
| Sabatino et al (2020)17 | Male C57BL/6 mice | DOX (5 mg/kg per week for 5 wk, i.p.) | EMPA (10 mg/kg, daily for 5 wk, oral gavage) | Improved SBP and DBP ↑ FS, EF ↓ Myocardial fibrosis and remodeling ↓ cTnT |
↓ ERK activity |
| Baris et al (2021)42 | Male Sprague-Dawley rats | DOX (2.5 mg/kg every other day for 14 d, i.p.) | EMPA (10 mg/kg daily for 14 d, oral gavage) | ↓ Prolongation of QT and QTc intervals ↑ EF, FS Preserved LVEDD and LVESD ↓ Myofibril loss |
↑ Mitochondrial biogenesis probably by PGC-1α up-regulation |
| Quagliariello et al (2021)43 | Female C57BL/6 mice | DOX (2.17 mg/kg/d for 7 d, i.p.) | EMPA (10 mg/kg/d, 3 d before DOX, oral gavage) | ↑ EF, FS ↓ Cardiac fibrosis |
↓ Ferroptosis and MDA content in the heart ↓ XO ↓ IL-1β, IL-6, and IL-8 cardiac expression ↓ MyD88 and NLRP3 ↓ Cardiac procollagen 1α1, MMP-9 ↓ Apoptosis |
| Chen (2023)49 | Male Sprague-Dawley rats | DOX (2.5 mg/kg twice a week for 4 wk, i.p.) | EMPA (30 mg/kg/d for 4 wk, intragastric) | ↓ LVEDD and LVESD, ↑ EF and FS ↓ CK-MB and NT-proBNP |
↓ Oxidative stress (↑ SOD and CAT) ↑ Energy metabolism (↑ serum ATP/ADP) ↑ AMPK/SIRT1/PGC-1α pathway |
| Chang et al (2021)44 | Male Sprague-Dawley diabetic rats | DOX (5 mg/kg/wk for 4 wk, i.p.) | DAPA (10 mg/kg/d for 6 wk, oral) followed by DOX | ↑ EF, FS ↑ Survival ↓ Hemodynamic declines (+dP/dt) and (−dP/dt) ↓ Fibrosis |
↓ ER stress (GRP78, PERK, eIF-2α, ATF-4, and CHOP) ↓ Apoptosis (Bax and cleaved caspase-3, ↑ Bcl-2) |
| Chang et al (2022)46 | Male Sprague-Dawley rats | DOX (5 mg/kg/wk for 4 wk i.p.) | DAPA (10 mg/kg/d for 6 wk oral) | ↑ Survival, ↑ EF, FS, ↓ myocardial fibrosis ↓ Hemodynamic changes (Ves, Ved ESPVR, +dP/dt, −dP/dt, and tau) |
↓ Apoptosis (mechanisms in vitro) |
| Hsieh et al (2022)47 | Male Sprague-Dawley rats | DOX (3 mg/kg for 4 wk, i.p.) | DAPA (0.1 mg/kg/d for 5 d for 4 wk, oral gavage) | ↑ EF, FS | ↓ Smad3, BNP, α-SMA, and phosphorylated p38 (mechanisms in vitro) |
| Belen et al (2022)45 | Male Sprague-Dawley albino rats | DOX (2.5 mg/kg/d every other day, 6 doses, i.p.) | DAPA (1 mg/kg/d, oral gavage) | ↓ cTnT, pro-BNP, TNF-α, FGF-21 levels | |
| Kim et al (2022)48 | C57BL/6J mice | DOX (15 mg/kg single dose, i.p.) | DAPA (1 mg/kg/d, oral) + high ARNI (sacubitril/valsartan 68 mg/kg/d) or low ARNI (34 mg/kg/d) 1 d after DOX | DAPA + low ARNI was the best regarding improved survival rate, cardiac function, hemodynamic change, and kidney function ↑ EF, FS ↑ β-MHC and MyBPC3 ↓ NT-proANP ↓ ANP and BNP |
Improved metabolic flexibility in DOX-injected heart through PPAR signaling pathway ↑ Genes related to fatty acid transport, fatty acid oxidation, ketogenesis, and gluconeogenesis-modulating metabolic pathway, including glucose, ketone bodies, and fatty acids |
| DOX (2.5 mg/kg every 4 d for 24 d, cumulative dose, 15 mg/kg, i.p.) | |||||
| Hu et al (2023)50 | Male C57BL/6 mice | DOX (5 mg/kg, once a week for 4 wk, i.p.) | DAPA (1.5 mg/kg/d for 4 wk, oral gavage) | ↓ LVIDd and LVIDs, ↑ EF and FS ↓ Remodeling |
↓ NLRP3 inflammasome ↓ Inflammatory markers (IL-6, IL-1β, and IL-18) |
| Satyam et al (2023)51 | Female Wistar rats | DOX (20 mg/kg, single dose, i.p.) | DAPA (0.9 mg/kg/d for 8 d, oral gavage) | Reversed DOX ECG changes ↓ CK-MB, AST |
|
| Quagliariello et al (2023)52 | Female C57BL/6 mice | DOX (2.17 mg/kg for 10 d, i.p.) | DAPA (12 mg/kg/d, oral gavage) | ↑ EF | ↓ Cardiac NLRP3, MyD88, DAMPs, and NF-κB ↓ Inflammatory markers (IL-1β, IL-6, TNF-α, G-CSF, and GM-CSF) ↑ Phosphorylated AMPK ↓ Calgranulin S100 and galectine-3 ↓ Myocardial and renal NF-κB |
| Ulusan et al (2023)53 | Male Wistar albino rats | DOX (2.15 mg/kg, every 3 d for 21 d, i.p.) | DAPA (10 mg/kg/d, oral gavage) | ↑ EF ↓ Prolongation of QRS, QT |
|
| Ren et al (2021)20 | Male C57BL/6J | Sunitinib (40 mg/kg/d for 28 consecutive days, oral gavage) | EMPA (10 mg/kg/d for 28 consecutive days, oral gavage) | ↓ HTN ↑ EF, FS Reversed left ventricular systolic and diastolic dysfunction ↑ CFR |
↓ cTnT, pro-BNP levels Autophagy restoration ↑ AMPK-mTOR signaling pathway |
| Min et al (2023)21 | Male C57BL/6J mice | Trastuzumab (10 mg/kg, once a week for 6 wk, i.p.) | EMPA (10 mg/kg twice per week for 6 wk, i.p.) | ↑ EF, FS, ↓ LVESD Improved cardiomyocyte mechanical and intracellular dysfunction Preserved mitochondrial integrity and function |
↓ Apoptosis, ferroptosis, DNA damage ↓ Oxidative stress ↓ Intracellular Ca2+ decay rate ↑ Mitochondrial biogenesis (PGC-1α) |
| Ali et al (2023)19 | Wistar albino rats | Cisplatin (7 mg/kg, single dose, i.p.) | CANA (10 mg/kg/d, oral) | ↓ AST, ALP, CK-MB, LDH | ↑ Nrf2 ↓ NO, MPO ↓ iNOS, NF-κB, TNF-a, IL-1β ↑ PI3K/AKT pathway ↓ Bax, Bcl-2, cytochrome c |
AKT = protein kinase B; ALP = alkaline phosphatase; α-SMA = α-smooth muscle actin; AMPK = adenosine monophosphate–activated protein kinase; ANP = atrial natriuretic peptide; ARN = angiotensin receptor neprilysin inhibitor; AST = aspartate aminotransferase; ATF-4 = activating transcription factor 4; Bax = Bcl-2-associated X protein; β-MHC = β-myosin heavy chain; βOHB = β-hydroxy butyrate; BNP = B-type natriuretic peptide; CAT = catalase; CFR = coronary flow reserve; CK-MB = creatine kinase MB; CRS = cardiorenal syndrome; cTnT = cardiac troponin T; DAMP = damage-associated molecular pattern; DOX = doxorubicin; ECG = electrocardiographic; EF = ejection fraction; eIF-2α = eukaryotic initiation factor 2α; ER = endoplasmic reticulum; ERK = extracellular signal-regulated kinase; FGF = fibroblast growth factor; FS = fractional shortening; G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; GRP78 = glucose-regulated protein 78; HTN = hypertension; i.p. = intraperitoneally; IL = interleukin; IL = interleukin; IL = interleukin; iNOS = inducible nitric oxide synthase; LDH = lactate dehydrogenase; LV = left ventricle; LVEDD = left ventricular end-diastolic diameter; LVESD = left ventricular end-systolic diameter; LVIDd = left ventricular internal diameter at end-diastole; LVIDs = left ventricular internal diameter at end-systole; MDA = malondialdehyde; MMP = matrix metallopeptidase; MPO = myeloperoxidase; mTOR = mammalian target of rapamycin; MyBPC3 = myosin-binding protein C3; MyD88 = myeloid differentiation primary response 88; NCD = normal chow diet; NF-κB = nuclear factor kappa-B; NLRP3 = nod-like receptor protein 3; NO = nitric oxide; NOX-1 = NADPH oxidase 1; NOX-2 = NADPH oxidase 2; Nrf2 = nuclear factor erythroid 2–related factor 2; NT-proANP = N-terminal pro–atrial natriuretic peptide; NT-proBNP = N-terminal pro–B-type natriuretic peptide; p38 = p38 mitogen-activated protein kinase; PARP = poly(adenosine diphosphate–ribose) polymerase; PERK = protein kinase R-like endoplasmic reticulum kinase; PGC = peroxisome proliferator–activated receptor-γ coactivator; PI3K = phosphoinositide 3-kinase; PPAR = peroxisome proliferator–activated receptor; QTc = corrected QT; SIRT1 = sirtuin 1; SIRT3 = sirtuin 3; Smad3 = mothers against decapentaplegic homolog 3; SOD = superoxide dismutase; TGF = transforming growth factor; TLR9 = Toll-like receptor 9; TNF = tumor necrosis factor; XO = xanthine oxidase; other abbreviations as in Table 1.
Table 3.
In Vitro Studies Demonstrating the Cardioprotective Effects of SGLT2 Inhibitors Against Cancer Treatment–Related Cardiotoxicity
| First Author (Year) | Cell Type | Anticancer Treatment | SGLT2 Inhibitor Treatment | Key Findings | Results and Proposed Mechanisms |
|---|---|---|---|---|---|
| Wang et al (2020)41 | Isolated neonatal rat ventricular cardiomyocytes and AC16 human cardiomyocytes | DOX (1 μM for 24 h) | EMPA (200 nM) | EMPA protected cells from DOX-induced apoptosis | ↑ Autophagic flux (↑ the LC3-II/LC3-I ratio) ↑ Binding of SIRT3 to the beclin 1-TLR9 complex through TLR9 ↓ ROS levels and DNA damage |
| Quagliariello et al (2021)43 | HL-1 adult mouse cardiomyocytes | DOX (0.1-50 μM for 24 h) DOX (100 nM for 12 h) |
EMPA (10, 50, 100, and 500 nM) | ↑ Viability ↓ Apoptosis |
↓ [Ca2+]i ↓ iROS, MDA 4-HNA, NO ↓ IL-8, IL-6, IL1-β ↓ Leukotrienes B4 ↓ p65/NF-κB ↓ MyD88 and NLRP3 |
| Lin et al (2023)82 | Isolated ventricular myocytes | DOX (1 μM for 120 min) | EMPA (1 μM 30 min before DOX) | ↓ Contraction malfunction | ↓ Mitochondrial ROS production ↓ Ca2+-handling disorders by ↑ Ca2+ transients, ↓ Ca2+ transient decay time, ↓ frequency of Ca2+ sparks, and ↑ Ca2+ content in the sarcoplasmic reticulum ↓ Oxidation of Ca2+/calmodulin-dependent protein kinase II (ox-CaMKII) and CaMKII-dependent phosphorylation of RyR2 |
| Chang et al (2021)44 | H9c2 rat cardiac myoblast | DOX (1 μM for 24 h) with high glucose | DAPA (10 μM 1 h before DOX) | ↓ Apoptosis | ↓ ROS generation ↓ ER stress |
| Chang et al (2022)46 | H9c2 rat cardiac myoblast | DOX (1 μM for 24 h) | DAPA (10 μM 1 h before DOX) | ↓ Apoptosis | ↓ ROS ↑ STAT3 expression |
| Hsieh et al (2022)47 | H9c2 rat cardiac myoblast | DOX (10 μM for 24 h) | DAPA (0-20 μM) | ↓ Apoptosis ↑ Viability |
↑ AKT/PI3K signaling ↑ Nrf2 nuclear translocation and activation ↑ Antioxidant HO-1, NQO1, and SOD activity ↓ Oxidative stress and mitochondrial dysfunction ↓ Smad3 activation ↓ ANP, BNP, collagen I, fibronectin, and α-SMA ↓ Fibrosis, inflammation ↓ p38 activation, nuclear NF-κB p65, IL-8 |
| Hu et al (2023)50 | H9c2 rat cardiac myoblast | DOX (5 μM for 24 h) | DAPA (5 μM) | ↓ NLRP3 inflammasome ↓ Inflammatory markers (IL-6, IL-1β, and TNF-α) ↓ Phosphorylated p38, phosphorylated ERK, and TLR4 |
|
| Quagliariello et al (2020)55 | AC16 human cardiomyocytes | Ipilimumab (50-500 nM for 72 h) for cell viability, then 100 nM for 12 h | EMPA (500 nM) | EMPA under hyperglycemic conditions ↓ cardiotoxicity of ipilimumab with ↑ responsiveness to ipilimumab in breast cancer cell lines | ↓ Leukotrienes type B4 production ↓ ROS production, MDA ↓ p65-NF-κB expression ↓ NLRP3 and MyD88 ↓ Cytokines and growth factors (IL-1β, IL-6, PDGF, VEGF, TGF-β) |
| Maurea et al (2021)54 | Coculture model of hPBMCs and cardiomyocytes | Ipilimumab (200 nM for 72 h) | DAPA | ↑ Viability | ↓ [Ca2+]i ↓ Lipid peroxidation ↓ p65/NF-κB ↓ IL-8, IL-6, IL1-β ↓ NLRP3 inflammasome |
| Ren et al (2021)20 | H9c2 rat cardiac myoblast | Sunitinib (1-20 μM for 48 h) | EMPA (50-1,000 nM for 48 h) | ↑ Viability | Autophagy restoration Restored the AMPK/mTOR signaling pathway Inhibition of AMPK or autophagy abolished EMPA effects |
| Madonna et al (2022)56 | HAECs | Ponatinib (1.7 nM) for 0-48 h | EMPA (100 or 500 nM) or DAPA (100 nM) for 0-48 h | EMPA ↑ the viability of cells exposed to ponatinib EMPA and DAPA ↓ the senescence of ponatinib- treated cells |
EMPA ↑ autophagic flux EMPA and DAPA ↑ proangiogenic function of endothelial cells EMPA and DAPA ↓ senescence of cells exposed to ponatinib |
| Min et al (2023)21 | Isolated neonatal mouse cardiomyocytes | Trastuzumab (2 μМ for 8 h) | EMPA (1 μM) | ↓ Lipid peroxidation ↓ Ferroptosis, DNA damage, cytosolic DNA accumulation |
|
| Dabour et al (2023)57 | HUVECs and EA.hy926 cells | Carfilzomib (0.5 μM for 24 h) | CANA, DAPA, EMPA (1-20 μM) 2 h before carfilzomib | Only CANA protected endothelial cells | AMPK restoration |
CaMKII = Ca2+/calmodulin-dependent protein kinase II; [Ca2+]i = intracellular calcium; HAEC = human aortic endothelial cell; hPBMC = human peripheral blood mononuclear cell; HUVEC = human umbilical vein endothelial cell; iROS = intracellular reactive oxygen species; NQO1 = NAD(P)H quinone dehydrogenase 1; PDGF = platelet-derived growth factor; STAT3 = signal transducer and activator of transcription 3; TLR4 = Toll-like receptor 4; VEGF = vascular endothelial growth factor; other abbreviations as in Tables 1 and 2.
Empagliflozin has demonstrated multifaceted cardioprotective effects against DOX cardiotoxicity,39, 40, 41, 42, 43,49 preserving cardiac function and inhibiting LV remodeling.40 This is evident through improvements in LV ejection fraction, fractional shortening,39,41,43,49,50 and the preservation of LV end-diastolic dimension and LV end-systolic dimension.49 Additionally, empagliflozin improved myocardial strain, and it reduced cardiac fibrosis in mice treated with DOX.17 Empagliflozin effectively prevented DOX-induced hypertrophic pathologic changes and perivascular and interstitial fibrosis, preserving LV mass.39 This protection was further strengthened by the prevention of DOX-induced prolongation of QT and corrected QT intervals.42 Similarly, empagliflozin ameliorated sunitinib-induced hypertension and cardiac dysfunction, reversing LV systolic and diastolic dysfunction while restoring coronary flow reserve.20 Administered alongside trastuzumab, empagliflozin preserved ejection fraction and fractional shortening and improved cardiomyocyte mechanical and intracellular function.21
Dapagliflozin also exhibited substantial cardioprotective effects against DOX cardiotoxicity.44, 45, 46, 47, 48,50, 51, 52, 53 Dapagliflozin improved ejection fraction and fractional shortening,44,46, 47, 48,50,52,53 mitigated DOX-induced hemodynamic declines (+dP/dt and −dP/dt), and reduced LV internal dimensions at end-diastole and end-systole.44,50 Additionally, it successfully reversed DOX-induced electrocardiographic changes.51 Furthermore, both empagliflozin and dapagliflozin demonstrated the ability to decrease the senescence of human aortic endothelial cells exposed to ponatinib.56 Intriguingly, only canagliflozin, not empagliflozin or dapagliflozin, protected endothelial cells from carfilzomib-induced apoptosis.57
The observed effects of SGLT2 inhibitors in reversing LV remodeling in animals treated with DOX align with recent clinical trials investigating the impact of SGLT2 inhibitors on LV remodeling, in which empagliflozin, in particular, led to favorable reverse LV remodeling in patients with HF with reduced ejection fraction, both with and without diabetes.58, 59, 60 These particular effects of SGLT2 inhibitors may partially explain their beneficial impact on clinical outcomes in patients with HF. In addition, a systematic review of 5 randomized controlled trials showed that SGLT2 inhibitors were associated with LV mass regression compared with placebo.61
Highlights
-
•
An extensive body of preclinical studies demonstrate the cardioprotective effects of SGLT2 inhibitors in preventing cardiotoxicity secondary to various cancer treatments.
The Molecular Mechanisms of the Cardioprotective Effects of SGLT2 Inhibitors Against Cancer Treatment–Related Cardiotoxicity
The molecular mechanisms underlying the protective effects of SGLT2 inhibitors against cancer treatment–related cardiotoxicity are multifactorial. In this section, we summarize the mechanisms that may contribute to the benefits of SGLT2 inhibitors in mitigating cardiotoxicity secondary to cancer treatment, emphasizing both direct and indirect myocardial effects related to inflammation, energetics, autophagic flux, oxidative and endoplasmic reticulum (ER) stress, ferroptosis, and endothelin-1 (ET-1) (Figure 1).
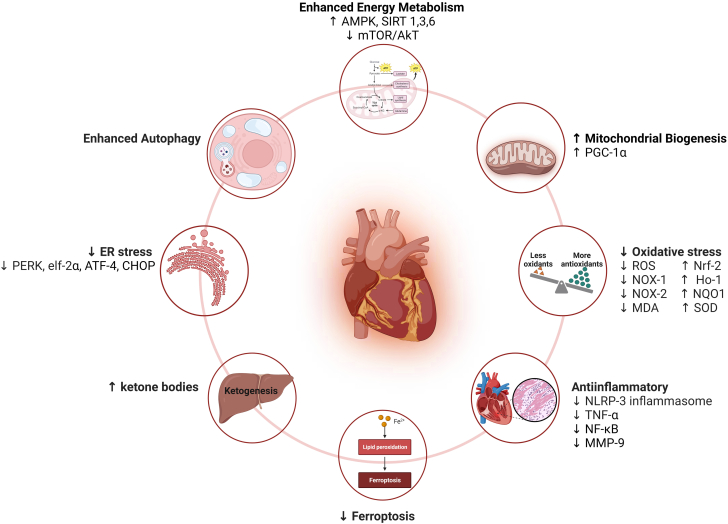
Figure 1.
Cardioprotective Mechanisms of SGLT2 Inhibitors Against Cancer Treatment–Induced Cardiotoxicity
Sodium-glucose cotransporter-2 (SGLT2) inhibitors protect against cardiovascular toxicity induced by anticancer drugs via multiple mechanisms. They enhance energy metabolism by increasing the activation of nutrient deprivation pathways (adenosine monophosphate-activated protein kinase [AMPK] and sirtuin [SIRT] 1, SIRT3, and SIRT6) while inhibiting the nutrient surplus pathways (AKT/mammalian target of rapamycin [mTOR]), resulting in enhanced autophagy and increased mitochondrial biogenesis (peroxisome proliferator–activated receptor-γ coactivator-1α [PGC-1α]). Additionally, SGLT2 inhibitors mitigate oxidative and endoplasmic reticulum (ER) stress, block inflammatory pathways, inhibit ferroptosis, and enhance ketogenesis. Created using BioRender.com. ATF-4 = activating transcription factor 4; CHOP = C/EBP homologous protein; eIF-2α = eukaryotic initiation factor 2α; Ho-1 = heme oxygenase 1; MDA = malondialdehyde; MMP = matrix metalloproteinase; NLRP-3 = nod-like receptor pyrin domain containing 3; NF-κB = nuclear factor κB; NOX-1 = NADPH oxidase 1; NOX-2 = NADPH oxidase 2; Nrf-2 = nuclear factor erythroid 2–related factor 2; NQO1 = NAD(P)H quinone dehydrogenase 1; PERK = protein kinase R-like endoplasmic reticulum kinase; ROS = reactive oxygen species; SOD = superoxide dismutase; TNF = tumor necrosis factor.
Anti-inflammatory effects of SGLT2 inhibitors
Inflammation played a central role in the onset and progression of HF, perpetuating a chronic and vicious cycle between inflammation and cardiac dysfunction.62 This inflammatory process has been implicated in the mechanisms that underlie cardiotoxicity secondary to cancer therapy and radiotherapy.63,64 Specifically, in DOX-induced HF, inflammation played a central role in both its onset and progression.65 DOX triggered inflammatory responses by up-regulating the levels of various inflammatory mediators, including interleukin-1 and tumor necrosis factor-α in the heart, activating immune responses, subsequently leading to cardiomyocyte damage.66 In addition, DOX activated the nod-like receptor pyrin domain containing 3 (NLRP3) inflammasome in cardiomyocytes, promoting cardiomyocyte apoptosis and cardiac remodeling and ultimately contributing to the development of HF.67
SGLT2 inhibitors exhibited considerable potential as anti-inflammatory agents, using indirect mechanisms that involve metabolic improvement, stress reduction, and direct modulation of inflammatory signaling pathways. These effects, which are largely independent of glucose levels, have established their clinical role in patients with HF, regardless of the presence of diabetes.12 SGLT2 inhibitors have been shown to mitigate cardiac inflammation and fibrosis by modulating the activation of nuclear factor κB and the NLRP3 inflammasome, thereby attenuating the synthesis of proinflammatory cytokines.12 Several studies have demonstrated the protective effects of SGLT2 inhibitors against DOX-induced myocardial inflammation.40,43,45,47,50,52 In these studies, SGLT2 inhibitors effectively attenuated the inflammatory response, as indicated by reduced levels of cytokines, including tumor necrosis factor-α and interleukins. For example, early administration of empagliflozin resulted in reduced myocardial inflammatory mediators (tumor necrosis factor-α, nuclear factor κB, interleukin-1β, and matrix metalloproteinase 9) in a model of DOX-induced cardiorenal syndrome.40 Additionally, empagliflozin43 and dapagliflozin45,50,52 consistently mitigated the expression of NLRP3 inflammasome and proinflammatory cytokines, demonstrated in both in vivo and in vitro settings across acute and chronic models of DOX cardiotoxicity. Furthermore, dapagliflozin protected H9c2 rat cardiomyoblasts from DOX-induced cytotoxicity by inhibiting the Toll-like receptor 4/NLRP3/nuclear factor κB inflammatory circuit.50
Similarly, empagliflozin attenuated the activation of NLRP3 inflammasome and proinflammatory cytokines in response to ipilimumab cardiotoxicity in AC16 human cardiomyocytes.55 Dapagliflozin showed similar attenuation in vitro in response to trastuzumab and ipilimumab cardiotoxicity.54 Recently, canagliflozin counteracted cisplatin-induced cardiotoxicity by reducing levels of nuclear factor κB, myeloperoxidase, inducible nitric oxide synthase, tumor necrosis factor-α, and interleukin-1β.19
The reported anti-inflammatory effects against cancer therapy–related cardiotoxicity are consistent with similar effects observed in other models of cardiometabolic diseases. Empagliflozin inhibited the expression and activation of nuclear factor κB in human cardiomyocytes exposed to high glucose concentrations.68 Additionally, both empagliflozin and dapagliflozin attenuated cardiac dysfunction in mice with HF, irrespective of the presence or absence of diabetes, by mitigating the activity of the NLRP3 inflammasome.69,70 Moreover, canagliflozin ameliorated the NLRP3 inflammasome–mediated release of cytokines in an experimental model of autoimmune myocarditis in mice.71
Although empagliflozin inhibited the activation of the NLRP3 inflammasome in macrophages of patients with T2DM,72 it did not exhibit significant alterations in inflammatory biomarkers, including plasma high-sensitivity C-reactive protein and midregional proadrenomedullin, as observed in some human studies involving patients with HF with reduced ejection fraction.59,73 This discrepancy may have been attributed to the difference in the assessment of inflammation, with clinical studies reporting at the blood level and preclinical studies measuring inflammatory markers at the tissue level.
Antioxidant effects of SGLT2 inhibitors
Strong evidence demonstrates that chemotherapy- and radiotherapy-induced cardiotoxicity involved the generation of reactive oxygen species and increased cellular oxidative stress.74,75 Particularly, DOX cardiotoxicity was associated with oxidative stress arising from an imbalance between reactive oxygen species and antioxidants.67
SGLT2 inhibitors have been shown to attenuate the generation of reactive oxygen species and enhance antioxidant mechanisms in various experimental models, including diabetic mice hearts,76 cardiomyocytes isolated from mice exposed to a high-fat diet,77 isolated murine cardiomyocytes undergoing hypoxia and reoxygenation,78 a nondiabetic mouse model of pressure overload–induced HF,79 and LV myocardial biopsies from patients with HF with preserved ejection fraction.80
In alignment with these findings, empagliflozin inhibited DOX-induced oxidative stress in a cardiorenal syndrome model by inhibiting NADPH oxidase 1, NADPH oxidase 2, and oxidized protein.40 It also enhanced the activity of antioxidant enzymes in DOX-treated rats.49 Furthermore, empagliflozin decreased malondialdehyde content in the heart, especially in the cardiac mitochondria, and reduced cardiac xanthine oxidase,43 which is deeply involved in DOX-induced oxidative stress and cardiotoxicity.81 Moreover, empagliflozin effectively mitigated DOX-induced mitochondrial reactive oxygen species production in isolated cardiomyocytes.82
Similarly, dapagliflozin has demonstrated similar effects on DOX-induced oxidative stress in H9c2 cardiomyoblasts.44,47 Dapagliflozin rescued DOX-inhibited antioxidant (heme oxygenase 1, NAD[P]H quinone dehydrogenase 1, and superoxide dismutase) activity and improved mitochondrial function via nuclear factor erythroid 2–related factor 2.47 Dapagliflozin prevented DOX-inhibited nuclear factor erythroid 2–related factor 2 nuclear translocation, and hence the expression of the nuclear factor erythroid 2–related factor 2–dependent antioxidant enzymes, including heme oxygenase 1 and NAD(P)H quinone dehydrogenase 1.47
Empagliflozin additionally counteracted oxidative stress induced by trastuzumab in vivo and, in isolated neonatal mouse cardiomyocytes, ameliorated DNA damage.21 In AC16 human cardiomyocytes, empagliflozin reduced the cardiotoxicity of ipilimumab by reducing reactive oxygen species production and lipid peroxidation.55 Furthermore, dapagliflozin protected human cardiomyocytes from ipilimumab cytotoxicity by reducing lipid peroxidation and intracellular calcium overload.54 Canagliflozin exhibited a cardioprotective effect against cisplatin-induced acute cardiotoxicity through the regulation of nuclear factor erythroid 2–related factor 2 expression.19
Mitigating ER stress
Mitochondrial and ER stresses both played critical roles in DOX cardiotoxicity through a mutual interaction.83 SGLT2 inhibitors have shown their potential to mitigate ER stress, which is particularly relevant in the treatment of cardiovascular events. Chang et al44 reported that dapagliflozin protected against DOX cardiotoxicity in diabetic rats and H9c2 cardiomyoblasts by reducing ER stress, as evidenced by the decreased expression of the ER-related proteins, including glucose-regulated protein 78, protein kinase R-like endoplasmic reticulum kinase, eukaryotic initiation factor 2α, activating transcription factor 4, and C/EBP homologous protein.44 SGLT2 inhibitors have shown similar findings in other cardiovascular disease models. In cardiomyocytes treated with angiotensin II, dapagliflozin has shown the ability to inhibit the protein kinase R-like endoplasmic reticulum kinase–eukaryotic initiation factor 2α–C/EBP homologous protein axis of the ER stress response via the activation of sirtuin 1.84 Additionally, empagliflozin attenuated myocardial ischemia-reperfusion injury and cardiomyocyte apoptosis by inhibiting ER stress.85
Increased ketone bodies (ketogenesis)
SGLT2 inhibitors, by promoting glycosuria, triggered a state resembling starvation marked by the hepatic production of ketone bodies, particularly β-hydroxy butyrate.86 Furthermore, patients with HF exhibited elevated levels of ketone bodies, potentially serving as a fuel source for the failing heart.87 Empagliflozin was shown to protect against acute and chronic DOX cardiomyopathy, presumably by increasing β-hydroxy butyrate levels.39 To validate this, Oh et al39 conducted experiments by incubating cardiac myocytes with β-hydroxy butyrate, resulting in reduced DOX cardiotoxicity.39 Similarly, the combination of dapagliflozin with a low dose of angiotensin receptor neprilysin inhibitor modulated metabolic pathways and increased β-hydroxy butyrate levels, contributing to their protective mechanisms against DOX-induced acute and chronic HF.48 Additionally, dapagliflozin modulated metabolic pathways, including glucose, ketone bodies, and fatty acids, by enhancing genes related to fatty acid transport, fatty acid oxidation, ketogenesis, and gluconeogenesis.48
Although dapagliflozin effectively modulated metabolic pathways, the role of ketone bodies in mediating the cardioprotective effects of SGLT2 inhibitors has been the subject of debate. First, empagliflozin increased cardiac adenosine triphosphate production without relying on the use of ketone bodies.88 Second, in diabetic, obese, hypertensive rats with mild HF, empagliflozin reduced myocardial ketone body use despite elevated blood β-hydroxy butyrate levels.88 Furthermore, β-hydroxy butyrate ameliorated mitochondrial dysfunction and inflammation in an HF with preserved ejection fraction mouse model independently of ketone oxidation.89 Additionally, the EMPA-VISION (Assessment of Cardiac Energy Metabolism, Function and Physiology in Patients With Heart Failure Taking Empagliflozin) trial showed that empagliflozin did not enhance cardiac energetics or alter the levels of circulating ketone bodies in patients with HF with reduced ejection fraction or HF with preserved ejection fraction.90 Despite the absence of conclusive evidence on whether the action of SGLT2 inhibitors in HF involves ketone metabolism, this does not preclude the potential therapeutic advantages from ketones. The available findings imply that the presence of ketones following SGLT2 inhibition might induce beneficial effects for cardiovascular protection. This could be attributed to their direct activation of nutrient deprivation signals rather than their function as a proficient source for adenosine triphosphate synthesis.91
Enhanced energy metabolism
Cells adjust their metabolic processes and functions in response to signals from their surroundings through the interplay of various regulatory proteins, including mammalian target of rapamycin (mTOR), sirtuins (sirtuin 1, sirtuin 3, sirtuin 6), and adenosine monophosphate–activated protein kinase (AMPK).91,92 The dysregulation of these proteins has been associated with cardiovascular disease.93 DOX-induced down-regulation of sirtuin 1 and AMPK activity was one of the key mechanisms of DOX cardiotoxicity, and AMPK activation by different approaches has demonstrated cardioprotective effects against DOX.93,94 AMPK down-regulation was implicated in various molecular pathways associated with DOX cardiotoxicity, including mitochondrial dysfunction, enhanced apoptosis, disrupted autophagy, and increased fibrosis. AMPK regulated mitochondrial biogenesis through peroxisome proliferator–activated receptor-γ coactivator-1α signaling, promoted oxidative mitochondrial metabolism, suppressed apoptosis via mTOR signaling inhibition, enhanced autophagy via Unc-51 like autophagy activating kinase 1 activation, and inhibited fibrosis by targeting transforming growth factor-β signaling.94
SGLT2 inhibitors enhance energy metabolism by up-regulating the expression or activity of nutrient deprivation signaling, such as AMPK, sirtuins, and peroxisome proliferator–activated receptor-γ coactivator-1α. Simultaneously, they down-regulate nutrient surplus signaling, such as mTOR activation, in various stressed tissues.91 SGLT2 inhibitors have been shown to activate AMPK via the inhibition of complex I of the mitochondrial respiratory chain.95,96 Although SGLT2 inhibitors demonstrated cardioprotection by activating AMPK in cardiac fibroblasts, cardiomyocytes,97,98 and murine hearts,96,99 only canagliflozin activated AMPK in endothelial cells.100,101 Empagliflozin and dapagliflozin have been shown to mitigate DOX cardiotoxicity through AMPK activation.49,52 Empagliflozin activated the AMPK/sirtuin 1/peroxisome proliferator–activated receptor-γ coactivator-1α–mediated mitochondrial biogenesis,49 and dapagliflozin attenuated short-term DOX cardiotoxicity by activating AMPK.52 In addition, the administration of empagliflozin in DOX-treated mice activated mitochondrial biogenesis, likely through the up-regulation of peroxisome proliferator–activated receptor-γ coactivator-1α, thereby preventing DOX-induced acute cardiotoxicity.42 Furthermore, empagliflozin mitigated cardiac dysfunction induced by sunitinib, both in vivo and in vitro, by activating AMPK, resulting in regulated cardiomyocyte autophagy.20 Recent findings have shown that canagliflozin, unlike empagliflozin or dapagliflozin, mitigated carfilzomib-induced endothelial apoptosis by restoring AMPK expression.57
Enhanced autophagy
Autophagic clearance of damaged cellular constituents reduced cellular stress, mitigated proinflammatory and profibrotic responses, preserved cellular integrity, and prevented apoptosis, thereby maintaining and restoring organ structure and function.91 As autophagy represents the primary cellular recycling mechanism, the dysregulation of autophagy became a critical mechanism of cancer therapy–related cardiotoxicity.102 Previous studies have noted that DOX blocked the autophagic flux in cardiomyocytes.103 Empagliflozin abrogated DOX cardiotoxicity by enhancing the autophagic flux in murine hearts and cardiomyocytes.41 In their study, Wang et al41 proposed that empagliflozin directly acts on sirtuin 3, facilitating the formation of a complex involving sirtuin 3, beclin 1, and Toll-like receptor 9, thereby enhancing autophagic flux. Likewise, empagliflozin ameliorated sunitinib-induced cardiac dysfunction by regulating cardiomyocyte autophagy, which was mediated by the AMPK/mTOR signaling pathway.20 Similarly, empagliflozin enhanced the autophagic flux in human aortic endothelial cells treated with ponatinib, resulting in reduced cellular senescence.56
These findings align with the role of SGLT2 inhibitors in enhancing autophagy in HF. There is extensive evidence linking autophagy to the pathogenesis of HF, as dysfunctional basic autophagy may lead to its onset, and excessive or maladaptive autophagy may aggravate the condition.104,105 Proteomic analyses of samples from the EMPEROR-Reduced and EMPEROR-Preserved clinical trials provide additional support for the notion that the effects of SGLT2 inhibitors are likely related to actions on the heart and kidney, promoting autophagic flux and nutrient deprivation signaling.106 A recent review examining the influence of SGLT2 inhibitors on the autophagic flux concluded that enhanced AMPK activity and autophagic flux contributed to the elevated adenosine triphosphate production in the heart following SGLT2 inhibition. This further supports the notion that the cardioprotective effects of SGLT2 inhibitors can be attributed to their modulation of nutrient and energy sensors, leading to the promotion of autophagy.91
Inhibiting ferroptosis
Ferroptosis, an iron-dependent and nonapoptotic type of cell death driven by excessive lipid reactive oxygen species and disrupted redox balance, has been linked to abnormal iron accumulation.107 Accumulating evidence suggests the crucial role of maintaining iron homeostasis for normal heart function, with ferroptosis playing a significant role in cardiovascular disease.108 Several anticancer medications, including DOX, etoposide, tyrosine kinase inhibitors, trastuzumab, and 5-fluorouracil, induce ferroptosis.108 Notably, SGLT2 inhibitors have shown their ability to mitigate cardiotoxicity secondary to DOX and trastuzumab by inhibiting ferroptosis.21,43 In a mouse model of DOX-induced acute cardiotoxicity, empagliflozin mitigated the deleterious cardiac effects of DOX by reducing iron overload and ferroptosis.43 Similarly, dapagliflozin protected against trastuzumab-induced cardiotoxicity by ameliorating ferroptosis.21
These effects were consistent in other models of HF, in which canagliflozin suppressed ferroptosis in mice with diabetic cardiomyopathy109 and in rats with HF with preserved ejection fraction.110 Canagliflozin reduced ferroptosis by regulating AMPK in cardiomyocytes,111 and dapagliflozin alleviated oxidative stress, lipid peroxidation, and ferrous iron overload. These effects were observed in a rat model of myocardial ischemia-reperfusion injury and in H9c2 cardiomyoblasts subjected to hypoxia and reoxygenation, achieved via mitogen-activated protein kinase signaling inhibition.112 Additionally, large-scale proteomics analyses from clinical trials involving SGLT2 inhibitors revealed differential expression of proteins associated with improved iron homeostasis.106,113
Inhibition of ET-1
ET-1, a potent vasoconstrictor, has been implicated in breast cancer–induced cardiac remodeling and cancer treatment–related cardiotoxicity.114,115 High-dose DOX significantly elevated serum and cardiac ET-1 in rats.116,117 Similarly, ET-1 mediated sunitinib-induced hypertension and cardiac fibrosis in tumor-bearing mice118 and induced systemic vasoconstriction in a swine model.119 Furthermore, the up-regulation of ET-1 may have served as a predictor for chemotherapy-related cardiotoxicity in women undergoing anthracycline-based chemotherapy followed by trastuzumab.114
Interestingly, there is strong preclinical and clinical evidence suggesting that SGLT2 inhibitors can reduce ET-1 concentration. In the DAPA-HF trial, after a 12-month treatment period, dapagliflozin caused a small decrease in serum ET-1 levels in patients with HF.120 In preclinical studies, empagliflozin inhibited ET-1 in human proximal tubular cells.121 Therefore, it is plausible that the inhibition of ET-1 mediated the protective effects of SGLT2 inhibitors in cardio-oncology. Nevertheless, as of now, there are no published studies reporting this mechanism.
Highlights
-
•
SGLT2 inhibitors offer multifactorial cardioprotective effects in cancer treatment–related cardiotoxicity.
-
•
Mechanisms include anti-inflammatory, antioxidant, ER stress mitigation, ketogenesis, enhanced energy metabolism, autophagy, inhibition of ferroptosis, and inhibition of ET-1.
Anticancer Effects of SGLT2 Inhibitors
Following the demonstration of the cardioprotective effects of SGLT2 inhibitors against cancer therapy–related cardiovascular toxicity, it is crucial to investigate their impact on the anticancer effects of cancer treatments. This investigation is essential to confirm that combining SGLT2 inhibitors with cancer treatments would not compromise their chemotherapeutic benefits.
Initially, concerns were raised regarding the risk for cancer with SGLT2 inhibitors, especially bladder cancer with dapagliflozin.122,123 However, subsequent studies and meta-analyses have examined these concerns, ultimately concluding that there was no overall risk for malignancy with SGLT2 inhibitors.124, 125, 126 Furthermore, recent epidemiologic studies have revealed a positive association between the use of SGLT2 inhibitors and improved overall survival among patients with non-small-cell lung cancer (NSCLC)127 and hepatocellular carcinoma128 with pre-existing diabetes, irrespective of patients’ demographics, tumor characteristics, and cancer treatments. Notably, a longer duration of SGLT2 inhibitor use, especially with canagliflozin, was associated with better survival. However, it is important to note that no randomized clinical trials have investigated the anticancer effects of SGLT2 inhibitors. To confirm these observations, several preclinical studies have provided evidence for potential anticancer effects of SGLT2 inhibitors on their own, particularly canagliflozin and dapagliflozin, in both experimental cell lines (Table 4) and animal models (Table 5).
Table 4.
In Vitro Studies Demonstrating the Anticancer Effects of SGLT2 Inhibitors
| First Author (Year) | Cancer Type | Cell Type | SGLT2i Treatment | Key Finding | Results and Proposed Mechanism |
|---|---|---|---|---|---|
| Villani et al (2016)24 | Prostate, lung, liver, breast cancer | Prostate (PC3, 22RV-1), lung (A549, H1299), liver (HepG2), and breast (MCF7) cancer cells | CANA DAPA (0-100 μM) |
Only CANA at clinically relevant concentrations ↓ proliferation and clonogenic survival of cancer cells alone and in combination with cytotoxic therapies | CANA: ↓ Glucose uptake, mitochondrial complex I–supported respiration ↓ ATP and lipogenesis ↑ Phosphorylation of AMPK |
| Li et al (2017)136 | Resistant NSCLC | HCC827, H1975 carrying specific EGFR mutations | CANA (0-100 μM) | ↓ Growth of NSCLC cell lines | ↑ Apoptosis ↓ EGFR phosphorylation ↓ Phosphorylation of Akt and ERK |
| Kuang et al (2017)141 | RCC | ACHN, A498, and CaKi-1 | DAPA (0-4 μM) | ↓ Cell growth in a dose- and time-dependent manner | ↑ G1 phase arrest ↑ Apoptosis ↓ SGLT2 expression and glucose uptake |
| Kaji et al (2018)129 | HCC | Huh7 and HepG2 cells | CANA (10 μM) | ↓ Liver cancer cell growth and angiogenic activity | ↓ Glycolytic metabolism ↑ G2/M arrest and apoptosis ↓ Phosphorylation of ERK, p38, and AKT |
| Hung et al (2019)130 | HCC | Huh-7 and Hep3B | CANA (0-50 μM) | ↓ Growth of HCC cells | ↓ Expression of β-catenin ↑ Proteasomal degradation of β-catenin protein by ↑ phosphorylation of β-catenin Direct inhibition of PP2A activity |
| Nasiri et al (2019)23 | Breast and colon cancer | E0771 breast tumors and MC38 colon tumors | CANA DAPA (5 μM and 5 mM) |
↓ Cancer cell growth rate | CANA ↓ both glucose uptake and oxidation at clinically relevant concentrations (5 μM) DAPA did not alter glucose uptake or tumor cell division at clinically relevant concentrations (0.5 μM) but did at a suprapharmacologic concentration (5 mM) |
| Nakano et al (2020)25 | HCC | Huh7 and HepG2 | CANA (3 μM, 10 μM) | ↓ Proliferation of HCC cells | Regulating metabolic reprogramming (alterations in metabolism of mitochondrial oxidative phosphorylation, fatty acid, and purine and pyrimidine) ↓ ATP synthase F1 subunit α Altered phosphorylation of AMPK |
| Zhou et al (2020)143 | Breast cancer | MCF-7, ZR-75-1 | CANA DAPA (0, 3.3, 11, 33, 100, 300 μM) |
Both ↓ human breast cancer cells proliferation and growth | ↑ Phosphorylation of AMPK ↓ Phosphorylation of p70S6K Cell cycle arrest in G1/G0 phase ↑ Cell apoptosis (AMPK-mediated cell cycle arrest and apoptosis) |
| Xu et al (2020)133 | Pancreatic cancer | Capan-1 and PANC-1 | CANA (20, 40, 60 and 80 μM) | ↓ Pancreatic cancer cell proliferation and colony formation | ↑ Apoptosis ↓ Glycolysis via the PI3K/AKT/mTOR pathway |
| Xie et al (2020)140 | Cervical cancer | HeLa and C33A | EMPA (50 μM) | ↓ Migration of cervical cancer cells and ↑ apoptosis | ↑ AMPK/FOXA1 pathway and ↓ expression of Shh |
| Komatsu et al (2020)144 | Breast cancer | MCF-7 cells | IPRA (1–50 μM) | ↓ Breast cancer cell proliferation | SGLT2 inhibition-dependent hyperpolarization of MCF-7 cell membrane Mitochondrial membrane instability ↓ DNA synthesis at high dose |
| Papadopoli et al (2021)145 | Breast cancer | MCF7, SKBR3 and BT-474, NT2197 | CANA DAPA (25, 50 μM) |
CANA ↓ proliferation of breast cancer cell lines DAPA showed modest effect |
Antiproliferative effects were not affected by glucose availability or the level of expression of SGLT2 ↓ Mitochondrial respiration and total ATP production ↓ Glutamine metabolism |
| Ren et al (2021)134 | Pancreatic cancer | PANC-1 and BxPC-3 | CANA (1 μM) SOTA (3 nM) |
↓ Proliferation and invasion of pancreatic cancer cells | ↓ Hippo pathway ↓ YAP1 expression |
| Yamamoto et al (2021)137 | Lung cancer | A549, H1975, and H520 | CANA (1-50 μM) | ↓ Growth of cells in a dose-dependent manner | ↓ DNA synthesis ↓ S phase entry (induced G1 arrest) ↓ ERK and MAPK phosphorylation Did not induce apoptosis Tumor weight was not decreased by CANA in vivo |
| Wu et al (2022)22 | Osteosarcoma | MNNG/HOS and MG-63 | CANA (0.5, 1, or 2 μM) | ↓ Osteosarcoma progression | Inducing immune cell infiltration ↑ STING/IRF3/IFN-β pathway ↓ AKT pathway |
| Wang et al (2022)142 | Papillary thyroid cancer | TPC-1 and BCPAP | CANA (10 μM) DAPA (0, 20, 40, 80 μM) |
↓ Thyroid cancer cells growth in a dose- and time-dependent manner DAPA ↓ proliferation of the same cells |
(Mechanisms only done with CANA) Dependent on SGLT2 expression ↓ Invasion of thyroid cancer cell ↓ Glucose uptake ↓ glycolysis ↑ AMPK pathway ↓ AKT/mTOR pathway ↑ Cell cycle arrest at G1/S checkpoint ↑ DNA damage and ATM/CHK2 pathway activation ↑ Apoptosis |
| Shoda et al (2023)132 | Glioblastoma | U251MG (human), U87MG (human), and GL261 (murine) | CANA (40 μM) | ↓ Glioblastoma cell proliferation | Dependent on SGLT2 expression ↓ Glucose uptake ↑ AMPK phosphorylation ↓ p70S6K phosphorylation |
| Ding et al (2023)139 | NSCLC, ovarian, pancreatic cancers | H292, SKOV3, MIA PaCa-2, primary NSCLC, ovarian and pancreatic cancer patient–derived cancer cells | CANA (20 μM) | SGLT2 is a positive regulator of PD-L1 (the interaction between SGLT2 and PD-L1 on the cell membrane is required for maintaining PD-L1 protein) | ↓ PD-L1 protein expression ↑ Proteasomal degradation of PD-L1 |
| Biziotis et al (2023)148 | Human adenocarcinoma, squamous cell, NSCLC | Human adenocarcinoma (A549, H1299 and H1975), squamous cell (SK-MES-1), and large cell (H460) NSCLC cells | CANA (5-30 μM) | ↓ Proliferation of all cell lines ↓ Clonogenic potential of A549, H1299, and H1975 cells |
↓ Oxygen consumption rate ↑ Extracellular acidification rate ↑ AMPK activity ↓ mTOR activity ↓ (MAPK) ERK1/2 ↓ HIF-1α ↓ HDAC2 |
ATP = adenosine triphosphate; EGFR = epidermal growth factor receptor; FOXA1 = forkhead box A1; HCC = hepatocellular carcinoma; HDAC2 = histone deacetylase 2; HIF-1α = hypoxia-inducible factor-1α; IFN = interferon; IPRA = ipragliflozin; IRF3 = interferon regulatory factor 3; MAPK = mitogen-activated protein kinase; NSCLC = non-small-cell lung cancer; PD-L1 = programmed cell death-ligand 1; PP2A = protein phosphatase 2A; Shh = sonic hedgehog signaling molecule; SOTA = sotagliflozin; STING = stimulator of interferon genes; YAP1 = YES-associated protein 1; other abbreviations as in Tables 1 and 2.
Table 5.
In Vivo Studies Demonstrating the Anticancer Effects of SGLT2 Inhibitors
| First Author (Year) | Cancer Type | Cancer Model | SGLT2 Inhibitor Treatment | Key Finding | Results and Proposed Mechanism |
|---|---|---|---|---|---|
| Scafoglio et al (2015)135 | Pancreatic cancer | NSG mice with ASPC-1 cells | CANA DAPA (30 mg/kg/d for 4 wk, oral gavage) |
CANA ↓ tumor growth rate equally with gemcitabine (positive control) DAPA caused modest insignificant ↓ in tumor growth, but a significant ↑ in tumor necrosis |
↓ Tumor growth ↑ Necrosis in the tumor center dependent on SGLT2 inhibition |
| Villani et al (2016)24 | Prostate cancer | BALB/c-Nude mice with PC3 cells | CANA (100 mg/kg, single dose, oral gavage) | ↓ Tumor growth rate (in vitro) | Activates AMPK in cancer cells |
| Kuang et al (2017)141 | RCC | CaKi-1 bearing athymic nude mice | DAPA (1.3 mg/kg, for 21 d, i.v.) | ↓ Tumor growth | ↑ Tumor necrosis ↓ SGLT2 expression |
| Kaji et al (2018)129 | HCC | Huh7- and HepG2-derived xenograft tumors in BALB/c nude mice | CANA (100 mg/kg/d for 4 wk, oral) | ↓ Human HCC xenograft tumor burden independently of glycemic status | ↓ Glycolytic metabolism and intratumor vascularization Inhibition of in vivo tumor growth is strongly associated with SGLT2 expression No effect on tumor growth, tumor weight in SGLT2-null HLE-derived xenograft models |
| Shiba et al (2018)131 | HCC in a mouse model of human NASH | MC4R-KO mice on high-fat diet for 1 y to develop HCC | CANA (30 mg/kg/d in diet for 8, 20, or 52 wk) | ↓ Number of tumors in the liver ↓ Maximum tumor size |
Antiproliferative in premalignant lesions ↓ Liver fibrosis ↓ Serum ALT ↓ Expression of Myc and Afp |
| Scafoglio et al (2018)142 | NSCLC | KPluc GEMMs (tumor induced by intranasal Adeno-Cre) and patient-derived xenografts | CANA (30 mg/kg/d, for 6 wk or 3 mo, oral gavage) | ↓ Tumor growth Delay onset of tumors ↑ Survival |
↓ Glucose uptake |
| Wu et al (2019)158 | HCC | Huh7 xenografted tumor model | CANA (100 mg/kg/d for 10 d then ↑ to 300 mg/d until day 30) | ↓ Tumor growth ↑ Survival |
↓ PP2A activity ↓ Glucose-influx-induced β-catenin up-regulation ↓ β-catenin expression |
| Nasiri et al (2019)23 | Breast cancer Colon cancer |
C57BL/6J mice with E0771 or MC38 cells with high-fat diet | DAPA (2.5 mg/kg/d, in drinking water) | ↓ Tumor growth | ↓ Tumor glucose uptake in an insulin-dependent manner Restoring hyperinsulinemia abrogated the effects of DAPA |
| Zhou et al (2020)143 | Breast cancer | BALB/C-nu/nu mice with MCF-7 cells | DAPA (100 mg/kg/d for 4 wk, oral) | ↓ Tumor growth | AMPK-mediated cell cycle arrest and apoptosis (mentioned only in vitro) |
| Xu et al (2020)133 | Pancreatic cancer | PANC-1-bearing nude mice | CANA (25, 50, and 100 mg/kg/d for 5 wk, oral) | ↓ Tumor growth | ↓ Glycolysis via ↓ protein levels of PI3K/AKT/mTOR pathway (↓GLUT-1 and LDHA expression) |
| Xie et al (2020)140 | Cervical cancer | HeLa cells treated with EMPA (50 μmol/L) transplanted into nude mice | EMPA | ↓ Growth of tumors in nude mice ↓ Proliferation of cervical cancer cells and ↑ their apoptosis |
↑ AMPK/FOXA1 pathway and ↓ the expression of Shh (dependent on SGLT2) |
| Ren et al (2021)134 | Pancreatic cancer | PANC-1 tumor-bearing athymic nude (nu/nu) mice | CANA (not mentioned) SOTA (30 mg/kg) |
↓ Growth of pancreatic tumor | |
| Wu et al (2022)22 | Osteosarcoma | K7M2 tumor-bearing C57BL/6 mice | CANA (30 mg/kg/d, oral) | ↓ Tumor growth | ↑ Immune cell infiltration by ↑ STING expression and activating the IRF3/IFN-β pathway |
| Wang et al (2022)142 | Papillary thyroid cancer | TPC-1 tumor in Balb/c nude mice | CANA (100 mg/kg/d for 28 d, oral) | ↓ Tumor growth | All mechanisms are in vitro only |
| Shoda et al (2023)132 | Glioblastoma | GL261-transplanted mice | CANA (100 mg/kg/d for 10 d, oral) | ↓ Glioblastoma tumor growth | Activation of AMPK (mentioned only in vitro) |
| Ding et al (2023)139 | NSCLC | CT26 mouse cancer model and NOD-PrkdcscidIl2rgem1/Smoc (NSG) mice humanized with PBMCs and Hu-SRC-SCID model | CANA (50 mg/kg/d for 1 wk, intragastric) | ↓ Tumor growth equivalent to anti-PD-1 antibody | ↓ PD-L1+ cells ↑ Infiltrating CD3+ T cells, activated CD8+ T cells, and IFN-γ production |
| Biziotis et al (2023)148 | NSCLC | Male athymic BALB/c nude mice with A549 and H1299 | CANA (60 mg/kg/d for 60 d for A549 and 36 d for H1299 tumors in the diet) | ↓ Tumor growth ↑ Survival |
↑ AMPK activity ↓ mTOR activity ↓ (MAPK) ERK1/2 ↓ HIF-1α ↓ HDAC2 |
Afp = alpha-fetoprotein; ALT = alanine transaminase; CD3 = cluster of differentiation 3; CD8 = cluster of differentiation 8; GEMM = genetically engineered murine model; IFN = interferon; Myc = myelocytomatosis oncogene; NASH = nonalcoholic steatohepatitis; NSG = NOD/SCID-ILR2-γ; RCC = renal cell carcinoma; other abbreviations as in Tables 1, 2, and 4.
These studies have consistently demonstrated the ability of SGLT2 inhibitors to impede the growth of tumors across the following cancer models: hepatocellular carcinoma,24,25,129, 130, 131 glioblastoma,132 osteosarcoma,22 pancreatic cancer,133, 134, 135 prostate cancer,24 lung cancer,24,136, 137, 138, 139 cervical cancer,140 renal cancer,141 papillary thyroid cancer,142 colon cancer,23 and breast cancer.23,24,143, 144, 145 Importantly, in preclinical studies, the combination of SGLT2 inhibitors with other chemotherapies and ionizing radiation enhanced their efficacy and increased cancer cell responsiveness to treatments (Table 6). This combination strategy carries the potential to lower the therapeutic doses of cancer treatments, thereby ameliorating their adverse effects, particularly cardiotoxicity. Furthermore, this combined approach holds promise in attenuating cancer cell resistance to treatment.
Table 6.
Preclinical Studies Demonstrating the Effect of Combining SGLT2 Inhibitors With Cancer Treatments
| First Author (Year) | Cell/Model Type | Anticancer Treatment | SGLT2 Inhibitor Treatment | Key Finding | Results and Proposed Mechanism |
|---|---|---|---|---|---|
| Villani et al (2016)24 | Human prostate cancer PC3 cells | Radiation or docetaxel | CANA (0-100 μM) | CANA ↑ the efficacy of both therapies in prostate cancer cells | ↓ Mitochondrial complex I |
| Quagliariello et al (2020)55 | Breast cancer MCF-7 and MDA-MB-231 cells | Ipilimumab (100-500 nM) | EMPA (500 nM) under hyperglycemic conditions | EMPA ↑ responsiveness to ipilimumab | ↑ ROS production ↑ MDA |
| Eliaa et al (2020)162 | Triple-negative breast cancers MDA-MB-231 | DOX (1.3 μM) | EMPA (50 μM) | DOX+EMPA showed a slightly ↓ cytotoxic activity against the MDA-MB-231 cell line compared with DOX alone, but more characteristic arrest in the growth of cells | ↑ Cell sensitivity to the DOX and ↑ antitumor effects: dose dependent ↓ Cancer growth, ↓ cell cycle proliferation, ↑ apoptosis ↓ Expression of MDR1 ↓ Bcl-2, JNK gene expression ↑ p21 gene expression |
| Zhong et al (2020)163 | Hepatocellular carcinoma HepG2, HepG2-ADR, breast cancer MCF7 cells | DOX (0-2.8 or 4.2 μM) | CANA (40 μM) | CANA ↑ the cytotoxic effect of DOX CANA ↑ intracellular accumulation of DOX in HepG2 cells |
↓ p-gp protein in HepG2 cells without affecting its gene expression ↓ Glucose uptake and ATP levels ↓ Autophagy at the early stage by ↑ ULK1 phosphorylation |
| HepG2-xenograft BALB/c nude mice | Stronger inhibition of DOX activity against tumor growth than DOX alone | ↑ Tumor necrosis | |||
| Quagliariello et al (2021)43 | Estrogen-responsive and triple-negative breast cancer cells (MCF-7 and MDA-MB-231 cell lines) | DOX (0.1-50 μM) | EMPA (10, 50, 500) | EMPA did not affect anticancer effects of DOX in breast cancer cells | |
| Biziotis et al (2023)148 | Human adenocarcinoma (A549, H1299, and H1975), squamous cell (SK-MES-1), and large cell (H460) NSCLC cells and male athymic BALB/c nude mice | Radiation | CANA (5-30 μM) | Additive antiproliferative effects in A549, H1299, and H1975 cells and synergistic effects in SK-MES-1 and H460 cells ↓ Clonogenic survival (additive in A549 and H1299 cells and synergistic in H1975 cells) ↓ Tumor growth in vivo |
↑ Anaerobic metabolism ↑ AMPK activity ↓ mTOR activity ↓ (MAPK) ERK1/2 ↓ HIF-1α ↓ HDAC2 |
Mechanisms of the anticancer effects of SGLT2 inhibitors
Several comprehensive review articles have discussed the potential anticancer mechanisms of SGLT2 inhibitors.146,147 Importantly, the presence of SGLTs (SGLT1 and/or SGLT2) has been confirmed in various types of cancer cells, including hepatocellular carcinoma; pancreatic, prostate, lung, and breast cancers; and brain, head, and neck tumors.22,129,132,135,138,140, 141, 142, 143 Consequently, some mechanisms underlying the anticancer effects of SGLT2 inhibitors are dependent upon the inhibition of SGLT2, whereas others are independent of SGLT2 inhibition. These mechanisms include the inhibition of complex I and the α subunit of adenosine triphosphate synthase F1 in the mitochondrial electron transport chain, cell cycle arrest, reduction in the adhesion of cancer cells, disruption of glutamine metabolism, and inhibition of several other pathways, including β-catenin action, mTOR, DNA and RNA synthesis, proangiogenic factors, cellular sodium influx, and L858R/T790M epidermal growth factor receptor kinase.146,147
Inhibition of mitochondrial complex I and AMPK activation
The mechanisms involved with the anticancer effects of SGLT2 inhibitors are depicted in Figure 2. The activation of AMPK was the most frequently reported mechanism underlying the anticancer properties of SGLT2 inhibitors,24,25,129,132,140,142,143,145,148 particularly with canagliflozin. Mechanistically, canagliflozin inhibited mitochondrial complex I–supported cellular respiration and oxidative phosphorylation, leading to reduced adenosine triphosphate production and an elevated adenosine monophosphate/adenosine triphosphate ratio, which in turn induced AMPK phosphorylation and activation. Activated AMPK resulted in the inhibition of the mTOR, inhibition of p70S6K, cell cycle arrest, and induction of apoptosis.129,133,142,143
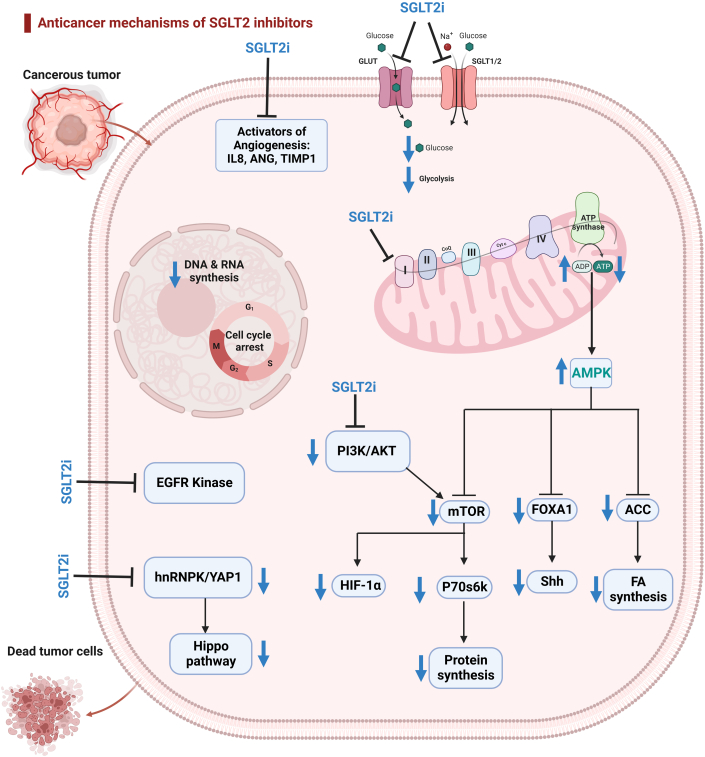
Figure 2.
Mechanisms of the Anticancer Effects of SGLT2i
SGLT2 inhibitors (SGLT2i) inhibit glucose uptake by inhibiting SGLTs (SGLT-1 and SGLT-2) and glucose transporters (GLUTs). SGLT2 inhibitors, especially canagliflozin, inhibit mitochondrial complex I–supported cellular respiration, reducing adenosine triphosphate production and activating AMPK. Activated AMPK inhibits acetyl–coenzyme A carboxylase (ACC), suppressing fatty acid synthesis. It also down-regulates forkhead box A1 (FOXA1) and sonic hedgehog signaling molecule (Shh) and inhibits mTOR and p70S6K, thereby suppressing protein synthesis. Inhibited mTOR results in the down-regulation of hypoxia-inducible factor-1α (HIF-1α). SGLT2 inhibitors inhibit the phosphoinositide 3-kinase (PI3K)/AKT pathway. SGLT2 inhibitors inhibit the Hippo signaling pathway by down-regulating the heterogeneous nuclear ribonucleoprotein K (hnRNPK)/YES-associated protein 1 (YAP1) axis. Additionally, SGLT2 inhibitors inhibit epidermal growth factor receptor (EGFR) kinase and angiogenesis activators. Created using BioRender.com. ANG = angiogenin; FA = fatty acid; IL8 = interleukin 8; TIMP1 = tissue inhibitor of metalloproteinase 1; other abbreviations as in Figure 1.
Notably, canagliflozin emerged as the most potent SGLT2 inhibitor in inhibiting mitochondrial complex I in vitro compared with other SGLT2 inhibitors.95 This may explain why, at clinically relevant concentrations, only canagliflozin exhibited antiproliferative properties,24,135,145 whereas dapagliflozin required suprapharmacologic concentrations to show the same effects.23 Further supporting this, maintaining cellular respiration in the presence of canagliflozin through the overexpression of the yeast mitochondrial NADH dehydrogenase (complex I) negated its antiproliferative effects.24 This underscores the critical role of inhibiting mitochondrial complex I–supported respiration in the antiproliferative effects of canagliflozin in cancer cells.
Activated AMPK also down-regulated forkhead box A1 and the sonic hedgehog signaling molecule.149 The sonic hedgehog signaling molecule pathway was highly expressed in several malignant tumors, facilitating tumor cell proliferation, resistance to chemotherapy, and tumor metastasis.150 Forkhead box A1 activated the sonic hedgehog signaling molecule pathway, inhibiting apoptosis and promoting abnormal cell proliferation.151 In cervical carcinoma cells and tumor-bearing mice, empagliflozin has been demonstrated to activate AMPK, and down-regulate the expression of forkhead box A1, thereby inhibiting the expression of sonic hedgehog signaling molecule, further inhibiting the malignant proliferation of cervical cancer cells, and inducing apoptosis.140 It is noteworthy that, to our knowledge, this study stands as the only report on the anticancer properties of empagliflozin.
Hypoxia-inducible factor-1α, a pivotal regulator of the hypoxic transcriptional response in cancer biology, plays a crucial role in various aspects, including glucose metabolism, initiation of the Warburg effect, angiogenesis, cell proliferation and survival, and invasion and metastasis, ultimately contributing to tumor resistance against cytotoxic agents.152 In a recent study, canagliflozin not only inhibited the proliferation and clonogenic survival of NSCLC in vitro and in vivo but also enhanced the effectiveness of radiotherapy to mediate these effects.148 Canagliflozin down-regulated hypoxia-inducible factor-1α protein levels and genes regulating its stability. Down-regulated hypoxia-inducible factor-1α has been attributed to the activation of AMPK and inhibition of mTOR, which serve as critical activators of hypoxia-inducible factor-1α signaling.153
Inhibition of glucose uptake
Inhibiting glucose uptake through SGLT2 inhibitors enhanced their anticancer effects.23,24,129,132,138,141,142 Notably, canagliflozin, in addition to inhibiting SGLT2, exhibited a higher affinity for inhibiting SGLT1 compared with other SGLT2 inhibitors154 and may also have inhibited the glucose transporter glucose transporter 1.133 Tumor cells of various types tended to overexpress both SGLTs (SGLT1 and SGLT2) and glucose transporters,155 relying heavily on aerobic glycolysis and accelerated glucose uptake, which is known as the Warburg effect.156 Inhibiting glucose uptake in cancer cells led to an energetic crisis and apoptosis, thereby decreasing cancer cell proliferation.156 However, inhibiting glucose uptake was unlikely to be the primary mechanism behind the antiproliferative effects of SGLT2 inhibitors. This is supported by findings showing that canagliflozin demonstrated similar antiproliferative effects under both low- and high-glucose conditions, as well as under pyruvate- or acetate-supplemented conditions.24 Furthermore, canagliflozin, and to a lesser extent dapagliflozin, inhibited the proliferation of breast cancer lines characterized by high rates of aerobic glycolysis, and glucose uptake remained consistent regardless of the presence or absence of glucose.145
Enhancing anticancer immune response
Intriguingly, 2 recent studies have demonstrated that SGLT2 inhibitors boosted tumor-immune response and increased infiltrating immune cells through 2 distinct mechanisms.22,139 In the first study, SGLT2 was identified as a positive regulator of programmed cell death-ligand 1 (PD-L1) in SGLT2-expressing cancer cells,139 with their interaction on the cell membrane proving essential for maintaining the PD-L1 protein level. Importantly, PD-L1, expressed on tumor cells, bound to programmed cell death 1 on active T cells, facilitating tumor immune escape by preventing the T lymphocyte from targeting and destroying the cancer cells.157 Canagliflozin disturbed the physical interaction between SGLT2 and PD-L1, triggering the ubiquitination and proteasome-mediated degradation of PD-L1. This led to the down-regulation of PD-L1 expression on the cell membrane by preventing the recycling of internalized PD-L1 in NSCLC, ovarian cancer, and pancreatic cancer cell lines.139 When tested in vivo, both canagliflozin and silencing SGLT2 reduced PD-L1 expression, resulting in a restriction of tumor progression equivalent to the effect of anti–programmed cell death 1 antibody.139 This effect correlated with increased activity of antitumor cytotoxic T cells.139
In the second in vivo study,22 canagliflozin inhibited osteosarcoma tumor growth and increased infiltration of immune cells. This effect was achieved by up-regulating the expression of stimulator of interferon genes and activating the interferon regulatory factor 3/interferon beta pathway, known for its ability to induce immune cells.158 In this study, the expression level of SGLT2 was negatively correlated with the infiltration level of immune cells in sarcoma, including CD8+ T cells, CD4+ T cells, and CD4+ T helper type 2 cells.22 As a result, inhibiting SGLT2 using canagliflozin or silencing increased the number of infiltrated immune cells, thereby inhibiting tumor growth.22 The investigators attributed the activation of the stimulator of interferon genes/interferon regulatory factor 3/interferon beta pathway via protein kinase B (AKT) inhibition by canagliflozin. This association is based on previous reports indicating a negative correlation between the stimulator of interferon genes pathway and the level of AKT phosphorylation.158
Inhibition of phosphoinositide 3-kinase/AKT pathway
SGLT2 inhibitors have been shown to inhibit the phosphoinositide 3-kinase/AKT pathway in cancer cells,22,129,133,136,142 which is the most commonly activated pathway in human cancers.159 The oncogenic activation of the phosphoinositide 3-kinase/AKT pathway in cancer cells induced metabolic reprogramming, promoting abnormal cell growth, whereas the inhibition of this pathway resulted in reduced cellular proliferation and enhanced cellular death.159
Inhibition of the Hippo pathway
The Hippo pathway is a highly conserved signaling pathway that controls organ size, tissue homeostasis, and cancer development.160 Recent studies have revealed that the Hippo signaling pathway might have contributed to tumorigenesis and cancer development.161 SGLT2, in particular, has been shown to interact with heterogeneous nuclear ribonucleoprotein K, promoting its nuclear translocation, which in turn promoted the expression of YES-associated protein 1, the downstream regulator of the Hippo pathway.134 SGLT2 inhibition by canagliflozin has been shown to inhibit the Hippo signaling pathway by down-regulating YES-associated protein 1 expression in pancreatic cancer.134
Highlights
-
•
Epidemiologic studies associate SGLT2 inhibitors with improved overall survival in specific cancers.
-
•
Preclinical studies demonstrate potential anticancer effects of SGLT2 inhibitors, whether used alone or in combination with standard cancer treatments.
-
•
Potential mechanisms underlying the anticancer effects of SGLT2 inhibitors include AMPK activation, inhibition of mitochondrial complex I, inhibition of glucose uptake, and enhancement of immune response.
Conclusions and Future Directions
In conclusion, considering the favorable tolerability profile and widespread use of SGLT2 inhibitors in the management of T2DM, HF, and chronic kidney disease, these agents hold promise as potential candidates for drug repurposing in cardio-oncology and cancer treatment. This review sheds light on abundant preclinical evidence supporting the use of SGLT2 inhibitors in cardio-oncology. Despite this progress, a multitude of unanswered questions persists, prompting the need for further in-depth exploration.
First, it is imperative to conduct further head-to-head comparisons among different SGLT2 inhibitors, examining both cardioprotective and anticancer effects. Most studies demonstrating cardioprotective effects have used empagliflozin and dapagliflozin, whereas those investigating anticancer effects have used canagliflozin. Furthermore, although the reviewed studies have consistently shown the cardioprotective effects of SGLT2 inhibitors against cancer treatment–related cardiotoxicity in tumor-free models, none has yet concurrently demonstrated the cardioprotective and the anticancer effects of SGLT2 inhibitors in tumor-bearing models. There is a pressing need to investigate these effects in tumor-bearing models to assess their efficacy and safety in a more clinically relevant context, aiming to safeguard the heart in the presence of cancer and its treatments. However, from a clinical prospective, the support for the use of SGLT2 inhibitors in cardio-oncology is grounded predominantly in anecdotal evidence derived from observational and epidemiologic studies. Given the inherent nature of these studies, which included primarily patients with both diabetes and cancer, there arises a critical need for randomized clinical trials. These trials should specifically investigate the safety and efficacy of SGLT2 inhibitors in patients undergoing potentially cardiotoxic cancer treatments, encompassing both those with and without diabetes.
Funding Support and Author Disclosures
Dr Zordoky is supported by the SURRGE Award from the University of Minnesota College of Pharmacy and by National Institutes of Health grant R01HL151740. Dr Dabour is supported by a scholarship from the Egyptian Ministry of Higher Education. Dr George is supported by the Fulbright Visiting Scholar Postdoctoral Program, U.S. Department of State, Bureau of Educational and Cultural Affairs. Dr Blaes is supported by the University of Minnesota Cancer Center grant (P30CA077598), and by National Institutes of Health grants R01CA267977, 1R01CA277714-01, R21AG080503, and R13CA278261. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Hsia D.S., Grove O., Cefalu W.T. An update on SGLT2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73. doi: 10.1097/MED.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 3.Packer M., Anker S.D., Butler J., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 5.McMurray J.J., Solomon S.D., Inzucchi S.E., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 6.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 7.FDA approves new treatment for a type of heart failure. U.S. Food and Drug Administration. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure Accessed June 2023.
- 8.FDA approves empagliflozin for adults with HFrEF. American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2021/08/23/11/39/fda-approves-empagliflozin-for-adults-with-hfref Accessed June 2023.
- 9.Larkin H.D. FDA expands empagliflozin heart failure indication. JAMA. 2022;327(13):1219. doi: 10.1001/jama.2022.3970. [DOI] [PubMed] [Google Scholar]
- 10.Farxiga extended in the US to reduce risk of cardiovascular death and hospitalisation for heart failure to a broader range of patients. AstraZeneca. https://www.astrazeneca.com/media-centre/press-releases/2023/farxiga-extended-in-the-us-for-heart-failure.html Accessed June 2023.
- 11.Chen S., Coronel R., Hollmann M.W., Weber N.C., Zuurbier C.J. Direct cardiac effects of SGLT2 inhibitors. Cardiovasc Diabetol. 2022;21(1):45. doi: 10.1186/s12933-022-01480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elrakaybi A., Laubner K., Zhou Q., Hug M.J., Seufert J. Cardiovascular protection by SGLT2 inhibitors–do anti-inflammatory mechanisms play a role? Mol Metab. 2022;64 doi: 10.1016/j.molmet.2022.101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopaschuk G.D., Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. J Am Coll Cardiol Basic Trans Science. 2020;5(6):632–644. doi: 10.1016/j.jacbts.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packer M. Molecular, cellular, and clinical evidence that sodium-glucose cotransporter 2 inhibitors act as neurohormonal antagonists when used for the treatment of chronic heart failure. J Am Heart Assoc. 2020;9(16) doi: 10.1161/JAHA.120.016270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essa H., Dobson R., Wright D., Lip G.Y. Hypertension management in cardio-oncology. J Hum Hypertens. 2020;34(10):673–681. doi: 10.1038/s41371-020-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gongora C.A., Drobni Z.D., Quinaglia Araujo Costa Silva T., et al. Sodium-glucose co-transporter-2 inhibitors and cardiac outcomes among patients treated with anthracyclines. Heart Fail. 2022;10(8):559–567. doi: 10.1016/j.jchf.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatino J., De Rosa S., Tamme L., et al. Empagliflozin prevents doxorubicin-induced myocardial dysfunction. Cardiovasc Diabetol. 2020;19(1):66. doi: 10.1186/s12933-020-01040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Qadir H., Carrasco R., Austin P.C., et al. The association of sodium-glucose cotransporter 2 inhibitors with cardiovascular outcomes in anthracycline-treated patients with cancer. J Am Coll Cardiol CardioOnc. 2023;5(3):318–328. doi: 10.1016/j.jaccao.2023.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali F.E., Hassanein E.H., Abd El-Ghafar O.A., Rashwan E.K., Saleh F.M., Atwa A.M. Exploring the cardioprotective effects of canagliflozin against cisplatin-induced cardiotoxicity: role of iNOS/NF-κB, Nrf2, and Bax/cytochrome C/Bcl-2 signals. J Biochem Mol Toxicol. 2023;37(4) doi: 10.1002/jbt.23309. [DOI] [PubMed] [Google Scholar]
- 20.Ren C., Sun K., Zhang Y., et al. Sodium–glucose cotransporter-2 inhibitor empagliflozin ameliorates sunitinib-induced cardiac dysfunction via regulation of AMPK–mTOR signaling pathway–mediated autophagy. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.664181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Min J., Wu L., Liu Y., et al. Empagliflozin attenuates trastuzumab-induced cardiotoxicity through suppression of DNA damage and ferroptosis. Life Sci. 2023;312 doi: 10.1016/j.lfs.2022.121207. [DOI] [PubMed] [Google Scholar]
- 22.Wu W., Zhang Z., Jing D., et al. SGLT2 inhibitor activates the STING/IRF3/IFN-beta pathway and induces immune infiltration in osteosarcoma. Cell Death Dis. 2022;13(6):523. doi: 10.1038/s41419-022-04980-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nasiri A.R., Rodrigues M.R., Li Z., Leitner B.P., Perry R.J. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. 2019;7:10. doi: 10.1186/s40170-019-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villani L.A., Smith B.K., Marcinko K., et al. The diabetes medication canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Mol Metab. 2016;5(10):1048–1056. doi: 10.1016/j.molmet.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakano D., Kawaguchi T., Iwamoto H., Hayakawa M., Koga H., Torimura T. Effects of canagliflozin on growth and metabolic reprograming in hepatocellular carcinoma cells: Multi-omics analysis of metabolomics and absolute quantification proteomics (iMPAQT) PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0232283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anker S.D., Butler J., Filippatos G., et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 27.Solomon S.D., McMurray J.J., Claggett B., et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;387(12):1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 28.Perkovic V., Jardine M.J., Neal B., et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 29.Heerspink H.J.L., Stefansson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 30.The EMPA-KIDNEY Collaborative Group. Herrington W.G., Staplin N., et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–127. doi: 10.1056/NEJMoa2204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenneman C.G., Sawyer D.B. Cardio-oncology: an update on cardiotoxicity of cancer-related treatment. Circ Res. 2016;118(6):1008–1020. doi: 10.1161/CIRCRESAHA.115.303633. [DOI] [PubMed] [Google Scholar]
- 32.Cardinale D., Iacopo F., Cipolla C.M. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. 2020;7:26. doi: 10.3389/fcvm.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henson K., McGale P., Taylor C., Darby S. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108(1):179–182. doi: 10.1038/bjc.2012.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Azambuja E., Procter M.J., van Veldhuisen D.J., et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32(20):2159–2165. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 35.Catino A.B., Hubbard R.A., Chirinos J.A., et al. Longitudinal assessment of vascular function with sunitinib in patients with metastatic renal cell carcinoma. Circ Heart Fail. 2018;11(3) doi: 10.1161/CIRCHEARTFAILURE.117.004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ederhy S., Cautela J., Ancedy Y., Escudier M., Thuny F., Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. J Am Coll Cardiol Img. 2018;11(8):1187–1190. doi: 10.1016/j.jcmg.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 37.Waxman A.J., Clasen S., Hwang W.T., et al. Carfilzomib-associated cardiovascular adverse events: a systematic review and meta-analysis. JAMA Oncol. 2018;4(3) doi: 10.1001/jamaoncol.2017.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Avula V., Sharma G., Kosiborod M.N., et al. SGLT2 inhibitor use and risk of clinical events in patients with cancer therapy-related cardiac dysfunction. J Am Coll Cardiol HF. 2024;12(1):67–78. doi: 10.1016/j.jchf.2023.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Oh C.M., Cho S., Jang J.Y., et al. Cardioprotective potential of an SGLT2 inhibitor against doxorubicin-induced heart failure. Korean Circ J. 2019;49(12):1183–1195. doi: 10.4070/kcj.2019.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang C.C., Chen Y.T., Wallace C.G., et al. Early administration of empagliflozin preserved heart function in cardiorenal syndrome in rat. Biomed Pharmacother. 2019;109:658–670. doi: 10.1016/j.biopha.2018.10.095. [DOI] [PubMed] [Google Scholar]
- 41.Wang C.Y., Chen C.C., Lin M.H., et al. TLR9 binding to beclin 1 and mitochondrial SIRT3 by a sodium-glucose co-transporter 2 inhibitor protects the heart from doxorubicin toxicity. Biology (Basel) 2020;9(11):369. doi: 10.3390/biology9110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baris V.O., Dincsoy A.B., Gedikli E., Zirh S., Muftuoglu S., Erdem A. Empagliflozin significantly prevents the doxorubicin-induced acute cardiotoxicity via non-antioxidant pathways. Cardiovasc Toxicol. 2021;21(9):747–758. doi: 10.1007/s12012-021-09665-y. [DOI] [PubMed] [Google Scholar]
- 43.Quagliariello V., De Laurentiis M., Rea D., et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovasc Diabetol. 2021;20(1):150. doi: 10.1186/s12933-021-01346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang W.T., Lin Y.W., Ho C.H., Chen Z.C., Liu P.Y., Shih J.Y. Dapagliflozin suppresses ER stress and protects doxorubicin-induced cardiotoxicity in breast cancer patients. Arch Toxicol. 2021;95(2):659–671. doi: 10.1007/s00204-020-02951-8. [DOI] [PubMed] [Google Scholar]
- 45.Belen E., Canpolat I., Yiğittürk G., Cetinarslan Ö. Cardio-protective effect of dapagliflozin against doxorubicin induced cardiomyopathy in rats. Eur Rev Med Pharmacol Sci. 2022;26:4403–4408. doi: 10.26355/eurrev_202206_29079. [DOI] [PubMed] [Google Scholar]
- 46.Chang W.T., Shih J.Y., Lin Y.W., et al. Dapagliflozin protects against doxorubicin-induced cardiotoxicity by restoring STAT3. Arch Toxicol. 2022;96(7):2021–2032. doi: 10.1007/s00204-022-03298-y. [DOI] [PubMed] [Google Scholar]
- 47.Hsieh P.L., Chu P.M., Cheng H.C., et al. Dapagliflozin mitigates doxorubicin-caused myocardium damage by regulating AKT-mediated oxidative stress, cardiac remodeling, and inflammation. Int J Mol Sci. 2022;23(17) doi: 10.3390/ijms231710146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim D., Jang G., Hwang J., et al. Combined therapy of low-dose angiotensin receptor-neprilysin inhibitor and sodium-glucose cotransporter-2 inhibitor prevents doxorubicin-induced cardiac dysfunction in rodent model with minimal adverse effects. Pharmaceutics. 2022;14(12):2629. doi: 10.3390/pharmaceutics14122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen M. Empagliflozin attenuates doxorubicin-induced cardiotoxicity by activating AMPK/SIRT-1/PGC-1alpha-mediated mitochondrial biogenesis. Toxicol Res (Camb) 2023;12(2):216–223. doi: 10.1093/toxres/tfad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu J., Xu J., Tan X., et al. Dapagliflozin protects against dilated cardiomyopathy progression by targeting NLRP3 inflammasome activation. Naunyn Schmiedebergs Arch Pharmacol. 2023;396(7):1461–1470. doi: 10.1007/s00210-023-02409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Satyam S.M., Bairy L.K., Shetty P., et al. Metformin and dapagliflozin attenuate doxorubicin-induced acute cardiotoxicity in Wistar rats: an electrocardiographic, biochemical, and histopathological approach. Cardiovasc Toxicol. 2023;23(2):107–119. doi: 10.1007/s12012-023-09784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quagliariello V., Paccone A., Iovine M., et al. C12 Dapagliflozin increases p-AMPK and reduces myocardial and renal NF-kB expression in preclinical models of short-term doxorubicin cardiotoxicity through MYD-188 and NLRP3 pathways. Eur Heart J Suppl. 2023:25. (Supplement_D):D5. [Google Scholar]
- 53.Ulusan S., Gulle K., Peynirci A., Sevimli M., Karaibrahimoglu A., Kuyumcu M.S. Dapagliflozin may protect against doxorubicin-induced cardiotoxicity. Anatol J Cardiol. 2023;27(6):339–347. doi: 10.14744/AnatolJCardiol.2023.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maurea N., Quagliariello V., Bonelli A., et al. TheSGLT-2 inhibitor dapagliflozin enhanced the anticancer activities and exerts cardioprotective effects during exposure to ipilimumab through NLRP3 inflammasome and pro-fibrotic cytokines. Eur Heart J. 2021;42(Supplement_1) [Google Scholar]
- 55.Quagliariello V., De Laurentiis M., Cocco S., et al. NLRP3 as putative marker of ipilimumab-induced cardiotoxicity in the presence of hyperglycemia in estrogen-responsive and triple-negative breast cancer cells. Int J Mol Sci. 2020;21(20):7802. doi: 10.3390/ijms21207802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Madonna R., Barachini S., Moscato S., et al. Sodium-glucose cotransporter type 2 inhibitors prevent ponatinib-induced endothelial senescence and disfunction: a potential rescue strategy. Vascul Pharmacol. 2022;142 doi: 10.1016/j.vph.2021.106949. [DOI] [PubMed] [Google Scholar]
- 57.Dabour M.S., Abdelgawad I.Y., Grant M.K., El-Sawaf E.S., Zordoky B.N. Canagliflozin mitigates carfilzomib-induced endothelial apoptosis via an AMPK-dependent pathway. Biomed Pharmacother. 2023;164 doi: 10.1016/j.biopha.2023.114907. [DOI] [PubMed] [Google Scholar]
- 58.Santos-Gallego C.G., Vargas-Delgado A.P., Requena-Ibanez J.A., et al. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. 2021;77(3):243–255. doi: 10.1016/j.jacc.2020.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Omar M., Jensen J., Kistorp C., et al. The effect of empagliflozin on growth differentiation factor 15 in patients with heart failure: a randomized controlled trial (Empire HF Biomarker) Cardiovasc Diabetol. 2022;21(1):34. doi: 10.1186/s12933-022-01463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee M.M.Y., Brooksbank K.J.M., Wetherall K., et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR-DM-HF) Circulation. 2021;143(6):516–525. doi: 10.1161/CIRCULATIONAHA.120.052186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dhingra N.K., Mistry N., Puar P., et al. SGLT2 inhibitors and cardiac remodelling: a systematic review and meta-analysis of randomized cardiac magnetic resonance imaging trials. ESC Heart Fail. 2021;8(6):4693–4700. doi: 10.1002/ehf2.13645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Linthout S., Tschöpe C. Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep. 2017;14:251–265. doi: 10.1007/s11897-017-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choksey A., Timm K.N. Cancer therapy-induced cardiotoxicity—a metabolic perspective on pathogenesis, diagnosis and therapy. Int J Mol Sci. 2021;23(1):441. doi: 10.3390/ijms23010441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boopathi E., Thangavel C. Dark side of cancer therapy: cancer treatment-induced cardiopulmonary inflammation, fibrosis, and immune modulation. Int J Mol Sci. 2021;22(18) doi: 10.3390/ijms221810126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pecoraro M., Del Pizzo M., Marzocco S., et al. Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol. 2016;293:44–52. doi: 10.1016/j.taap.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Prathumsap N., Shinlapawittayatorn K., Chattipakorn S.C., Chattipakorn N. Effects of doxorubicin on the heart: from molecular mechanisms to intervention strategies. Eur J Pharmacol. 2020;866 doi: 10.1016/j.ejphar.2019.172818. [DOI] [PubMed] [Google Scholar]
- 67.Shi S., Chen Y., Luo Z., Nie G., Dai Y. Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy. J Cell Commun Signal. 2023;21(1):1–20. doi: 10.1186/s12964-023-01077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scisciola L., Taktaz F., Fontanella R.A., et al. Targeting high glucose-induced epigenetic modifications at cardiac level: the role of SGLT2 and SGLT2 inhibitors. Cardiovasc Diabetol. 2023;22(1):1–14. doi: 10.1186/s12933-023-01754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Byrne N.J., Matsumura N., Maayah Z.H., et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Heart Fail. 2020;13(1) doi: 10.1161/CIRCHEARTFAILURE.119.006277. [DOI] [PubMed] [Google Scholar]
- 70.Ye Y., Bajaj M., Yang H.-C., Perez-Polo J.R., Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovasc Drugs Ther. 2017;31:119–132. doi: 10.1007/s10557-017-6725-2. [DOI] [PubMed] [Google Scholar]
- 71.Long Q., Li L., Yang H., et al. SGLT2 inhibitor, canagliflozin, ameliorates cardiac inflammation in experimental autoimmune myocarditis. Int Immunopharmacol. 2022;110 doi: 10.1016/j.intimp.2022.109024. [DOI] [PubMed] [Google Scholar]
- 72.Kim S.R., Lee S.-G., Kim S.H., et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nat Commun. 2020;11(1):2127. doi: 10.1038/s41467-020-15983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fuchs Andersen C., Omar M., Glenthøj A., et al. Effects of empagliflozin on erythropoiesis in heart failure: data from the Empire HF trial. Eur J Heart Fail. 2023;25(2):226–234. doi: 10.1002/ejhf.2735. [DOI] [PubMed] [Google Scholar]
- 74.Angsutararux P., Luanpitpong S., Issaragrisil S. Chemotherapy-induced cardiotoxicity: overview of the roles of oxidative stress. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/795602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ping Z., Peng Y., Lang H., et al. Oxidative stress in radiation-induced cardiotoxicity. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/3579143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li C., Zhang J., Xue M., et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc Diabetol. 2019;18:1–13. doi: 10.1186/s12933-019-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun X., Han F., Lu Q., et al. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat diet–induced obese mice. Diabetes. 2020;69(6):1292–1305. doi: 10.2337/db19-0991. [DOI] [PubMed] [Google Scholar]
- 78.Lu Q., Liu J., Li X., et al. Empagliflozin attenuates ischemia and reperfusion injury through LKB1/AMPK signaling pathway. Mol Cell Endocrinol. 2020;501 doi: 10.1016/j.mce.2019.110642. [DOI] [PubMed] [Google Scholar]
- 79.Li X., Flynn E.R., do Carmo J.M., et al. Direct cardiac actions of sodium-glucose cotransporter 2 inhibition improve mitochondrial function and attenuate oxidative stress in pressure overload-induced heart failure. Front Cardiovasc Med. 2022;9:1159. doi: 10.3389/fcvm.2022.859253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kolijn D., Pabel S., Tian Y., et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res. 2021;117(2):495–507. doi: 10.1093/cvr/cvaa123. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka Y., Nagoshi T., Yoshii A., et al. Xanthine oxidase inhibition attenuates doxorubicin-induced cardiotoxicity in mice. Free Radic Biol Med. 2021;162:298–308. doi: 10.1016/j.freeradbiomed.2020.10.303. [DOI] [PubMed] [Google Scholar]
- 82.Lin R., Peng X., Li Y., et al. Empagliflozin attenuates doxorubicin-impaired cardiac contractility by suppressing reactive oxygen species in isolated myocytes. Mol Cell Biochem. 2023 doi: 10.1007/s11010-023-04830-z. Published online August 30. [DOI] [PubMed] [Google Scholar]
- 83.Bagchi A.K., Malik A., Akolkar G., et al. Study of ER stress and apoptotic proteins in the heart and tumor exposed to doxorubicin. Biochim Biophys Acta Mol Cell Res. 2021;1868(7) doi: 10.1016/j.bbamcr.2021.119039. [DOI] [PubMed] [Google Scholar]
- 84.Ren F., Xie Z., Jiang Y., et al. Dapagliflozin attenuates pressure overload-induced myocardial remodeling in mice via activating SIRT1 and inhibiting endoplasmic reticulum stress. Acta Pharmacol Sin. 2022;43(7):1721–1732. doi: 10.1038/s41401-021-00805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang C.C., Li Y., Qian X.Q., et al. Empagliflozin alleviates myocardial I/R injury and cardiomyocyte apoptosis via inhibiting ER stress-induced autophagy and the PERK/ATF4/Beclin1 pathway. J Drug Target. 2022;30(8):858–872. doi: 10.1080/1061186X.2022.2064479. [DOI] [PubMed] [Google Scholar]
- 86.Herring R.A., Shojaee-Moradie F., Stevenage M., et al. The SGLT2 inhibitor dapagliflozin increases the oxidation of ingested fatty acids to ketones in type 2 diabetes. Diabetes Care. 2022;45(6):1408–1415. doi: 10.2337/dc21-2043. [DOI] [PubMed] [Google Scholar]
- 87.Voros G., Ector J., Garweg C., et al. Increased cardiac uptake of ketone bodies and free fatty acids in human heart failure and hypertrophic left ventricular remodeling. Circ Heart Fail. 2018;11(12) doi: 10.1161/CIRCHEARTFAILURE.118.004953. [DOI] [PubMed] [Google Scholar]
- 88.Verma S., Rawat S., Ho K.L., et al. Empagliflozin increases cardiac energy production in diabetes: novel translational insights into the heart failure benefits of SGLT2 inhibitors. J Am Coll Cardiol Basic Trans Science. 2018;3(5):575–587. doi: 10.1016/j.jacbts.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng Y., Xie M., Li Q., et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ Res. 2021;128(2):232–245. doi: 10.1161/CIRCRESAHA.120.317933. [DOI] [PubMed] [Google Scholar]
- 90.Hundertmark M.J., Adler A., Antoniades C., et al. Assessment of cardiac energy metabolism, function, and physiology in patients with heart failure taking empagliflozin: the randomized, controlled EMPA-VISION trial. Circulation. 2023;147(22):1654–1669. doi: 10.1161/CIRCULATIONAHA.122.062021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Packer M. Critical reanalysis of the mechanisms underlying the cardiorenal benefits of SGLT2 inhibitors and reaffirmation of the nutrient deprivation signaling/autophagy hypothesis. Circulation. 2022;146(18):1383–1405. doi: 10.1161/CIRCULATIONAHA.122.061732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sadria M., Layton A.T. Interactions among mTORC, AMPK and SIRT: a computational model for cell energy balance and metabolism. J Cell Commun Signal. 2021;19(1):1–17. doi: 10.1186/s12964-021-00706-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J., Zhang J., Xiao M., et al. Molecular mechanisms of doxorubicin-induced cardiotoxicity: novel roles of sirtuin 1-mediated signaling pathways. Cell Mol Life Sci. 2021;78:3105–3125. doi: 10.1007/s00018-020-03729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Timm K.N., Tyler D.J. The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc Drugs Ther. 2020;34(2):255–269. doi: 10.1007/s10557-020-06941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hawley S.A., Ford R.J., Smith B.K., et al. The Na+/glucose co-transporter inhibitor canagliflozin activates AMP-activated protein kinase by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65(9):2784–2794. doi: 10.2337/db16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nikolaou P.E., Mylonas N., Makridakis M., et al. Cardioprotection by selective SGLT-2 inhibitors in a non-diabetic mouse model of myocardial ischemia/reperfusion injury: a class or a drug effect? Basic Res Cardiol. 2022;117(1):27. doi: 10.1007/s00395-022-00934-7. [DOI] [PubMed] [Google Scholar]
- 97.Ye Y., Jia X., Bajaj M., Birnbaum Y. Dapagliflozin attenuates Na+/H+ exchanger-1 in cardiofibroblasts via AMPK activation. Cardiovasc Drugs Ther. 2018;32(6):553–558. doi: 10.1007/s10557-018-6837-3. [DOI] [PubMed] [Google Scholar]
- 98.Tsai K.-L., Hsieh P.-L., Chou W.-C., Cheng H.-C., Huang Y.-T., Chan S.-H. Dapagliflozin attenuates hypoxia/reoxygenation-caused cardiac dysfunction and oxidative damage through modulation of AMPK. Cell Biosci. 2021;11(1):44. doi: 10.1186/s13578-021-00547-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aragon-Herrera A., Feijoo-Bandin S., Santiago M.O., et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochem Pharmacol. 2019;170 doi: 10.1016/j.bcp.2019.113677. [DOI] [PubMed] [Google Scholar]
- 100.Mancini S.J., Boyd D., Katwan O.J., et al. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and-independent mechanisms. Sci Rep. 2018;8(1):1–14. doi: 10.1038/s41598-018-23420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uthman L., Kuschma M., Römer G., et al. Novel anti-inflammatory effects of canagliflozin involving hexokinase II in lipopolysaccharide-stimulated human coronary artery endothelial cells. Cardiovasc Drugs Ther. 2021;35:1083–1094. doi: 10.1007/s10557-020-07083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li M., Russo M., Pirozzi F., Tocchetti C.G., Ghigo A. Autophagy and cancer therapy cardiotoxicity: from molecular mechanisms to therapeutic opportunities. Biochim Biophys Acta Mol Cell Res. 2020;1867(3) doi: 10.1016/j.bbamcr.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 103.Li D.L., Wang Z.V., Ding G., et al. Doxorubicin blocks cardiomyocyte autophagic flux by inhibiting lysosome acidification. Circulation. 2016;133(17):1668–1687. doi: 10.1161/CIRCULATIONAHA.115.017443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ajoolabady A., Tuomilehto J., Lip G.Y., Klionsky D.J., Ren J. Deciphering the role of autophagy in heart failure. Cardiol Plus. 2021;6(2):92–101. [Google Scholar]
- 105.Yamaguchi O. Autophagy in the heart. Circ J. 2019;83(4):697–704. doi: 10.1253/circj.CJ-18-1065. [DOI] [PubMed] [Google Scholar]
- 106.Zannad F., Ferreira J.P., Butler J., et al. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights into the EMPEROR programme. Eur Heart J. 2022;43(48):4991–5002. doi: 10.1093/eurheartj/ehac495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fang X., Ardehali H., Min J., Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20(1):7–23. doi: 10.1038/s41569-022-00735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Beretta G.L. Ferroptosis-induced cardiotoxicity and antitumor drugs. Curr Med Chem. 2023 doi: 10.2174/0929867331666230719124453. Published August 7. [DOI] [PubMed] [Google Scholar]
- 109.Du S., Shi H., Xiong L., Wang P., Shi Y. Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front Endocrinol (Lausanne) 2022;13 doi: 10.3389/fendo.2022.1011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma S., He L.-L., Zhang G.-R., et al. Canagliflozin mitigates ferroptosis and ameliorates heart failure in rats with preserved ejection fraction. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(8):945–962. doi: 10.1007/s00210-022-02243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang W., Lu J., Wang Y., et al. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway. Int J Mol Sci. 2023;24(1):858. doi: 10.3390/ijms24010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen W., Zhang Y., Wang Z., et al. Dapagliflozin alleviates myocardial ischemia/reperfusion injury by reducing ferroptosis via MAPK signaling inhibition. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1078205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ferrannini E., Murthy A.C., Lee Y.H., et al. Mechanisms of sodium-glucose cotransporter 2 inhibition: insights from large-scale proteomics. Diabetes Care. 2020;43(9):2183–2189. doi: 10.2337/dc20-0456. [DOI] [PubMed] [Google Scholar]
- 114.Krishnarao K., Bruno K.A., Di Florio D.N., et al. Upregulation of endothelin-1 may predict chemotherapy-induced cardiotoxicity in women with breast cancer. J Clin Med. 2022;11(12):3547. doi: 10.3390/jcm11123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maayah Z.H., Ferdaoussi M., Boukouris A.E., et al. Endothelin receptor blocker reverses breast cancer–induced cardiac remodeling. J Am Coll Cardiol CardioOnc. 2023;5(5):686–700. doi: 10.1016/j.jaccao.2023.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Atas E., Kismet E., Kesik V., et al. Cardiac troponin-I, brain natriuretic peptide and endothelin-1 levels in a rat model of doxorubicin-induced cardiac injury. J Cancer Res Ther. 2015;11(4):882–886. doi: 10.4103/0973-1482.144636. [DOI] [PubMed] [Google Scholar]
- 117.El-Boghdady N.A. Increased cardiac endothelin-1 and nitric oxide in adriamycin-induced acute cardiotoxicity: protective effect of Ginkgo biloba extract. Indian J Biochem Biophys. 2013;50(3):202–209. [PubMed] [Google Scholar]
- 118.Sourdon J., Facchin C., Certain A., et al. Sunitinib-induced cardiac hypertrophy and the endothelin axis. Theranostics. 2021;11(8):3830. doi: 10.7150/thno.49837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kappers M.H., de Beer V.J., Zhou Z., et al. Sunitinib-induced systemic vasoconstriction in swine is endothelin mediated and does not involve nitric oxide or oxidative stress. Hypertension. 2012;59(1):151–157. doi: 10.1161/HYPERTENSIONAHA.111.182220. [DOI] [PubMed] [Google Scholar]
- 120.Yeoh S.E., Docherty K.F., Campbell R.T., et al. Endothelin-1, outcomes in patients with heart failure and reduced ejection fraction, and effects of dapagliflozin: findings from DAPA-HF. Circulation. 2023;147(22):1670–1683. doi: 10.1161/CIRCULATIONAHA.122.063327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pirklbauer M., Bernd M., Fuchs L., et al. Empagliflozin inhibits basal and IL-1beta-mediated MCP-1/CCL2 and endothelin-1 expression in human proximal tubular cells. Int J Mol Sci. 2020;21(21):8189. doi: 10.3390/ijms21218189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shi N., Shi Y., Xu J., et al. SGLT-2i and risk of malignancy in type 2 diabetes: a meta-analysis of randomized controlled trials. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.668368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia M., Arteche-Martinez U., Lertxundi U., Aguirre C. SGLT2 inhibitors and bladder cancer: analysis of cases reported in the European Pharmacovigilance Database. J Clin Pharmacol. 2021;61(2):187–192. doi: 10.1002/jcph.1722. [DOI] [PubMed] [Google Scholar]
- 124.Gallo M., Monami M., Ragni A., Renzelli V. Cancer related safety with SGLT2-i and GLP1-RAs: should we worry? Diabetes Res Clin Pract. 2023;198 doi: 10.1016/j.diabres.2023.110624. [DOI] [PubMed] [Google Scholar]
- 125.Spiazzi B.F., Naibo R.A., Wayerbacher L.F., et al. Sodium-glucose cotransporter-2 inhibitors and cancer outcomes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2023;198 doi: 10.1016/j.diabres.2023.110621. [DOI] [PubMed] [Google Scholar]
- 126.Pelletier R., Ng K., Alkabbani W., Labib Y., Mourad N., Gamble J.M. The association of sodium-glucose cotransporter 2 inhibitors with cancer: an overview of quantitative systematic reviews. Endocrinol Diabetes Metab. 2020;3(3) doi: 10.1002/edm2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Luo J., Hendryx M., Dong Y. Sodium-glucose cotransporter 2 (SGLT2) inhibitors and non-small cell lung cancer survival. Br J Cancer. 2023;128(8):1541–1547. doi: 10.1038/s41416-023-02177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hendryx M., Dong Y., Ndeke J.M., Luo J. Sodium-glucose cotransporter 2 (SGLT2) inhibitor initiation and hepatocellular carcinoma prognosis. PLoS One. 2022;17(9) doi: 10.1371/journal.pone.0274519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaji K., Nishimura N., Seki K., et al. Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer. 2018;142(8):1712–1722. doi: 10.1002/ijc.31193. [DOI] [PubMed] [Google Scholar]
- 130.Hung M.H., Chen Y.L., Chen L.J., et al. Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose-influx-induced beta-catenin activation. Cell Death Dis. 2019;10(6):420. doi: 10.1038/s41419-019-1646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shiba K., Tsuchiya K., Komiya C., et al. Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep. 2018;8(1):2362. doi: 10.1038/s41598-018-19658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shoda K., Tsuji S., Nakamura S., et al. Canagliflozin inhibits glioblastoma growth and proliferation by activating AMPK. Cell Mol Neurobiol. 2023;43(2):879–892. doi: 10.1007/s10571-022-01221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu D., Zhou Y., Xie X., et al. Inhibitory effects of canagliflozin on pancreatic cancer are mediated via the downregulation of glucose transporter-1 and lactate dehydrogenase A. Int J Oncol. 2020;57(5):1223–1233. doi: 10.3892/ijo.2020.5120. [DOI] [PubMed] [Google Scholar]
- 134.Ren D., Sun Y., Zhang D., et al. SGLT2 promotes pancreatic cancer progression by activating the Hippo signaling pathway via the hnRNPK-YAP1 axis. Cancer Lett. 2021;519:277–288. doi: 10.1016/j.canlet.2021.07.035. [DOI] [PubMed] [Google Scholar]
- 135.Scafoglio C., Hirayama B.A., Kepe V., et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A. 2015;112(30):E4111–E4119. doi: 10.1073/pnas.1511698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li H., Tong C.W., Leung Y., Wong M.H., To K.K., Leung K.S. Identification of clinically approved drugs indacaterol and canagliflozin for repurposing to treat epidermal growth factor tyrosine kinase inhibitor-resistant lung cancer. Front Oncol. 2017;7:288. doi: 10.3389/fonc.2017.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yamamoto L., Yamashita S., Nomiyama T., et al. Sodium-glucose cotransporter 2 inhibitor canagliflozin attenuates lung cancer cell proliferation in vitro. Diabetol Int. 2021;12(4):389–398. doi: 10.1007/s13340-021-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Scafoglio C.R., Villegas B., Abdelhady G., et al. Sodium-glucose transporter 2 is a diagnostic and therapeutic target for early-stage lung adenocarcinoma. Sci Transl Med. 2018;10(467) doi: 10.1126/scitranslmed.aat5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ding L., Chen X., Zhang W., et al. Canagliflozin primes antitumor immunity by triggering PD-L1 degradation in endocytic recycling. J Clin Invest. 2023;133(1) doi: 10.1172/JCI154754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xie Z., Wang F., Lin L., et al. An SGLT2 inhibitor modulates SHH expression by activating AMPK to inhibit the migration and induce the apoptosis of cervical carcinoma cells. Cancer Lett. 2020;495:200–210. doi: 10.1016/j.canlet.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 141.Kuang H., Liao L., Chen H., Kang Q., Shu X., Wang Y. Therapeutic effect of sodium glucose co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med Sci Monit. 2017;23:3737–3745. doi: 10.12659/MSM.902530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Y., Yang L., Mao L., et al. SGLT2 inhibition restrains thyroid cancer growth via G1/S phase transition arrest and apoptosis mediated by DNA damage response signaling pathways. Cancer Cell Int. 2022;22(1):74. doi: 10.1186/s12935-022-02496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou J., Zhu J., Yu S.-J., et al. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110821. [DOI] [PubMed] [Google Scholar]
- 144.Komatsu S., Nomiyama T., Numata T., et al. SGLT2 inhibitor ipragliflozin attenuates breast cancer cell proliferation. Endocr J. 2020;67(1):99–106. doi: 10.1507/endocrj.EJ19-0428. [DOI] [PubMed] [Google Scholar]
- 145.Papadopoli D., Uchenunu O., Palia R., et al. Perturbations of cancer cell metabolism by the antidiabetic drug canagliflozin. Neoplasia. 2021;23(4):391–399. doi: 10.1016/j.neo.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dutka M., Bobiński R., Francuz T., et al. SGLT-2 inhibitors in cancer treatment—mechanisms of action and emerging new perspectives. Cancers (Basel) 2022;14(23):5811. doi: 10.3390/cancers14235811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lau K.T., Ng L., Wong J.W., et al. Repurposing sodium-glucose co-transporter 2 inhibitors (SGLT2i) for cancer treatment—a review. Rev Endocr Metab Disord. 2021;22(4):1121–1136. doi: 10.1007/s11154-021-09675-9. [DOI] [PubMed] [Google Scholar]
- 148.Biziotis O.D., Tsakiridis E.E., Ali A., et al. Canagliflozin mediates tumor suppression alone and in combination with radiotherapy in non-small cell lung cancer (NSCLC) through inhibition of HIF-1α. Mol Oncol. 2023;17(11):2235–2256. doi: 10.1002/1878-0261.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun Y., Tao C., Huang X., et al. Metformin induces apoptosis of human hepatocellular carcinoma HepG2 cells by activating an AMPK/p53/miR-23a/FOXA1 pathway. Onco Targets Ther. 2016:2845–2853. doi: 10.2147/OTT.S99770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jeng K.-S., Chang C.-F., Lin S.-S. Sonic hedgehog signaling in organogenesis, tumors, and tumor microenvironments. Int J Mol Sci. 2020;21(3):758. doi: 10.3390/ijms21030758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kugler M.C., Joyner A.L., Loomis C.A., Munger J.S. Sonic hedgehog signaling in the lung. From development to disease. Am J Respir Cell Mol Biol. 2015;52(1):1–13. doi: 10.1165/rcmb.2014-0132TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Masoud G.N., Li W. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dodd K.M., Yang J., Shen M.H., Sampson J.R., Tee A.R. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2015;34(17):2239–2250. doi: 10.1038/onc.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scheen A.J. Pharmacodynamics, efficacy and safety of sodium–glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 155.Szablewski L. Expression of glucose transporters in cancers. Biochim Biophys Acta Rev Cancer. 2013;1835(2):164–169. doi: 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 156.Potter M., Newport E., Morten K.J. The Warburg effect: 80 years on. Biochem Soc Trans. 2016;44(5):1499–1505. doi: 10.1042/BST20160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Han Y., Liu D., Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020;10(3):727. [PMC free article] [PubMed] [Google Scholar]
- 158.Wu S., Zhang Q., Zhang F., et al. HER2 recruits AKT1 to disrupt STING signalling and suppress antiviral defence and antitumour immunity. Nat Cell Biol. 2019;21(8):1027–1040. doi: 10.1038/s41556-019-0352-z. [DOI] [PubMed] [Google Scholar]
- 159.Hoxhaj G., Manning B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20(2):74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xiao Y., Dong J. The hippo signaling pathway in cancer: a cell cycle perspective. Cancers (Basel) 2021;13(24):6214. doi: 10.3390/cancers13246214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zygulska A., Krzemieniecki K., Pierzchalski P. Hippo pathway—brief overview of its relevance in cancer. J Physiol Pharmacol. 2017;68(3):311–335. [PubMed] [Google Scholar]
- 162.Eliaa S.G., Al-Karmalawy A.A., Saleh R.M., Elshal M.F. Empagliflozin and doxorubicin synergistically inhibit the survival of triple-negative breast cancer cells via interfering with the mTOR pathway and inhibition of calmodulin: in vitro and molecular docking studies. ACS Pharmacol Transl Sci. 2020;3(6):1330–1338. doi: 10.1021/acsptsci.0c00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhong J., Sun P., Xu N., et al. Canagliflozin inhibits p-gp function and early autophagy and improves the sensitivity to the antitumor effect of doxorubicin. Biochem Pharmacol. 2020;175 doi: 10.1016/j.bcp.2020.113856. [DOI] [PubMed] [Google Scholar]