Abstract
Introduction
Brachial plexus birth injury is the most common birth injury causing permanent disability in Finland. This study aimed to assess risk factors of a permanent brachial plexus birth injury and calculate the incidence.
Material and methods
This is a retrospective population‐based study including all deliveries between 2006 and 2022 in Southern Finland. The number of children born, obstetric data, and migrant status were gathered from the registries of the Finnish Institute for Health and Welfare, and Statistics Finland. Race of the mothers of children with a permanent brachial plexus birth injury was recorded. The severity of permanent brachial plexus birth injury was assessed using the 3‐month Toronto test score. A lower score was indicative of a more severe injury (scored 0–10).
Results
One hundred of the 298 428 children born during the 17‐year study period sustained a permanent brachial plexus birth injury (0.34 per 1000). Mothers of children with a permanent brachial plexus birth injury had a higher body mass index (29 vs. 24 kg/m2) and their pregnancies were more often complicated by diabetes (28% vs. 12%), shoulder dystocia (58% vs. 0.3%), and/or assisted deliveries (45% vs. 10%) compared with all other mothers (p < 0.001). Thirty two of the 52 725 children born to migrant mothers had a permanent brachial plexus birth injury (0.61 per 1000). The incidence of permanent brachial plexus birth injury was 5.7 times higher among children of Black migrants from Africa (18/11 738, 1.53 per 1000) compared with children of native mothers (0.27 per 1000). Black mothers had a higher body mass index at the start of pregnancy (29 vs. 26 kg/m2, p = 0.02) compared with Caucasians. Children of Black mothers had a more severe injury compared with all others (p = 0.007) with a mean 3‐month Toronto test score of 4.2 (range 0.0–6.5, SD ±1.6) vs. 5.6 (range 0.0–9.3, SD ±2.2).
Conclusions
Shoulder dystocia and assisted delivery are the most important risk factors for a permanent brachial plexus birth injury. Black race was associated with a higher rate and a more severe permanent brachial plexus birth injury.
Keywords: birth injury, brachial plexus birth injury, incidence, migrant, race, risk factor
Mothers of children with a permanent brachial plexus birth injury (BPBI) had a higher body mass index and their pregnancies were more often complicated by diabetes, shoulder dystocia, and/or assisted deliveries compared with all other mothers (p < 0.001). The incidence was higher for a permanent BPBI in the migrant vs. the native population (0.61 vs. 0.27, p = 0.01) with African migrants having the highest incidence of 1.53 in comparison to the native population (p = 0.006). Children of Black mothers had a more severe injury compared with all others (p = 0.007).
Abbreviations
- 3MTS
3‐month Toronto test Score
- BMI
body mass index
- BPBI
brachial plexus birth injury
- CI
confidence interval
- DM
diabetes mellitus
- GDM
gestational diabetes mellitus
- HUS
Helsinki University Hospital
- ICD‐10
International Classification of Diseases 10th revision
- NHS
UK National Health Service
- OR
odds ratio
- SD
standard deviation
- THL
Finnish Institute for Health and Welfare
Key message.
The incidence of permanent brachial plexus birth injury is on the rise in the non‐white population for unknown reasons. The injury appears to be worse in children of Black women.
1. INTRODUCTION
Brachial plexus birth injury (BPBI) is the most common severe birth injury in Finland, with an overall incidence of 2.5 per 1000 live births. 1 The incidence is declining in many countries. 1 , 2 , 3 , 4 Several pregnancy‐related risk factors for BPBI have been identified, but as many as half of BPBI still occur without any identifiable risk factor. 2 Shoulder dystocia is the most important known risk factor leading to BPBI in 6%–40%. 2 , 5 Other risk factors include instrumental delivery and factors, such as diabetes mellitus (DM), causing fetal macrosomia. 2 , 3 , 4 Socioeconomic and racial factors also appear to play a role with women residing in poorer areas or areas with lower availability of health care, as well as non‐white women, showing an increased risk for delivering a child with a BPBI. 2 , 3 , 4 , 6
Possible explanations for the declining incidence of BPBI in some areas are thought to be the rising rate of cesarean sections and differences in the availability and standard of antenatal care, especially with regards to DM. In addition, timely identification and treatment of shoulder dystocia births appear to have a lowering effect on the incidence. 2 , 3 , 4 , 5 , 7 As a consequence many birth hospitals strive to educate the delivery staff by giving regular shoulder dystocia simulation trainings, which have proven to decrease the prevalence of permanent BPBI as well as other delivery complications. 5 , 7 Most BPBI resolve spontaneously during the first 3 months. 8 , 9 , 10 In about one‐fifth of the children, the injury is permanent. 3 , 4 A permanent BPBI injury causes lifelong disability of varying degrees. 11 , 12 , 13 In the most severe cases, the affected limb is short and painful, with a limited range of motion and diminished sensation. 14 , 15 The incidence of permanent BPBI is between 0.1 and 1.6 per 1000 births. 5 , 8 , 9 , 16 , 17
This study aimed to assess risk factors leading to a permanent BPBI in Southern Finland. In addition, the incidence of both permanent and transient injury was calculated.
2. MATERIAL AND METHODS
This retrospective population‐based study analyzed risk factors for a permanent BPBI. Helsinki University Hospital (HUS) Women's Hospital, with its three satellite units, represents the only birth center in Southern Finland, serving a population of two million inhabitants. HUS New Children's Hospital is the only primary care center for patients with BPBI in the area. STROBE guidelines were followed.
2.1. Data collection
The Finnish Institute for Health and Welfare (THL) (www.thl.fi) has gathered standardized essential information on all pregnancies, including data on antenatal care, delivery characteristics, and information regarding the newborn. Race is not registered. For this study, the following maternal data were collected from the THL database: personal identity code (for data linkage), maternal age, parity, comorbidities and type of delivery, gestational age at delivery, mode and characteristics of delivery, and complications during pregnancy and delivery. Assisted deliveries are documented by the performing obstetrician, and data regarding the procedure duration (minutes) and difficulty, number of pulls, and the difficulty in delivering shoulders (1 = easy, 2 = moderate, 3 = difficult) are registered. Data collected from the newborn included personal identity code, sex, birthweight, and birth injuries as International Classification of Diseases 10th revision (ICD‐10) codes.
Statistics Finland (www.stat.fi) collects data regarding Finland's migrant population including country of birth, residential area, parity, and maternal age at delivery year. The data from the Statistics Finland database are only available in aggregated format. In this study a migrant is defined as a person with foreign (non‐Finnish) background up to second generation.
HUS New Children's Hospital hosts a prospective BPBI registry storing information on all children with a permanent BPBI as well as maternal birth characteristics. The registry includes data on newborn birthweight, sex, side of injury, and severity of the injury. In addition, it includes information on possible parental migrant status and departure country, race, and knowledge of the national language (Finnish or Swedish). Race is described as the US National Institutes of Health recommends (Black, Asian, Caucasian, and Hispanic). From 2006 HUS Women's Hospital and Children's Hospital have been using the same electronic patient record system.
Any child presenting with BPBI in Southern Finland is examined by the referral center's pediatrician at 0–2 days of age. All children with an injury that does not resolve itself during the first month are referred to the HUS New Children's Hospital's BPBI clinic for further evaluation by a specialized team consisting of a hand surgeon with a minimum level 4 expertise, an occupational therapist, a physiotherapist, and a nurse. 18 , 19 The same team regularly examines patients at set intervals from 1 month to 18 years. The 3‐month Toronto test score (3MTS) is calculated to assess the injury's severity. 13 The 3MTS is graded from 0 to 10, where 3.5 or lower indicates a severe type of permanent injury where the child may benefit from plexus reconstruction. A permanent BPBI is defined as clinically evident limited active or passive range of motion or decreased strength of the affected limb that is still detectable at 1 year.
2.2. Statistical analyses
Maternal and delivery characteristics between cases with and without BPBI were compared using the chi‐squared and t tests. A similar comparison was used to detect differences between migrant and native women as well as women and children of different racial backgrounds. Associations with the severity of the injury was investigated by fitting linear regression models for the 3MTS score. Univariate and multivariate models were used to identify correlations between maternal risk factors (including race and migration status) and permanent BPBI. The level of significance was set at p < 0.05. Odds ratios (OR) with 95% confidence intervals (CI) for having a severe injury (3MTS ≤3.5) were calculated. An external biostatistician was consulted.
3. RESULTS
3.1. Study population
All women giving birth between 2006 and 2022 in Southern Finland and their live‐born children were included in the study (Table 1). During the study period, 298 428 live births occurred in the study area, of which 52 725 were births by migrants (17.7%) (Table 2). The rate of cesarean deliveries increased from 18.3% in 2006 to 22.7% in 2022, with an overall frequency of 18.5% (n = 54 788). Of all newborns, 600 sustained a BPBI (ICD‐10 codes: P14.0, P14.1, and P14.3), of which 100 (51 girls) were permanent (16.7%). Of the children with a permanent injury, 98 were born vaginally (six breech) and two via emergency cesareans (breech n = 1, placental abruption n = 1). All except four were full‐term deliveries with a mean pregnancy duration of 39.9 weeks (range 37.0–42.3, SD ±2.1). Of the four premature newborns, two were breech births at 28 and 29 weeks of gestation and two were cephalic births at week 35.
TABLE 1.
Maternal and delivery characteristics.
| All births (excl. BPBI) | Births resulting in a permanent BPBI | p value BPBI vs. no BPBI | |
|---|---|---|---|
| Deliveries/newborns | 298 328/299119 | 100/100 | |
| Age (years) | 31.4 (12.3–58.1, SD ±5.2) | 31.0 (18.8–41.9, SD ±5.2) | 0.399 |
| BMI | 24.2 (12.0–68.0, SD ±4.7) | 26.9 (17.5–41.5, SD ±4.9) | <0.001 |
| Nulliparity | 45.5% (n = 134 553) | 47.0% (n = 47) | 0.770 |
| Diabetes (all) | 12.3% (n = 36 379) | 28% (n = 28) | <0.001 |
| Gestational diabetes | 11.3% (n = 33 515) | 24% (n = 24) | <0.001 |
| Diabetes type I | 0.6% (n = 1784) | 4% (n = 4) | <0.001 |
| Duration of pregnancy (weeks) | 39.8 (21.6–44.3, SD ±1.8) | 39.9 (28.9–42.3, SD ±2.1) | 0.648 |
| Cesarean delivery | 18.5% (n = 54 788) | 2% b (n = 2) | <0.001 |
| Induction of labor | 24.6% (67 575/274 893) | 26% (n = 26) | 0.115 |
| Breech delivery | 0.7% (n = 2023) | 6.1% (n = 6) | <0.001 |
| Stage II duration (minutes) | 30.5 (0–90, SD ±27.7) | 31.2 (4–81, SD ±18.6) | 0.07 |
| Assisted delivery | 10.1% a (29 704 vacuum, 26 forceps) | 44.6% a (41/92, all vacuum) | <0.001 |
| Shoulder dystocia | 0.3% a (663/240 640) | 57.6% a (n = 53/92) | <0.001 |
| Birthweight | 3477 (400–6150, SD ±573) | 4107 (780–5580, SD ±677) | <0.001 |
| Migrant mother | 17.6% (n = 52 725) | 32% (n = 32/100) | <0.001 |
| African | 3.9% (n = 11 738) | 18% (18/100) | <0.001 |
Note: Obstetric data from all deliveries (n = 298 428) in Southern Finland from 2006 to 2022. BMI as reported at the start of the pregnancy. Comparisons between the groups were made using the chi‐squared and t tests. Results are expressed in mean with range and SD or rate (%) and number (n).
Abbreviations: BMI, body mass index; BPBI, brachial plexus birth injury; SD, standard deviation.
Breech and cesarean deliveries were excluded.
Both were emergency cesarean deliveries (placental abruption and unexpected breech in spontaneously started deliveries).
TABLE 2.
Incidence of permanent brachial plexus birth injury by maternal migration background continent.
| Native | Asia | Africa | America a | Europe | Oceania | Unknown | All | |
|---|---|---|---|---|---|---|---|---|
| Live births (n) | 245 703 | 15 711 | 11 738 | 1377 | 23 133 | 63 | 703 | 298 428 |
| Permanent BPBI (n) | 68 | 5 | 18 | 1 | 8 | 0 | 0 | 100 |
| Incidence | 0.27 | 0.32 | 1.53 | NA | 0.35 | NA | NA | 0.34 |
Note: The difference in the incidence of permanent BPBI compared with native population by maternal migration background continent during the study period from 2006 to 2022. Cesarean deliveries have not been removed as mode of birth is not gathered in the Statistics Finland database.
Abbreviation: BPBI, brachial plexus birth injury.
America represents North, Central and South America in the database, which is why one Hispanic mother could not be referenced to her background continent (South America).
In 54/100 children the injury was right‐sided (two bilateral, both breech). The mean 3MTS was 5.6 (range 0–9.3, SD ±2.2). Thirty‐two (32%) of the children with a permanent injury were born to migrant mothers, eighteen (56%) of which were Black, and of African descent. The remaining 14 children were born to migrants from Europe (n = 8, Caucasian), Asia (n = 5, Asian), and South America (n = 1, Hispanic). All native mothers (n = 68) were Caucasian. Tables 1 through 3 present further birth and maternal characteristics.
TABLE 3.
Differences in delivery characteristics between Caucasian and non‐white mothers.
| Caucasian | Non‐white | p value | |
|---|---|---|---|
| Number of deliveries | 76 | 24 | NA |
| Age (years) | 31.7 (19.4–41.9, SD ±5.0) | 29.2 (18.8–40.3, SD ±5.6) | 0.08 |
| BMI | 26.1 (19.3–41.5, SD ±4.6) | 28.3 (20.1–40.0, SD ±5.3) | 0.03 |
| Nulliparity | 47.4% (n = 36) | 25.0% (n = 6) | 0.01 |
| Diabetes (all) | 26.3% (n = 20) | 33.3% (n = 8) | 0.54 |
| Gestational diabetes | 22.3% (n = 17) | 29.2% (n = 7) | 0.49 |
| Diabetes type I | 3.9% (n = 3) | 4.1% (n = 1) | 0.9 |
| Pregnancy duration (weeks) | 40.0 (28.9–42.3, SD ±1.9) | 39.8 (38.0–42.1, SD ±2.6) | 0.78 |
| Induction of labor | 26.3% (n = 20) | 25.0% (n = 6) | 0.89 |
| Cesarean delivery | 2.6% b (n = 2) | 0.0% (n = 0) | NA |
| Breech delivery | 5.2% (n = 4) | 8.3% (n = 2) | 0.04 |
| Assisted delivery | 46.4% a (n = 32) | 40.9% a (n = 9) | 0.69 |
| Duration (minutes) | 7.4 (1–20, SD ±4.7) | 8.8 (5–16, SD ±3.7) | 0.51 |
| Number of pulls | 3.4 (1–10, SD ±2.0) | 4.7 (1–8, SD ±2.2) | 0.11 |
| Shoulder delivery difficulty (1–3) | 2.7 (1–3, SD ±0.6) | 2.6 (1–3, SD ±0.6) | 0.74 |
| Overall difficulty (1–3) | 1.6 (1–3, SD ±0.7) | 2.3 (1–3, SD ±1.0) | 0.046 |
| Stage II duration (minutes) | 32.6 (4–81, SD ±18.3) | 25.6 (4–65, SD ±17.1) | 0.09 |
| Shoulder dystocia | 57.9% a (n = 40) | 59.1% a (n = 13) | 0.87 |
| Birthweight (grams) | 4062 (780–5010, SD ±656) | 3913 (1510–5580, SD ±743) | 0.38 |
Note: The differences in pregnancy and delivery characteristics between Caucasian and non‐white mothers to children with a permanent brachial plexus birth injury. Comparisons between the groups were made using the chi‐squared and t tests. Results were expressed in mean with range and SD or rate (%) and number (n).
Abbreviations: BMI, body mass index; BPBI, brachial plexus birth injury; SD standard deviation.
Breech and cesarean deliveries were excluded; all assisted deliveries were vacuum extractions.
Both were emergency cesarean deliveries (placental abruption and unexpected breech in spontaneously started deliveries).
3.2. Risk factors
Mothers of children with a permanent BPBI had a higher body mass index (BMI) at the start of pregnancy and more often presented with any type of DM (p < 0.001 for both). In comparison with all other mothers, mothers of children with a permanent BPBI had a significantly higher occurrence of deliveries complicated by shoulder dystocia (59% vs. 0.3%) and/or assisted deliveries (45% vs. 10%) (p < 0.001 for both). There was no difference in maternal age, parity, gestation at delivery, incidence of induction, or duration of stage 2 (active labor) between the forementioned groups (Table 1). Children with a permanent injury were heavier at birth (p < 0.001).
The migrant mothers were younger (mean 29.4 vs. 32.1 years, p = 0.02) than the native mothers. However, no difference was found in BMI, DM, nulliparity, duration of pregnancy, type of delivery, occurrence of shoulder dystocia, or birthweight between the two (p > 0.05) groups. Non‐white women (Asian, Black, Hispanic, n = 24) had a higher BMI at the start of pregnancy (mean 28.3 vs. 26.1 kg/m2, p = 0.03) and a lower rate of nulliparity (25% vs. 47%, p = 0.01) in comparison with Caucasians (Table 3). This difference was more pronounced in Black mothers (mean BMI 28.7 kg/m2, p = 0.02 and 16.7% nulliparity rate, p < 0.01). Race did not impact gestation at delivery nor the incidence of induced deliveries; however, the incidence of breech deliveries was higher in the non‐white group (p = 0.04) (Table 3). Among the 41 assisted deliveries, the non‐white (n = 9) women's procedures were categorized as more difficult (p = 0.046) in comparison with Caucasians (Table 3). Of the 32 migrant mothers, only 10 spoke one of the national languages at the time of delivery.
3.3. Severity of the injury
Neither the duration of delivery stage 2 nor duration of the vacuum extraction correlated with the injury's severity (p > 0.05). Children of Black mothers had a more severe injury compared with all others (p = 0.007) with a mean 3MTS of 4.2 (range 0.0–6.5, SD ±1.6) vs. 5.6 (range 0.0–9.3, SD ±2.2) (Figure 1). Neither migrant Caucasians (mean 6.3, range 0.3–7.6, SD ±1.3, p = 0.7) nor Asians (mean 7.2, range 5.4–8.6, SD ±1.9, p = 0.3) showed any statistically significant difference to the native population (mean 5.9, range 0.0–9.3, SD ±2.3) with regards to the 3MTS.
FIGURE 1.
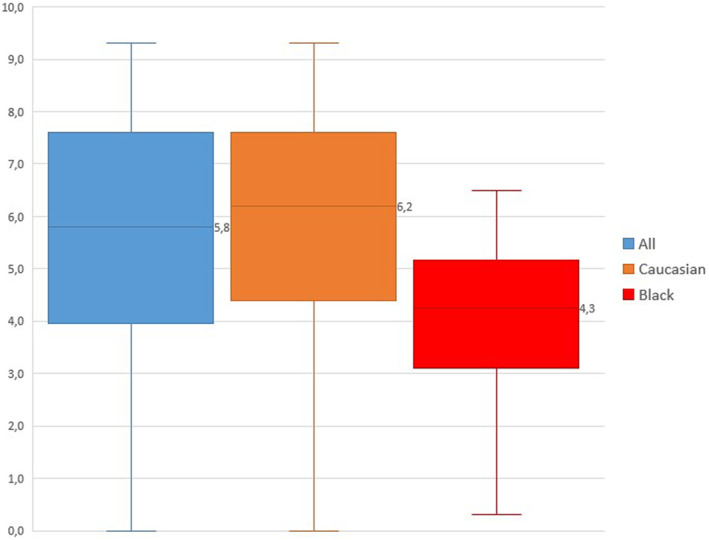
Box‐plot figure showing the difference in the 3‐month Toronto test scores. A lower score indicates a more severe injury. All children's scores are included in group All (n = 100), both native and migrant Caucasians in group Caucasians (n = 76), and all Black (n = 18) in group Black. The median (line in box) separates the first and third quartiles. The difference is statistically significant between the Black and All as well as Black and Caucasian (p = 0.003 and p = 0.004).
The OR of having a child with a 3MTS of 3.5 or lower was trending higher in the migrant population (OR 2.2, 95% CI 0.8–6.0) when compared with the native population. When analyzing the effect of race, Black mothers had the highest OR of having a child with a 3MTS of 3.5 or lower (OR 2.3, 95% CI 0.8–6.8) when compared with all others. Knowledge of national language at delivery did not correlate with the severe injury (p = 0.4).
3.4. Uni‐ and multivariate models
In the univariate linear regression analysis, non‐white race was associated with a more severe injury expressed by 3MTS (β = −1.12, range −2.17 to −0.07, p = 0.004). For Black women, the correlation was strongest (β = −1.64, range −2.79 to −0.49, p = 0.005). In the multivariate model adjusted for possible risk factors leading to BPBI, only Black race negatively impacted the outcome defined as lower 3MTS (β = −1.52, range −2.88 to −0.15, p = 0.03) (Table 4). No other associations between migration status, race, or birth characteristics, as described in Table 1 in relation to 3MTS, were found in the uni‐ or multivariate models (p > 0.05).
TABLE 4.
Multivariate model used to determine the effect of different maternal risk factors on a more severe permanent brachial plexus birth injury defined as a 3‐month Toronto test score (3MTS) ≤3.5.
| Maternal variable | Beta (range) | p value |
|---|---|---|
| Body mass index | −0.02 (−0.14 to 0.09) | 0.71 |
| Diabetes mellitus (all) | −0.94 (−2.16 to 0.27) | 0.13 |
| Shoulder dystocia | −0.57 (−1.62 to 0.48) | 0.29 |
| Age | −0.03 (−0.13 to 0.07) | 0.52 |
| Birthweight | 0.05 (−0.05 to 0.16) | 0.33 |
| Duration | 0.12 (−0.19 to 0.43) | 0.44 |
| Nulliparous | 0.22 (−0.98 to 1.42) | 0.71 |
| African | −1.52 (−2.88 to −0.15) | 0.03 |
Note: Only Black race associated with a worse 3‐month Toronto Score.
3.5. Trend in incidence
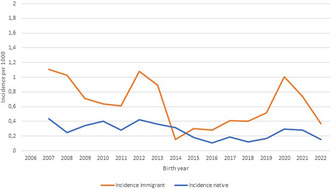
The mean calculated risk for a permanent BPBI in Southern Finland over the study period was 0.40 per 1000 vaginal live births, whereas the same for all deliveries was 0.34 (Table 2). The overall incidence showed a decreasing trend with considerable annual variation (Figure 2). The incidence was higher in the migrant vs. the native population (0.61 vs. 0.27, p = 0.01) with African migrants having the highest incidence of 1.53 in comparison to the native population (p = 0.006) (Table 2). Over the past 10 years (2013–2022) the incidence has been increasing in the immigrant population in comparison with the native population (p = 0.02) (Table 2). During the last 5 years of the study (2017–2022), more than half of all children with a permanent (n = 22) injury were born to migrant mothers (n = 12) (Figure 3).
FIGURE 2.
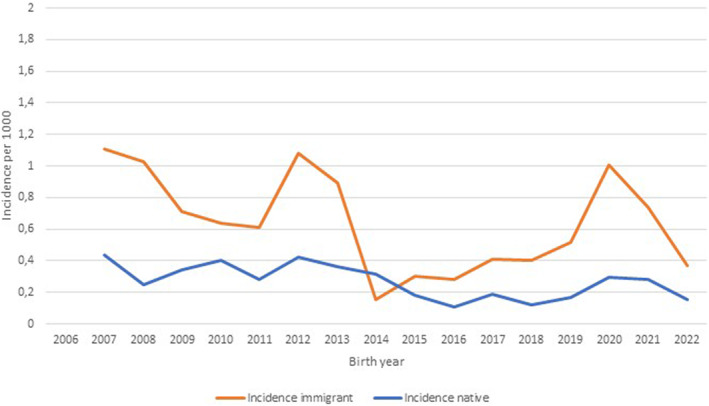
The incidence of permanent brachial plexus injury in migrant (mean 0.61 ± 0.49 SD, range 0.0–1.8) and native (mean 0.27 ± 0.16 SD, range 0–0.62) births over the study period expressed as two‐period moving averages. The mean total incidence during the study was 0.34 ± 0.18 SD per 1000 births (range 0–1.8). Cesarean deliveries have not been removed as they are not gathered in the Statistics Finland database. The difference in the incidence of permanent injuries between native and migrant populations was statistically significant (p = 0.003).
FIGURE 3.
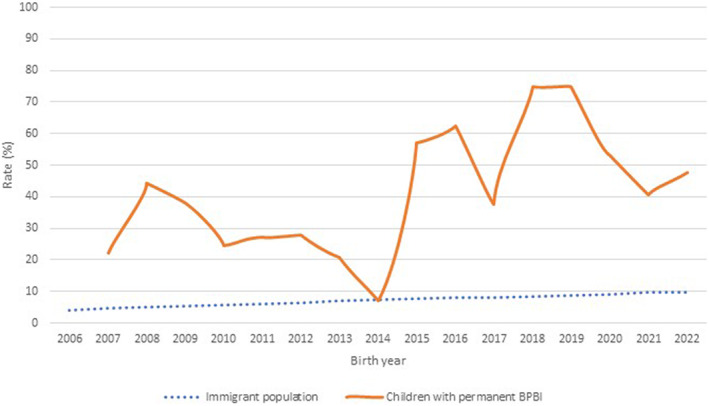
An increasing rate (%) of children with a permanent brachial plexus birth injury are born to migrant mothers presented as a two‐period moving average. The migrant population (rate) during the study period from 2006 to 2022 in Southern Finland is also shown.
4. DISCUSSION
Migrant women, especially Black women had a higher risk for having a child with a permanent and more severe type of BPBI (p < 0.05). Racial variations in the prevalence of BPBI have been reported both from the UK and the USA, suggesting socioeconomic factors as well as lack of access to high‐quality perinatal care may play a role. 2 , 3 , 4 The UK National Health Service (NHS) maternity statistics from 2022 showed a prevalence of BPBI in Black women of 0.8 in 1000 live births, whereas the same for Caucasians was 0.5. 20 In Finland, all women are entitled to the same free‐of‐charge prenatal care, including regular appointments with a midwife or physician to monitor maternal and fetal well‐being and screen for various risk factors. All deliveries are treated in public hospitals with highly trained delivery staff; private obstetricians are unavailable for delivery. It is possible that although the health care is free, it might be used differently by the immigrant population. It is not possible to know with high reliability whether the treatment of immigrant women was equal to that of native women. Unfortunately, we did not assess provision of antenatal care at the time of childbirth. However, major discrepancies in either utilization or treatment would lead to more pronounced pregnancy‐related complications such as a higher rate of DM and fetal macrosomia, or differences in induction or assisted deliveries, which were not identified in this study. We believe that variation in maternity care due to socioeconomic status is unlikely to explain the differences in the incidences of this study.
Gestational DM (GDM) is a known risk factor for fetal macrosomia. In this study, mothers to children with a permanent BPBI had a significantly higher frequency of GDM as well as pregestational diabetes compared with the background population (p < 0.001 for both). The diagnostic criteria for GDM have been changing over time. 21 More recent reports evaluating the relationship between maternal glycemia and fetal growth trajectories have confirmed the early impact of maternal glycemia on excess fetal growth before the diagnosis of standard GDM (from 24 weeks) suggesting the importance of early identification of mothers at risk. 21 Dietary acculturation appears to increase the risk of GDM in non‐white women. 22 In addition, African migrant women are reported to have the worst pregnancy outcomes among migrants and appear to develop GDM more often. 23 A strong inverse association between a woman's own birthweight and her risk for later developing GDM has been found, which appears to be independent of BMI. 24 According to this “fetal origin” hypothesis, intrauterine malnutrition—which occurs in many developing countries—impairs the function of pancreatic beta cells increasing the women's risk of developing GDM when exposed to a high‐energy diet later in life. 24 Unfortunately, we had no information on the number of mothers in this study who underwent an oral glucose tolerance test, and therefore we were unable to analyze possible differences in glucose levels between the mothers of children with a permanent BPBI and all mothers.
BPBI is declining in Finland, the UK, and the parts of USA. 1 , 2 , 3 , 4 Shoulder dystocia simulation training has yielded excellent results in lowering the prevalence of BPBI—nationally and globally—and is possibly the main reason for the decline together with the increase in cesarean births. 2 , 3 , 4 , 5 , 7 Nevertheless, the incidence in the migrant population is increasing, with almost 60% of children with a permanent injury born to migrant mothers during the last 5 years of the study. Also other studies have noted a greater prevalence of severe maternal comorbidities as well as an elevated risk of death during childbirth in migrants and non‐White women. 25 , 26 , 27 , 28 Many risk factors for these complications are the same as those of shoulder dystocia. 5 , 7 In parts of Sweden the incidence of stillbirths appears higher in women from sub‐Saharan countries in comparison with women originating from other areas. 28 Similarly, according to the UK NHS Maternity Statistics 2021–2022 Asian and Black women have a significantly higher risk for neonatal deaths (3.0 vs. 1.3 per 1000 live births) and emergency cesarean sections (25% vs. 18%) compared with Caucasians. 20 , 29 According to the UK NHS Maternity Statistics 2021–2022 Asian and Black women have a significantly higher risk for neonatal deaths (3.0 vs. 1.3 per 1000 live births) and emergency cesarean sections (25% vs. 18%) compared with Caucasians. 20 , 29 The same statistics show that Caucasian women's deliveries progress spontaneously slightly more often than in other ethnic groups (52.2% vs. 50.0%). 20 , 29 As there was no difference in the prevalence of the known risk factors for shoulder dystocia or BPBI between the groups of this study, the answer to the injury's predominance in the non‐white population could lie in pelvic anatomy. Women from the European gene pool often present with a transversally oval inlet compatible, while East Asian and African women, on average, have a rounder inlet, often oval in the anterior–posterior direction, matching the anthropoid pelvic shape. 30 , 31 , 32 It has been suggested that delivery progresses slightly differently in different pelvis types, and some argue that an anthropoid pelvis more often leads to a persistent occiput posterior position (OR 1.4 in Black vs. Caucasian women), increasing the risk for maternal and newborn morbidities. 33 , 34 In births complicated by shoulder dystocia, the occiput posterior position has been reported to increase the risk of BPBI. 33 Unfortunately, occiput posterior position is not registered in the birth registry in Finland.
As most of the migrant women (22/32) did not speak either of the two national languages at the time of childbirth, it is possible that lack of a common language as well as different cultural background influenced the expecting mother's ability to understand dietary as well as pregnancy‐ and delivery‐related instructions during pregnancy as well as during the progression of labor. Interpreting services could be beneficial during pre‐birth evaluation to confirm that the expecting mother knows when and whom to contact, and in addition to improve the communication with the delivery staff. Training the delivery staff in cultural differences during childbirth could be of benefit.
We have defined risk factors for a permanent BPBI and reported the population‐based incidence with special emphasis on the mothers' racial background. According to our knowledge, this is the first study to analyze a defined population for risk factors leading to a permanent BPBI using the participating hospitals' long tradition of prospective data gathering and the registry of THL, which has been shown to have high completeness and validity. 35 As information on race must not be registered in the THL database for legal reasons, risk factor analysis mirroring the whole population based on racial subgroups could not be done. Instead, we had to use aggravated data as reported by Statistics Finland, making the rather blunt assumption that most migrants from Africa are Black and those from Asia are Asians. Complete subgroup analysis was unachievable because of the small number of patients from some continents. DM during pregnancy is clearly of importance and we strongly suggest that future studies analyze the relationship between glucose levels during pregnancy and outcome.
5. CONCLUSION
Care should be taken, especially in the migrant population, to prevent and promptly recognize and treat known risk factors leading to birth complications. The incidence of a permanent BPBI is on the rise in the migrant population in Southern Finland.
AUTHOR CONTRIBUTIONS
The following contributions are reported in alphabetical order. All authors have approved the final version of the article. Concept and development of idea: Marja Kaijomaa, Petra Grahn and Yrjänä Nietosvaara. Design and literature search: Marja Kaijomaa and Petra Grahn. Critical revision and approval of design: Mika Gissler and Yrjänä Nietosvaara. Acquisition of data: Mika Gissler, Marja Kaijomaa, Petra Grahn. Funding and resources, analysis and statistics, tables and drafting of paper: Petra Grahn. Critical revision of the paper for intellectual content: Mika Gissler, Marja Kaijomaa and Yrjänä Nietosvaara. All authors interpreted the data and approved the final version of the article.
FUNDING INFORMATION
PG received a research grant for research leave from Finska Läkaresällskapet (www.fls.fi). None of the other authors report any funding.
CONFLICT OF INTEREST STATEMENT
None of the authors report any conflict of interest.
ETHICS STATEMENT
Institutional approval for the study was received from HUS Regional Committee on Medical Research Ethics (registration number 79/E7/2001) on March 2, 2001 and HUS/3082/2023 on April 26, 2023.
Grahn P, Gissler M, Nietosvaara Y, Kaijomaa M. Ethnic background as a risk factor for permanent brachial plexus birth injury: A population‐based study. Acta Obstet Gynecol Scand. 2024;103:1201‐1209. doi: 10.1111/aogs.14817
REFERENCES
- 1. Kekki M, Salonen A, Tihtonen K, Mattila V, Gissler M, Huttunen T. The incidence of birth injuries decreased in Finland between 1997 and 2017: a nationwide register study. Acta Paediatr. 2020;109:2562‐2569. [DOI] [PubMed] [Google Scholar]
- 2. DeFrancesco C, Shah D, Rogers B, Shah A. The epidemiology of brachial plexus birth palsy in the United States: declining incidence and evolving risk factors. J Pediatr Orthop. 2019;39:134‐140. [DOI] [PubMed] [Google Scholar]
- 3. Lalka A, Gralla J, Sibbel S. Brachial plexus birth injury: epidemiology and birth weight impact on risk factors. J Pediatr Orthop. 2020;40:460‐465. [DOI] [PubMed] [Google Scholar]
- 4. Merrison H, Mangtani A, Quick T. The shifting demographics of birth‐related brachial plexus injury: the impact of socio‐economic status and ethnic groups. J Plast Reconstr Aesthet Surg. 2021;74:560‐568. [DOI] [PubMed] [Google Scholar]
- 5. Kaijomaa M, Gissler M, Äyräs O, Sten A, Grahn P. Impact of simulation training on the management of shoulder dystocia and incidence of permanent brachial plexus birth injury: an observational study. BJOG. 2023;130:70‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans‐Jones G, Kay S, Weindling A, et al. Congenital brachial palsy: incidence, causes, and outcome in the United Kingdom and Republic of Ireland. Arch Dis Child Fetal Neonatal Ed. 2003;88:185‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crofts J, Lenguerrand E, Bentham G, et al. Prevention of brachial plexus injury—12 years of shoulder dystocia training: an interrupted time‐series study. BJOG. 2016;123:111‐118. [DOI] [PubMed] [Google Scholar]
- 8. Laurent J, Lee R, Shenaq S, Parke J, Solis I, Kowalik L. Neurosurgical correction of upper brachial plexus birth injuries. J Neurosurg. 1993;79:197‐203. [DOI] [PubMed] [Google Scholar]
- 9. Hoeksma A, ter Steeg A, Nelissen R, van Ouwerkerk W, Lankhorst G, de Jong B. Neurological recovery in obstetric brachial plexus injuries: a historical cohort study. Dev Med Child Neurol. 2004;46:76‐83. [DOI] [PubMed] [Google Scholar]
- 10. Pondaag W, Malessy M, Gert van Dijk J, Thomeer R. Natural history of obstetric brachial plexus palsy: a systematic review. Dev Med Child Neurol. 2004;46:138‐144. [DOI] [PubMed] [Google Scholar]
- 11. Waters P, Smith G, Jaramillo D. Glenohumeral deformity secondary to brachial plexus birth palsy. J Bone Joint Surg Am. 1998;80:668‐677. [DOI] [PubMed] [Google Scholar]
- 12. Waters P. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg Am. 1999;81:649‐659. [DOI] [PubMed] [Google Scholar]
- 13. Michelow B, Clarke H, Curtis C, Zuker R, Seifu Y, Andrews D. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675‐680. [PubMed] [Google Scholar]
- 14. Bae D, Ferretti M, Waters P. Upper extremity size difference in brachial plexus birth palsy. Hand (N Y). 2008;3:297‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruxelle J, Travers V, Thiebaut J. Occurrence and treatment of pain after brachial plexus injury. Clin Orthop Relat Res. 1988;237:87‐95. [PubMed] [Google Scholar]
- 16. Pöyhiä T, Lamminen A, Peltonen J, Kirjavainen M, Willamo P, Nietosvaara Y. Brachial plexus birth injury: US screening for glenohumeral joint instability. Radiology. 2010;254:253‐260. [DOI] [PubMed] [Google Scholar]
- 17. Chauhan S, Blackwell S, Ananth C. Neonatal brachial plexus palsy: incidence, prevalence, and temporal trends. Semin Perinatol. 2014;38:210‐218. [DOI] [PubMed] [Google Scholar]
- 18. Tang J, Giddins G. Why and how to report surgeons' levels of expertise. J Hand Surg Eur. 2016;41:365‐366. [DOI] [PubMed] [Google Scholar]
- 19. Grahn P, Sommarhem A, Nietosvaara Y. A protocol‐based treatment plan to improve shoulder function in children with brachial plexus birth injury: a comparative study. J Hand Surg Eur. 2022;47:248‐256. [DOI] [PubMed] [Google Scholar]
- 20. National Health Service (NHS) . Maternity Statistics 2021–2022 published November 2022 on. https://digital.nhs.uk/
- 21. Sweeting A, Wong J, Murphy H, Ross G. A clinical update on gestational diabetes mellitus. Endocr Rev. 2022;43:763‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hedderson M, Darbinian J, Ferrara A. Disparities in the risk of gestational diabetes by race‐ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010;24:441‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salvador S, Bertozzi S, Londero A, Driul L, Da Riol R, Marchesoni D. L'outcome della gravidanza nella donna immigrata: studio retrospettivo [Outcome of pregnancy for immigrant women: a retrospective study]. Minerva Ginecol. 2010;62:277‐285. [PubMed] [Google Scholar]
- 24. Innes K, Byers T, Marshall J, Barón A, Orleans M, Hamman R. Association of a woman's own birth weight with subsequent risk for gestational diabetes. JAMA. 2002;287:2534‐2541. Erratum in: JAMA 2002; 287: 3212. [DOI] [PubMed] [Google Scholar]
- 25. Nair M, Kurinczuk J, Knight M. 2014 ethnic variations in severe maternal morbidity in the UK—a case‐control study. PLoS One. 2014;9:e95086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Knight M, Bunch K, Tuffnell D, et al. Saving Lives, Improving mothers' Care – Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2014–16. National Perinatal Epidemiology Unit, University of Oxford; 2018. [Google Scholar]
- 27. Fleszar L, Bryant A, Johnson C, et al. Trends in state‐level maternal mortality by racial and ethnic group in the United States. JAMA. 2023;330:52‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lundén M, Hulthén Varli I, Kopp Kallner H, Åmark H. Incidence of stillbirth among women with different risk profiles in Stockholm 2001–2020: a repeated cross‐sectional study. Acta Obstet Gynecol Scand. 2023;00:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith L, on behalf of MBRRACE‐UK . Perinatal Mortality Surveillance Report UK Perinatal Deaths for Births from January to December 2020. National Perinatal Epidemiology Unit, University of Oxford; 2021. [Google Scholar]
- 30. Caldwell W, Moloy H. Anatomical variations in the female pelvis: their classification and obstetrical significance. Proc R Soc Med. 1938;32:1‐30. [PMC free article] [PubMed] [Google Scholar]
- 31. Kurki H. Bony pelvic canal size and shape in relation to body proportionality in humans. Am J Phys Anthropol. 2013;151:88‐101. [DOI] [PubMed] [Google Scholar]
- 32. Betti L, Manica A. Human variation in the shape of the birth canal is significant and geographically structured. Proc Biol Sci. 2018;285:20181807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng Y, Shaffer B, Caughey A. Associated factors and outcomes of persistent occiput posterior position: a retrospective cohort study from 1976 to 2001. J Matern Fetal Neonatal Med. 2006;19:563‐568. [DOI] [PubMed] [Google Scholar]
- 34. Barth W. Persistent occiput posterior. Obstet Gynecol. 2015;125(3):695‐709. [DOI] [PubMed] [Google Scholar]
- 35. Gissler M, Teperi J, Hemminki E, Meriläinen J. Data quality after restructuring a nationwide medical birth registry. Scand J Soc Med. 1995;23:75‐80. [DOI] [PubMed] [Google Scholar]


