Abstract
Introduction
The role of multiple high‐risk human papillomavirus (HR‐HPV) infections on the occurrence of persistence/recurrence of high‐grade squamous intraepithelial lesion (HSIL) after conization/surgery for cervical intraepithelial neoplasia was evaluated.
Material and methods
A systematic search of Pubmed/Medine, Scopus, Cochrane databases from inception to June 30, 2023 was performed. Three reviewers independently screened the abstracts of the selected studies and extracted data from full‐text articles. The data were subsequently tabulated and compared for consistency. The bias associated with each included study was evaluated according to the OSQE method. PROSPERO registration number CRD42023433022.
Results
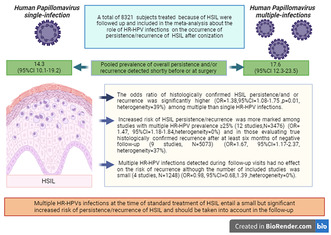
Out of 1606 records screened, 22 full text articles met the inclusion criteria. A total of 8321 subjects treated (loop electrosurgical excision, laser or surgery) because of HSIL were followed‐up and included in the meta‐analysis. The pooled prevalence of overall persistence and/or recurrence was 17.6 (95% CI: 12.3–23.5) in multiple and 14.3 (95% CI: 10.1–19.2) in single HR‐HPV infections detected shortly before or at surgery. The pooled rate of multiple HR‐HPV infections was 25% (95% CI: 20.4–30). The odds ratio of histologically confirmed HSIL persistence and/or recurrence was significantly higher (OR: 1.38, 95% CI:1.08–1.75, p = 0.01, heterogeneity = 39%) among multiple than single HR‐HPV infections. Increased risk of HSIL persistence/recurrence was more marked among studies with multiple HR‐HPVs prevalence ≥25% (12 studies, N = 3476) (OR: 1.47, 95% CI: 1.18–1.84, heterogeneity = 0%) and in those evaluating true histologically confirmed recurrence after at least 6 months of negative follow‐up (9 studies, N = 5073) (OR: 1.67, 95% CI: 1.17–2.37, heterogeneity = 37%). Multiple HR‐HPVs infection detected during follow‐up visits had no effect on the risk of recurrence although the number of included studies was small (4 studies, N = 1248) (OR: 0.98, 95% CI: 0.68–1.39, heterogeneity = 0%). The risk of bias was rated as high in 10 and low‐moderate in 12 studies, respectively. In subgroup analysis, the risk of bias of the included studies (low/moderate vs. high), had a small, although not significant effect on the odds ratios of persistence/recurrence of HSIL (OR: 1.57, 95% CI: 1.23–2 for low‐moderate risk of bias and OR: 1.06, 95% CI: 0.65–1.75 for high risk of bias; p‐value for subgroup differences = 0.17).
Conclusions
Multiple HR‐HPVs infections at the time of standard treatment of HSIL entail a small but significant increased risk of persistence/recurrence of HSIL and should be taken into account in the follow‐up plan.
Keywords: CIN, HPV, HR‐HPV, HSIL, meta‐analysis
Multiple HR‐HPVs infection among women treated for HSIL is common, involving 25% of the subjects, and is associated with a small but significantly increased risk of persistence/recurrence of high‐grade cervical lesions. In the future multiple HR‐HPV infections should be considered into the follow‐up strategy of women treated for HSIL.
Abbreviations
- CIN
cervical intraepithelial neoplasia
- CIN2+
high grade cervical intraepithelial neoplasia
- HR‐HPV
high‐risk Human papillomavirus
- HSIL
high grade squamous intraepithelial lesion.
- LEEP
loop electrosurgical excision procedure
- OR
odds ratio
Key message.
Multiple high‐risk human papillomavirus infections should be considered into the follow‐up strategy of women treated for high grade squamous intraepithelial lesion.
1. INTRODUCTION
In prevalence surveys among general population, coinfection with multiple high‐risk (HR) human papillomavirus infection (HPV) accounted for 4%–15% of invasive cervical cancer and up to 46% of HPV infection. 1 , 2 Multiple HR‐HPV has been consistently related with an increased risk of high grade cervical intraepithelial neoplasia (CIN2+) and invasive cervical cancer among African human immunodeficiency virus (HIV) positive. 3 , 4 The strong association between multiple HR‐HPV, precancer lesions and invasive cervical cancer among HIV‐positive women has been attributed to epidemiological factors such as sexual behavior and to a direct interaction between HIV and HPV. 5 , 6 Despite being common, the role of coinfection with multiple HPV in cervical oncogenesis is controversial. Estimating the proportion of precancer lesions or invasive cervical cancer attributable to multiple infection is difficult from an epidemiological standpoint. 1 The clustering mechanism that allows different HPVs to associate is largely unknown, and the effects of multiple HR‐HPVs on the occurrence or progression of lesions may be modulated by the phylogeny of HPVs involved in the coinfection. 7 , 8 Investigations using laser microdissection technique in CIN2 lesions reveal that one virus causes one lesion, even if colliding lesions are often associated with different HPV types. 9 The detection of viral transcripts in invasive cervical cancer suggests that in coinfection a single HPV genome is preferentially expressed, whereas the role of the other HPVs involved in the infection remains unknown. 10 Regardless of biological mechanisms, some studies suggest that multiple HR‐HPV infections may have a predictive role following therapy in CIN2+ lesions as well as invasive cervical malignancies. 1 , 2 , 11 The purpose of this meta‐analysis was to summarize the findings of studies that investigated the prognostic role of multiple HR‐HPV infections in the treatment of high‐grade cervical lesions.
2. MATERIAL AND METHODS
We searched Pubmed/Medine, Scopus, Cochrane database from inception to June 30, 2023. The terms used for searches included “Multiple Human Papillomavirus” OR “Human Papillomavirus” OR “Squamous Intraepithelial Neoplasia” OR “Cervical Intraepithelial Neoplasia” Or “Cervical Dysplasia” AND “Treatment” OR “Persistence” OR “Recurrence” OR “Failure”. We included only studies with an initial histological diagnosis of HSIL on cone or, when not available, in cervical biopsies preceding conization. In addition, we included only retrospective and prospective case–control and cohort studies with available follow‐up of at least 6 months, while case reports were excluded. Given the strong and consistent association between multiple HR‐HPVs infection, cervical intraepithelial neoplasia, invasive cervical cancer and HIV infection, studies on HIV seropositive women were excluded from the analysis. 2 , 3 , 4 , 5 , 6 Recurrence was defined as the presence of histologically confirmed HSIL after 6 months of follow‐up and at least one negative cytological result after conization. For persistence/recurrence category we adopted the definitions used in the included studies which comprised either persistence/residual of histologically proven HSIL within 6 months following conization or a recurrence subsequently. Three reviewers (AS, MD, CC) screened independently abstracts of the selected studies and extracted data from full‐text articles. Data were subsequently tabulated and compared for consistency. The bias associated with each included study was evaluated according to the OSQE method of Drukker et al. 12 This is a bias evaluation method developed for both case–control and cohort studies and include several domains adapted from Newcastle‐Ottawa scale, Strobe, and ROBINS‐I methods. Quality items were independently assigned by three investigators (MD, CC, AS), and discrepancies were discussed with the other authors to reach concordance.
We used the packages meta and metaphor of R to analyze the data. 13 Random‐effect models were used to compute pooled prevalences and odds ratios (OR) and 95% confidence intervals (CI) of the outcomes studied. Heterogeneity in the effects was evaluated by the I 2 statistics. Additional events (persistence and/or recurrence) associated with multiple HR‐HPVs infection were computed by using the subroutine nnt of metafor on R. When heterogeneity was significant, we used subgroup meta‐analysis. We also tested by using meta‐regression for the effect of different moderators such as the rates of margin positivity, CIN3 of the cone and the rate of HPV 16 infection at the time of conization. Finally, we checked for publication bias (small study effects) using the Egger test.
3. RESULTS
We screened 1606 records for potential inclusions. After exclusion of records because of irrelevancy, duplications, HIV seropositive samples, and case reports, 22 studies were included in the quantitative analysis (Figure 1). 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 Multiple HR‐HPVs were checked at the time or shortly before treatment of HSIL in 20 studies, 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 34 and during follow‐up visits in four 21 , 27 , 33 , 35 ; in two cases multiple HR‐HPVs were checked both at conization and during follow‐up. 21 , 27 A total of 14 papers were from Asia, 14 , 16 , 19 , 22 , 23 , 24 , 26 , 27 , 28 , 30 , 31 , 32 , 34 , 35 and eight from Europe. 15 , 17 , 18 , 20 , 21 , 25 , 29 , 33 The design of the study included 15 retrospective cohort papers, 14 , 19 , 22 , 23 , 24 , 25 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 35 six prospective cohort studies, 15 , 16 , 17 , 18 , 21 , 26 , 32 and one case control study. 20 Treatment modalities included exclusively loop electrosurgical excision procedure (LEEP) in 12 studies, 15 , 17 , 18 , 19 , 21 , 22 , 24 , 25 , 27 , 28 , 33 , 35 exclusive hysterectomy in three studies, 23 , 30 , 34 and a mixed of LEEP, laser or surgery in the remaining seven reports. 14 , 16 , 20 , 26 , 29 , 31 , 32 None of the subjects included had received cryotherapy. Out of 8321 patients included in the studies, the pooled prevalences of overall persistence and/or recurrence were 17.6 (95% CI: 12.3–23.5) in multiple and 14.3 (95% CI: 10.1–19.2) in single HR‐HPV infections (Figure 2). Overall, the pooled rates of HR‐HPV in the studies examined was 25% (95% CI: 20.4–30). The odds ratio (OR) of HSIL persistence and/or prevalence was significantly higher (OR: 1.38, 95% CI: 1.08–1.75, p = 0.01) among multiple than single HR‐HPV infections. On the basis of these results, testing positive for multiple HR‐HPVs at the time of conization was associated with the occurrence of one additional HSIL event every 20.8 (95% CI: 11–93.4) cases of recurrence/persistence. The heterogeneity of the model, although low (38%) was statistically significant (p = 0.04). For this reason, we performed a subgroup analysis on the basis of outcomes (true recurrence vs. persistence/recurrence) (Figure 3). The association between multiple HR‐HPVs was significant for true recurrence of HSIL (OR: 1.67, 95% CI : 1.17–2.37, p = 0.004), but not for persistence/recurrence (OR: 1.2, 95% CI: 0.88–1.62, p = 0.25); the model had low heterogeneity for both outcomes and the difference between the two outcomes was significant only in fixed effects. Nevertheless, in this model multiple HR‐HPVs infection was associated with an additional event every 13.8 (95% CI: 7.3–49.4) true HSIL recurrences. The rates of histologically confirmed CIN 3 on surgical specimens (cone or hysterectomy) (16 studies) ranged from 50.9% to 100%, whereas margin involvement ranged from 0% to 66% of the participants studied (13 studies). Finally, the proportion of HPV 16 positive cervical samples ranged from 23% to 51% (19 studies). Rates of CIN 3 on surgical specimens (β = −0.0035 ± 0.008, p = 0.4), margins involvement (β = 0.002 ± 0.008, p = 0.8) and HPV16 prevalence (β = −1.46 ± 1.22, p = 0.24) had no effect on the relationship between multiple HR‐HPVs and HSIL persistence/recurrence. Since the proportion of multiple HR‐HPVs detected depends on the characteristics of the population studied but also on the sensitivity of the molecular method used, we performed a subgroup analysis on the pooled ORs of overall persistence/recurrence according to the rates of multiple HR‐HPV (≥25%, <25%) (Figure 4). The increased risk of persistence/recurrence among individuals with multiple HR‐HPV was confirmed among populations with prevalence ≥25% (OR: 1.47, 95% CI: 1.18–1.84, p = 0.001, hetereogeneity = 0%) but not in those with prevalence <25% (OR: 1.11, 95% CI: 0.63–1.94, heterogeneity = 63%). However, the difference between the two odds ratios was not significant (p = 0.36). Eventually, Figure 5 reports pooled ORs of recurrence when multiple HR‐HPVs infection was detected during follow‐up visits, at least 6 months after conization. The number of the studies was low but multiple HR‐HPVs detected during follow‐up visits had no effect on the likelihood of recurrence (OR: 0.98, 95% CI: 0.68–1.39, p = 0.89).
FIGURE 1.
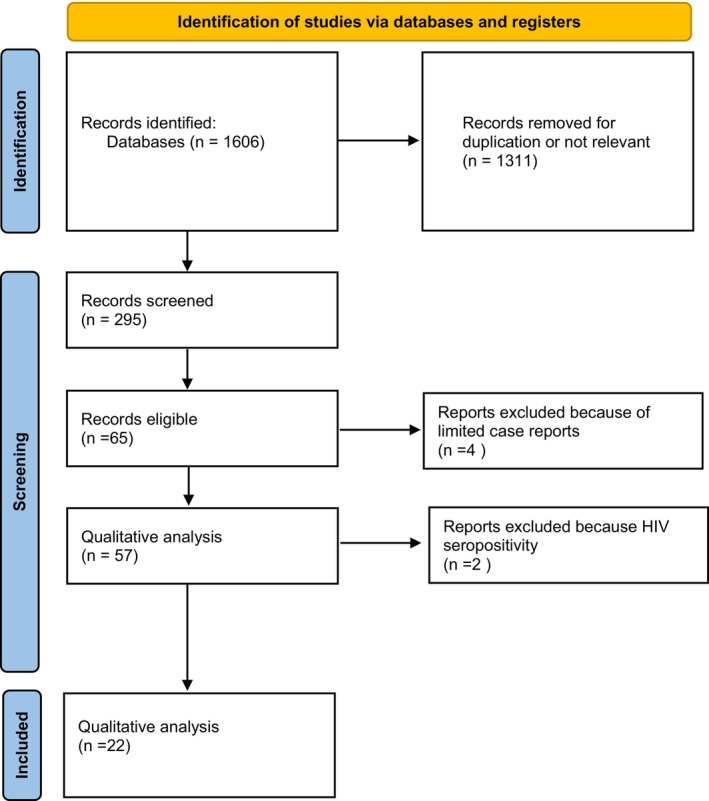
Literature screening of studies including multiple high risk human papillomavirus.
FIGURE 2.
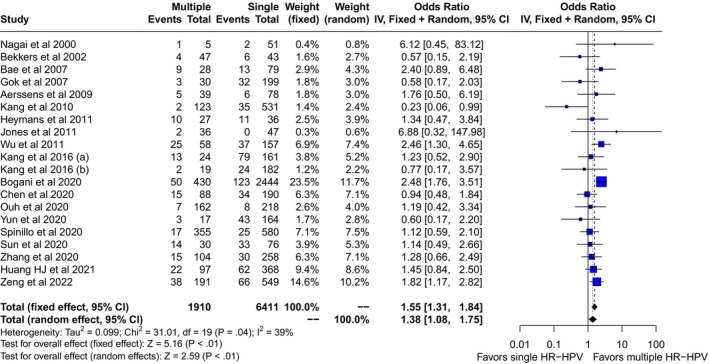
Pooled odds ratio (OR) and 95% confidence intervals (CI) of persistence and or recurrence of high grade squamous intraepithelial lesion associated with multiple high risk papillomavirus infection detected at or shortly before standard treatment.
FIGURE 3.
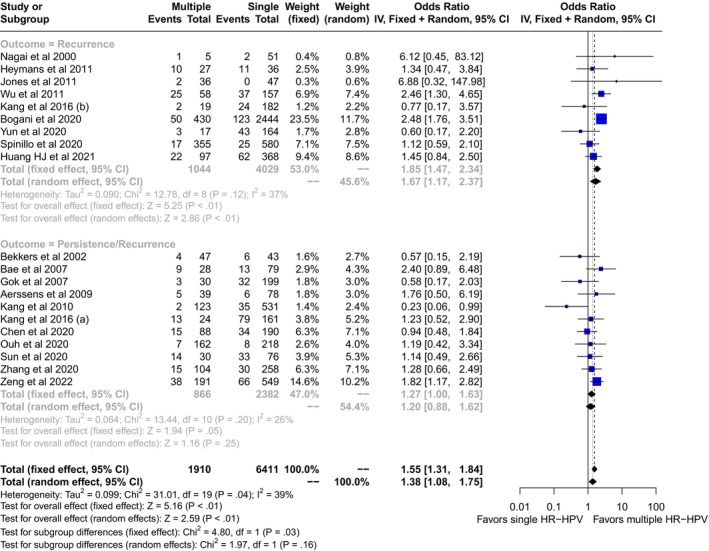
Pooled odds ratio (OR) and 95% confidence intervals (CI) of true recurrence and of persistence/recurrence of high grade squamous intraepithelial lesion associated with multiple high risk papillomavirus infection detected at or shortly before standard treatment.
FIGURE 4.
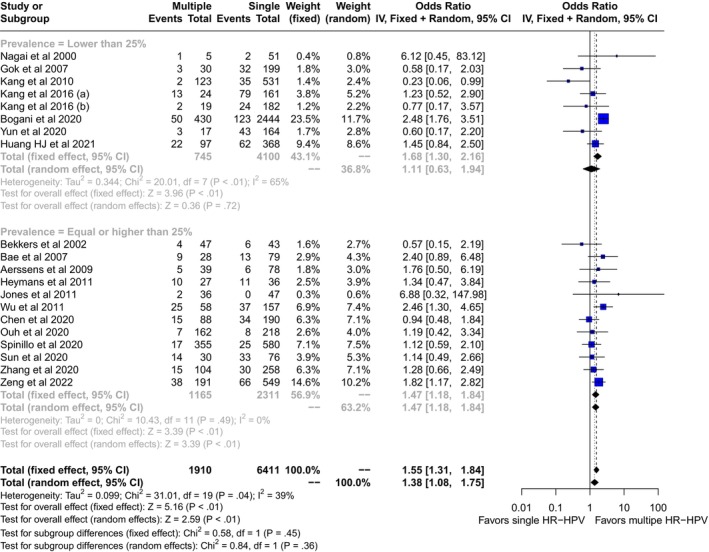
Pooled odds ratio (OR) and 95% confidence intervals (CI) of persistence and/or recurrence of high grade squamous intraepithelial lesion associated with multiple high risk papillomavirus infection (HR‐HPV) detected at or shortly before standard treatment in studies with multiple HR‐HPV prevalence ≥25% and <25%.
FIGURE 5.
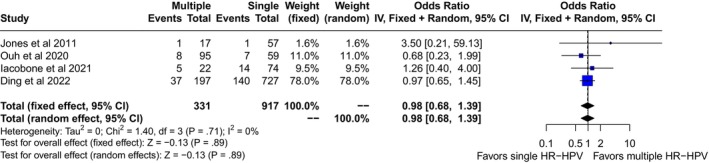
Pooled odds ratio (OR) and 95% confidence intervals (CI) of recurrence of high grade squamous intraepithelial lesion associated with multiple high risk papillomavirus infection detected during follow‐up visits.
The risk of bias evaluated by the QSQE method for all studies included is reported as supplementary material (Table S1). Twelve studies were considered at low‐intermediate risk of bias and 10 at high‐risk of bias. In the subgroup analysis evaluating the risk of overall prevalence/recurrence according to the quality of the studies included (10 at low‐moderate risk of bias and 10 at high risk of bias) odds ratios of outcome associated with multiple HR‐HPV were 1.5 (95% CI: 1.23–2, p < 0.001, heterogeneity = 34%, N = 6635) and 1.06 (95% CI: 0.65–1.75, p = 0.8, heterogeneity = 32%, N = 1686) in studies with low‐moderate and high risk of bias, respectively (Figure S1). The risk of outcome between the two groups differed significantly only on the fixed effect method.
4. DISCUSSION
The results of this meta‐analysis of 8321 individuals with histologically proven HSIL suggest that multiple HR‐HPV infections detected at the time of treatment or shortly before, are associated with a small, but significant increase in the risk of persistence and/or recurrence of high‐grade cervical lesions. These results were more pronounced with low heterogeneity, in studies evaluating true recurrence, among those with a prevalence of multiple infection greater than 25% and in those judged of low‐moderate as compared to those with high risk of bias. Multiple HR‐HPVs detected during follow‐up of treated HSIL, on the other hand, had no effect on the likelihood of recurrence, although the number of studies evaluating this association was small.
The role of multiple HR‐HPV infections in cervical oncogenesis is controversial. Among HIV infected women, multiple HR‐HPVs are rather common and have been consistently linked to an increased risk of both high‐grade CIN and invasive cervical cancer. 3 , 4 This association is less consistent in general population; some large prevalence studies have found increased rates of multiple HR‐HPV in HSIL, 36 , 37 but this association has not been validated by others. 38 The occurrence of multiple HR‐HPVs is influenced by the sensitivity of the molecular methods used as well as by various epidemiological risk factors (age and immune status of the host, sexual history and behavior, distribution of different HR‐HPVs in the population studied). 7 , 9 , 10 , 35 , 37 The assessment of the causal relationship between multiple HR‐HPVs infection and the development or progression of CIN is complicated by the large number of covariates involved. 37 On the other hand, age, virological factors (type of virus, persistence of infection, viral loads), completeness of excision, and endocervical involvement are the key risk factors for persistence/recurrence or worsening of cervical lesions. 39 , 40
A recent long‐term follow‐up study conducted by Bogani et al. has suggested a notable correlation between HPV persistence and an increased risk of CIN2+ recurrence disease after conization for up to 1 year (recurrence risk of 13.1%). 41 Another study revealed that, in a high‐risk population undergoing cervical conization for high‐grade cervical lesions with positive margins and persistent HPV infection after 6 months, positive endocervical margins rather than ectocervical margins were associated with poorer outcomes (HR: 4.56, 95% CI: 1.23–7.95; p = 0.021). 42 These results emphasize the significance of monitoring HPV persistence and considering specific margin types in predicting the likelihood of recurrence in individuals with high‐grade cervical lesions.
Given the low and well‐defined number of risk factors, women treated for CIN represent a suitable population to study the prognostic role of multiple HR‐HPVs. Previous metanalyses on risk factors for CIN recurrence focused mainly of the role of incomplete excision, persistence of HPV infection and on the protective role of adjuvant HPV vaccination. 39 , 40 , 41 , 42 , 43 Although there are a significant number of studies which have evaluated the role of HR‐HPVs on recurrence after standard treatment of CIN, no pooled data have been published.
The retrospective design, use of molecular methods with different sensitivities, and lack of complete information on sexual history of participants of the included studies are the main limitations of this meta‐analysis. In addition, we had no information on the duration/persistence of HR‐HPV infection which could influence recurrence or progression of high grade SIL. 16 , 37 A detailed risk of bias assessment was performed for all studies to critically address potential confounding. Although the heterogeneity of the included studies was low (<50%) in all comparisons, subgroup analysis suggests that the increased risk of persistence/recurrence was more consistent among studies rated at low‐moderate risk of bias, reinforcing the conclusions of the analysis. On the other hand, the use of a well‐defined histological diagnosis of HSIL both at enrollment and during follow‐up, the assessment of the role of cofactors like the prevalence of multiple infection or the prevalence of CIN3 in histological samples in the populations under study are the main strengths of the analysis.
Multiple sexual partners, early sexual debut, immunodeficiency, and younger age are all linked to higher rates of multiple HR‐HPVs infection. 1 , 2 , 3 , 4 Although invasive cervical cancers are associated with predominantly single HR‐HPV genotypes and only a minority of physically colliding preinvasive cervical lesions are caused by multiple genotypes, 9 multiple HR‐HPV infections could still play a role in cervical oncogenesis. 10 According to a number of studies, infections from multiple HR‐HPV genotypes are largely independent and multiple lesions seem to be caused by different viruses 2 , 9 ; in this situation multiple infection may serve as a kind of reservoir of HR‐HPV sustaining HPV infection. 9 Multiple HR‐HPV infections are also associated with increased viral loads and, in follow‐up studies, with the persistence of HPV infection. 9 , 11 , 16 , 26 , 34 On the other hand, both increased viral loads and persistent HPV infection have been convincingly linked to an increased risk of high‐grade CIN and progression to invasive cervical cancer. 5 , 16 , 26 , 44 These data support the role of multiple HR‐HPVs as predictors of increased risk of recurrence after standard treatment of high‐grade SIL.
The risk factors for recurrence of HSIL after therapy are well documented and current guidelines do not mention multiple HR‐HPVs infection as a significant risk factor. 45 Among women treated for high‐grade HSIL, the risk of recurrence remains elevated for 10–25 years and is higher in women with persistent HR‐HPV infections. 40 , 41 , 42 , 43 , 44 , 45 , 46 During follow‐up, these women should have a co‐test (cytology and HR‐HPV test) performed at 12 months and every 1–3 years afterwards. 45 , 46 The findings of this meta‐analysis suggest that, while the risk of HSIL persistence/recurrence associated with multiple HR‐HPV infections is small (one additional case every 14 true recurrences), it should be considered in the clinical assessment of the risk of post‐treatment failure.
5. CONCLUSION
The results of this study suggest that multiple HR‐HPVs infection among women treated for HSIL is common, involving 25% of the individuals, and is associated with a small but significantly increased risk of persistence/recurrence of high‐grade cervical lesions. Given these findings, in the future multiple HR‐HPV infections should be considered into the follow‐up strategy of women treated for HSIL.
AUTHOR CONTRIBUTIONS
All authors participated in the study design. Arsenio Spinillo retrieved data and performed data analysis. All authors participated in data interpretation. Chiara Cassani, Mattia Domioni, Marianna Francesca Pasquali and Barbara Gardella drafted the initial version of the manuscript, which was revised by all authors who also approved the final article.
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Supporting information
Figure S1:
Table S1:
ACKNOWLEDGMENTs
Open access funding provided by BIBLIOSAN.
Cassani C, Dominoni M, Pasquali MF, Gardella B, Spinillo A. The role of multiple high‐risk human papillomavirus infection on the persistence recurrence of high‐grade cervical lesions after standard treatment: A systematic review and a meta‐analysis. Acta Obstet Gynecol Scand. 2024;103:1028‐1035. doi: 10.1111/aogs.14827
REFERENCES
- 1. Li N, Yang L, Zhang K, Zhang Y, Zheng T, Dai M. Multiple human papillomavirus infections among Chinese women with and without cervical abnormalities: a population‐based multi‐center cross‐sectional study. Front Oncol. 2011;1:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaccarella S, Franceschi S, Snijders PJ, et al. Concurrent infection with multiple human papillomavirus types: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2010;19:503‐510. [DOI] [PubMed] [Google Scholar]
- 3. Clifford GM, de Vuyst H, Tenet V, Plummer M, Tully S, Franceschi S. Effect of HIV infection on human papillomavirus types causing invasive cervical cancer in Africa. J Acquir Immune Defic Syndr. 2016;73:332‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okoye JO, Ofodile CA, Adeleke OK, Obioma O. Prevalence of high‐risk HPV genotypes in sub‐Saharan Africa according to HIV status: a 20‐year systematic review. Epidemiol Health. 2021;43:e2021039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marima R, Hull R, Lolas G, et al. The catastrophic HPV/HIV dual viral oncogenomics in concert with dysregulated alternative splicing in cervical cancer. Int J Mol Sci. 2021;22:10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Williamson AL. The interaction between human immunodeficiency virus and human papillomaviruses in heterosexuals in Africa. J Clin Med. 2015;4:579‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pimenoff VN, Tous S, Benavente Y, et al. Distinct geographic clustering of oncogenic human papillomaviruses multiple infections in cervical cancers: results from a worldwide cross‐sectional study. Int J Cancer. 2019;144:2478‐2488. [DOI] [PubMed] [Google Scholar]
- 8. Spinillo A, Dal Bello B, Alberizzi P, et al. Clustering patterns of human papillomavirus genotypes in multiple infections. Virus Res. 2009;142:154‐159. [DOI] [PubMed] [Google Scholar]
- 9. Quint W, Jenkins D, Molijn A, et al. One virus, one lesion—individual components of CIN lesions contain a specific HPV type. J Pathol. 2012;227:62‐71. [DOI] [PubMed] [Google Scholar]
- 10. Brant AC, Menezes AN, Felix SP, Almeida LM, Moreira MAM. Preferential expression of a HPV genotype in invasive cervical carcinomas infected by multiple genotypes. Genomics. 2020;112:2942‐2948. [DOI] [PubMed] [Google Scholar]
- 11. de Brot L, Pellegrini B, Moretti ST, et al. Infections with multiple high‐risk HPV types are associated with high‐grade and persistent low‐grade intraepithelial lesions of the cervix. Cancer Cytopathol. 2017;125:138‐143. [DOI] [PubMed] [Google Scholar]
- 12. Drukker M, Weltens I, van Hooijdonk CFM, Vandenberk E, Bak M. Development of a methodological quality criteria list for observational studies: the observational study quality evaluation. Front Res Metr Anal. 2021;6:675071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36:1‐48. [Google Scholar]
- 14. Nagai Y, Maehama T, Asato T, Kanazawa K. Persistence of human papillomavirus infection after therapeutic conization for CIN 3: is it an alarm for disease recurrence? Gynecol Oncol. 2000;79:294‐299. [DOI] [PubMed] [Google Scholar]
- 15. Bekkers RL, Melchers WJ, Bakkers JM, et al. The role of genotype‐specific human papillomavirus detection in diagnosing residual cervical intraepithelial neoplasia. Int J Cancer. 2002;102:148‐151. [DOI] [PubMed] [Google Scholar]
- 16. Bae JH, Kim CJ, Park TC, Namkoong SE, Park JS. Persistence of human papillomavirus as a predictor for treatment failure after loop electrosurgical excision procedure. Int J Gynecol Cancer. 2007;17:1271‐1277. [DOI] [PubMed] [Google Scholar]
- 17. Gök M, Coupé VM, Berkhof J, et al. HPV16 and increased risk of recurrence after treatment for CIN. Gynecol Oncol. 2007;104:273‐275. [DOI] [PubMed] [Google Scholar]
- 18. Aerssens A, Claeys P, Beerens E, et al. Prediction of recurrent disease by cytology and HPV testing after treatment of cervical intraepithelial neoplasia. Cytopathology. 2009;20:27‐35. [DOI] [PubMed] [Google Scholar]
- 19. Kang WD, Oh MJ, Kim SM, Nam JH, Park CS, Choi HS. Significance of human papillomavirus genotyping with high‐grade cervical intraepithelial neoplasia treated by a loop electrosurgical excision procedure. Am J Obstet Gynecol. 2010;203(72):e1‐e6. [DOI] [PubMed] [Google Scholar]
- 20. Heymans J, Benoy IH, Poppe W, Depuydt CE. Type‐specific HPV geno‐typing improves detection of recurrent high‐grade cervical neoplasia after conisation. Int J Cancer. 2011;129:903‐909. [DOI] [PubMed] [Google Scholar]
- 21. Jones J, Saleem A, Rai N, et al. Human papillomavirus genotype testing combined with cytology as a ‘test of cure’ post treatment: the importance of a persistent viral infection. J Clin Virol. 2011;52:88‐92. [DOI] [PubMed] [Google Scholar]
- 22. Wu D, Zheng Y, Chen W, et al. Prediction of residual/recurrent disease by HPV genotype after loop excision procedure for high‐grade cervical intraepithelial neoplasia with negative margins. Aust N Z J Obstet Gynaecol. 2011;51:114‐118. [DOI] [PubMed] [Google Scholar]
- 23. Kang WD, Ju UC, Kim SM. A human papillomavirus (HPV)‐16 or HPV‐18 genotype is a reliable predictor of residual disease in a subsequent hysterectomy following a loop electrosurgical excision procedure for cervical intraepithelial neoplasia 3. J Gynecol Oncol. 2016;27:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kang WD, Kim SM. Human papillomavirus genotyping as a reliable prognostic marker of recurrence after loop electrosurgical excision procedure for high‐grade cervical intraepithelial neoplasia (CIN2‐3) especially in postmenopausal women. Menopause. 2016;23:81‐86. [DOI] [PubMed] [Google Scholar]
- 25. Bogani G, Pinelli C, Chiappa V, et al. Age‐specific predictors of cervical dysplasia recurrence after primary conization: analysis of 3,212 women. J Gynecol Oncol. 2020;31:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen L, Dong B, Zhang Q, et al. HR‐HPV viral load quality detection provide more accurate prediction for residual lesions after treatment: a prospective cohort study in patients with high‐grade squamous lesions or worse. Med Oncol. 2020;37:37. [DOI] [PubMed] [Google Scholar]
- 27. Ouh YT, Cho HW, Kim SM, et al. Risk factors for type‐specific persistence of high‐risk human papillomavirus and residual/recurrent cervical intraepithelial neoplasia after surgical treatment. Obstet Gynecol Sci. 2020;63:631‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yun HN, Kang WD, Ju UC, Kim SM. Human papillomavirus genotyping is a reliable prognostic marker of recurrence for high‐grade cervical intraepithelial neoplasia (CIN2‐3) with positive margins after loop electrosurgical excision procedure. Eur J Gynaecol Oncol. 2020;41:969‐974. [Google Scholar]
- 29. Spinillo A, Dominoni M, Boschi AC, et al. Clinical significance of the interaction between human papillomavirus (HPV) type 16 and other high‐risk human papillomaviruses in women with cervical intraepithelial neoplasia (CIN) and invasive cervical cancer. J Oncol. 2020;2020:6508180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun X, Lei H, Xie X, Ruan G, An J, Sun P. Risk factors for residual disease in hysterectomy specimens after conization in post‐menopausal patients with cervical intraepithelial neoplasia grade 3. Int J Gen Med. 2020;13:1067‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang Q, Dong B, Chen L, et al. Evaluation of PCR‐reverse dot blot human papillomavirus genotyping test in predicting residual/recurrent CIN 2+ in posttreatment patients in China. Cancer Manag Res. 2020;12:2369‐2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huang HJ, Tung HJ, Yang LY, et al. Role of human papillomavirus status after conization for high‐grade cervical intraepithelial neoplasia. Int J Cancer. 2021;148:665‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iacobone AD, Radice D, Sandri MT, et al. Human papillomavirus same genotype persistence and risk of cervical intraepithelial Neoplasia2+ recurrence. Cancers (Basel). 2021;13:3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeng Y, Jiang T, Zheng Y, et al. Risk factors predicting residual lesion in subsequent hysterectomy following cold knife conization (CKC) for high‐grade squamous intraepithelial lesion (HSIL). BMC Womens Health. 2022;22:358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding T, Li L, Duan R, Chen Y, Yang B, Xi M. Risk factors analysis of recurrent disease after treatment with a loop electrosurgical excision procedure for high‐grade cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2023;160:538‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaturvedi AK, Katki HA, Hildesheim A, et al. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. 2011;203:910‐920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schmitt M, Depuydt C, Benoy I, et al. Multiple human papillomavirus infections with high viral loads are associated with cervical lesions but do not differentiate grades of cervical abnormalities. J Clin Microbiol. 2013;51:1458‐1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Y, Ang Q, Wu H, et al. Prevalence of human papillomavirus genotypes and precancerous cervical lesions in a screening population in Beijing, China: analysis of results from China's top 3 hospital, 2009–2019. Virol J. 2020;17:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bogani G, Sopracordevole F, Di Donato V, et al. High‐risk HPV‐positive and ‐negative high‐grade cervical dysplasia: analysis of 5‐year outcomes. Gynecol Oncol. 2021;161:173‐178. [DOI] [PubMed] [Google Scholar]
- 40. Santesso N, Mustafa RA, Wiercioch W, et al. Systematic reviews and meta‐analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2016;132:266‐271. [DOI] [PubMed] [Google Scholar]
- 41. Bogani G, Sopracordevole F, Ciavattini A, et al. Duration of human papillomavirus persistence and its relationship with recurrent cervical dysplasia. Eur J Cancer Prev. 2023;32:525‐532. [DOI] [PubMed] [Google Scholar]
- 42. Giannini A, Di Donato V, Sopracordevole F, et al. Outcomes of high‐grade cervical dysplasia with positive margins and HPV persistence after cervical Conization. Vaccines (Basel). 2023;11:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eriksen DO, Jensen PT, Schroll JB, Hammer A. Human papillomavirus vaccination in women undergoing excisional treatment for cervical intraepithelial neoplasia and subsequent risk of recurrence: a systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2022;101:597‐607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta‐analysis. Am J Epidemiol. 2008;168:123‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP risk‐based management consensus guidelines. J Low Genit Tract Dis. 2020;24:132‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perkins RB, Guido RL, Saraiya M, et al. Summary of current guidelines for cervical cancer screening and Management of Abnormal Test Results: 2016–2020. J Womens Health (Larchmt). 2021;30:5‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1:
Table S1:


