Abstract
Introduction
Women with polycystic ovary syndrome (PCOS) have more pregnancy complications like gestational diabetes, hypertension, and preterm labor than other women. Metformin has been used in an attempt to improve pregnancy outcomes. Our study aims to explore childbirth experiences in women with PCOS compared with a reference population. It also explores the potential influence of metformin, obesity, pregnancy complications, and the duration and mode of birth on childbirth experiences.
Material and methods
This study is a cohort study combining data from two randomized trials conducted in Norway, Sweden and Iceland. The PregMet2 study (ClinicalTrials.gov, NCT01587378) investigated the use of metformin vs. placebo in pregnant women with PCOS. The Labour Progression Study (ClinicalTrials.gov, NCT02221427) compared the WHO partograph to Zhang's guidelines for progression of labor and were used as the reference population. A total of 365 women with PCOS and 3604 reference women were included. Both studies used the Childbirth Experience Questionnaire (CEQ). Main outcome measures were total CEQ score and four domain scores. The CEQ scores were compared using Mann–Whitney U test for women in Robson group 1 with PCOS (n = 131) and reference women (n = 3604). CEQ scores were also compared between metformin‐treated (n = 180) and placebo‐treated (n = 185) women with PCOS, and for different subgroups of women with PCOS.
Results
There was no difference in total CEQ score between women with PCOS and reference women—Wilcoxon–Mann–Whitney (WMW)‐odds 0.96 (95% confidence interval [CI] 0.78–1.17). We detected no difference in CEQ scores between the metformin‐ and placebo‐treated women with PCOS (WMW‐odds 1.13, 95% CI 0.89–1.43). Complications in pregnancy did not affect CEQ (WMW‐odds 1, 95% CI 0.76–1.31). Higher body mass index (WMW‐odds 0.75, 95% CI 0.58–0.96), longer duration of labor (WMW‐odds 0.69, 95% CI 0.49–0.96), and cesarean section (WMW‐odds 0.29, 95% CI 0.2–0.42) were associated with lower CEQ scores in women with PCOS.
Conclusions
Women with PCOS experience childbirth similarly to the reference women. Metformin did not influence childbirth experience in women with PCOS, neither did pregnancy complications. Obesity, long duration of labor or cesarean section had a negative impact on childbirth experience.
Keywords: Childbirth Experience Questionnaire, childbirth experiences, polycystic ovary syndrome
Women with polycystic ovary syndrome have similar childbirth experiences to other women, despite their increased risk of complications. Their childbirth experiences are negatively influenced by high BMI, long duration of labor, and operative delivery.
Abbreviations
- BMI
body mass index
- CEQ
Childbirth Experience Questionnaire
- LaPS
Labour Progression Study
- PCOS
polycystic ovary syndrome
- SD
standard deviation
- WMW‐odds
Wilcoxon–Mann–Whitney odds
Key message.
This study provides novel knowledge that women with polycystic ovary syndrome have similar childbirth experiences to other women, despite their increased risk of complications. Their childbirth experiences are negatively influenced by high BMI, long duration of labor, and operative delivery.
1. INTRODUCTION
Polycystic ovary syndrome (PCOS) is a heterogeneous condition with four phenotypes. 1 It affects 5%–20% of women, depending on the diagnostic criteria used and population studied. 2 Women with PCOS often suffer from comorbidities related to metabolic, psychological, and reproductive factors. Compared with women without PCOS, they have higher prevalence of insulin resistance, type 2 diabetes, metabolic syndrome, non‐alcoholic fatty liver disease, obesity, hypertension, and risk for cardiovascular diseases. 3 , 4 , 5 Obesity exacerbates most co‐morbidities and symptoms associated with PCOS. 6 Anxiety, depression, and eating disorders are also more common among women with PCOS compared with healthy women. 7 , 8 Pregnancy‐induced hypertension, preeclampsia, gestational diabetes, preterm birth, and cesarean section are more prevalent among women with PCOS. 9
Metformin is a drug predominantly used in type 2 diabetes to increase insulin sensitivity. 10 It has no known teratogenic effects and is also used to treat gestational diabetes. 11 , 12 We have previously shown that metformin during pregnancy prevents late miscarriages and preterm delivery but has no effect on the incidence or severity of gestational diabetes in women with PCOS. 13
Childbirth experiences are influenced by a woman's previous experiences in life, her preparedness for birth, fear of childbirth, experience of pain and pain control during birth, and the mode and outcome of the delivery. 14 , 15 , 16 Furthermore, the experiences are influenced by her satisfaction with support during labor and her involvement in decision‐making. 14 , 15 Childbirth experiences may have immediate as well as long‐term positive or negative effects on well‐being and health. 17 , 18 , 19 , 20 , 21 A positive childbirth experience contributes to increased consecutive vaginal deliveries, whereas negative experiences contribute to future fear of giving birth and an increased risk of cesarean section. 22
According to World Health Organization recommendations on intrapartum care, the care provided should not only be safe, but also contribute to a positive childbirth experience. 23 Labor care in the Nordic countries aims towards one‐to‐one care, with a midwife present during the entire active labor. 24 The public health system is considered of high quality and is free of charge. 25
Our primary aim is to explore childbirth experiences in women with PCOS compared with a reference population. Secondary aims are to explore whether metformin in pregnancy affects childbirth experience in women with PCOS, and to explore the association between childbirth experiences and body mass index (BMI), pregnancy complications and duration and mode of birth.
2. MATERIAL AND METHODS
2.1. Design and participants
Our study is a cohort study consisting of women with PCOS from the PregMet2 study 13 and women from the Labour Progression Study (LaPS) 26 as a reference group. The study participation is illustrated in Figure 1.
FIGURE 1.
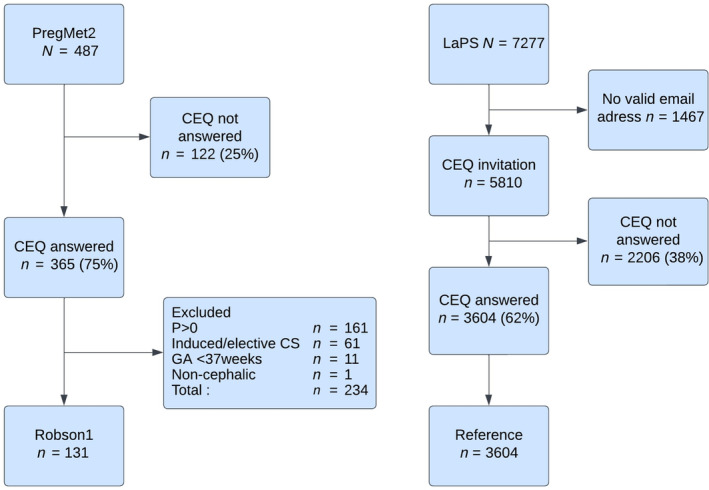
Flowchart for the comparison of women with polycystic ovary syndrome and reference women. CEQ, Childbirth Experience Questionnaire; CS, cesarean section; GA, gestational age; LaPS, Labour Progression Study; P>0, multiparous; PregMet2, PregMet2 Study; Robson1, Robson group 1.
The PregMet2 study was a randomized, placebo controlled, double‐blinded multicenter trial in Norway, Sweden, and Iceland on the effect of metformin on preterm birth in women with PCOS. 13 In total, 487 women from 11 hospitals were included and treated with metformin or placebo from first trimester until delivery. Inclusion and exclusion criteria, and the randomization process are described in detail elsewhere. 13 The PregMet2 study found that metformin prevents preterm birth and late miscarriages in women with PCOS. The present study includes the 365 (75%) women from the PregMet2 who answered the Childbirth Experience Questionnaire (CEQ). For the primary aim, we included only the 131 participants in Robson group 1 (nulliparous, singleton fetus in cephalic presentation, spontaneous onset of labor at term) to maximize comparability with the LaPS population.
As a reference population we used the LaPS population, a Norwegian cluster‐randomized, controlled, multicenter study with the aim of evaluating the effects of the WHO partograph vs Zhang's guideline for labor progression among nulliparous women. 26 In total, 7277 women in Robson group 1 were included from 14 hospitals, and 5810 were invited to answer the CEQ. In the present study, 3604 (62%) women from the LaPS study who answered the CEQ were included. 27 Further inclusion and exclusion criteria as well as the randomization process and results of the study are described elsewhere. 26
2.2. Data collection
Data for the PregMet2 study were collected between October 2012 and September 2017. At inclusion, data on demographics, maternal anthropometry, and previous and present obstetric and medical history were recorded. The participants had follow‐up visits in gestational weeks 19, 28, 32, and 36 including oral glucose tolerance tests, blood sampling for later analyses, additional ultrasound examinations, breast size measurements, and Edinburgh Postnatal Depression Scale. A telephone interview was conducted 8 weeks postpartum. 13 Data from delivery and 8 weeks postpartum were gathered from medical records and via telephone interviews and recorded in an online Case Report Form (WebCRF, version2). Most participants returned the CEQ on paper within 8 weeks postpartum, but some questionnaires were filled in by a study nurse/midwife during the telephone interview.
For the LaPS study, data were collected between December 2014 and January 2017. Baseline and birth data were recorded from medical records and registered into an online Case Report Form (WebCRF) by a local investigator. The participants who had provided a valid email address (n = 5810) were sent a link to an online version of the CEQ 4 weeks after inclusion. Among those who completed the CEQ (n = 3604), 97% of the answers were returned electronically between 4 and 8 weeks postpartum. 27 We consider a response rate over 60% to be high. 28
2.3. The Childbirth Experience Questionnaire
The CEQ was developed and validated in Sweden by Dencker et al. 29 The CEQ (version one) used in our study measures childbirth experiences in four domains: own capacity, participation, perceived safety, and professional support. 29 It consists of 22 items, 19 of which are answered on a four‐point Likert scale where one point is total agreement with the statement and four points are total disagreement. The remaining three items are answered on a visual analog scale from 0 to 100, categorized as 1 (0–40), 2 (41–60), 3 (61–80), and 4 (81–100). 29 Before the analyses, all CEQ items with positive statements had their scores reversed so that a high score reflected a positive scoring. 29 The questionnaire was available in English, Swedish, and Norwegian, which excluded participants not fluent in any of these languages.
2.4. Statistical analyses
SPSS version 28.0 (IBM Corp.) was used for statistical analyses. Descriptive statistics are presented as means and standard deviations for continuous variables and numbers and percentages for categorical variables. Basic characteristics were compared between the two study populations using t test for continuous variables and chi‐squared test or Fisher's exact test for categorical variables.
To address the primary aim, we compared the birth experiences among PCOS women (PregMet2) to the reference population (LaPS). For comparisons of total CEQ score and individual domain scores we used Mann–Whitney U test because of the skewed distribution of these data. The Wilcoxon–Mann–Whitney odds ratio (WMW‐odds) with a 95% confidence interval (CI) were calculated as an effect measure, see supporting material(Appendix S1). 30 Any missing items in the CEQ were imputed with the mean value for the relevant domain score, provided the respondent had answered at least half of the items in the given domain as described by Dencker et al. 29 When more than half the items were missing from any domain, we decided to exclude the case from the total CEQ score (n = 5).
Secondary aims were addressed using data from the PregMet2 study. The effect of metformin on childbirth experience in women with PCOS was compared using the Mann–Whitney U test. Possible factors associated with childbirth experiences were identified from literature, discussed and chosen in the research group and included BMI, pregnancy complications, duration and mode of birth delivery. For the analyses, BMI was dichotomized <30 kg/m2 (normal and overweight) or ≥30 kg/m2 (obesity). The presence of complications in pregnancy was also a dichotomous variable defined as the presence of any of the following: gestational diabetes, pregnancy‐induced hypertension and preeclampsia, preterm labor, venous thromboembolism, and hyperemesis gravidarum. Active labor was defined as at least 4 cm dilated cervix and regular, painful contractions, and duration of active labor was dichotomized as <8 h or ≥8 h. The 8‐hour cut‐off was discussed thoroughly in the research team and chosen following expected progress of 1 cm/h from 4 cm to 10 cm dilatation and adding 2 h for the descent and expulsion phases in line with current Norwegian guidelines. 31 Mode of delivery was classified in spontaneous vaginal delivery, operative vaginal delivery (including both forceps and ventouse delivery), and cesarean section. The Mann–Whitney U test was used to investigate associations between childbirth experiences and the dichotomous variables. Associations between mode of delivery and childbirth experience were assessed using Kruskal–Wallis test, and subsequent pairwise testing using Mann–Whitney U test with a Holm–Bonferroni correction to account for multiple testing between the three mode of delivery categories.
Values of p less than 0.05 were considered statistically significant.
3. RESULTS
Women with PCOS had higher BMI, maternal age, and level of education, and their infants were more often transferred to the neonatal intensive care unit, compared with reference women (Table 1). Baseline characteristics of metformin‐ and placebo‐treated women with PCOS in the PregMet2 study were comparable (Table S1).
TABLE 1.
Baseline and birth characteristics for Robson group 1: Women with polycystic ovary syndrome compared with reference women.
| PCOS | Reference | p value | |
|---|---|---|---|
| (n = 131) | (n = 3604) | ||
| Age (years, at birth), mean ± SD | 29.4 ± 4.1 | 27.6 ± 4.5 | <0.001 |
| Civil status, n (%) | |||
| Married/co‐habitant | 128 (98) | 3408 (95) | 0.115 |
| Other | 3 (2) | 196 (5) | |
| Education, n (%) | |||
| Elementary school | 7 (5) | 158 (4) | 0.004 |
| High school | 27 (21) | 1242 (35) | |
| College/University | 97 (74) | 2204 (61) | |
| Body mass index (kg/m2), n (%) | |||
| BMI <25 | 58 (44) | 2571 (72)11 | <0.001 |
| BMI 25–30 | 44 (34) | 725 (20) | |
| BMI >30 | 29 (22) | 297 (8) | |
| Body mass index (kg/m2), mean ± SD | 26.6 ± 5.4 | 23.7 ± 4.3 | <0.001 |
| Smoking, n (%) | 4 (3) | 202 (6)33 | 0.202 |
| Mode of delivery, n (%) | |||
| Spontaneous vaginal | 89 (68) | 2699 (75) | 0.131 |
| Operative vaginal | 29 (22) | 676 (19) | |
| Cesarean section | 13 (10) | 228 (6) | |
| Estimated bleeding at birth/CS (mL), mean ± SD | 385 ± 2051 | 417 ± 304 | 0.242 |
| Duration active labor (hours) mean ± SD | 6,7 ± 3.920 | 7,0 ± 4.3 | 0.529 |
| Apgar score < 7 at 5 min, n (%) | 3 (2)1 | 44 (1) | 0.227 |
| Baby transferred to NICU, n (%) | 15 (12) | 131 (4) | <0.001 |
Note: Numbers in n (%)m or mean ± SDm as appropriate where “m” is number missing in column. Statistical analyses performed with independent samples t test for continuous data and Pearson's chi‐squared test, Likelihood ratio, or Fisher's exact test as appropriate for categorical data.
Abbreviations: BMI, body mass index; CS, cesarean section; NICU, neonatal intensive care unit; PCOS, polycystic ovary syndrome; SD, standard deviation.
Among women in Robson group 1, we found no difference in total CEQ score between women with PCOS and the reference population (mean ± SD 3.19 ± 0.43 vs. 3.21 ± 0.42, p = 0.660, WMW‐odds 0.96, 95% CI 0.78–1.17). Women with PCOS had a higher domain score for perceived safety and a lower score for own capacity compared with the reference population, but there was no difference in the domains professional support or participation (Table 2).
TABLE 2.
Childbirth Experience Questionnaire total and domain scores in Robson1: Women with polycystic ovary syndrome compared with reference women.
| Score | PCOS (n = 131) | Reference (n = 3604) | p value | WMW odds (95% CI) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Total score CEQ | 3.19 ± 0.43 | 3.21 ± 0.42 | 0.660 | 0.96 (0.78–1.17) |
| Own capacity | 2.71 ± 0.52 | 2.80 ± 0.46 | 0.037 | 0.81 (0.66–0.99) |
| Professional support | 3.68 ± 0.52 | 3.68 ± 0.49 | 0.668 | 1.04 (0.86–1.26) |
| Perceived safety | 3.36 ± 0.62 | 3.27 ± 0.63 | 0.038 | 1.24 (1.01–1.51) |
| Participation | 3.35 ± 0.66 | 3.39 ± 0.65 | 0.360 | 0.91 (0.75–1.11) |
Note: p values from Mann–Whitney U test as appropriate for non‐normally distributed data. Missing values imputed if 50% or more of domain score is answered.
Abbreviations: CEQ, Childbirth Experience Questionnaire; CI, confidence interval; PCOS, polycystic ovary syndrome; Robson1, Robson group 1; SD, standard deviation; WMW‐odds, Wilcoxon–Mann–Whitney odds.
Total CEQ score and the domain scores were similar for the metformin and placebo groups (Table 3), and for women who experienced pregnancy complications vs. no complications (Table S2).
TABLE 3.
Childbirth Experience Questionnaire total score and domain scores in women with polycystic ovary syndrome: metformin compared with placebo.
| Score | Metformin (n = 180) | Placebo (n = 185) | p‐Value | WMW‐odds (95% CI) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Total score CEQ | 3.22 ± 0.45 | 3.17 ± 0.46 | 0.340 | 1.13 (0.89–1.43) |
| Own capacity | 2.79 ± 0.56 | 2.75 ± 0.56 | 0.440 | 1.1 (0.87–1.39) |
| Professional support | 3.70 ± 0.54 | 3.64 ± 0.58 | 0.142 | 1.18 (0.95–1.47) |
| Perceived safety | 3.44 ± 0.59 | 3.41 ± 0.63 | 0.637 | 1.06 (0.84–1.34) |
| Participation | 3.10 ± 0.96 | 3.02 ± 0.94 | 0.290 | 1.14 (0.9–1.44) |
Note: p values from Mann–Whitney U test as appropriate for non‐normally distributed data. Missing values imputed if 50% or more of domain score is answered.
Abbreviations: CEQ, Childbirth Experience Questionnaire; CI, confidence interval; PCOS, polycystic ovary syndrome; Robson1, Robson group 1; SD, standard deviation; WMW‐odds, Wilcoxon‐Mann–Whitney‐odds.
Women with PCOS and BMI >30 kg/m2 had a large variation in childbirth experiences (Figure S1) but scored overall significantly lower on total CEQ score compared with those with BMI <30 kg/m2 (mean ± SD, 3.10 ± 0.52 vs. 3.24 ± 0.40, p = 0.025, WMW‐odds 0.75, 95% CI 0.58–0.96). They also had lower scores on the two domain scores professional support and participation, respectively, whereas there was no difference in the domains own capacity or perceived safety (Table 4).
TABLE 4.
Childbirth Experience Questionnaire total and domain scores in women with polycystic ovary syndrome: BMI ≥30 kg/m2 compared with BMI <30 kg/m2.
| Score | BMI ≥30 kg/m2 (n = 127) | BMI <30 kg/m2 (n = 238) | P value | WMW‐odds (95% CI) |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Total score CEQ | 3.10 ± 0.52 | 3.24 ± 0.40 | 0.025 | 0.75 (0.58–0.96) |
| Own capacity | 2.71 ± 0.58 | 2.80 ± 0.54 | 0.223 | 0.86 (0.67–1.1) |
| Professional support | 3.59 ± 0.64 | 3.72 ± 0.51 | 0.049 | 0.79 (0.63–1) |
| Perceived safety | 3.36 ± 0.66 | 3.46 ± 0.57 | 0.127 | 0.83 (0.64–1.06) |
| Participation | 2.81 ± 1.08 | 3.19 ± 0.84 | 0.004 | 0.69 (0.54–0.89) |
Note: p values from Mann–Whitney U test as appropriate for non‐normally distributed data. Missing values imputed if 50% or more of domain score is answered.
Abbreviations: CEQ, Childbirth Experience Questionnaire; CI, confidence interval; PCOS, polycystic ovary syndrome; Robson1, Robson group 1; SD, standard deviation; WMW‐odds, Wilcoxon–Mann–Whitney odds.
Women with PCOS with duration of labor >8 h scored significantly lower on total CEQ score than those with shorter duration of labor (mean ± SD 3.13 ± 0.36 vs. 3.21 ± 0.46, p = 0.028, WMW‐odds 0.69, 95% CI 0.49–0.96). They had a lower score for own capacity than women with shorter labor. We found no difference for the domain scores participation, professional support, or perceived safety (Table S3). We observed a larger spread in CEQ values among women with shorter duration of labor (Figure S2).
Women with PCOS who had a cesarean section or operative vaginal birth scored lower than women with a spontaneous vaginal birth for total CEQ, and on the domains own capacity, perceived safety, and participation (Table S4). In pairwise comparisons, we found that total CEQ was statistically significantly higher for those with a spontaneous vaginal delivery compared with both operative vaginal delivery and cesarean section (p < 0.001) (Table S5a–c).
4. DISCUSSION
Women with PCOS seemed to have childbirth experiences similar to those of the reference women, but they reported lower own capacity and higher perceived safety. Among women with PCOS, treatment with metformin during pregnancy did not appear to influence childbirth experiences, nor did the presence of pregnancy complications. On the other hand, obesity, long‐lasting labor, and operative delivery had a negative influence on childbirth experiences.
This cohort study is based on two large studies, which both used the validated CEQ in the same period. The PregMet2 study was a Nordic multicenter randomized controlled trial. A high number of participants, low dropout from the study, and a high response rate (75%) on CEQ contribute to the solidity. The LaPS study, which served as a reference population, was a large multicenter cluster randomized controlled trial in Norway. It had a high number of participants and a high response rate (62%) for the CEQ.
The pregnancy follow up was closer in the PCOS group participating in a clinical study. However, both groups received standard care at birth. For participants in the PregMet2 study who did not return the CEQ form, the CEQ was filled by a study midwife/nurse during a telephone interview at 8 weeks postpartum and this may have influenced the answers provided. Additionally, the present study is based on previously collected data and was therefore not planned based on an a priori sample size calculation. Nonetheless, the large sample size, particularly of the LaPS study, means that the primary analyses are sufficiently powered to detect differences as small as 0.11 CEQ units, assuming a standard deviation of 0.42 as seen in the total CEQ 32 (https://homepage.univie.ac.at/robin.ristl/samplesize.php?test=wilcoxon).
To the best of our knowledge this is the first study to report on childbirth experiences in women with PCOS. Despite having higher pregestational BMI and a higher risk of comorbidity and pregnancy complications, women with PCOS in Robson group 1 overall seem to be as satisfied with their childbirth experience as the reference population. Interestingly they score lower on own capacity and higher on perceived safety than the reference women.
Given the higher rate of pregnancy complications and comorbidities among women with PCOS, and also the higher rate of admissions to neonatal intensive care unit among their offspring, the lack of overall difference in childbirth experience was unexpected. Possible explanations are the high quality of pregnancy and labor care in the Nordic countries, 25 which achieves a high level of care also for more complicated pregnancies, or that the CEQ focuses more on the experience of active birth and not the postpartum period. Women in the PregMet2 study had close follow up by study midwives and doctors from first trimester until 8 weeks postpartum. We wonder if this may partially explain the finding of a higher score on perceived safety, even if the women received standard care from a different/unfamiliar midwife during childbirth.
The lower score for own capacity may be associated with the fact that women with PCOS experience more body dissatisfaction compared with women without PCOS. 33 Unwanted weight gain, hirsutism, and infertility/subfertility contribute to less body confidence. 33 To believe in her body's innate ability is important for a woman's confidence for physiological birth. 34 Obstetricians and midwives working with women with PCOS should be aware of this and contribute to strengthening the women's confidence in their ability to give birth. Integrating the philosophy from a midwifery model of woman‐centered childbirth care into our birthing units may assist in keeping our focus on the woman and her actual resources, and not merely on her diagnosis or increased risk. 35
Although the difference between women with PCOS and the reference women for perceived safety and own capacity was statistically significant, the mean difference in these domain scores was small at only 0.09. A recent study assumed a difference in mean CEQ score of more than 0.10 between groups to be clinically significant. 36 However, further studies are needed to estimate which difference in CEQ scores should be considered clinically meaningful.
The PregMet2 study was used to take a closer look at factors affecting birth experience in women with PCOS. Metformin reducing gestational weight gain and preterm birth could have had a modifying effect on childbirth experiences, but we found no evidence to support this possibility. 13 The average difference in gestational weight gain between the metformin group and the placebo group of 2.6 kg is probably not enough to influence childbirth experiences, 37 and the same is probably true for the total reduction in preterm birth in the metformin group. 13 In line with this, complications during pregnancy did not influence the childbirth experiences among women with PCOS.
Being obese in pregnancy is associated with an increased risk for both somatic and psychological comorbidities. 38 Women with obesity may feel vulnerable and under scrutiny as they cannot hide their obesity. 39 Among women with PCOS in this study, obesity was associated with lower total CEQ score as well as lower scores on the domains professional support and participation. There were more multiparous women in the group with high BMI than in the low BMI group. We think this strengthens the finding of an association between high BMI and poorer childbirth experiences, as one normally expects that parous women have faster and easier labors. Midwives and obstetricians are often hesitant to address excessive weight and may want to avoid the extra work and risk associated with women with obesity. 40 Inappropriate comments or jokes about obesity are reported by patients. 40 We can speculate whether this may contribute to the experience of less professional support during childbirth. Women with obesity are informed and aware of their increased risks in pregnancy and are submitted to procedures that may interfere with their preferred birth plan. 39 This may further lead to the experience of less participation and a poorer overall childbirth experience.
Longer duration of labor is associated with operative delivery which in turn is associated with a wish for cesarean section in the next pregnancy. 41 From our data we have no reason to believe that women with PCOS in Robson 1 have a longer active phase of labor than other women. However, we found that, among women with PCOS, a longer duration of active labor resulted in a poorer overall childbirth experience, and a lower score of own capacity. Other Nordic studies have found that women with longer duration of labor scored lower on the CEQ domains own capacity and perceived safety, 41 or had a poorer overall childbirth experience. 42
In line with other studies, we found that the mode of birth influenced childbirth experiences. 14 , 16 In addition, the relationship between BMI, duration of labor and childbirth experiences observed in our study may be mediated by mode of birth. Women with obesity have higher risk for any operative delivery, 38 and prolonged labor is a reason to perform emergency cesarean section. 43 The comparatively high variance in CEQ scores for both duration of labor and for BMI suggests that the picture is ambiguous and confirms that childbirth experiences are multifaceted and influenced by many factors.
5. CONCLUSION
All in all, women with PCOS reported similar childbirth experiences as reference women. Metformin did not influence childbirth experience, nor did pregnancy complications. Obesity, long duration of labor and any operative delivery had negative impact on childbirth experiences. Future research should further explore the childbirth experiences of women with PCOS in terms of their overall experience and the aspects identified in the CEQ domains using both qualitative and quantitative methods.
AUTHOR CONTRIBUTIONS
Eszter Vanky conceived the idea of the study. Eszter Vanky, Tone Shetelig Løvvik, Rebecka Dalbye, Melanie Rae Simpson, and Anne Engtrø Husby contributed to the design of the study. Eszter Vanky, Tone Shetelig Løvvik, Rebecka Dalbye, and Anne Engtrø Husby were responsible for the data collection. Anne Engtrø Husby performed the statistical analyses with help from Melanie Rae Simpson and Eszter Vanky. Analysis and results were discussed in the group. Anne Engtrø Husby wrote the first draft, and all authors critically revised it. All authors approved the final version of the manuscript.
FUNDING INFORMATION
The Liaison committee between the Central Norway Health Authorities and the universities and colleges in Central Norway funded this project. The PregMet2 study was funded by the Research Council of Norway, Novo Nordisk Foundation, St Olav's University Hospital, and Norwegian University of Science and Technology. The LaPS study was funded by Østfold Hospital Trust.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflicting interests.
ETHICS STATEMENT
Ethical approvals for the PregMet2 study were obtained from the Regional Committee for Health Research Ethics of Central Norway (REC no 2011/1434), the Regional Ethical Review Board in Stockholm (Dmnb: 2012/1200–31/2), and the National Bioethics Committee in Iceland (VSNb2012100011/03.10). Clinical trial authorization was obtained from the Norwegian Medicines Agency, also approved in Iceland and Sweden, and registered with ClinicalTrials.gov, number NCT01587378. The LaPS study was approved by the Regional Committee for Medical and Health Research Ethics on December 11, 2013 (2013/1862/REK Sør‐Øst) and registered with ClinicalTrials.gov, number NCT02221427. Informed consent was obtained from all participants in both studies.
Supporting information
Figures S1–S2.
Tables S1–S5.
Appendix S1.
ACKNOWLEDGMENTS
We would like to thank the co‐investigators, midwives, and study nurses in the two original studies, PregMet2 and LaPS for their relentless work. We want to express our sincere appreciation to all women who participated and by that contribute.
Husby AE, Simpson MR, Dalbye R, Larsen M, Vanky E, Løvvik TS. Childbirth experiences in women with polycystic ovary syndrome: A cohort study. Acta Obstet Gynecol Scand. 2024;103:1092‐1100. doi: 10.1111/aogs.14800
Eszter Vanky and Tone Shetelig Løvvik share last authorship.
REFERENCES
- 1. The Rotterdam ESHRE/ASRM‐sponsored PCOS consensus workshop group . Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41‐47. [DOI] [PubMed] [Google Scholar]
- 2. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova‐Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106:6‐15. [DOI] [PubMed] [Google Scholar]
- 3. Amiri M, Ramezani Tehrani F, Behboudi‐Gandevani S, Bidhendi‐Yarandi R, Carmina E. Risk of hypertension in women with polycystic ovary syndrome: a systematic review, meta‐analysis and meta‐regression. Reprod Biol Endocrinol. 2020;18:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lazaridou S, Dinas K, Tziomalos K. Prevalence, pathogenesis and management of prediabetes and type 2 diabetes mellitus in patients with polycystic ovary syndrome. Hormones. 2017;16:373‐380. [DOI] [PubMed] [Google Scholar]
- 5. Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long‐term health. Fertil Steril. 2018;110:794‐809. [DOI] [PubMed] [Google Scholar]
- 6. Teede HJ, Joham AE, Paul E, et al. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring). 2013;21:1526‐1532. [DOI] [PubMed] [Google Scholar]
- 7. Brutocao C, Zaiem F, Alsawas M, Morrow AS, Murad MH, Javed A. Psychiatric disorders in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Endocrine. 2018;62:318‐325. [DOI] [PubMed] [Google Scholar]
- 8. Lee I, Cooney LG, Saini S, Sammel MD, Allison KC, Dokras A. Increased odds of disordered eating in polycystic ovary syndrome: a systematic review and meta‐analysis. Eat Weight Disord. 2019;24:787‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu H‐F, Chen H‐S, Rao D‐P, Gong J. Association between polycystic ovary syndrome and the risk of pregnancy complications: a PRISMA‐compliant systematic review and meta‐analysis. Medicine. 2016;95:e4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naderpoor N, Shorakae S, de Courten B, Misso ML, Moran LJ, Teede HJ. Metformin and lifestyle modification in polycystic ovary syndrome: systematic review and meta‐analysis. Hum Reprod Update. 2015;21:560‐574. [DOI] [PubMed] [Google Scholar]
- 11. NICE . Diabetes in pregnancy: management from preconception to the postnatal period. 2015. NICE guideline [NG3]. https://www.nice.org.uk/guidance/ng3/chapter/Recommendations [PubMed]
- 12. Cassina M, Donà M, Di Gianantonio E, Litta P, Clementi M. First‐trimester exposure to metformin and risk of birth defects: a systematic review and meta‐analysis. Hum Reprod Update. 2014;20:656‐669. [DOI] [PubMed] [Google Scholar]
- 13. Løvvik TS, Carlsen SM, Salvesen Ø, et al. Use of metformin to treat pregnant women with polycystic ovary syndrome (PregMet2): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7:256‐266. [DOI] [PubMed] [Google Scholar]
- 14. Chabbert M, Panagiotou D, Wendland J. Predictive factors of women's subjective perception of childbirth experience: a systematic review of the literature. J Reprod Infant Psychol. 2021;39:43‐66. [DOI] [PubMed] [Google Scholar]
- 15. Henriksen L, Grimsrud E, Schei B, Lukasse M. Factors related to a negative birth experience – a mixed methods study. Midwifery. 2017;51:33‐39. [DOI] [PubMed] [Google Scholar]
- 16. Dencker A, Nilsson C, Begley C, et al. Causes and outcomes in studies of fear of childbirth: a systematic review. Women Birth. 2019;32:99‐111. [DOI] [PubMed] [Google Scholar]
- 17. Olza I, Leahy‐Warren P, Benyamini Y, et al. Women's psychological experiences of physiological childbirth: a meta‐synthesis. BMJ Open. 2018;8:e020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lundgren I, Karlsdottir SI, Bondas T. Long‐term memories and experiences of childbirth in a Nordic context—a secondary analysis. Int J Qual Stud Health Well‐being. 2009;4(2):115‐128. [Google Scholar]
- 19. Michels A, Kruske S, Thompson R. Women's postnatal psychological functioning: the role of satisfaction with intrapartum care and the birth experience. J Reprod Infant Psychol. 2013;31:172‐182. [Google Scholar]
- 20. McKelvin G, Thomson G, Downe S. The childbirth experience: a systematic review of predictors and outcomes. Women Birth. 2021;34:407‐416. [DOI] [PubMed] [Google Scholar]
- 21. Fenech G, Thomson G. Tormented by ghosts from their past': a meta‐synthesis to explore the psychosocial implications of a traumatic birth on maternal well‐being. Midwifery. 2014;30:185‐193. [DOI] [PubMed] [Google Scholar]
- 22. Gottvall K, Waldenström U. Does a traumatic birth experience have an impact on future reproduction? BJOG. 2002;109:254‐260. [DOI] [PubMed] [Google Scholar]
- 23. WHO . WHO recommendations: intrapartum care for a positive childbirth experience. 2018. [PubMed]
- 24. Helsedirektoratet . Et trygt fødetilbud. Kvalitetskrav til fødselsomsorgen. [A safe food offer. Quality requirements for maternity care]. In: Helsedirektoratet, editor. 2010.
- 25. Daníelsdóttir SaI J. The First 1000 Days in the Nordic Countries: A Situation Analysis. Nordic Council of Ministers; 2020. [Google Scholar]
- 26. Bernitz S, Dalbye R, Zhang J, et al. The frequency of intrapartum caesarean section use with the WHO partograph versus Zhang's guideline in the Labour Progression Study (LaPS): a multicentre, cluster‐randomised controlled trial. Lancet. 2019;393:340‐348. [DOI] [PubMed] [Google Scholar]
- 27. Rozsa DJ, Dalbye R, Bernitz S, et al. The effect of Zhang's guideline vs the WHO partograph on childbirth experience measured by the Childbirth Experience Questionnaire in the labor progression study (LaPS): a cluster randomized trial. Acta Obstet Gynecol Scand. 2022;101:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ruseckaite R, Mudunna C, Caruso M, Ahern S. Response rates in clinical quality registries and databases that collect patient reported outcome measures: a scoping review. Health Qual Life Outcomes. 2023;21:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dencker A, Taft C, Bergqvist L, Lilja H, Berg M. Childbirth Experience Questionnaire (CEQ): development and evaluation of a multidimensional instrument. BMC Pregnancy Childbirth. 2010;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fay MP, Malinovsky Y. Confidence intervals of the Mann‐Whitney parameter that are compatible with the Wilcoxon‐Mann‐Whitney test. Stat Med. 2018;37:3991‐4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norsk gynekologisk forening (Norwegian Gynecological Association) . Veileder i fødselshjelp 2020 [Supervisor in obstetric care 2020] [ePub ISBN 978–82–692382‐2‐8].
- 32. Noether GE. Sample size determination for some common nonparametric tests. J Am Stat Assoc. 1987;82:645‐647. [Google Scholar]
- 33. Davitadze M, Malhotra K, Khalil H, et al. Body image concerns in women with polycystic ovary syndrome: a systematic review and meta‐analysis. Eur J Endocrinol. 2023;189:R1‐R9. [DOI] [PubMed] [Google Scholar]
- 34. Neerland CE. Maternal confidence for physiologic childbirth: a concept analysis. J Midwifery Womens Health. 2018;63:425‐435. [DOI] [PubMed] [Google Scholar]
- 35. Berg M, Asta Ólafsdóttir Ó, Lundgren I. A midwifery model of woman‐centred childbirth care – in Swedish and Icelandic settings. Sex Reprod Healthc. 2012;3:79‐87. [DOI] [PubMed] [Google Scholar]
- 36. Waldum ÅH, Lukasse M, Staff Anne C , Falk RS, Sørbye Ingvil K, Jacobsen AF. Intrapartum pudendal nerve block analgesia and childbirth experience in primiparous women with vaginal birth: a cohort study. Birth. 2023;50:182‐191. [DOI] [PubMed] [Google Scholar]
- 37. Molin J, Vanky E, Løvvik TS, Dehlin E, Bixo M. Gestational weight gain, appetite regulating hormones, and metformin treatment in polycystic ovary syndrome: a longitudinal, placebo‐controlled study. BJOG. 2022;129:1112‐1121. [DOI] [PubMed] [Google Scholar]
- 38. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16:621‐638. [DOI] [PubMed] [Google Scholar]
- 39. Nyman VMK, Prebensen ÅK, Flensner GEM. Obese women's experiences of encounters with midwives and physicians during pregnancy and childbirth. Midwifery. 2010;26:424‐429. [DOI] [PubMed] [Google Scholar]
- 40. Christenson A, Torgerson J, Hemmingsson E. Attitudes and beliefs in Swedish midwives and obstetricians towards obesity and gestational weight management. BMC Pregnancy Childbirth. 2020;20:755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gaudernack LC, Michelsen TM, Egeland T, Voldner N, Lukasse M. Does prolonged labor affect the birth experience and subsequent wish for cesarean section among first‐time mothers? A quantitative and qualitative analysis of a survey from Norway. BMC Pregnancy Childbirth. 2020;20:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carlhäll S, Nelson M, Svenvik M, Axelsson D, Blomberg M. Maternal childbirth experience and time in labor: a population‐based cohort study. Sci Rep. 2022;12:11930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. da Silva CP, Hansson Bittár M, Vladic SY. Indications for increase in caesarean delivery. Reprod Health. 2019;16:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S2.
Tables S1–S5.
Appendix S1.


