Key Points
-
•
After initial clinical response, flares of acute GVHD are common and associated with higher NRM.
-
•
MAGIC biomarkers at first CR/VGPR can predict GVHD flares.
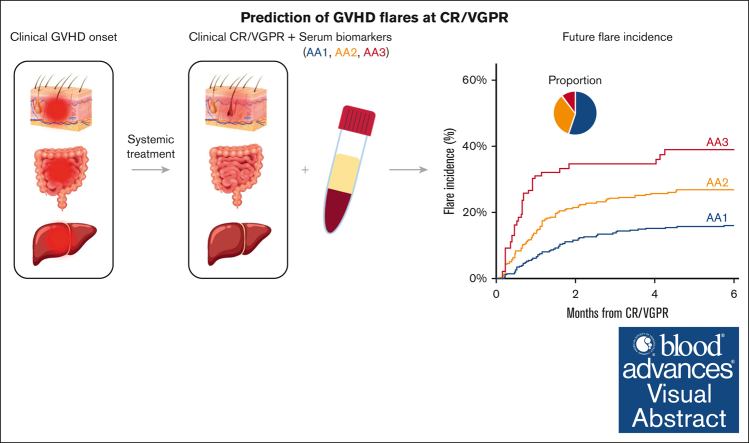
Visual Abstract
Abstract
The absence of a standardized definition for graft-versus-host disease (GVHD) flares and data on its clinical course are significant concerns. We retrospectively evaluated 968 patients across 23 Mount Sinai Acute GVHD International Consortium (MAGIC) transplant centers who achieved complete response (CR) or very good partial response (VGPR) within 4 weeks of treatment. The cumulative incidence of flares within 6 months was 22%, and flares were associated with a higher risk of nonrelapse mortality (NRM; adjusted hazard ratio [aHR], 4.84; 95% confidence interval [CI], 3.19-7.36; P < .001). Flares were more severe (grades 3/4, 41% vs 16%; P < .001) and had more frequent lower gastrointestinal (LGI) involvement (55% vs 32%; P < .001) than the initial GVHD. At CR/VGPR, elevated MAGIC biomarkers predicted the future occurrence of a flare, along with its severity and LGI involvement. In multivariate analyses, higher Ann Arbor (AA) biomarker scores at CR/VGPR were significant risk factors for flares (AA2 vs AA1: aHR, 1.81 [95% CI, 1.32-2.48; P = .001]; AA3 vs AA1: aHR, 3.14 [95% CI, 1.98-4.98; P < .001]), as were early response to initial treatment (aHR, 1.84; 95% CI, 1.21-2.80; P = .004) and HLA-mismatched unrelated donor (aHR, 1.74; 95% CI, 1.00-3.02; P = .049). MAGIC biomarkers also stratified the risk of NRM both at CR/VGPR and at the time of flare. We conclude that GVHD flares are common and carry a significant mortality risk. The occurrence of future flares can be predicted by serum biomarkers that may serve to guide adjustment and discontinuation of immunosuppression.
Introduction
Acute graft-versus-host disease (GVHD) is a common life-threatening complication after allogeneic hematopoietic cell transplantation (HCT).1,2 The current standard first-line treatment is systemic steroids, which can induce a clinical response in a majority of patients.3, 4, 5, 6, 7 However, acute GVHD symptoms often recur (flare) after the tapering or discontinuation of steroids.8 Indeed, in a recent randomized, phase 3 trial of ruxolitinib for steroid-refractory acute GVHD (REACH 2 trial), one-third of enrolled patients were eligible due to inability to taper systemic corticosteroids.9 But to date, limited data are available regarding the incidence, clinical presentations, and outcomes of flares of acute GVHD. These fundamental knowledge gaps hinder the progress toward risk-adapted patient management including tapering of immunosuppressive agents.
In this analysis from the Mount Sinai Acute GVHD International Consortium (MAGIC), we first defined a flare of acute GVHD according to expert consensus in our consortium. Using this definition, we sought to characterize the incidence, clinical presentation, and long-term outcomes of flares in a large multicenter cohort with prospectively collected clinical and laboratory data. We also evaluate the risk factors for flares at the time of initial clinical response to treatment, with a focus on the ability of MAGIC serum biomarkers to predict long-term outcomes when clinical symptoms are minimal or absent.
Methods
Patient selection
The MAGIC database and biorepository collects detailed clinical information and blood samples from patients who undergo allogeneic transplants, at 23 international HCT centers in North America, Europe, and Asia. Patients are prospectively monitored for acute GVHD symptoms and treatment every week through day 100 after HCT and for 4 weeks after the initiation of systemic treatment and then less frequently up until 2 years after their HCT. GVHD symptoms are adjudicated according to a rigorous prospective-specimen-collection, retrospective-blinded-evaluation study design.10, 11, 12 Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Adult and pediatric patients who received their first HCT between 2014 and 2021 were included in this study if they achieved complete response (CR) or very good partial response (VGPR) to systemic steroid treatment for acute GVHD (at least methylprednisolone 0.1 mg/kg per day or equivalent), within 4 weeks without primary disease relapse, and if a serum sample was available at the first achievement of CR/VGPR. Responses were assessed without regard to treatment given for GVHD. Supplemental Table 1 shows the number of patients from each participating center, which includes 324 patients who were included in a prior analysis of biomarkers as a response end point.13
Definitions
Acute GVHD was diagnosed and staged according to the published MAGIC consensus criteria.12 CR was defined as the complete resolution of acute GVHD manifestations. VGPR was defined as any improvement that approximates CR but has residual stage 1 skin disease.14 Flares were defined as the earliest date that 2 criteria were both met: symptom severity increased by at least 1 stage in at least 1 organ and intensified treatment (methylprednisolone increased by at least 0.25 mg/kg or equivalent or addition of another systemic immunosuppressive agent). Flares after primary disease relapse, donor lymphocyte infusion or the onset of chronic GVHD15 were not included in the analysis. HCT-specific comorbidity index (HCT-CI) scores and intensity of conditioning regimens were defined as per published criteria.16,17 Death was considered related to acute GVHD if GVHD symptoms were present at the time of death or if death occurred from a complication such as infection while receiving systemic treatment for acute GVHD (≥10 mg methylprednisolone equivalent [MPE] per day).18 The weekly steroid taper rate was calculated as described in the supplemental Methods, and patients were assigned to rapid and slow taper groups as follows: the slow taper group included patients who were tapered <20% per week if the maximum steroid dose was ≤1 mg/kg or <30% per week if the maximum steroid dose was >1mg/kg. All other patients were included in the rapid taper group.
Serum samples
Serial serum samples were prospectively collected, cryopreserved, and stored at a central laboratory. Serum concentrations of suppressor of tumorigenicity 2 (ST2)19 and regenerating islet-derived protein 3-α (REG3α)20 were analyzed by enzyme-linked immunosorbent assays, as previously reported.21, 22, 23 The MAGIC algorithm probability (MAP) was calculated as a single value between 0.001 and 0.999 according to the formula: log(–log[1 – MAP]) = –11.263 + 1.844(log10ST2) + 0.577(log10REG3α) and validated thresholds for Ann Arbor (AA) scores were used (AA1 < 0.14; 0.14 ≤ AA2 < 0.29; AA3 ≥ 0.29).18,21,22,24, 25, 26
Statistical analysis
Grouped variables were compared using the Fisher exact test, and continuous variables were compared by the Mann-Whitney U test. The analyses of flare as a time-dependent covariate for the risk of nonrelapse mortality (NRM) used cause-specific Cox proportional hazards regression models.27 The cumulative incidence of flare and NRM were estimated and plotted according to the Gray method. Predictors of flares were evaluated using the method of Fine and Gray in multivariate analysis. Competing risks for the cumulative incidence of flare were the relapse of primary and death without flare of GVHD, whereas the competing risk for NRM was relapse. All outcomes were censored at 6 months from the starting point (either at CR/VGPR or at the onset of flare).
We included the following variables as potential risk factors for GVHD flare in a multivariate analysis: maximum GVHD grade, maximum steroid dose before CR/VGPR, time to CR/VGPR, use of immunosuppressive agents other than steroids before CR/VGPR, response to treatment, and serum biomarkers MAP at CR/VGPR. We included additional covariates in the multivariate analysis based on their known prognostic significance on GVHD outcomes:24,28,29 recipient age at HCT, sex mismatch, donor type, GVHD prophylaxis, HCT-CI, use of antithymocyte globulin or alemtuzumab, and conditioning regimen intensity. In the multivariate analysis which treated a flare as a time-dependent covariate for NRM, we reduced the number of covariates because of the small number of events. These included maximum GVHD grade, maximum steroid dose before CR/VGPR, timing of achievement of CR/VGPR, use of immunosuppressive agents other than steroids before CR/VGPR, response to treatment, recipient age,24 and HCT-CI.29 Correlations among the variables in the multivariate models were tested to avoid multicollinearity (supplemental Table 2).30
The interaction between AA scores and steroid taper rate was measured using additive scales.31 The additive scale interaction was evaluated by calculating the relative risk caused by interaction, and its confidence interval (CI) was estimated based on the delta method.32
Statistical significance was set at .05, and all P values were 2-tailed. Statistical analyses were performed with R or EZR version 1.61 (Jichi Medical University Saitama Medical Center, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 4.2.2, Vienna, Austria).33
Results
Patient characteristics and incidence of flares
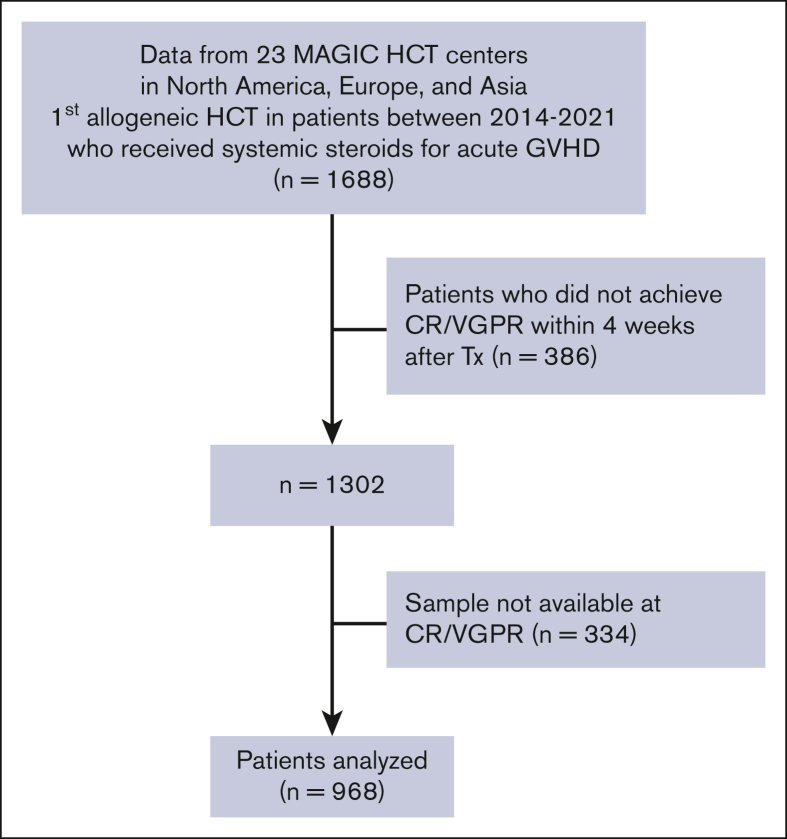
The CONSORT diagram for this analysis shows that the MAGIC database included 1302 patients who received systemic corticosteroids for acute GVHD and who achieved CR/VGPR within 4 weeks (Figure 1). Serum samples at the time of CR/VGPR were not available in 334 patients. The 6 month NRM of the remaining 968 patients did not differ from the total group (10% vs 10%, P = .889). Because the cumulative incidence of flare and of NRM were similar after CR and VGPR in these 968 patients, we combined them as a single clinical response to first-line therapy (supplemental Figure 1). The median maximum daily dose of corticosteroids before CR/VGPR in these patients was 1 mg/kg methylprednisolone or equivalent (range, 0.1-3.2 mg/kg; Table 1; supplemental Table 3). Most patients were treated for maximum of grade 1/2 GVHD (81%) and achieved CR/VGPR (82%) within 2 weeks.
Figure 1.
CONSORT diagram. Tx, treatments.
Table 1.
Patient characteristics
| Overall |
|
|---|---|
| n = 968 (%) | |
| Median age at HCT, y (range) | 55 (0-79) |
| Recipient age, y | |
| <18 | 106 (11) |
| 18-54 | 377 (39) |
| ≥55 | 485 (50) |
| Primary disease | |
| Acute leukemia | 524 (54) |
| MDS/MPN | 238 (25) |
| Lymphoma | 85 (9) |
| Other | 121 (13) |
| Sex mismatch | |
| Female to male | 153 (16) |
| Others | 815 (84) |
| Donor type | |
| HLA-matched related | 172 (18) |
| HLA-matched unrelated | 540 (56) |
| HLA-mismatched unrelated | 104 (11) |
| Haploidentical | 116 (12) |
| Umbilical cord blood | 36 (4) |
| GVHD prophylaxis | |
| CNI based | 738 (76) |
| PTCy based | 188 (19) |
| Others | 42 (4) |
| HCT-CI | |
| 0-2 | 652 (67) |
| ≥3 | 316 (33) |
| Use of ATG or alemtuzumab | |
| No | 553 (57) |
| Yes | 415 (43) |
| Donor source | |
| Bone marrow | 202 (21) |
| Peripheral blood | 730 (75) |
| Umbilical cord blood | 36 (4) |
| Conditioning | |
| MAC (TBI <8 Gy) | 421 (44) |
| MAC (TBI ≥8 Gy) | 156 (16) |
| RIC | 391 (40) |
| Median year of HCT (range) | 2018 (2014-2021) |
| Max grades before CR/VGPR | |
| Grades 1-2 | 788 (81) |
| Grades 3-4 | 180 (19) |
| Timing of achievement of CR/VGPR | |
| Late response (>14 d) | 172 (18) |
| Early response (≤14 d) | 796 (82) |
| Max steroid dose before CR/VGPR | |
| <1 mg/kg | 460 (48) |
| ≥1 mg/kg | 508 (53) |
| GVHD treatment before CR/VGPR | |
| Steroid alone | 864 (89) |
| Steroid + ruxolitinib | 22 (2.0) |
| Steroid + other∗ | 82 (8.5) |
| Treatment response | |
| CR | 682 (71) |
| VGPR | 286 (30) |
ATG, antithymocyte globulin; CNI, calcineurin inhibitor; MAC, myeloablative conditioning; MDS/MPN, myelodysplastic syndromes/myeloproliferative neoplasms; RIC, reduced intensity conditioning; TBI, total body irradiation.
Excludes ruxolitinib.
Association of flare with long-term outcomes
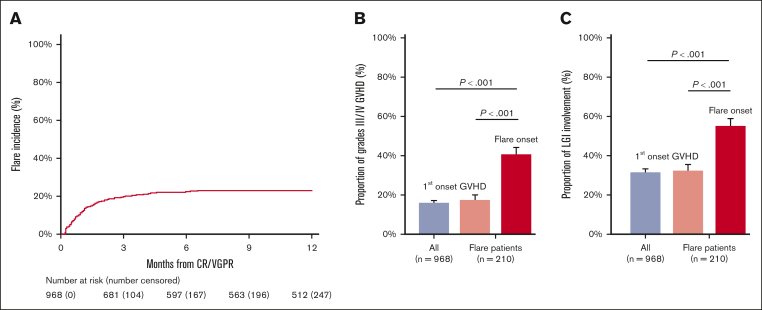
The overall cumulative incidence of NRM at 6 months after CR/VGPR was 10% (95% CI, 8-12), with a median follow-up of survivors after CR/VGPR of 22 months (range, 1-39). Follow-up was less than 6 months for 22 survivors (2%) after CR/VGPR. The 6 month cumulative incidence of flares after first CR/VGPR was 22% (95% CI, 19-24; Figure 2A). The median time to flare was 28 days (range, 2-448), and 87% of flares occurred within 3 months from CR/VGPR. Of the 210 patients experiencing flares, 138 (66%) were treated with an escalation of steroids, 47 (22%) received second-line agents, and 25 (12%) were treated with an increase of steroids in addition to second-line therapy. The cumulative incidence of 6-month NRM after flares was 27% (95% CI, 21-33). Six-month NRM was not significantly different for patients whose flares were treated by increasing steroid dose (23%; 95% CI, 16.0-30.0), adding a second-line agent (33%; 95% CI, 19.5-46.3), or both (40%; 95% CI, 20.8-58.6; P = .086). Patients with grade 3/4 GVHD at the time of flare, however, were more likely to experience NRM than patients with grade 1/2 GVHD (47% vs 12%; P < .001). The development of a flare treated as a time-dependent covariate was significantly associated with higher risk of NRM in a multivariate analysis (hazard ratio [HR], 4.79; P < .001; supplemental Table 4). As expected, the greatest contributor to NRM after flares was acute GVHD or complications from its treatment (54/71 [76%]). The time to flare did not affect NRM; patients with early flares (defined as occurring <28 days from CR/VGPR) experienced the same 6-month NRM as patients with late flares (27% [95% CI, 19.1-35.9] vs 27% [95% CI, 18.4-35.5]; P = .962). Similarly, flares during steroid tapers (151/210 [72%]) and after steroid discontinuation (59/210 [28%]) had similar 6-month NRM from the onset of flares (26% [95% CI, 19.3-33.3] vs 29% [95% CI, 18.0-41.0]; P = .638).
Figure 2.
Incidence, severity, and LGI involvement of flares. (A) The cumulative incidence of flares after CR/VGPR; the cumulative incidence at 6 month was 21.6% (95% CI, 19.0-24.2). (B) The proportion of grades 3/4 acute GVHD at first onset in the entire population (n = 968, 16.3%), in patients with flare (n = 210, 18.1%), and at the time of flare (n = 210, 41.4%). (C) The proportion of LGI involvement at first onset in the entire population (n = 968, 31.6%), in patients with flare (n = 210, 32.9%), and at the time of flare (n = 210, 55.2%). P values for pairwise comparisons were adjusted using a Bonferroni method. The error bars represent standard error.
Consistent with the unexpectedly high risk of NRM, the clinical severity of acute GVHD at the onset of flare was greater than that of the original episode (grade 3-4, 41% vs 16%; P = .001; Figure 2B; supplemental Table 5). This increased severity was mainly because of greater lower gastrointestinal (LGI) involvement (55% vs 32%; P < .001; Figure 2C; supplemental Table 5). Grade 3/4 GVHD and/or LGI involvement were not more frequent at onset of GVHD among patients who had a subsequent flare than among those who did not.
Prediction of flares at CR/VGPR
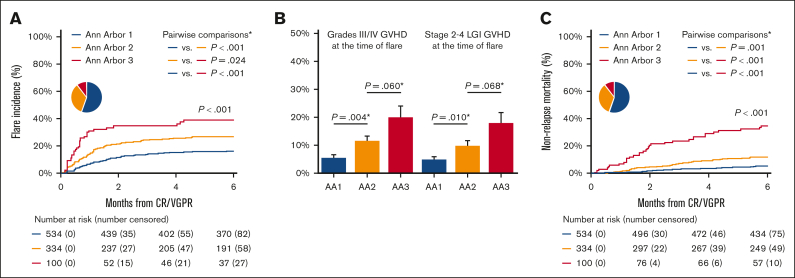
The MAP combines the concentrations of 2 serum biomarkers into a single value that can be considered a “liquid biopsy” of crypt damage throughout the GI tract.13,23 The MAP value determines the risk of 6-month NRM and is used to calculate the AA score that has been validated as a predictive biomarker by several research groups.34,35 The MAP detects damage to GI crypts even before symptoms appear,21 and we, therefore, measured MAPs in all patients at the time of CR/VGPR. The incidence of eventual flare increased with each increase in AA score (AA1, 16%; AA2, 26%; AA3, 39%; P < .001; Figure 3A), as did the severity of flare and the proportion of patients with LGI involvement (Figure 3B). In an exploratory analysis, we calculated the Akaike information criterion for predicting GVHD flares based on the AA score at treatment initiation (2510.3), at CR/VGPR (2492.5), and at both time points (2494.1). The lowest Akaike information criterion was at CR/VGPR, suggesting that the time point closest to flare was the strongest predictor. A multivariate analysis confirmed AA scores at CR/VGPR as significant risk factors for that development of GVHD flares (AA2 vs AA1: HR, 1.80 [P = .001]; AA3 vs AA1: HR, 3.13 [P < .001]; Table 2). The AA score also predicted 6-month NRM at the time of first CR/VGPR (AA1, 5%; AA2, 11%; AA3, 34%; P < .001; Figure 3C).
Figure 3.
Association of biomarkers at CR/VGPR on GVHD flare, its severity, and NRM. (A) The cumulative incidence of flares after CR/VGPR stratified by the AA score at CR/VGPR; the cumulative incidence at 6 month was 15.8% (95% CI, 12.8-19.0) in AA1, 26.1% (95% CI, 21.4-30.9) in AA2, and 38.6% (95% CI, 28.6-48.5) in AA3. (B) The proportion of patients who developed grades 3/4 GVHD flare stratified by the AA scores at CR/VGPR (left panel); 5.4% (29/534) in AA1, 11.4% (38/334) in AA2, and 20.0% (20/100) in AA3. The proportion of patients who developed GVHD flare with stage 2-4 LGI involvement stratified by the AA scores at CR/VGPR (right panel); 4.9% (26/534) in AA1, 9.9% (33/334) in AA2, and 18.0% (18/100) in AA3. The error bars represent standard error. (C) The cumulative incidence of NRM after CR/VGPR stratified by MAP biomarkers at CR/VGPR; the cumulative incidence at 6 months was 4.7% (95% CI, 3.2-6.8) in AA1, 11.0% (95% CI, 7.9-14.7) in AA2, and 33.5% (95% CI, 24.4-43.3) in AA3. The pie chart shows the percentage of AA1 (blue), AA2 (yellow), and AA3 (red). ∗P values for pairwise comparisons were adjusted using a Bonferroni method.
Table 2.
Risk factors of flares within 6 months after CR/VGPR
| Without steroid taper rates |
With steroid taper rates |
|||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Biomarker score at CR/VGPR | ||||
| AA1 | 1 | Ref | 1 | Ref |
| AA2 | 1.80 (1.31-2.45) | <.001 | 1.80 (1.31-2.47) | <.001 |
| AA3 | 3.13 (1.97-4.96) | <.001 | 3.17 (1.99-5.06) | <.001 |
| Recipient age, y | ||||
| 18-54 | 1 | Ref | 1 | Ref |
| <18 | 1.22 (0.72-2.06) | .460 | 0.99 (0.57-1.71) | .970 |
| ≥55 | 1.04 (0.75-1.44) | .820 | 0.99 (0.71-1.37) | .950 |
| Sex mismatch | ||||
| Others | 1 | Ref | 1 | Ref |
| Female to male | 1.45 (0.99-2.13) | .055 | 1.43 (0.97-2.11) | .067 |
| Donor type | ||||
| HLA-matched related | 1 | Ref | 1 | Ref |
| HLA-matched unrelated | 1.16 (0.76-1.78) | .490 | 1.23 (0.79-1.90) | .360 |
| HLA-mismatched unrelated | 1.74 (1.00-3.02) | .049 | 1.72 (0.98-3.03) | .060 |
| Haploidentical | 1.14 (0.52-2.48) | .740 | 1.36 (0.61-3.03) | .450 |
| Umbilical cord blood | 1.50 (0.69-3.26) | .300 | 1.89 (0.88-4.06) | .100 |
| GVHD prophylaxis | ||||
| CNI based | 1 | Ref | 1 | Ref |
| PTCy based | 1.01 (0.56-1.81) | .990 | 0.92 (0.50-1.70) | .800 |
| Others | 1.30 (0.64-2.61) | .470 | 1.25 (0.63-2.49) | .520 |
| HCT-CI | ||||
| 0-2 | 1 | Ref | 1 | Ref |
| ≥3 | 1.15 (0.85-1.56) | .370 | 1.19 (0.87-1.62) | .280 |
| Use of ATG or alemtuzumab | ||||
| No | 1 | Ref | 1 | Ref |
| Yes | 0.95 (0.70-1.30) | .760 | 0.81 (0.58-1.12) | .200 |
| Conditioning | ||||
| MAC (TBI <8 Gy) | 1 | Ref | 1 | Ref |
| MAC (TBI ≥8 Gy) | 0.76 (0.49-1.18) | .220 | 0.73 (0.46-1.15) | .180 |
| RIC | 1.07 (0.77-1.48) | .680 | 0.98 (0.71-1.34) | .870 |
| Max grades before CR/VGPR | ||||
| Grades 1-2 | 1 | Ref | 1 | Ref |
| Grades 3-4 | 1.02 (0.69-1.49) | .930 | 1.00 (0.68-1.45) | .990 |
| Timing of achievement of CR/VGPR | ||||
| Late response (>14 d) | 1 | Ref | 1 | Ref |
| Early response (≤14 d) | 1.80 (1.17-2.77) | .007 | 2.15 (1.40-3.30) | <.001 |
| Max steroids dose before CR/VGPR∗ | ||||
| ≥1 mg/kg | 1 | Ref | 1 | Ref |
| <1 mg/kg | 1.07 (0.78-1.48) | .670 | 1.40 (0.99-1.98) | .053 |
| GVHD treatment before CR/VGPR | ||||
| Steroid alone | 1 | Ref | 1 | Ref |
| Steroid + ruxolitinib | 0.77 (0.22-2.68) | .680 | 0.98 (0.28-3.44) | .970 |
| Steroid + other† | 1.22 (0.77-1.92) | .400 | 1.44 (0.91-2.27) | .120 |
| Treatment response | ||||
| CR | 1 | Ref | 1 | Ref |
| VGPR | 1.11 (0.81-1.52) | .510 | 1.21 (0.88-1.66) | .250 |
| Steroid taper rates | ||||
| Slow tapers | 1 | Ref | ||
| Rapid tapers | 2.65 (1.93-3.64) | <.001 | ||
ATG, antithymocyte globulin; CNI, calcineurin inhibitor; MAC, myeloablative conditioning; Ref, reference; RIC, reduced intensity conditioning; TBI, total body irradiation.
Dose of methylprednisolone or equivalent.
Excludes ruxolitinib.
Surprisingly, flares occurred more frequently among patients with early achievement of CR/VGPR (within 14 days) than those who responded later (>14 days; 23% vs 14%; P = .016), which was confirmed by a multivariate analysis (HR, 1.84; P = .004; Table 2). Other than HLA-mismatched unrelated donors (HR, 1.74; P = .049; Table 2), no additional clinical variables were associated with the development of flare (Table 2; supplemental Table 6). We reasoned that time to achieve CR/VGPR might influence steroid exposure for 2 reasons. First, as expected, the cumulative doses of steroids before CR/VGPR were significantly higher in patients who were slower to respond to treatment (median cumulative dose of steroids in mg/kg MPE, 21.3 vs 9.34; P < .001). Second, the time to achievement of CR/VGPR might influence how steroids were tapered; for example, patients whose GVHD was more sensitive to steroid treatment (ie, early responders) might be tapered more rapidly. To explore this possibility, we estimated the rate of steroid dose reduction for each patient using the difference in 2 steroid doses: at first CR/VGPR and at 4 weeks later, flare, death, or discontinuation of steroids, whichever occurred first; we expressed this value as a weekly reduction rate. We observed an interaction between the taper rate and the maximum steroid dose for GVHD flares (supplemental Figure 2), and thus, we incorporated the maximum steroid dose in the determination of slow and rapid taper groups (supplemental Methods). The slow taper group included patients who were tapered <20% per week if the maximum steroid dose was ≤1 mg/kg MPE or <30% per week if the maximum steroid dose was >1 mg/kg MPE; all other patients were included in the rapid taper group. Patients whose steroids were tapered rapidly were nearly twice as likely to experience a flare compared with patients who were tapered slowly (30% vs 17%; P < .001; Figure 4A). Interestingly, patients who achieved CR/VGPR <14 days were just as likely to be tapered slowly as patients who took longer to achieve CR/VGPR (504/796 [63%] vs 97/172 [56%]; P = .100). A multivariate analysis revealed that the rate of steroid taper (HR, 2.65; P < .001), AA scores, and time to CR/VGPR were all significant and independent risk factor of flares (Table 2). We further explored the relationship among these risk factors in a stepwise fashion. First, graphical visualization of the 2 strongest risk factors, AA score and steroid taper, showed that patients tapered rapidly were more likely to flare than patients tapered slowly, and the difference among taper groups was larger for patients with AA2/3 GVHD (Figure 4B-C). The interaction between AA score at CR/VGPR and steroid taper was highly significant (P = .008), that is, the benefit of slow tapers was greater for patients with AA2/3 GVHD (supplemental Table 7). Finally, we examined the relationship among all 3 risk factors (supplemental Table 8). In this analysis, an early CR/VGPR was associated with higher incidence of flares in patients with AA1 GVHD at the time of response whether they were tapered slowly or rapidly, but the effect was not observed for patients with AA2/3 GVHD at the time of response.
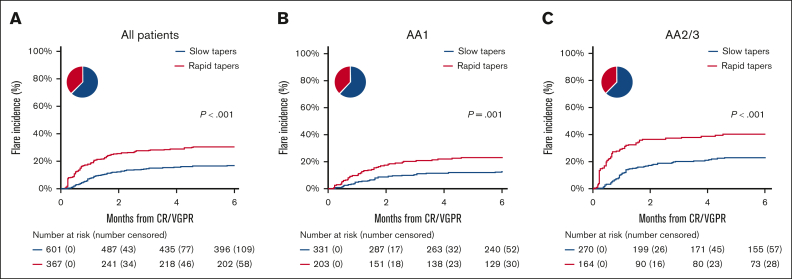
Figure 4.
Associations of steroid tapers on flares in all patients and patients with AA1 and AA2/3. (A) The cumulative incidence of flares was 16.5% (95% CI, 13.6-19.6) in slow tapers and 30.0% (95% CI, 25.3-34.8) in rapid tapers. (B) Only patients with AA1 at CR/VGPR. The cumulative incidence of flares was 11.9% (95% CI, 8.7-15.7) in slow tapers and 22.1% (95% CI, 16.6-28.1) in rapid tapers. (C) Only patients with AA2/3 at CR/VGPR. The cumulative incidence of flares was 22.3% (95% CI, 17.4-27.5) in slow tapers and 39.8% (95% CI, 32.1-37.4) in rapid tapers. Tapers were defined as rapid if the weekly steroid reduction rate ≥ 30% per week when the maximum steroid dose before CR/VGPR ≥1 mg/kg or ≥ 20% per week when the maximum steroid dose before CR/VGPR <1 mg/kg. The pie chart shows the percentage of patients whose steroid taper was slow (blue) and rapid (red).
Biomarkers at the onset of flare
Biomarker scores predict NRM at several time points other than GVHD onset,13,22,24 and we, therefore, assessed their predictive value at the time of flare. Serum samples were available in 98 of 210 patients (47%) at the time of flare. The difference in 6-month cumulative incidence of NRM was not statistically significant for patients with and without samples (22% vs 32%; P = .108). The 6-month incidence of NRM from the time of flare steadily increased with each increase in AA score (AA1, 6%; AA2, 19%; AA3, 42%; P = .001; supplemental Figure 3).
Discussion
Flares of acute GVHD are often observed during tapering or after discontinuation of immunosuppressive therapy, however their significance with respect to overall outcomes is not well understood. Although flares are recognized as important in clinical GVHD trials either as an end point (ie, duration of response)36,37 or as an eligibility criterion for steroid-refractory/dependent GVHD,9,38, 39, 40 there is no established definition. Relying on expert consensus in our consortium, in this study, we define a flare of acute GVHD as worsening of symptoms by at least 1 stage in at least 1 organ after an initial CR/VGPR that prompted an increase or restart of immunosuppressive treatment.
In this large multicenter study that used granular clinical data of weekly GVHD grading and treatments uniformly collected by the MAGIC, the incidence of flares was 22% at 6-months from initial treatment response. Flares were more severe than the initial episode of acute GVHD, mainly due to more LGI involvement, which resulted in a significantly higher risk of NRM. At the time of CR/VGPR, elevated MAP predicted the future occurrence of a flare and its severity. At flare onset, MAGIC biomarkers also successfully stratify patients for the risk of NRM similarly to their performance in de novo acute GVHD.23, 24, 25,41 These findings are consistent with prior studies showing that biomarkers can predict outcomes at specified time points after initiation of treatment (eg, day 28)13 and emphasize that the MAP reflects subclinical damage to the GI crypts when clinical symptoms are well controlled.
In addition to an increase in serum MAP scores, 3 clinical risk factors for GVHD flare were identified. One was the use of an HLA-mismatched unrelated donor, a well-known factor of severe acute GVHD.28 Given the lack of significant difference in posttransplant cyclophosphamide (PTCy) haploidentical donor HCT in our analysis, GVHD prophylaxis with PTCy might overcome the risk of flares in HLA-mismatched settings, but we could not perform subgroup analysis stratified by GVHD prophylaxis because of limited sample size of nonhaploidentical HCT with PTCy. The 2 other clinical risk factors, earlier achievement of clinical response and a faster steroid taper, may be surrogates for cumulative steroid exposure before flare. These results should be interpreted cautiously because we calculated the weekly steroid reduction rate from limited data over a short time period. Our estimate assumed that the rate of steroid tapering was constant in the first month after CR/VGPR, when, in fact, physicians often adjust the rate when the initial steroid dose is higher42 and continue to modify the rate as the dose of steroids decreases.3 Nevertheless, our findings suggest that inadequate exposure to corticosteroids might contribute for GVHD flare, a possibility supported by the observation that the risk of flares increases when a rapid taper is combined with greater subclinical disease (ie, high MAP score). Taken together, our data suggest that the risk of flares may be modifiable using biomarkers to stratify the steroid tapering schedule. We recently initiated a prospective trial to test aggressive steroid tapers in responding patients guided by serial monitoring of biomarker parameters (registered at www.ClinicalTrials.gov as #NCT05090384). Notably, other clinical characteristics including higher peak GVHD severity, higher peak daily dose of steroids, or the use of additional immunosuppressive agents in combination with steroids were not significantly associated with increased risk of GVHD flare.
Our study has several limitations. First, we did not empirically derive our definition of flare, and it is possible that other definitions of flare may associate more strongly with NRM. Second, the treatment of these patients with GVHD was at the physician’s discretion and reflected wide variation in practice. Patients who experienced a mild symptom increase (eg, skin stage 1 to 2) and who received an increase in immunosuppression were included, whereas patients with the same increase in GVHD symptoms who were not treated with increased immunosuppression were excluded because they did not meet the definition of flare. Third, patients who did not respond to primary treatment for GVHD within 28 days, who are considered poor prognostic populations,5,14,43, 44, 45 were also excluded because our data/sample collection protocol is focused on the first 28 days from the onset of GVHD, with very limited samples available beyond 28 days. Fourth, treatment decisions regarding steroid dose and duration may be influenced by factors other than GVHD such as the presence of minimal residual disease,46, 47, 48, 49 concurrent infections,50, 51, 52 and inflammatory conditions such as transplant-associated thrombotic microangiopathy53, 54, 55 that are not consistently included in the database and, therefore, could not be considered in these analyses. Fifth, treatment decisions varied among physicians and transplant centers and reflect real world clinical practice. In our cohort, treatment for grade 1 acute GVHD was common, and there was wide variation in initial steroid dose, as has been observed elsewhere.42,56,57 However, neither GVHD severity nor steroid dose before CR/VGPR correlated with the incidence of flares, underscoring the importance of careful management after clinical responses even in such cases. Finally, we were not able to model the kinetics of MAP over time in this study with limited data/samples available, although such dynamic and mathematical modeling may improve the prediction of GVHD flare in the future.
In conclusion, this study described incidence, risk factors, and outcomes of GVHD flare after initial clinical response. GVHD flares among patients who achieved CR/VGPR are common and result in higher NRM. This study emphasizes the significance of GVHD flares in clinical practice, and our consensus definition of flares enables future clinical studies to accurately report the incidence and impact of experimental treatments on flares. MAGIC biomarkers, which can detect residual acute GVHD activity and damage, help quantify the risk for flares and their severity and may guide future treatment strategies including steroid tapers.
Conflict-of-interest disclosure: S.A.G. reports receiving research and/or clinical trial support from Novartis, Servier, Vertex, Cellectis, and Tmunity/Kite, and being a member of study steering committees, consulting, or scientific advisory boards for Novartis, Allogene, Adaptimmune, Juno/Bristol Myers Squibb, CRISPR/Vertex, Jazz, Kyttaro, and Cabaletta. M.W. received consulting fees from Amgen (Munich, Germany) and speaker’s fees from Novartis (Nürnberg, Germany). J.E.L. reports research support from Equillium, Incyte, MaaT Pharma, and Mesoblast, and consulting fees from bluebird bio, Editas, Equillium, Inhibrx, Kamada, Mesoblast, Sanofi, and X4 Pharmaceuticals. J.E.L. and J.L.M.F. are coinventors on a GVHD biomarker patent. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank the patients, their families, medical staffs, and data managers in the Mount Sinai Acute GVHD International Consortium centers.
Y.A. is a recipient of the Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad. This work was supported by the National Institutes of Health, National Cancer Institute (grant PO1CA03942), the Pediatric Cancer Foundation, and the German Jose Carreras Leukemia Foundation (grants DJCLS 01 GVHD 2016 and DJCLS 01 GVHD 2020).
Authorship
Contribution: Y.A. designed the study, collected the clinical data, conducted the statistical analysis, and wrote the manuscript; N.S. collected the clinical data, advised statistical methods, and reviewed and revised the manuscript; M.H., P.A.-H., F.A., C.C., H.K.C., M.E., A.M.E., S.A.G., E.O.H., W.J.H., C.L.K., S. Kraus, M.M.A.M., P.M., M.Q., R.R., T.S., E.U., I.V., M.W., R.Z., and Z.D. collected the clinical data, and reviewed and revised the manuscript; J.B., G.E., S.G., N.K., and R.Y. collected and reviewed the clinical data; S. Kasikis, R.B., S. Kowalyk, and G.M. performed the laboratory analysis; J.E.L., J.L.M.F., and R.N. interpreted data, advised methods, reviewed and revised the manuscript, and organized this project; and all authors contributed to the writing of the report and approved the final version of the article.
Footnotes
J.L.M.F., J.E.L., and R.N. contributed equally to this work.
Data will be available upon reasonable request from the corresponding author, John E. Levine (john.levine@mssm.edu) or Yu Akahoshi (akahoshiu@gmail.com).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin PJ. How I treat steroid-refractory acute graft-versus-host disease. Blood. 2020;135(19):1630–1638. doi: 10.1182/blood.2019000960. [DOI] [PubMed] [Google Scholar]
- 3.Martin PJ, Rizzo JD, Wingard JR, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012;18(8):1150–1163. doi: 10.1016/j.bbmt.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8(7):387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 5.MacMillan ML, DeFor TE, Weisdorf DJ. The best endpoint for acute GVHD treatment trials. Blood. 2010;115(26):5412–5417. doi: 10.1182/blood-2009-12-258442. [DOI] [PubMed] [Google Scholar]
- 6.MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761–767. doi: 10.1016/j.bbmt.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeiser R, Socie G, Schroeder MA, et al. Efficacy and safety of itacitinib versus placebo in combination with corticosteroids for initial treatment of acute graft-versus-host disease (GRAVITAS-301): a randomised, multicentre, double-blind, phase 3 trial. Lancet Haematol. 2022;9(1):e14–e25. doi: 10.1016/S2352-3026(21)00367-7. [DOI] [PubMed] [Google Scholar]
- 8.Mohty M, Holler E, Jagasia M, et al. Refractory acute graft-versus-host disease: a new working definition beyond corticosteroid refractoriness. Blood. 2020;136(17):1903–1906. doi: 10.1182/blood.2020007336. [DOI] [PubMed] [Google Scholar]
- 9.Zeiser R, von Bubnoff N, Butler J, et al. Ruxolitinib for glucocorticoid-refractory acute graft-versus-host disease. N Engl J Med. 2020;382(19):1800–1810. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 10.Pepe MS, Feng Z, Janes H, Bossuyt PM, Potter JD. Pivotal evaluation of the accuracy of a biomarker used for classification or prediction: standards for study design. J Natl Cancer Inst. 2008;100(20):1432–1438. doi: 10.1093/jnci/djn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine JE, Hogan WJ, Harris AC, et al. Improved accuracy of acute graft-versus-host disease staging among multiple centers. Best Pract Res Clin Haematol. 2014;27(3-4):283–287. doi: 10.1016/j.beha.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris AC, Young R, Devine S, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016;22(1):4–10. doi: 10.1016/j.bbmt.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinagesh HK, Ozbek U, Kapoor U, et al. The MAGIC algorithm probability is a validated response biomarker of treatment of acute graft-versus-host disease. Blood Adv. 2019;3(23):4034–4042. doi: 10.1182/bloodadvances.2019000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin PJ, Bachier CR, Klingemann HG, et al. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a joint statement. Biol Blood Marrow Transplant. 2009;15(7):777–784. doi: 10.1016/j.bbmt.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorror ML, Storer B, Storb RF. Validation of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in single and multiple institutions: limitations and inferences. Biol Blood Marrow Transplant. 2009;15(6):757–758. doi: 10.1016/j.bbmt.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aziz MD, Shah J, Kapoor U, et al. Disease risk and GVHD biomarkers can stratify patients for risk of relapse and nonrelapse mortality post hematopoietic cell transplant. Leukemia. 2020;34(7):1898–1906. doi: 10.1038/s41375-020-0726-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Ramadan AM, Griesenauer B, et al. ST2 blockade reduces sST2-producing T cells while maintaining protective mST2-expressing T cells during graft-versus-host disease. Sci Transl Med. 2015;7(308) doi: 10.1126/scitranslmed.aab0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao D, Kim YH, Jeong S, et al. Survival signal REG3alpha prevents crypt apoptosis to control acute gastrointestinal graft-versus-host disease. J Clin Invest. 2018;128(11):4970–4979. doi: 10.1172/JCI99261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartwell MJ, Ozbek U, Holler E, et al. An early-biomarker algorithm predicts lethal graft-versus-host disease and survival. JCI Insight. 2018;3(16) doi: 10.1172/jci.insight.124015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Major-Monfried H, Renteria AS, Pawarode A, et al. MAGIC biomarkers predict long-term outcomes for steroid-resistant acute GVHD. Blood. 2018;131(25):2846–2855. doi: 10.1182/blood-2018-01-822957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Etra A, Gergoudis S, Morales G, et al. Assessment of systemic and gastrointestinal tissue damage biomarkers for GVHD risk stratification. Blood Adv. 2022;6(12):3707–3715. doi: 10.1182/bloodadvances.2022007296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akahoshi Y, Spyrou N, Hogan WJ, et al. Incidence, clinical presentation, risk factors, outcomes, and biomarkers in de novo late acute GVHD. Blood Adv. 2023;7(16):4479–4491. doi: 10.1182/bloodadvances.2023009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spyrou N, Akahoshi Y, Ayuk F, et al. The utility of biomarkers in acute GVHD prognostication. Blood Adv. 2023;7(17):5152–5155. doi: 10.1182/bloodadvances.2023009929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Malki MM, London K, Baez J, et al. Phase 2 study of natalizumab plus standard corticosteroid treatment for high-risk acute graft-versus-host disease. Blood Adv. 2023;7(17):5189–5198. doi: 10.1182/bloodadvances.2023009853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Austin PC, Latouche A, Fine JP. A review of the use of time-varying covariates in the Fine-Gray subdistribution hazard competing risk regression model. Stat Med. 2020;39(2):103–113. doi: 10.1002/sim.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117(11):3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorror ML, Martin PJ, Storb RF, et al. Pretransplant comorbidities predict severity of acute graft-versus-host disease and subsequent mortality. Blood. 2014;124(2):287–295. doi: 10.1182/blood-2014-01-550566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vatcheva KP, Lee M, McCormick JB, Rahbar MH. Multicollinearity in regression analyses conducted in rpidemiologic studies. Epidemiology (Sunnyvale) 2016;6(2) doi: 10.4172/2161-1165.1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36(5):1111–1118. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 32.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3(5):452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotta M, Satake A, Yoshimura H, et al. Elevation of early plasma biomarkers in patients with clinical risk factors predicts increased nonrelapse mortality after allogeneic hematopoietic stem cell transplantation. Transplant Cell Ther. 2021;27(8):660.e1–660.e8. doi: 10.1016/j.jtct.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 35.Pidala J, Hamadani M, Dawson P, et al. Randomized multicenter trial of sirolimus vs prednisone as initial therapy for standard-risk acute GVHD: the BMT CTN 1501 trial. Blood. 2020;135(2):97–107. doi: 10.1182/blood.2019003125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolanos-Meade J, Logan BR, Alousi AM, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124(22):3221–3227. doi: 10.1182/blood-2014-06-577023. quiz 3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etra A, Capellini A, Alousi A, et al. Effective treatment of low-risk acute GVHD with itacitinib monotherapy. Blood. 2023;141(5):481–489. doi: 10.1182/blood.2022017442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YB, Perales MA, Li S, et al. Phase 1 multicenter trial of brentuximab vedotin for steroid-refractory acute graft-versus-host disease. Blood. 2017;129(24):3256–3261. doi: 10.1182/blood-2017-03-772210. [DOI] [PubMed] [Google Scholar]
- 39.Magenau JM, Goldstein SC, Peltier D, et al. alpha(1)-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease. Blood. 2018;131(12):1372–1379. doi: 10.1182/blood-2017-11-815746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jagasia M, Perales MA, Schroeder MA, et al. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label phase 2 trial. Blood. 2020;135(20):1739–1749. doi: 10.1182/blood.2020004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine JE, Braun TM, Harris AC, et al. A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study. Lancet Haematol. 2015;2(1):e21–e29. doi: 10.1016/S2352-3026(14)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mielcarek M, Furlong T, Storer BE, et al. Effectiveness and safety of lower dose prednisone for initial treatment of acute graft-versus-host disease: a randomized controlled trial. Haematologica. 2015;100(6):842–848. doi: 10.3324/haematol.2014.118471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saliba RM, Couriel DR, Giralt S, et al. Prognostic value of response after upfront therapy for acute GVHD. Bone Marrow Transplant. 2012;47(1):125–131. doi: 10.1038/bmt.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine JE, Logan B, Wu J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16(12):1693–1699. doi: 10.1016/j.bbmt.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inamoto Y, Martin PJ, Storer BE, Mielcarek M, Storb RF, Carpenter PA. Response endpoints and failure-free survival after initial treatment for acute graft-versus-host disease. Haematologica. 2014;99(2):385–391. doi: 10.3324/haematol.2013.093062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim T, Moon JH, Ahn JS, et al. Next-generation sequencing-based posttransplant monitoring of acute myeloid leukemia identifies patients at high risk of relapse. Blood. 2018;132(15):1604–1613. doi: 10.1182/blood-2018-04-848028. [DOI] [PubMed] [Google Scholar]
- 47.Bader P, Salzmann-Manrique E, Balduzzi A, et al. More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv. 2019;3(21):3393–3405. doi: 10.1182/bloodadvances.2019000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spyridonidis A. How I treat measurable (minimal) residual disease in acute leukemia after allogeneic hematopoietic cell transplantation. Blood. 2020;135(19):1639–1649. doi: 10.1182/blood.2019003566. [DOI] [PubMed] [Google Scholar]
- 49.Akahoshi Y, Nishiwaki S, Mizuta S, et al. Tyrosine kinase inhibitor prophylaxis after transplant for Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer Sci. 2019;110(10):3255–3266. doi: 10.1111/cas.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akahoshi Y, Kimura SI, Inamoto Y, et al. Effect of cytomegalovirus reactivation with or without acute graft-versus-host disease on the risk of nonrelapse mortality. Clin Infect Dis. 2021;73(3):e620–e628. doi: 10.1093/cid/ciaa1871. [DOI] [PubMed] [Google Scholar]
- 51.Akahoshi Y, Kimura SI, Tada Y, et al. Cytomegalovirus gastroenteritis in patients with acute graft-versus-host disease. Blood Adv. 2022;6(2):574–584. doi: 10.1182/bloodadvances.2021005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akahoshi Y, Nakasone H, Takenaka K, et al. CMV reactivation after allogeneic HCT is associated with a reduced risk of relapse in acute lymphoblastic leukemia. Blood Adv. 2023;7(12):2699–2708. doi: 10.1182/bloodadvances.2022009376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dietrich S, Falk CS, Benner A, et al. Endothelial vulnerability and endothelial damage are associated with risk of graft-versus-host disease and response to steroid treatment. Biol Blood Marrow Transplant. 2013;19(1):22–27. doi: 10.1016/j.bbmt.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Young JA, Pallas CR, Knovich MA. Transplant-associated thrombotic microangiopathy: theoretical considerations and a practical approach to an unrefined diagnosis. Bone Marrow Transplant. 2021;56(8):1805–1817. doi: 10.1038/s41409-021-01283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Epperla N, Li A, Logan B, et al. Incidence, risk factors for and outcomes of transplant-associated thrombotic microangiopathy. Br J Haematol. 2020;189(6):1171–1181. doi: 10.1111/bjh.16457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reshef R, Saber W, Bolanos-Meade J, et al. Acute GVHD diagnosis and adjudication in a multicenter trial: a report from the BMT CTN 1202 Biorepository Study. J Clin Oncol. 2021;39(17):1878–1887. doi: 10.1200/JCO.20.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low-dose prednisone does not compromise patient outcomes. Blood. 2009;113(13):2888–2894. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.