Abstract
Close monitoring for cardiotoxicity during anthracycline chemotherapy is crucial for early diagnosis and therapy guidance. Currently, monitoring relies on cardiac imaging and serial measurement of cardiac biomarkers like cardiac troponin and natriuretic peptides. However, these conventional biomarkers are nonspecific indicators of cardiac damage. Exploring new, more specific biomarkers with a clear link to the underlying pathomechanism of cardiotoxicity holds promise for increased specificity and sensitivity in detecting early anthracycline-induced cardiotoxicity. miRNAs (microRNAs), small single-stranded, noncoding RNA sequences involved in epigenetic regulation, influence various physiological and pathological processes by targeting expression and translation. Emerging as new biomarker candidates, circulating miRNAs exhibit resistance to degradation and offer a direct pathomechanistic link. This review comprehensively outlines their potential as early biomarkers for cardiotoxicity and their pathomechanistic link.
Key Words: cardiomyopathy, cardiotoxicity, chemotherapy, circulating biomarkers, epigenetics, heart failure
Central Illustration
Highlights
-
•
Circulating microRNAs (cmiRNAs), epigenetic regulators, are stable and provide a link to the mechanisms of anthracycline-induced cardiotoxicity.
-
•
Several cmiRNAs, mainly miR-34a, have a cardiac origin and a role in cardiotoxicity.
-
•
Currently, the use of cmiRNAs in clinical practice is limited by lack of standardization.
-
•
Future research should focus on uniform protocols for cmiRNA detection and quantification, and larger studies for biomarker validation.
Anthracyclines are one of the most frequently used and effective chemotherapeutic agents in the treatment of breast cancer, leukemia, and lymphoma.1 Within cancer tissues, anthracyclines bind with topoisomerase 2 alfa (Top2α) and DNA to form a ternary complex, inducing double-stranded DNA breaks, the inhibition of DNA replication, cell cycle arrest, and apoptosis.1
The main concern associated with anthracycline chemotherapy is the potential for cardiotoxicity, leading to the clinical entity known as cancer therapy–related cardiac dysfunction (CTRCD).2 In a recent registry of adult cancer patients undergoing anthracycline treatment, cardiotoxicity was identified in 37.5% of patients during follow-up. Moreover, up to 9% experienced a clinically significant decrease in left ventricular ejection fraction (LVEF) within the first year after completing anthracycline chemotherapy.3,4 The administration of preventive measures such as renin angiotensin aldosterone system (RAAS) blockers, beta-blockers, or mineralocorticoid receptor antagonists remains debatable, given the inconclusive results from randomized controlled trials failing to demonstrate a protective effect.2 Nevertheless, in cases where CTRCD has developed, timely interventions, such as guideline-directed medical therapy for heart failure, could halt and even fully reverse the cardiac dysfunction. This underscores the therapeutic potential and highlights the significance of avoiding premature interruption of anthracycline chemotherapy, directly affecting cancer prognosis.
Recognizing the vital importance of early detection in CTRCD, the 2022 guidelines from the European Society of Cardiology in the field of cardio-oncology recommend close monitoring during anthracycline chemotherapy to facilitate early diagnosis and guide therapy recommendations.2 In addition to using cardiac imaging, monitoring practices include the serial measurement of cardiac biomarkers, such as cardiac troponin (cTn) and natriuretic peptides (NP).2 Nevertheless, it is crucial to note that these guidelines acknowledge the potential limitations associated with the use of these proposed biomarkers. Reference values for cTn and NP have not been established for patients with cancer or those undergoing cancer treatment. Additionally, both cTn and NP, being nonspecific markers of cardiac damage, are susceptible to influences by patient characteristics (eg, sex, race, body mass index) and comorbidities (eg, renal disease, infections).2 Therefore, improving the specificity and sensitivity in detecting early anthracycline-induced cardiotoxicity necessitates the exploration of more specific biomarkers, preferably those with a well-established association with the underlying pathomechanism.
Mechanisms of anthracycline-induced cardiotoxicity
The pathomechanisms of anthracycline-induced cardiotoxicity are multifactorial (Figure 1).
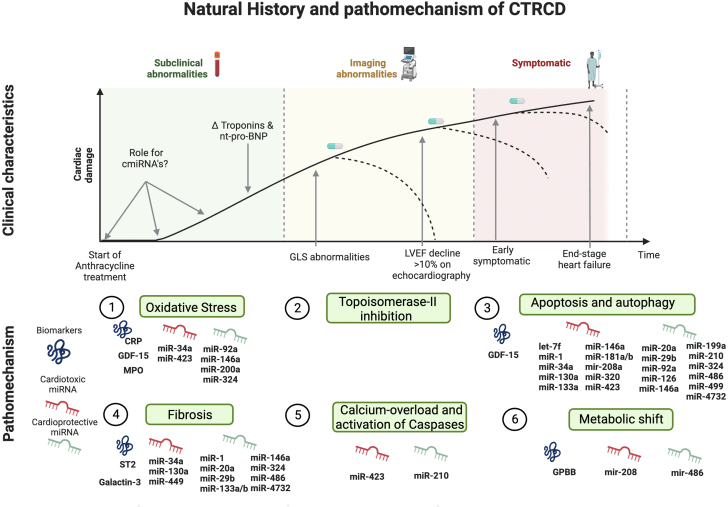
Figure 1.
Position of miRNAs in the Diagnostic Landscape and Mechanisms of CTRCD
As cardiac damage develops over time, detection methods vary to detect the presence of cancer therapy–related cardiac dysfunction (CTRCD). Serum cardiac biomarkers such as (hs-)troponins I and T and natriuretic peptides are early, but nonspecific, markers. As cardiac damage progresses, cardiac dysfunction emerges, starting with a decrease in global longitudinal change (GLS) and followed by a more pronounced systolic dysfunction and reduction of left ventricular ejection fraction (LVEF). Patients will become symptomatic at a later stage. Circulating microRNAs (cmiRNAs) are epigenetic regulators and more specific for anthracycline-induced cardiotoxicity, mechanistically linked to the mode of action of anthracyclines, and are detectable before irreversible cardiac damage occurs (potentially reversible stage). Created with BioRender.com. CRP = C-reactive protein; GDF = growth differentiation factor; GPBB = glycogen phosphorylase BB; miRNA = micro-RNA; MPO = myeloperoxidase; NT-proBNP = N terminal pro–B-type natriuretic peptide; ST2 = soluble interleukin 1 receptor-like 1.
First, production of reactive oxygen species (ROS) occur during anthracycline therapy, increasing oxidative stress. Anthracyclines combine with NAD(P)H to produce superoxide in the absence of any enzymatic activity.5 Additionally, they form irreversible complexes with cardiolipins of the mitochondrial membrane, disrupting their function in the electron transport chain and resulting in the formation of ROS.6,7 Because the heart is one of the most metabolically active organs, possessing the highest mitochondrial content among all tissues, this disruption in mitochondrial function significantly affects cardiac function.8 Moreover, anthracyclines form complexes with iron, altering iron metabolism by stabilizing transferrin and producing ROS through interaction with free oxygen.9, 10, 11 This explains the reason iron overload in patients can increase the risk of cardiotoxicity.11 Furthermore, the binding of anthracyclines to endothelial nitric oxide (NO) synthase (eNOS) reductase disrupts the NO–ROS balance through endothelium–cardiomyocyte crosstalk.11
Second, anthracyclines inhibit topoisomerase 2 beta (Top2β), leading to DNA intercalation, double-strand breaks, and subsequent inhibition of replication and transcription. Consequently, cell death pathways are activated in cardiomyocytes.1 Third, anthracyclines increase intracellular calcium levels, resulting in apoptosis and the activation of calcium-dependent proteases known as calpains and caspases.12 The doxorubicin metabolite, doxorubinicol, inhibits the sodium–calcium exchanger channel (NCX), disrupting the normal transport of calcium out of the cell.11 Furthermore, anthracyclines induce alterations in genes that regulate calcium exchange and storage, leading to impaired cardiac systolic and diastolic function.11 The activation of calpains also induced titin degradation, compromising the structure and contraction of cardiomyocytes.12 Fourth, anthracyclines disturb apoptosis and autophagy by activating both intrinsic and extrinsic apoptosis pathways.11 ROS-activated heat shock factor (HSF)-1, leading to the stabilization of p53 and generation of proapoptotic proteins.13 The p53-mediated suppression of GATA binding protein 4 (GATA-4), a transcription factor, hindered mitochondrial biogenesis, impeding the synthesis and function of B cell lymphoma 2 (Bcl-2), an anti-apoptotic protein.14,15 Autophagy was induced by inhibiting the protein kinase B phosphorylation (PKB or Akt)/mammalian target of rapamycin (mTOR) pathway and down-regulating beclin 1.16
Fifth, anthracyclines affect cardiac energy metabolism. Oxidative stress leads to a shift in cardiac metabolism from aerobic to anaerobic by reducing the oxidation of long-chain fatty acids and increasing anaerobic glycolysis.11,17 Additionally, anthracyclines inhibited the adenosine monophosphate–activated (AMP-activated) protein kinase (AMPK) signaling pathway,11 thereby reducing the activity of acetyl coenzyme A (acetyl-CoA) carboxylase, an important enzyme for the biosynthesis of fatty acids.18
Sixth, anthracyclines impair the transcription and translation of matrix metalloprotease (MMP-2 and -9), leading to a decrease in collagen degradation. Consequently, this process activates transforming growth factor (TGF)-beta and suppressor of mothers against decapentaplegic homolog 3 (SMAD) signaling, resulting in increased fibrosis.11,19,20 Aside from the effects on cardiomyocytes, toxic effects on vascular smooth muscle cells and cardiac endothelial cells have been described.21 One example involves increased endothelin-1 production in endothelial cells, inducing vasoconstriction and increased afterload.22,23
Circulating proteins as biomarkers for anthracycline cardiotoxicity
In addition to NP and cardiac Tn, both of which have demonstrated their potential as biomarkers in several studies, research has explored other proteins as potential biomarkers for anthracycline cardiotoxicity.24, 25, 26 As shown in Figure 1, C-reactive protein (CRP) and myeloperoxidase (MPO), both markers of inflammation and oxidative stress, exhibited increased levels after anthracycline therapy, with higher levels observed in patients experiencing a more significant decrease in LVEF.24,25,27,28 Growth differentiation factor (GDF)-15, known for its anti-apoptotic and antihypertrophic effects through the TGF-β pathway, was up-regulated by cell injury, inflammation, oxidative stress, and hypoxia.24,29 The administration of anthracyclines resulted in increased GDF-15 levels, again, directly correlating with the degree of LVEF reduction.27
Glycogen phosphorylase BB (GPBB), an essential enzyme in the glycogenolysis and energy metabolism of cardiomyocytes,24 showed elevated levels after anthracycline therapy, correlating with left ventricular diastolic dysfunction.30 Placental growth factor (PlGF), a member of the vascular endothelial growth factor family, showed elevated levels after anthracycline chemotherapy and served as a predictive marker for CTRCD development in those receiving anthracyclines followed by trastuzumab.24,27 Both asymmetric dimethylarginine (ADMA) and N-monomethyl arginine (NMMA), metabolites of the arginine-NO pathway,24 increased after anthracycline chemotherapy and were associated with an increased risk of CTRCD in patients with breast cancer.31
Circulating microRNA as biomarkers for anthracycline-induced cardiotoxicity
Because cardiotoxic effects may manifest before protein synthesis, the emergence of epigenetic regulators has recently gained attention as potential biomarkers. microRNAs (miRNAs) are short, single-stranded RNA sequences consisting of 18 to 24 nucleotides. As noncoding RNA molecules, they play a crucial role in the regulation of gene expression. Through their influence on the expression and translation of 1 or more target messenger RNAs (mRNAs), miRNAs are implicated in various physiological and pathological processes. While abundant in the cytoplasm, miRNAs can also be released into the blood through 3 main ways. First, they can be shed in miRNA-enriched extracellular microparticles, such as microvesicles and exosomes. The second, and most common, mechanism involves the binding of miRNAs to RNA-binding protein complexes, such as Ago2, a key intracellular protein in miRNA-mediated RNA silencing.32 Third, miRNAs can also bind to high-density lipid proteins.32 Despite the presence of RNases, miRNAs remain stable in peripheral blood.
The distribution of miRNAs in circulating exosomes differed from intracellular miRNA production, indicating that certain miRNAs were actively secreted, whereas others were favored for intracellular processes.33 Various sorting mechanisms come into play, allowing cells to guide which specific intracellular miRNAs are secreted in exosomes. Sorting based on the miRNA sequence relies on characteristics or distinct motives on the 3′ end, facilitating recognition by ribonucleoproteins and enabling selective secretion. Likewise, sequence motifs induce cellular retention. The preferred motifs for this sorting process varied from cell type to cell type, resulting in a difference in retained and secreted miRNAs between cell types.33 Sequence-independent pathways were also present, with various proteins, including Ago2, having been suggested to play a role.34 This sorting was influenced by several (pathological) conditions; for example, patients with atherosclerosis showed increased secretion of miR-92a-3p in endothelial microvesicles compared with those without atherosclerosis.35 Therefore, circulating miRNAs emerge as an attractive prospect for diagnostic biomarkers in cardiotoxicity due to their resistance to degradation and their ability to provide direct pathomechanistic information36 (Figure 1).
Although most studies have primarily focused on anthracycline-induced epigenetic dysregulation inside cardiomyocytes, this review shifts its focus to existing studies involving circulating miRNA (cmiRNA). This shift is essential for translating findings into clinically relevant and readily applicable biomarkers. We investigate the dynamics of cmiRNA profiles in response to anthracycline therapy, exploring differences between patients who develop cardiotoxicity and those who do not (Central Illustration). We include reports encompassing all cancer types, anthracyclines, and age categories to provide an extensive overview. We begin by summarizing the findings of these reports and the observed early and late cmiRNA dynamics after chemotherapy. Subsequently, we establish a link between the use of cmiRNAs as circulating biomarkers and their (patho)mechanistic roles, offering a more in-depth overview of their involvement in distinct mechanisms.
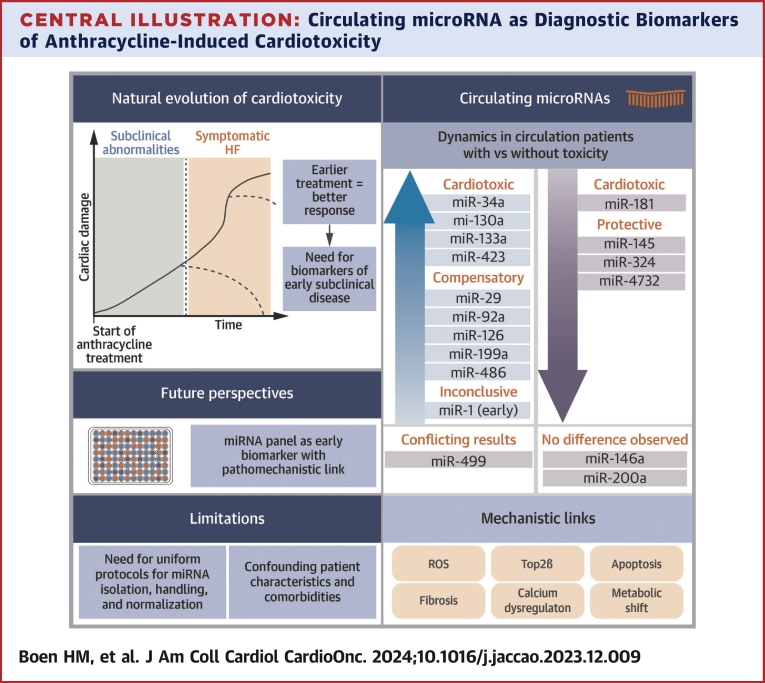
Central Illustration.
Circulating microRNA as Diagnostic Biomarkers of Anthracycline-Induced Cardiotoxicity
This schematic representation highlights the need for new biomarkers, the dynamics of circulating microRNA (miRNA), and their pathomechanistic link, and explores future perspectives and remaining limitations for their routine use. HF = heart failure; ROS = reactive oxygen species.
Circulating miRNA in response to anthracyclines in animal models
An overview of published studies (5 in mice, 3 in rats, 1 in dogs) and their main characteristics are given in Supplemental Table 1. Six of 9 studies used an array-based approach, providing hypothesis-free insights into the cmiRNA profile after exposure to doxorubicin (DOX) in clinically relevant doses.
Most studies have focused on dynamic changes in cmiRNA after treatment. In all mouse studies, an increase in miR-34a levels was observed in both plasma37, 38, 39, 40 and myocardial tissue, suggesting a cardiac origin.37,38 Similarly, rats exposed to repeated low doses of DOX showed increased miR-34a levels.41 In mice, a dose-dependent rise in miR-34a levels appeared early, starting from day 6 after treatment, preceding any clinical signs of cardiotoxicity, whereas cTnT levels rose from day 7 onwards.40 miR-34a levels remained elevated during treatment and persisted even after cessation of treatment.39,40 In close association with miR-34a, a rise in miR-34c levels after DOX treatment was also described.39,40 Additionally, an increase in miR-133a levels was observed after DOX treatment in both mice and rats.37,38,42,43 In rats, an early rise in miR-1 and miR-133b levels was seen in the first 24 hours after a single high dose of anthracyclines.42,43 Conversely, in mice, miR-1 levels increased 24 hours after 4 DOX doses.44 For all other cmiRNAs, data rely on single studies and require confirmation in future studies.
Three studies compared cmiRNA profiles between animals with and without cardiotoxicity.39,45 The up-regulation of miR-34a was more evident in mice manifesting cardiotoxicity compared with those without cardiotoxicity.39 miR-34a values correlated with cTnT.40 Additionally, when rats were cotreated with dexrazoxane, an iron chelator preventing ROS formation, the increase in miR-34a levels was abolished in both plasma and myocardial tissue, maintaining normal cardiac function.41 These data might suggest that cmiR-34a reflects early cardiac damage. The rise in miR-133a levels after DOX was attenuated in mice with cardiotoxicity compared to those without cardiotoxicity, potentially indicating a cardioprotective effect.39 In dogs with sarcoma, an increase in cmiR-181d levels was observed in those with a significant decline in LVEF after anthracycline treatment.45 Notably, in mice, miR-181d was down-regulated after DOX, demonstrating the possibility of interspecies differences in some less-conserved miRNAs.
Apparent contradictory results among miRNA array studies can likely be attributed to the heterogeneity of study protocols (involving different species, strains, sexes, and/or DOX dosages), miRNA array technique (including array, real-time quantitative polymerase chain reaction [RT-qPCR], normalization methods), and the timing of cmiRNA profiling.46 Intriguingly, miR-499 exhibited elevated levels in 2 female mouse models after DOX,38,44 whereas the levels decreased in male mice displaying signs of cardiotoxicity,39 suggesting sex differences in response to DOX. Additionally, several studies applied a single high-dose protocol, hindering the translation of findings into the clinical setting.
Circulating miRNA in response to anthracyclines in patients
Although most studies in human cohorts mainly focus on the dysregulation of miRNAs after DOX treatment, some studies also link these specific disturbances to the development of anthracycline-induced cardiotoxicity. Establishing a link between changes in cmiRNA and the development of cardiotoxicity is important because it allows the distinction of observed effects from treatment-related changes in cmiRNA associated with the tumor. An overview of dysregulated miRNAs and their mechanisms is provided in Table 1. For a more in-depth exploration, Supplemental Table 2 presents an overview of the 19 available clinical studies profiling miRNAs after anthracycline treatment.
Table 1.
Overview of cmiRNAs, Their Dynamic Changes in Relation to Cardiotoxicity or Cardioprotection, and the Assumed Mechanism
| miRNA | Dynamic in Circulation After Anthracyclinesa | Relation With Cardiotoxicity (TOX vs No TOX) | ROS | Topo2β Inhibition | Apoptosis | Fibrosis | Calcium Activation of Caspases | Metabolic Shift & Structural Abnormalities | Overall CT Effect |
|---|---|---|---|---|---|---|---|---|---|
| Let-7f | ? | ↓ at baseline59,60 | — | — | ↑77 | — | — | — | CT |
| miR-1 | ↑44,51,52,56; ↓53 | ↑52; late ↓ after end of chemotherapy47 | — | — | ↑79,80 | ↓105 | — | — | CT/protective |
| miR-20a | ? | ↓ at baseline59,60 | — | ↓95,96 | ↓95 | — | Protective | ||
| miR-29 | ↑50,51,55 | ↑51 | — | — | ↓/↑87 | ↓104 | — | — | Protective |
| miR-34a | ↑41,47, 48, 49, 50 | ↑50 | — | — | ↑41,65,74 | ↑74,101,102 | — | — | CT |
| miR-92a | ↑55 | ↑47 | ↓69 | — | ↓69 | — | — | — | Protective |
| miR-126 | ↑48,50 | ↑50 ↓ at baseline59,60 |
— | — | ↓100,138 | — | — | — | Protective |
| miR-130a | ↑61 | ↑61 ↓ at baseline60 |
— | — | ↑76,139,140 | ↑141 | — | — | CT |
| miR-133a/b | ↑52,↓47 | ↑54 | — | — | ↑54 | ↓103 | — | — | CT |
| miR-145 | ↑47; ↓55 | ↓55 | — | — | — | — | — | ↓142 | Protective |
| miR-146a | ↑52; ↓55 | No relation | — | — | ↓75 ↑66 | ↓75 | — | — | Protective |
| miR-181a/b | ↓55 | ↓55,57 | — | — | (↑81,82) | — | — | — | CT |
| miR-199a-3p | ↑53 | ↑53 | — | — | ↓92 | — | — | — | Protective |
| miR-200a | ↑53 | No relation | ↓73 | — | — | — | — | — | Protective |
| miR-208a | Undetectable in plasma48,52,54,56 | — | — | — | ↑143 | — | — | ↑106 | CT |
| miR-210 | ↑55 | ↓ at baseline59,60 | — | — | ↓88,89 | — | ↓88 | — | Protective |
| miR-320a | ↑55,62 | Not described | — | — | ↑62 | — | — | — | CT |
| miR-324 | ↓53 | ↓47 | ↓70 | — | ↓144 | ↓145 | — | — | Protective |
| miR-423 | ↑48,50,52 | ↑48 | ↑68 | — | ↑68 | — | ↑68 | — | CT |
| miR-486 | ↑55 | ↑47 | — | — | ↓90,91 | ↓91 | — | ↓90,91 | Protective |
| miR-499 | ↑44,50,51,55 followed by ↓55; not always detectable54,56 | ↑51; ↓55 ↑ at baseline49 |
— | — | ↓97 | ↑146 | — | — | Protective |
| miR-4732-3p | ? | ↓63 | ↓63 | — | ↓63 | ↓63 | — | — | Protective |
Only miRNAs for which circulatory changes were observed in ≥2 clinical studies are described.
cmiRNA = circulating microRNA; CT = cardiotoxicity; ROS = reactive oxygen species; Topo2β = topoisomerase 2β; TOX = cardiotoxicity.
The oncological background of each studied population is described in Supplemental Table 2. It should be noted that oncological background and response to chemotherapy also influence circulating microRNAs (miRNAs).
miR-34a
Similar to preclinical studies, human investigations also highlight miR-34a as a potential circulating biomarker for CTRCD. In patients with breast cancer, an early rise in plasma miR-34a levels was observed after the first and second doses of anthracyclines,47,48 and this rise persisted consistently up to 12 months after the completion of cancer treatment.49 Likewise, adult patients with lymphoma exhibited increased miR-34a levels compared with baseline after the initiation of treatment.41 Patients with HER2+ breast cancer also exhibited elevated levels of serum miR-34 after anthracycline therapy.50 This rise in serum miR-34a levels, observed after the completion of anthracycline treatment, was found to correlate with a rise in cardiac troponin I (cTnI) levels during the course of anthracycline treatment.50 However, no correlation between miR-34a levels and changes in LVEF was reported.41,47, 48, 49, 50
miR-1 cluster
Circulating miR-1 showed early elevation, observed as early as 6 hours after a single anthracycline dose.44,47,51 This rise in cmiR-1 persisted and correlated with a decline in LVEF, proving to have greater predictive value for cardiotoxicity compared with cTnI.52 However, long-term follow-up in childhood cancer survivors revealed lower miR-1 levels compared with a control cohort, observed 5 years after completion of therapy.53 Both miR-133a and miR-133b, part of the miR-1 cluster, exhibited changes after DOX treatment. A decline in miR-133a/b levels after the first DOX cycle has been reported,47 and an elevation in miR-133b levels after DOX treatment has been described.52 However, only a rise in plasma miR-133a levels after DOX treatment could discriminate between breast cancer patients with and without cardiotoxicity.54
Myomers
The muscle-specific cluster comprising miR-208a/b and miR-449 is often referred to as the myomers. Contradictory findings on miR-449 have been reported. Both early (observed 6 hours after the first dose) and late up-regulation in miR-499 were observed after anthracycline treatment in patients with breast cancer, sarcoma, or lymphoma, and this rise correlated with cTnT levels.44,49, 50, 51 However, in children with diverse malignancies, a decline in miR-499 levels was associated with a ≥10% decline in LVEF.55 To add complexity, some researchers could not detect miR-499 at all in samples of patients with breast cancer and children with leukemia treated with anthracycline chemotherapy.54,56 Two other muscle-specific miRNAs, miR-208a and miR-208b, were undetectable in patient samples.48,52,54
miR-29
Circulating miR-29a, miR-29b, and miR-29c levels increased after anthracycline treatment in both children and adults, correlating with a rise in cTnT (for miR-29b) and cTnI (for mir-29a) levels. The increase in both miR-29c and cTnT levels could be observed as early as 6 hours after the first dose.50,51,55 Interestingly, miR-29a was down-regulated early in a mouse model,40 highlighting the need for caution when translating data from animal models to humans and underscoring the importance of validation in human cohorts.
miR-423
In patients with breast cancer, miR-423 was up-regulated after 2 anthracycline cycles, remaining elevated for up to 6 months after the end of chemotherapy.48,50,52 This early rise in miR-423 at 3 months correlated with the decline in LVEF and cTnT at 6 months.48,50
miR-486-5p
Children and adult patients with breast cancer exhibited elevated levels of serum miR-486-5p early after the first anthracycline dose.47,55 However, this miRNA was not found to be elevated later in the treatment, and no association with cardiotoxicity was observed.
miR-181
In children, a late decline in miR-181a-5p levels was observed 1 year after treatment, and this decline was associated with a decline in LVEF of >10%.55 Additionally, increased miR-181a-3p levels at baseline, before the start of chemotherapy, were associated with less LVEF recovery 6 months after the end of treatment in adults.57 Moreover, in adult patients with breast cancer, a decline in miR-181b/c levels was associated with a rise in cTnI and/or cTnT.49
miR-126
Patients with breast cancer exhibited elevated levels of miR-126, a miRNA linked to vascular function,58 throughout the treatment period, and these levels remained elevated at 3 months after the end of treatment.48,50 This rise in miR-126 correlated with both circulating cTnI and cTnT levels at 3 and 6 months after the initiation of treatment.50 Additionally, in patients with breast cancer who developed cardiotoxicity, baseline values of miR-126, recorded before the start of treatment, were lower compared with their treated counterparts who did not develop cardiotoxicity.59,60
miR-130a
Patients with breast cancer exhibited increased levels of plasma miR-130a from 3 months after treatment with anthracyclines up to 15 months after treatment in all patients.61 Within this study population, baseline miR-130a negatively correlated with LVEF and positively correlated with cTnI. During follow-up, miR-130a levels were higher in patients who developed cardiotoxicity.61 However, in a different cohort of patients with breast cancer, levels of plasma miR-130a at baseline were negatively correlated with N terminal pro–B-type natriuretic peptide (NT-proBNP).60
miR-320a
miR-320a levels increased after anthracycline chemotherapy in adult patients with leukemia and in the serum of pediatric patients 1 year after completion of chemotherapy.55,62 Unfortunately, no data on its relationship with cardiotoxicity were reported.
mir-324
In adults, a decline in plasma miR-324 levels from baseline was observed after the first cycle in patients with breast cancer who developed cardiotoxicity.47 In long-term childhood cancer survivors, miR-324 plasma levels declined compared with healthy control subjects,53 but this decline showed no relation to cardiotoxicity.53
miR-4732-3p
Serum values were lower at the end of treatment in patients with breast cancer experiencing cardiotoxicity compared with matched control subjects without cardiotoxicity.63
miR-199a-3p and miR200
miR-199a-3p and 200a-3p levels increased in extracellular vesicles of children with leukemia treated with DOX compared with healthy control subjects.53 However, no difference was observed between children with and without cardiotoxicity. In adult patients with breast cancer, an early rise in miR-199a-3p levels was seen after 2 cycles of chemotherapy, and this rise correlated with cTnT values.48
For certain miRNAs, only a predictive value based on their baseline values was described. Lower baseline values for miRNA let-7f were observed in patients with breast cancer who developed cardiotoxicity.59,60 Additionally, baseline let-7f negatively correlated with cTnI and NT-proBNP values after treatment.60 Similarly, lower baseline miR-210 levels were seen in patients who developed cardiotoxicity after anthracycline treatment,41,59 and this was negatively correlated to the rise in circulating cTnI after treatment.41
miR-17-92 cluster
Patients with breast cancer who developed cardiotoxicity exhibited lower baseline values of miR-20a.59,60 miR-20a, along with miR-17, emerged as a significant protective factor in a multivariable analysis for cardiotoxicity,59 indicating a potential predictive role for these miRNAs. An early rise in miR-92a levels after the first dose of DOX was observed in patients with breast cancer who developed CTRCD.47 In children with diverse malignancies, miR-92a levels increased directly after the first dose and 1 year after treatment, irrespective of the development of CTRCD.55 In adults with lymphoma, miR-221-3p was reduced at baseline in patients with poor recovery of LVEF after the end of DOX treatment, compared to those with good recovery.57
Contradictory findings were reported for some miRNAs between pediatric and adult cohorts. For example, cmiR-146a levels were found to decrease in serum after the first anthracycline dose in children,55 whereas they increased in the plasma of adult patients with breast cancer during DOX treatment.52 Similarly, an early decline in miR-145 levels was seen in anthracycline-treated children, correlating with the development of a significant (>10%) decline in LVEF.55 By contrast, both adults with and without cardiotoxicity exhibited a rise in miR-145 levels after the first cycle of DOX.47
In summary, a distinctive profile of dysregulated miRNA is associated with anthracycline chemotherapy and CTRCD (Table 1). Nevertheless, discrepancies persist among individual studies, which could be attributed to the differences in patient population (such as age and sex), DOX dosing, timing, and analysis techniques.
Overview of potential mechanisms of cardiotoxicity associated with specific miRNAs
Figure 1 and Table 1 provide an overview of the effects of different miRNAs in the context of cardiotoxicity. For a comprehensive and in-depth exploration of the mechanisms of specific miRNAs in other cardiac conditions, refer to Supplemental Table 3. As described earlier, due to the sorting of miRNA, changes in cmiRNA do not always mirror intracellular changes in miRNA concentration.33 Therefore, an increase in cmiRNA in cardiotoxicity does not necessarily indicate an increased action of this miRNA in its originating cell. Additionally, these miRNAs can influence other cell types when taken up from the circulation.
Oxidative stress
Oxidative stress can up-regulate the expression of certain miRNAs, and these miRNAs, in turn, can alter ROS production. miR-34a and miR-146a serve as 2 examples of miRNAs induced by ROS. Anthracycline-induced ROS leads to DNA damage and an increase in the expression of tumor protein p53 and miR-34a, creating a positive feedback loop that results in apoptosis.64,65 Similarly, the increased myocardial expression of miR-146a after anthracycline treatment may have been attributed to increased ROS levels, given that nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a downstream mediator of ROS, has been reported to induce its expression.66,67 Conversely, miR-423 is an example of a miRNA that can induce ROS; studies have shown its capacity to induce ROS in H9C2 cells subjected to hypoxia/reoxygenation.68
Several miRNAs have also demonstrated the ability to prevent ROS production. In a type 2 diabetic rat model, the overexpression of miR-92a-2-5p attenuated oxidative stress.69 Additionally, transfecting human endothelial progenitor cells from myocardial infarction patients and healthy controls with a miR-324-5p mimic resulted in a significant reduction in ROS levels.70 miR-181c targets mitochondrial function by altering the expression of mitochondrial genes, such as mt-COX1, a subunit of complex IV of the respiratory chain, leading to increased ROS production.71,72 Additionally, miR-200a, found to be down-regulated in DOX-treated mice, exhibited antioxidant properties by targeting Kelch-like ECH associating protein 1 (Keap1). Keap1, a scaffolding protein of nuclear factor-like 2 (Nrf2), inhibited Nfr2 from performing its antioxidant function.73
Top2β inhibition
Although the inhibition of Top2β is an important pathomechanism in cardiotoxicity, current literature lacks studies linking specific cmiRNAs (or other circulating biomarkers) to this pathway. Several miRNAs have been identified to target TOP2β, the gene responsible for encoding TopIIβ; however, none of these miRNAs have been reported as dysregulated after anthracycline treatment.
Apoptosis and autophagy
miR-34a, a direct target of p53, enhances apoptosis by targeting cyclin D1 (CCND1) and Bcl-2, key regulators of the cell cycle and anti-apoptotic proteins, respectively (Figure 2). Additionally, miR-34a suppressed SIRT1 activity, resulting in reduced deacetylation of p53 and, consequently, a more active p53.41,65,74 Overexpression of miR-34a in rat cardiomyocytes increased the expression of Bcl-2-associated X protein (BAX) in the setting of DOX treatment.41 Conversely, inhibiting miR-34a led to decreased apoptosis in both rat cardiac progenitor cells treated with DOX and in the myocardium of a DOX-treated rat model.65,74 Moreover, inhibiting miR-34a in this rat model attenuated cardiac dysfunction.74
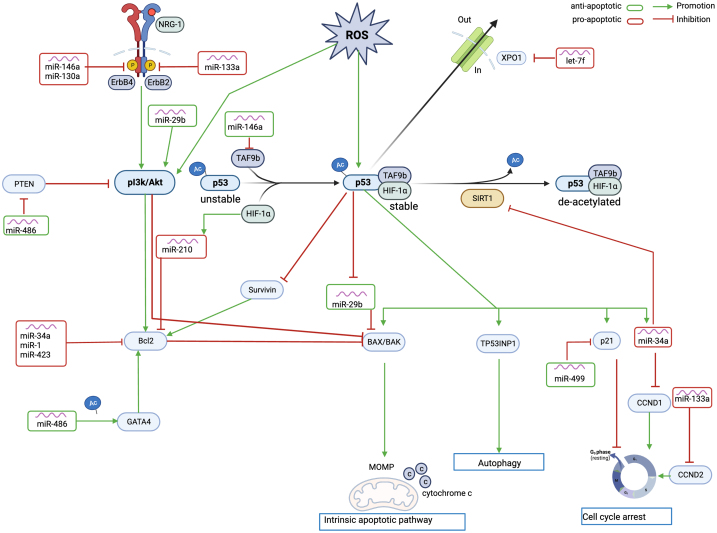
Figure 2.
Role of miRNAs and Apoptosis in Cardiotoxicity
Schematic overview of the effects of various miRNAs on apoptosis in response to doxorubicin (DOX) treatment. An increase in reactive oxygen species (ROS) due to doxorubicin triggers the activation of the p53 pathway. Antiapoptotic miRNAs are displayed in green; proapoptotic miRNAs are displayed in red. Created with BioRender.com. Ac = acylated protein; BAX/BAK = Bcl-2-associated X protein; Bcl2 = B cell lymphoma 2; CCND1 = cyclin D1; CCND2 = cyclin D2; GATA4 = GATA binding protein 4; HIF = hypoxia inducible factor; miR = microRNA; MOMP = mitochondrial outer membrane permeabilization; p21 = cyclin-dependent kinase inhibitor 1; p53 = tumor protein p53; SIRT1 = sirtuin; TAF9B = TATA-binding protein associated factor 9b; TP53INP1 = tumor protein P53 inducible nuclear protein 1.
Several other miRNAs influence the p53 pathway by targeting factors that stabilize p53. For instance, miR-146a targeted the TATA-binding protein associated factor 9b (TAF9b), a coactivator and stabilizer of p53.75 In a long-term DOX-treated mouse model, miR-146 knockout led to increased apoptosis and impaired autophagy.75 However, there are contrasting observations indicating that miR-146 affected the anti-apoptotic neuregulin-1 (NRG-1) and epidermal growth factor receptor (ErbB) signaling pathway.66 In neonatal rat cardiomyocytes treated with DOX, miR-146a overexpression increased apoptosis.66 Another miRNA, miR-130a, targeted Erbb4 in neonatal rat ventricular cardiomyocytes.76 This interaction with the Erbb4 pathway may explain the increased toxicity observed when anthracyclines are combined with trastuzumab, a drug targeting ErbB2. Interestingly, ErbB2 was identified as a target of miR-133a in patients with breast cancer undergoing DOX treatment.54
Exportin-1 (XPO1), a transporter crucial for the nucleus-to-cytosol transport of p53, is targeted by let-7f.77 The suppression of XPO1 leads to enhanced nuclear availability of p53, thereby increasing apoptosis activity. In rat cardiomyocytes, let-7f levels increased after exposure to DOX, and the inhibition of let-7f resulted in the prevention of cardiotoxicity in a rat model.77
Other pathways of apoptosis are influenced by miRNAs. In the setting of hypoxia/reperfusion, miR-423 stimulated apoptosis and mitochondrial damage in rat cardiomyocytes by targeting the Wnt/β-catenin signaling pathway and Myb-related protein B (MYBL2), a central regulator of cell survival, resulting in a decrease in Bcl-2.68 Another miRNA, miR-200b, affected the Wnt/β-catenin signaling by inhibiting ZEB1, leading to an increase in cardiotoxicity in mice models.78 Although a decrease in miR-200b has been described in exosomes derived from trophoblast stem cells, there are no available data on circulating miR-200b in the setting of cardiotoxicity.78
Similar to miR-34a, miR-1 directly targeted Bcl-2 in a rat model of hypoxia/reperfusion injury.79 Additionally, miR-1 inhibited histone deacetylase 4 (HDAC4), leading to the inhibition of myocardial cell proliferation.80 miR-181 played a stimulating role in apoptosis and decreased mitochondrial homeostasis of different cell types by targeting Bcl-2 and its associated receptor myeloid leukemia cell differentiation protein (Mcl-1).81, 82, 83 Furthermore, Bcl-2 played a protective role for autophagy by binding to and stabilizing beclin1. The cardioprotective miR-30e, which is declined in rat cardiomyocytes after DOX, inhibited beclin 1, leading to a decrease in autophagy.84
Additionally, miR-181a/b targeted phosphate and tensin homolog (PTEN), opposing the PI3K/AKT pathway involved in the switch to an anabolic metabolism, resulting in a decrease in glycolysis.71,85,86 Several miRNAs may protect against apoptosis. In a rat model of DOX cardiotoxicity, local delivery of miR-29b improved cardiac function by decreasing the expression of BAX.87 In animal models of ischemia, miR-210 overexpression rescued cardiac function, increased cardiomyocyte proliferation, and induced angiogenesis by targeting the cell cycle inhibitor APC.88,89
A rise in miR-486 resulted in the activation of SRF and GATA4, transcription factors that promote cardiomyocyte proliferation and growth.90,91 Although GATA4 has antiapoptotic properties, it also functioned as a senescence marker.92 Due to its direct targeting of GATA4, a decline in miR-199a after DOX resulted in an increase in senescence of cardiomyocytes.
Additionally, miR-30e, miR-486, and miR-495-3p target PTEN, which opposes the PI3K/AKT pathway, resulting in decreased apoptosis.90,93,94 In a mouse model of hypoxia/reperfusion injury, miR-486 overexpression resulted in a decrease in apoptotic cardiomyocytes and infarct size.90 miR-20a protected cardiomyocytes from apoptosis in vitro, and in diabetic rats, overexpression of miR-20a attenuated cardiac dysfunction by targeting Rock2 and Egln3.95,96 Finally, miR-499 appeared to play a protective role against apoptosis by regulating mitochondrial fission and targeting cyclin-dependent kinase inhibitor 1 (p21), a cell cycle inhibitor activated by p53.97 miR-221-3p targeted Sirt-2, leading to the inhibition of the AMPK pathway, increasing senescence and decreasing hypertrophy.98 miR-4732-3p showed prosurvival properties by activating the Hippo pathway, but its direct initiator for this activation is still unknown.63
In this setting, it is important to note that autophagy-related proteins and genes play a role in sorting and secreting miRNAs. For example, the LC3-conjugation machinery, an integral part of the membrane of autophagosome, is also involved in selecting small noncoding RNAs, including miRNAs in extracellular vesicles.99 This mechanism can lead to a change in cmiRNA levels during autophagy activation. miR-126 exerts its antiapoptotic properties in endothelial cells through interactions with autophagic vesicles. By binding to Ago2 and forming a complex with Mex3a on the surface of autophagic vesicles, miR-126 was guided into the cell's nucleus. In the nucleus, miR-126 dissociated from Ago2 and bound to caspase-3, inhibiting its dimerization and limiting apoptosis.100
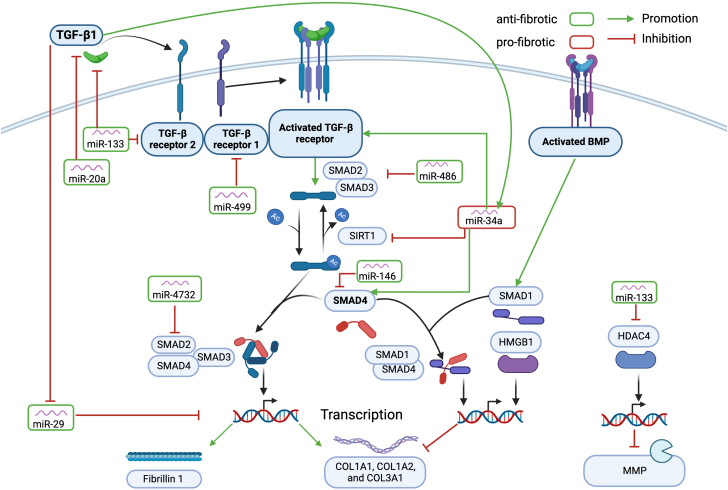
Fibrosis
miR-34a down-regulated SIRT1, resulting in an increase in acetylated SMAD2/SMAD3 and increased fibrosis through the activation of the TGF-β pathway (Figure 3).74 Additionally, miR-34a directly increased SMAD4.101 In a positive feedback loop, murine cardiac tissue showed increased miR-34a levels after myocardial infarction through the activation of TGF-β1, resulting in worsened cardiac function and increased fibrosis.101,102 Conversely, other miRNAs exerted protective effects against fibrosis. miR-486 exhibited antifibrotic properties through direct targeting of SMAD2/3.91 miR-146 played a regulatory role in fibrosis and cardiac remodeling by reducing SMAD4 expression,75 and miR-4732 reduced fibrosis by directly targeting SMAD2.63 The overexpression of miR-20a led to a decrease in the expression of TGF-β in the cardiac tissue of diabetic rats.95 miR-133 has been suggested to play a regulatory role in fibrosis by targeting TGFβ and TGFβ receptor II, affecting collagen production in atrial canine fibroblasts.103 miR-29b prevented fibrosis by targeting elastin, fibrillin 1, and different collagens, all elements of the extracellular matrix.104 In a myocardial infarction mouse model, the inhibition of miR-29b resulted in increased collagen deposition and myocardial fibrosis.104 Finally, in a hypertensive rat model, miR-1 inhibited the proliferation of cardiac fibroblasts.105
Figure 3.
Role of miRNAs and Fibrosis in Cardiotoxicity
Schematic overview of the effects of different miRNAs on fibrosis in response to DOX treatment. Doxorubicin activates the TGF-β pathway. Antifibrotic miRNAs are displayed in green; profibrotic miRNAs are displayed in red. Created with BioRender.com. BMP = bone morphogenetic protein; COL = collagen; HDAC4 = histone deacetylase 4; HMGB1 = high mobility group box 1; MMP = matrix metalloproteinase; SMAD = suppressor of mothers against decapentaplegic; TGF = transforming growth factor; other abbreviations as in Figures 1 and 2.
Other miRNA-driven pathomechanisms involved in cardiotoxicity
In addition to the major pathomechanisms discussed earlier, the described miRNAs (Table 1) can interfere with other pathways. First, several miRNAs influenced calcium homeostasis and caspase activation. For instance, both miR-210 and miR-30e have demonstrated cardioprotective properties by targeting caspase 3.84,88 On the other hand, miR-423 has exhibited cardiotoxic properties by increasing caspase 3 and caspase 7.68
Second, miRNAs targeting the expression of structural proteins in cardiomyocytes play a crucial role. The muscle-specific miR-208 family regulated cardiac remodeling in response to stress through isomorphic switches of myosin heavy chain.106 miR-221-3p decreased α-tubulin, a structural protein of microtubule.98 As described earlier, miR-486 targets PTEN,90 a pathway involved in not only apoptosis, as previously mentioned, but also in the switch to an anabolic metabolism.90 Additionally, miR-486 targeted Forkhead box O 1 (FoxO1), a transcription factor involved in differentiation and metabolism.91 The extensively studied miR-1 had several effects on cardiac development and function.107 miR-1 played a role in arrhythmogenesis: its overexpression in myocardial tissue impaired cardiac conduction through the up-regulation of Irx5, a transcription factor that regulates the expression of KCNJ2 (which encodes a potassium-channel subunit) and GJA1 (which encodes connexin 43 gap junction channels).108,109 Both miR-1 and miR-133 are necessary for skeletal and cardiac muscle development and function.43,110
Third, besides affecting cardiomyocytes, anthracyclines can affect other cell types, including endothelial cells. miR-29b promoted angiogenesis through the up-regulation of PKB/Akt phosphorylation, resulting in increased endothelial cell proliferation and migration.111 The miR-17-92 cluster, including miR-20a and miR-92a, has been linked to the regulation of endothelial function.112 miR-320a played a regulatory role in promoting endothelial cell proliferation and reducing microvessel density.62 Further in-depth analysis elucidated that miR-320a targeted VEGF-A, resulting in endothelial cell apoptosis, migration, and a decrease in NO release.62 Additionally, miR-126, a miRNA with important proangiogenic activity,58,113 was induced by hypoxia and shear-stress (KLF2).114 Similarly, miR-145 expression was induced by both shear stress and statin therapy.114 miR-210, produced by cardiomyocytes under ischemic stress, exerted paracrine and long-distance effects on endothelial cells, activating protective mechanisms in these target cells.112
Fourth, several miRNAs are involved in the inflammatory response and activation of a post-injury immune response. miR-146, for instance, was shown to induce cytokine production and leukocyte migration in a dose-dependent manner by interacting with TLR-7.115 Likewise, members of the let-7 family exhibited the capability to activate TLR-7 as well.116 Additionally, the cardiotoxic miR-200b induced elevated levels of IL-6 and IL-8.78
Technical considerations regarding sampling and analysis protocols
As stated earlier, cmiRNAs are involved in numerous physiological and pathophysiological processes. The magnitude of their role in cardiovascular disease might even be overshadowed by their role in cancer. Therefore, the influence of these cancer- or treatment-related disturbances in cmiRNA profiles cannot be underestimated. Specific reviews are available for readers with a special interest in cmiRNA dysregulation in oncologic patients.117,118
Different methods are used for the identification and quantification of cmiRNAs. Next-generation sequencing offers the advantage of unbiased miRNA identification, including unannotated miRNAs, but is the most expensive. Microarrays have a lower sensitivity and specificity than RT-qPCR and are primarily suited for screening rather than quantitative analysis. Screening results from next-generation sequencing or microarrays should, therefore, always be validated by RT-qPCR or digital droplet PCR due to their higher sensitivity and specificity.36 Digital droplet PCR has a lower limit of detection and higher reproducibility than RT-qPCR.119
RT-qPCR protocols differ among vendors and labs, complicating the comparisons of individual studies. For example, various approaches are available for normalizing RT-qPCR results. These methods include the use of exogenic spike-in cel-miR, normalization to a single endogenous RNA such as U6, or using multiple endogenous controls determined through an independent experiment.46 The performance of these various methods has been described in other sources.46 In brief, U6 is an intracellular, small nuclear RNA involved in the spliceosome.120 However, comparative testing for normalization methods revealed the use of U6 to be highly variable, and as such, its application should be avoided.46 Conversely, normalization to multiple endogenous controls using a mathematical normalization technique, such as geNorm, showed the least variability.46,121 Moreover, miRNA quantification can be performed using both serum and plasma. However, because serum is obtained after platelet activation, it may contain platelet-derived miRNAs, which could potentially influence results and pose a limitation when comparing findings across different studies.122 Existing papers often provide limited descriptions of sample handling, which can affect miRNA quantification. Factors such as the collection method, processing, presence of hemolysis, and circadian variation all have the potential to influence miRNA quantification.123,124 These examples highlight the importance of establishing uniform protocols for miRNA isolation, handling, and normalization.
Future prospects
The role of specific miRNAs, most notably miR-34a, as circulating biomarkers of early cardiac damage after anthracycline treatment, has been confirmed in various preclinical models and clinical settings. The cardiac origin of several cmiRNAs has been reported, contributing, at least in part, to our understanding of their role in the pathogenesis of cardiotoxicity.
Despite promising findings, several barriers must be overcome before cmiRNAs can be routinely implemented in the daily monitoring of cardiotoxicity (Central Illustration). Notably, only a few studies have focused on the differential expression of cmiRNAs in patients with and without cardiotoxicity. The observed dysregulation in cmiRNAs after anthracycline treatment also extends to patients who do not develop cardiotoxicity. This may reflect a treatment response or a more general effect of anthracyclines unrelated to cardiac damage. To address this, future studies must consider treatment response effects, potentially through stratification—an aspect not explored thus far—and focus on linking specific cmiRNA changes to the development of cardiotoxicity.
Second, a large heterogeneity persists across studies, highlighting the need for a more uniform approach to facilitate translation. Differences exist in anthracycline-dosing protocols, sampling techniques, miRNA-quantification/identification techniques, and normalization methods. Although recommendations for miRNA isolation and normalization are available in the literature,46,125 the field lacks consensus guidelines. There is an urgent need for comprehensive guidelines, such as those recently published for RNA quantification by the cardioRNA consortium.126 Third, the impact of underlying comorbidities and patient characteristics on cmiRNA profiles remains insufficiently understood. Previous studies have shown correlations between the expression of certain miRNAs and factors such as age,127 gender,128 and race.129 Additionally, comorbidities and risk factors such as diabetes, arterial hypertension, and smoking, as well as cardiac medication, might have influenced miRNA expression.124,130, 131, 132, 133, 134 Finally, concomitant anticancer treatments, such as trastuzumab, might have influenced the levels and expression of circulating biomarkers.124,135
Fourth, a shift is needed from focusing on individual miRNAs to adopting a network-based approach. Many cmiRNAs are highly correlated, providing an opportunity to identify disease-specific or pathomechanism-specific cmiRNA patterns of co-expressing miRNAs. Using a cmiRNA pattern or signature would provide better diagnostic value compared with individual cmiRNAs and is likely to offer more reliable insights. Additionally, a network-based analysis would allow for more in-depth clarification of the processes involved. Future research should aim to improve network analysis methodologies and their interpretation to fully realize the potential of cmiRNAs in cardiotoxicity monitoring.136,137
Considering these factors, the creation of a cmiRNA panel seems feasible. The selection of miRNAs for such a panel should depend on the specific research question being addressed, taking into account the involved mechanisms and the protective or cardiotoxic properties of the miRNAs. A comprehensive panel should include miRNAs capable of distinguishing between patients with and without cardiotoxicity, as well as miRNAs that assess the various pathomechanisms at play.
Conclusions
cmiRNAs emerge as novel candidate biomarkers for anthracycline-induced cardiotoxicity, given their stability in plasma and ability to establish a direct pathomechanistic link (Central Illustration). Future research should focus on discerning the differences between patients with and without cardiotoxicity, as well as the time course of cmiRNA deviations. Additionally, even prior to chemotherapy, assessing cmiRNA could identify patients who are particularly susceptible and warrant closer follow-up. Given the correlation of various cmiRNA disturbances with cardiotoxicity, the creation of a carefully selected cmiRNA panel for assessing early cardiotoxicity diagnosis may hopefully be feasible with additional research, provided technical limitations are duly taken into account.
Funding Support and Author Disclosures
This work was supported by the Flanders Research Foundation - FWO (research grant G055821N, doctoral research grant 1192420N to Dr Boen and senior clinical investigator grant 1804320N to Dr Van Craenenbroeck) and by the Belgian Foundation against Cancer (research grant C/2020/1374 and clinical mandate grant to CF 2021/1594). Ms Cherubin was an Early Stage Researcher fellow on the INSPIRE project, which has received funding from the European Union's Horizon 2020 Research and Innovation Program (H2020-MSCA-ITN program, Grant Agreement: No858070), and has received a SEP-grant from the Research Council of the University of Antwerp. Mr Bosman was supported as a predoctoral fellow by the Fund for Scientific Research (FWO) Flanders (grant number: 1S33720N). Dr Loeys holds a consolidator grant from the European Research Council (Genomia – ERC-COG-2017-771945). Dr Gevaert has received institutional lecture/advisory board fees from Abbott, AstraZeneca, Boehringer Ingelheim, Novartis, and Menarini outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.McGowan J.V., Chung R., Maulik A., Piotrowska I., Walker J.M., Yellon D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31:63–75. doi: 10.1007/s10557-016-6711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyon A.R., López-Fernández T., Couch L.S., et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 3.López-Sendón J., Álvarez-Ortega C., Zamora Auñon P., et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J. 2020;41:1720–1729. doi: 10.1093/eurheartj/ehaa006. [DOI] [PubMed] [Google Scholar]
- 4.Cardinale D., Colombo A., Bacchiani G., et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 5.Octavia Y., Tocchetti C.G., Gabrielson K.L., Janssens S., Crijns H.J., Moens A.L. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Goormaghtigh E., Chatelain P., Caspers J., Ruysschaert J.M. Evidence of a specific complex between adriamycin and negatively-charged phospholipids. Biochim Biophys Acta. 1980;597:1–14. doi: 10.1016/0005-2736(80)90145-5. [DOI] [PubMed] [Google Scholar]
- 7.Goormaghtigh E., Huart P., Praet M., Brasseur R., Ruysschaert J.M. Structure of the adriamycin-cardiolipin complex. Role in mitochondrial toxicity. Biophys Chem. 1990;35:247–257. doi: 10.1016/0301-4622(90)80012-v. [DOI] [PubMed] [Google Scholar]
- 8.Brown D.A., Perry J.B., Allen M.E., et al. Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–250. doi: 10.1038/nrcardio.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutteridge J.M. Lipid peroxidation and possible hydroxyl radical formation stimulated by the self-reduction of a doxorubicin-iron (III) complex. Biochem Pharmacol. 1984;33:1725–1728. doi: 10.1016/0006-2952(84)90340-x. [DOI] [PubMed] [Google Scholar]
- 10.Minotti G., Recalcati S., Mordente A., et al. The secondary alcohol metabolite of doxorubicin irreversibly inactivates aconitase/iron regulatory protein-1 in cytosolic fractions from human myocardium. FASEB J. 1998;12:541–552. doi: 10.1096/fasebj.12.7.541. [DOI] [PubMed] [Google Scholar]
- 11.Rawat P.S., Jaiswal A., Khurana A., Bhatti J.S., Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111708. [DOI] [PubMed] [Google Scholar]
- 12.Lim C.C., Zuppinger C., Guo X., et al. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem. 2004;279:8290–8299. doi: 10.1074/jbc.M308033200. [DOI] [PubMed] [Google Scholar]
- 13.Vedam K., Nishijima Y., Druhan L.J., et al. Role of heat shock factor-1 activation in the doxorubicin-induced heart failure in mice. Am J Physiol Heart Circ Physiol. 2010;298:H1832–H1841. doi: 10.1152/ajpheart.01047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi S., Volden P., Timm D., Mao K., Xu X., Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velez J.M., Miriyala S., Nithipongvanitch R., et al. p53 regulates oxidative stress-mediated retrograde signaling: a novel mechanism for chemotherapy-induced cardiac injury. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu W., Qin X., Zhang Y., et al. Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc Diagn Ther. 2020;10:752–769. doi: 10.21037/cdt-19-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho R.A., Sousa R.P., Cadete V.J., et al. Metabolic remodeling associated with subchronic doxorubicin cardiomyopathy. Toxicology. 2010;270:92–98. doi: 10.1016/j.tox.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Timm K.N., Tyler D.J. The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc Drugs Ther. 2020;34:255–269. doi: 10.1007/s10557-020-06941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetzenich A., Hatam N., Zernecke A., et al. Alteration of matrix metalloproteinases in selective left ventricular adriamycin-induced cardiomyopathy in the pig. J Heart Lung Transplant. 2009;28:1087–1093. doi: 10.1016/j.healun.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Spallarossa P., Altieri P., Garibaldi S., et al. Matrix metalloproteinase-2 and -9 are induced differently by doxorubicin in H9c2 cells: the role of MAP kinases and NAD(P)H oxidase. Cardiovasc Res. 2006;69:736–745. doi: 10.1016/j.cardiores.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Luu A.Z., Chowdhury B., Al-Omran M., Teoh H., Hess D.A., Verma S. Role of endothelium in doxorubicin-induced cardiomyopathy. J Am Coll Cardiol Basic Trans Science. 2018;3:861–870. doi: 10.1016/j.jacbts.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bien S., Riad A., Ritter C.A., et al. The endothelin receptor blocker bosentan inhibits doxorubicin-induced cardiomyopathy. Cancer Res. 2007;67:10428–10435. doi: 10.1158/0008-5472.CAN-07-1344. [DOI] [PubMed] [Google Scholar]
- 23.Yang L.L., Gros R., Kabir M.G., et al. Conditional cardiac overexpression of endothelin-1 induces inflammation and dilated cardiomyopathy in mice. Circulation. 2004;109:255–261. doi: 10.1161/01.CIR.0000105701.98663.D4. [DOI] [PubMed] [Google Scholar]
- 24.Xiao H., Wang X., Li S., Liu Y., Cui Y., Deng X. Advances in biomarkers for detecting early cancer treatment-related cardiac dysfunction. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.753313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ky B., Putt M., Sawaya H., et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipshultz S.E., Miller T.L., Scully R.E., et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042–1049. doi: 10.1200/JCO.2010.30.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Putt M., Hahn V.S., Januzzi J.L., et al. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem. 2015;61:1164–1172. doi: 10.1373/clinchem.2015.241232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todorova V.K., Hsu P.C., Wei J.Y., et al. Biomarkers of inflammation, hypercoagulability and endothelial injury predict early asymptomatic doxorubicin-induced cardiotoxicity in breast cancer patients. Am J Cancer Res. 2020;10:2933–2945. [PMC free article] [PubMed] [Google Scholar]
- 29.Bouabdallaoui N., Claggett B., Zile M.R., et al. Growth differentiation factor-15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2018;20:1701–1709. doi: 10.1002/ejhf.1301. [DOI] [PubMed] [Google Scholar]
- 30.Horacek J.M., Jebavy L., Vasatova M., et al. Glycogen phosphorylase BB as a potential marker of cardiac toxicity in patients treated with anthracyclines for acute leukemia. Bratisl Lek Listy. 2013;114:708–710. doi: 10.4149/bll_2013_149. [DOI] [PubMed] [Google Scholar]
- 31.Finkelman B.S., Putt M., Wang T., et al. Arginine-nitric oxide metabolites and cardiac dysfunction in patients with breast cancer. J Am Coll Cardiol. 2017;70:152–162. doi: 10.1016/j.jacc.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Creemers E.E., Tijsen A.J., Pinto Y.M., Rooij E.V. Circulating microRNAs. Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Martin R., Wang G., Brandão B.B., et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446–451. doi: 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu D., Di K., Fan B., et al. MicroRNAs in extracellular vesicles: sorting mechanisms, diagnostic value, isolation, and detection technology. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.948959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Li Q., Hosen M.R., et al. Atherosclerotic conditions promote the packaging of functional microRNA-92a-3p into endothelial microvesicles. Circ Res. 2019;124:575–587. doi: 10.1161/CIRCRESAHA.118.314010. [DOI] [PubMed] [Google Scholar]
- 36.Gareev I., Beylerli O., Yang G., et al. The current state of MiRNAs as biomarkers and therapeutic tools. Clin Exp Med. 2020;20:349–359. doi: 10.1007/s10238-020-00627-2. [DOI] [PubMed] [Google Scholar]
- 37.Hanousková B., Skála M., Brynychová V., et al. Imatinib-induced changes in the expression profile of microRNA in the plasma and heart of mice--a comparison with doxorubicin. Biomed Pharmacother. 2019;115 doi: 10.1016/j.biopha.2019.108883. [DOI] [PubMed] [Google Scholar]
- 38.Gioffré S., Ricci V., Vavassori C., et al. Plasmatic and chamber-specific modulation of cardiac microRNAs in an acute model of DOX-induced cardiotoxicity. Biomed Pharmacother. 2019;110:1–8. doi: 10.1016/j.biopha.2018.11.042. [DOI] [PubMed] [Google Scholar]
- 39.Ruggeri C., Gioffré S., Chiesa M., et al. A specific circulating microRNA cluster is associated to late differential cardiac response to doxorubicin-induced cardiotoxicity in vivo. Dis Markers. 2018;2018 doi: 10.1155/2018/8395651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai V.G., Vijay V., Lee T., et al. MicroRNA-34a-5p as a promising early circulating preclinical biomarker of doxorubicin-induced chronic cardiotoxicity. J Appl Toxicol. 2022;42:1477–1490. doi: 10.1002/jat.4309. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J.-N., Fu Y.-H., Hu Z.-Q., et al. Activation of miR-34a-5p/Sirt1/p66shc pathway contributes to doxorubicin-induced cardiotoxicity. Sci Rep. 2017;7 doi: 10.1038/s41598-017-12192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nishimura Y., Kondo C., Morikawa Y., et al. Plasma miR-208 as a useful biomarker for drug-induced cardiotoxicity in rats. J Appl Toxicol. 2015;35:173–180. doi: 10.1002/jat.3044. [DOI] [PubMed] [Google Scholar]
- 43.Calvano J., Achanzar W., Murphy B., et al. Evaluation of microRNAs-208 and 133a/b as differential biomarkers of acute cardiac and skeletal muscle toxicity in rats. Toxicol Appl Pharmacol. 2016;312:53–60. doi: 10.1016/j.taap.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 44.Jeyabal P., Bhagat A., Wang F., et al. Circulating microRNAs and cytokines as prognostic biomarkers for doxorubicin-induced cardiac injury and for evaluating the effectiveness of an exercise intervention. Clin Cancer Res. 2023;29:4430–4440. doi: 10.1158/1078-0432.CCR-23-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beaumier A., Robinson S.R., Robinson N., et al. Extracellular vesicular microRNAs as potential biomarker for early detection of doxorubicin-induced cardiotoxicity. J Vet Intern Med. 2020;34:1260–1271. doi: 10.1111/jvim.15762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gevaert A.B., Witvrouwen I., Vrints C.J., et al. MicroRNA profiling in plasma samples using qPCR arrays: recommendations for correct analysis and interpretation. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Todorova V.K., Makhoul I., Wei J., Klimberg V.S. Circulating miRNA profiles of doxorubicin-induced cardiotoxicity in breast cancer patients. Ann Clin Lab Sci. 2017;47:115–119. [PubMed] [Google Scholar]
- 48.Frères P., Bouznad N., Servais L., et al. Variations of circulating cardiac biomarkers during and after anthracycline-containing chemotherapy in breast cancer patients. BMC Cancer. 2018;18:102. doi: 10.1186/s12885-018-4015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gioffré S., Chiesa M., Cardinale D.M., et al. Circulating microRNAs as potential predictors of anthracycline-induced troponin elevation in breast cancer patients: diverging effects of doxorubicin and epirubicin. J Clin Med. 2020;9(5):1418. doi: 10.3390/jcm9051418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lakhani H.V., Pillai S.S., Zehra M., et al. Detecting early onset of anthracyclines-induced cardiotoxicity using a novel panel of biomarkers in West-Virginian population with breast cancer. Sci Rep. 2021;11:7954. doi: 10.1038/s41598-021-87209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leger K.J., Leonard D., Nielson D., de Lemos J.A., Mammen P.P., Winick N.J. Circulating microRNAs: potential markers of cardiotoxicity in children and young adults treated with anthracycline chemotherapy. J Am Heart Assoc. 2017;6(4) doi: 10.1161/JAHA.116.004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rigaud V.O., Ferreira L.R., Ayub-Ferreira S.M., et al. Circulating miR-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget. 2017;8:6994–7002. doi: 10.18632/oncotarget.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Totoń-Żurańska J., Sulicka-Grodzicka J., Seweryn M.T., et al. MicroRNA composition of plasma extracellular vesicles: a harbinger of late cardiotoxicity of doxorubicin. Mol Med. 2022;28:156. doi: 10.1186/s10020-022-00588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alves M.T., da Conceição I., de Oliveira A.N., et al. microRNA miR-133a as a biomarker for doxorubicin-induced cardiotoxicity in women with breast cancer: a signaling pathway investigation. Cardiovasc Toxicol. 2022;22:655–662. doi: 10.1007/s12012-022-09748-4. [DOI] [PubMed] [Google Scholar]
- 55.Oatmen K.E., Toro-Salazar O.H., Hauser K., et al. Identification of a novel microRNA profile in pediatric patients with cancer treated with anthracycline chemotherapy. Am J Physiol Heart Circ Physiol. 2018;315:H1443–H1452. doi: 10.1152/ajpheart.00252.2018. [DOI] [PubMed] [Google Scholar]
- 56.Cheung Y.-F., Li V.W.-Y., Lai C.T.-M., et al. Circulating high-sensitivity troponin T and microRNAs as markers of myocardial damage during childhood leukaemia treatment. Pediatr Res. 2021;89:1245–1252. doi: 10.1038/s41390-020-1049-5. [DOI] [PubMed] [Google Scholar]
- 57.Harries I., Biglino G., Ford K., et al. Prospective multiparametric CMR characterization and MicroRNA profiling of anthracycline cardiotoxicity: a pilot translational study. Int J Cardiol Heart Vasc. 2022;43 doi: 10.1016/j.ijcha.2022.101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S., Olson E.N. AngiomiRs--key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19:205–211. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin X., Chang F., Wang Z., Jiang W. Correlation of circulating pro-angiogenic miRNAs with cardiotoxicity induced by epirubicin/cyclophosphamide followed by docetaxel in patients with breast cancer. Cancer Biomark. 2018;23(4):473–484. doi: 10.3233/CBM-181301. [DOI] [PubMed] [Google Scholar]
- 60.Zhu Z., Li X., Dong H., Ke S., Zheng W.H. Let-7f and miRNA-126 correlate with reduced cardiotoxicity risk in triple-negative breast cancer patients who underwent neoadjuvant chemotherapy. Int J Clin Exp Pathol. 2018;11:4987–4995. [PMC free article] [PubMed] [Google Scholar]
- 61.Feng Q., Ren Y., Hou A., et al. MicroRNA-130a increases and predicts cardiotoxicity during adjuvant chemotherapy in human epidermal growth factor receptor-2-positive breast cancer. J Breast Cancer. 2021;24(2):153–163. doi: 10.4048/jbc.2021.24.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin Z., Zhao Y., Li H., et al. miR-320a mediates doxorubicin-induced cardiotoxicity by targeting VEGF signal pathway. Aging (Albany NY) 2016;8:192–207. doi: 10.18632/aging.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sánchez-Sánchez R., Reinal I., Peiró-Molina E., et al. MicroRNA-4732-3p is dysregulated in breast cancer patients with cardiotoxicity, and its therapeutic delivery protects the heart from doxorubicin-induced oxidative stress in rats. Antioxidants (Basel) 2022;11(10):1955. doi: 10.3390/antiox11101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Angelis A., Piegari E., Cappetta D., et al. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121:276–292. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piegari E., Russo R., Cappetta D., et al. MicroRNA-34a regulates doxorubicin-induced cardiotoxicity in rat. Oncotarget. 2016;7:62312–62326. doi: 10.18632/oncotarget.11468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horie T., Ono K., Nishi H., et al. Acute doxorubicin cardiotoxicity is associated with miR-146a-induced inhibition of the neuregulin-ErbB pathway. Cardiovasc Res. 2010;87:656–664. doi: 10.1093/cvr/cvq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taganov K.D., Boldin M.P., Chang K.J., Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhu X., Lu X. MiR-423-5p inhibition alleviates cardiomyocyte apoptosis and mitochondrial dysfunction caused by hypoxia/reoxygenation through activation of the wnt/β-catenin signaling pathway via targeting MYBL2. J Cell Physiol. 2019;234:22034–22043. doi: 10.1002/jcp.28766. [DOI] [PubMed] [Google Scholar]
- 69.Yu M., Sun Y., Shan X., et al. Therapeutic overexpression of miR-92a-2-5p ameliorated cardiomyocyte oxidative stress injury in the development of diabetic cardiomyopathy. Cell Mol Biol Lett. 2022;27:85. doi: 10.1186/s11658-022-00379-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen P., Zhong J., Ye J., et al. miR-324-5p protects against oxidative stress-induced endothelial progenitor cell injury by targeting Mtfr1. J Cell Physiol. 2019;234:22082–22092. doi: 10.1002/jcp.28771. [DOI] [PubMed] [Google Scholar]
- 71.Das S., Kohr M., Dunkerly-Eyring B., et al. Divergent effects of miR-181 family members on myocardial function through protective cytosolic and detrimental mitochondrial microRNA targets. J Am Heart Assoc. 2017;6(3) doi: 10.1161/JAHA.116.004694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Das S., Ferlito M., Kent O.A., et al. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res. 2012;110:1596–1603. doi: 10.1161/CIRCRESAHA.112.267732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu X., Liu H., Wang Z., Hu Z., Li L. miR-200a attenuated doxorubicin-induced cardiotoxicity through upregulation of Nrf2 in mice. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/1512326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piegari E., Cozzolino A., Ciuffreda L.P., et al. Cardioprotective effects of miR-34a silencing in a rat model of doxorubicin toxicity. Sci Rep. 2020;10 doi: 10.1038/s41598-020-69038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan J.-A., Tang Y., Yu J.-Y., et al. miR-146a attenuates apoptosis and modulates autophagy by targeting TAF9b/P53 pathway in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2019;10(9):668. doi: 10.1038/s41419-019-1901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feyen E., Ricke-Hoch M., Fraeyenhove J.V., et al. ERBB4 and multiple microRNAs that target ERBB4 participate in pregnancy-related cardiomyopathy. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.120.006898. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y., Duan C., Liu W., et al. Upregulation of let-7f-2-3p by long noncoding RNA NEAT1 inhibits XPO1-mediated HAX-1 nuclear export in both in vitro and in vivo rodent models of doxorubicin-induced cardiotoxicity. Arch Toxicol. 2019;93:3261–3276. doi: 10.1007/s00204-019-02586-4. [DOI] [PubMed] [Google Scholar]
- 78.Ni J., Liu Y., Kang L., et al. Human trophoblast-derived exosomes attenuate doxorubicin-induced cardiac injury by regulating miR-200b and downstream Zeb1. J Nanobiotechnol. 2020;18:171. doi: 10.1186/s12951-020-00733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhai C., Tang G., Peng L., et al. Inhibition of microRNA-1 attenuates hypoxia/re-oxygenation-induced apoptosis of cardiomyocytes by directly targeting Bcl-2 but not GADD45Beta. Am J Transl Res. 2015;7:1952–1962. [PMC free article] [PubMed] [Google Scholar]
- 80.Diniz G.P., Lino C.A., Moreno C.R., Senger N., Barreto-Chaves M.L.M. MicroRNA-1 overexpression blunts cardiomyocyte hypertrophy elicited by thyroid hormone. J Cell Physiol. 2017;232:3360–3368. doi: 10.1002/jcp.25781. [DOI] [PubMed] [Google Scholar]
- 81.Ouyang Y.B., Lu Y., Yue S., Giffard R.G. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12:213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qian Y., Jiang J., Peng J., Wang Q., Shen Y. Evaluation of MIR-181a as a potential therapeutic target in osteoarthritis. Trop J Pharm Res. 2017;16:1069. [Google Scholar]
- 83.Indrieri A., Carrella S., Romano A., et al. miR-181a/b downregulation exerts a protective action on mitochondrial disease models. EMBO Mol Med. 2019;11 doi: 10.15252/emmm.201708734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lai L., Chen J., Wang N., Zhu G., Duan X., Ling F. MiRNA-30e mediated cardioprotection of ACE2 in rats with Doxorubicin-induced heart failure through inhibiting cardiomyocytes autophagy. Life Sci. 2017;169:69–75. doi: 10.1016/j.lfs.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 85.Wu X.F., Zhou Z.H., Zou J. MicroRNA-181 inhibits proliferation and promotes apoptosis of chondrocytes in osteoarthritis by targeting PTEN. Biochem Cell Biol. 2017;95:437–444. doi: 10.1139/bcb-2016-0078. [DOI] [PubMed] [Google Scholar]
- 86.Henao-Mejia J., Williams A., Goff L.A., et al. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013;38:984–997. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jing X., Yang J., Jiang L., Chen J., Wang H. MicroRNA-29b regulates the mitochondria-dependent apoptotic pathway by targeting Bax in doxorubicin cardiotoxicity. Cell Physiol Biochem. 2018;48:692–704. doi: 10.1159/000491896. [DOI] [PubMed] [Google Scholar]
- 88.Arif M., Pandey R., Alam P., et al. MicroRNA-210-mediated proliferation, survival, and angiogenesis promote cardiac repair post myocardial infarction in rodents. J Mol Med (Berl) 2017;95:1369–1385. doi: 10.1007/s00109-017-1591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan Z.G., Qu X.L., Chu P., et al. MicroRNA-210 promotes angiogenesis in acute myocardial infarction. Mol Med Rep. 2018;17:5658–5665. doi: 10.3892/mmr.2018.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bei Y., Lu D., Bär C., et al. miR-486 attenuates cardiac ischemia/reperfusion injury and mediates the beneficial effect of exercise for myocardial protection. Mol Ther. 2022;30:1675–1691. doi: 10.1016/j.ymthe.2022.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lange S., Banerjee I., Carrion K., et al. miR-486 is modulated by stretch and increases ventricular growth. JCI Insight. 2019;4(19) doi: 10.1172/jci.insight.125507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xia W., Chang B., Li L., et al. MicroRNA therapy confers anti-senescent effects on doxorubicin-related cardiotoxicity by intracellular and paracrine signaling. Aging (Albany NY) 2021;13:25256–25270. doi: 10.18632/aging.203743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meng J., Xu C. MicroRNA-495-3p diminishes doxorubicin-induced cardiotoxicity through activating AKT. J Cell Mol Med. 2022;26:2076–2088. doi: 10.1111/jcmm.17230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Yin Y., Jiang H. miR-30e-5p alleviates inflammation and cardiac dysfunction after myocardial infarction through targeting PTEN. Inflammation. 2021;44:769–779. doi: 10.1007/s10753-020-01376-w. [DOI] [PubMed] [Google Scholar]
- 95.Liu X., Guo B., Zhang W., Ma B., Li Y. MiR-20a-5p overexpression prevented diabetic cardiomyopathy via inhibition of cardiomyocyte apoptosis, hypertrophy, fibrosis and JUNK/NF-κB signalling pathway. J Biochem. 2021;170:349–362. doi: 10.1093/jb/mvab047. [DOI] [PubMed] [Google Scholar]
- 96.Frank D., Gutenberg J., Boomgaarden I., et al. MicroRNA-20a inhibits stress-induced cardiomyocyte apoptosis involving its novel target Egln3/PHD3. J Mol Cell Cardiol. 2012;52(3):711–717. doi: 10.1016/j.yjmcc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 97.Wan Q., Xu T., Ding W., et al. miR-499-5p attenuates mitochondrial fission and cell apoptosis via p21 in doxorubicin cardiotoxicity. Front Genet. 2019;9:734. doi: 10.3389/fgene.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhuang L., Xia W., Chen D., et al. Exosomal LncRNA–NEAT1 derived from MIF-treated mesenchymal stem cells protected against doxorubicin-induced cardiac senescence through sponging miR-221-3p. J Nanobiotechnol. 2020;18:157. doi: 10.1186/s12951-020-00716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leidal A.M., Huang H.H., Marsh T., et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Bio.l. 2020;22:187–199. doi: 10.1038/s41556-019-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santovito D., Egea V., Bidzhekov K., et al. Noncanonical inhibition of caspase-3 by a nuclear microRNA confers endothelial protection by autophagy in atherosclerosis. Sci Transl Med. 2020;12(546):eaaz2294. doi: 10.1126/scitranslmed.aaz2294. [DOI] [PubMed] [Google Scholar]
- 101.Huang Y., Qi Y., Du J.Q., Zhang D.F. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4. Expert Opin Ther Targets. 2014;18:1355–1365. doi: 10.1517/14728222.2014.961424. [DOI] [PubMed] [Google Scholar]
- 102.Yang Y., Cheng H.-W., Qiu Y., et al. MicroRNA-34a plays a key role in cardiac repair and regeneration following myocardial infarction. Circ Res. 2015;117:450–459. doi: 10.1161/CIRCRESAHA.117.305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shan H., Zhang Y., Lu Y., et al. Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc Res. 2009;83:465–472. doi: 10.1093/cvr/cvp130. [DOI] [PubMed] [Google Scholar]
- 104.van Rooij E., Sutherland L.B., Thatcher J.E., et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valkov N., King M.E., Moeller J., Liu H., Li X., Zhang P. MicroRNA-1-mediated inhibition of cardiac fibroblast proliferation through targeting Cyclin D2 and CDK6. Front Cardiovasc Med. 2019;6:65. doi: 10.3389/fcvm.2019.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Rooij E., Quiat D., Johnson B.A., et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17:662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Safa A., Bahroudi Z., Shoorei H., et al. miR-1: a comprehensive review of its role in normal development and diverse disorders. Biomed Pharmacother. 2020;132 doi: 10.1016/j.biopha.2020.110903. [DOI] [PubMed] [Google Scholar]
- 108.Ai J., Zhang R., Li Y., et al. Circulating microRNA-1 as a potential novel biomarker for acute myocardial infarction. Biochem Biophys Res Commun. 2010;391:73–77. doi: 10.1016/j.bbrc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 109.Yang B., Lin H., Xiao J., et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 110.Yu H., Lu Y., Li Z., Wang Q. microRNA-133: expression, function and therapeutic potential in muscle diseases and cancer. Curr Drug Targets. 2014;15:817–828. doi: 10.2174/1389450115666140627104151. [DOI] [PubMed] [Google Scholar]
- 111.Zhu M.L., Yin Y.L., Ping S., et al. Berberine promotes ischemia-induced angiogenesis in mice heart via upregulation of microRNA-29b. Clin Exp Hypertens. 2017;39:672–679. doi: 10.1080/10641963.2017.1313853. [DOI] [PubMed] [Google Scholar]
- 112.Boen J.R.A., Gevaert A.B., De Keulenaer G.W., Van Craenenbroeck E.M., Segers V.F.M. The role of endothelial miRNAs in myocardial biology and disease. J Mol Cell Cardiol. 2020;138:75–87. doi: 10.1016/j.yjmcc.2019.11.151. [DOI] [PubMed] [Google Scholar]
- 113.Gryshkova V., Lushbough I., Palmer J., et al. microRNAs signatures as potential biomarkers of structural cardiotoxicity in human-induced pluripotent stem-cell derived cardiomyocytes. Arch Toxicol. 2022;96:2033–2047. doi: 10.1007/s00204-022-03280-8. [DOI] [PubMed] [Google Scholar]
- 114.Hergenreider E., Heydt S., Tréguer K., et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 115.Feng Y., Zou L., Yan D., et al. Extracellular microRNAs induce potent innate immune responses via TLR7/MyD88-dependent mechanisms. J Immunol. 2017;199:2106–2117. doi: 10.4049/jimmunol.1700730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lehmann S.M., Krüger C., Park B., et al. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 117.Maryam M., Naemi M., Hasani S.S. A comprehensive review on oncogenic miRNAs in breast cancer. J Genet. 2021:100. [PubMed] [Google Scholar]
- 118.Orso F., Quirico L., Dettori D., et al. Role of miRNAs in tumor and endothelial cell interactions during tumor progression. Semin Cancer Biol. 2020;60:214–224. doi: 10.1016/j.semcancer.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 119.Robinson S., Follo M., Haenel D., et al. Droplet digital PCR as a novel detection method for quantifying microRNAs in acute myocardial infarction. Int J Cardiol. 2018;257:247–254. doi: 10.1016/j.ijcard.2017.10.111. [DOI] [PubMed] [Google Scholar]
- 120.Didychuk A.L., Butcher S.E., Brow D.A. The life of U6 small nuclear RNA, from cradle to grave. RNA. 2018;24:437–460. doi: 10.1261/rna.065136.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vandesompele J., De Preter K., Pattyn F., et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lombardi G., Perego S., Sansoni V., Banfi G. Circulating miRNA as fine regulators of the physiological responses to physical activity: pre-analytical warnings for a novel class of biomarkers. Clin Biochem. 2016;49:1331–1339. doi: 10.1016/j.clinbiochem.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 123.Heegaard N.H.H., Carlsen A.L., Lilje B., et al. Diurnal variations of human circulating cell-free micro-RNA. PLoS One. 2016;11 doi: 10.1371/journal.pone.0160577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Becker N., Lockwood C.M. Pre-analytical variables in miRNA analysis. Clin Biochem. 2013;46:861–868. doi: 10.1016/j.clinbiochem.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 125.Witvrouwen I., Gevaert A.B., Van Craenenbroeck E.M., Van Craenenbroeck A.H. MicroRNA isolation from plasma for real-time qPCR array. Curr Protoc Hum Genet. 2018;99:e69. doi: 10.1002/cphg.69. [DOI] [PubMed] [Google Scholar]
- 126.de Gonzalo-Calvo D., Marchese M., Hellemans J., et al. Consensus guidelines for the validation of qRT-PCR assays in clinical research by the CardioRNA consortium. Mol Ther Methods Clin Dev. 2022;24:171–180. doi: 10.1016/j.omtm.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jazbutyte V., Fiedler J., Kneitz S., et al. MicroRNA-22 increases senescence and activates cardiac fibroblasts in the aging heart. Age (Dordr) 2013;35:747–762. doi: 10.1007/s11357-012-9407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sharma S., Eghbali M. Influence of sex differences on microRNA gene regulation in disease. Biol Sex Differ. 2014;5(1):3. doi: 10.1186/2042-6410-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Te J.L., Dozmorov I.M., Guthridge J.M., et al. Identification of unique microRNA signature associated with lupus nephritis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weber M., Baker M.B., Patel R.S., Quyyumi A.A., Bao G., Searles C.D. MicroRNA expression profile in CAD patients and the impact of ACEI/ARB. Cardiol Res Pract. 2011;2011 doi: 10.4061/2011/532915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Takahashi Y., Satoh M., Minami Y., Tabuchi T., Itoh T., Nakamura M. Expression of miR-146a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: effect of renin-angiotensin system blockade and statins on miRNA-146a/b and Toll-like receptor 4 levels. Clin Sci (Lond) 2010;119:395–405. doi: 10.1042/CS20100003. [DOI] [PubMed] [Google Scholar]
- 132.Solayman M.H., Langaee T.Y., Gong Y., et al. Effect of plasma MicroRNA on antihypertensive response to beta blockers in the Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR) studies. Eur J Pharm Sci. 2019;131:93–98. doi: 10.1016/j.ejps.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schembri F., Sridhar S., Perdomo C., et al. MicroRNAs as modulators of smoking-induced gene expression changes in human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:2319–2324. doi: 10.1073/pnas.0806383106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gupta S.K., Bang C., Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:484–488. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 135.Yang F., Fu Z., Yang M., et al. Expression pattern of microRNAs related with response to trastuzumab in breast cancer. J Cell Physiol. 2019;234(9):16102–16113. doi: 10.1002/jcp.28268. [DOI] [PubMed] [Google Scholar]
- 136.Zampetaki A., Willeit P., Drozdov I., Kiechl S., Mayr M. Profiling of circulating microRNAs: from single biomarkers to re-wired networks. Cardiovasc Res. 2012;93:555–562. doi: 10.1093/cvr/cvr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mayr M., Zampetaki A., Willeit P., Willeit J., Kiechl S. MicroRNAs within the continuum of postgenomics biomarker discovery. Arterioscler Thromb Vasc Biol. 2013;33:206–214. doi: 10.1161/ATVBAHA.112.300141. [DOI] [PubMed] [Google Scholar]
- 138.Yang H.-H., Chen Y., Gao C.-Y., Cui Z.-T., Yao J.-M. Protective effects of microRNA-126 on human cardiac microvascular endothelial cells against hypoxia/reoxygenation-induced injury and inflammatory response by activating PI3K/Akt/eNOS signaling pathway. Cell Physiol Biochem. 2017;42:506–518. doi: 10.1159/000477597. [DOI] [PubMed] [Google Scholar]
- 139.Liu H., Huan L., Yin J., et al. Role of microRNA-130a in myocardial hypoxia/reoxygenation injury. Exp Ther Med. 2017;13:759–765. doi: 10.3892/etm.2016.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pakravan G., Foroughmand A.M., Peymani M., et al. Downregulation of miR-130a, antagonized doxorubicin-induced cardiotoxicity via increasing the PPARγ expression in mESCs-derived cardiac cells. Cell Death Dis. 2018;9:758. doi: 10.1038/s41419-018-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mazurek S.R., Calway T., Harmon C., Farrell P., Kim G.H. MicroRNA-130a regulation of desmocollin 2 in a novel model of arrhythmogenic cardiomyopathy. Microrna. 2017;6(2):143–150. doi: 10.2174/2211536605666161109111031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xin M., Small E.M., Sutherland L.B., et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009;23:2166–2178. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tony H., Yu K., Qiutang Z. MicroRNA-208a silencing attenuates doxorubicin induced myocyte apoptosis and cardiac dysfunction. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/597032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang K., Zhang D.L., Long B., et al. NFAT4-dependent miR-324-5p regulates mitochondrial morphology and cardiomyocyte cell death by targeting Mtfr1. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu J., Lei S., Sun S., et al. MiR-324-3p regulates fibroblast proliferation via targeting TGF-β1 in atrial fibrillation. Int Heart J. 2020;61:1270–1278. doi: 10.1536/ihj.20-423. [DOI] [PubMed] [Google Scholar]
- 146.Wu J., Yue B., Lan X., et al. MiR-499 regulates myoblast proliferation and differentiation by targeting transforming growth factor β receptor 1. J Cell Physiol. 2019;234:2523–2536. doi: 10.1002/jcp.26903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.