Abstract
Epilepsy is a common and debilitating neurological disorder, and approximately one-third of affected individuals have ongoing seizures despite appropriate trials of two anti-seizure medications. This population with drug-resistant epilepsy (DRE) may benefit from neurostimulation approaches, such as vagus nerve stimulation (VNS), deep brain stimulation (DBS) and responsive neurostimulation (RNS). In some patient populations, these techniques are FDA-approved for treating DRE. VNS is used as adjuvant therapy for children and adults. Acting via the vagus afferent network, VNS modulates thalamocortical circuits, reducing seizures in approximately 50 % of patients. RNS uses an adaptive (closed-loop) system that records intracranial EEG patterns to activate the stimulation at the appropriate time, being particularly well-suited to treat seizures arising within eloquent cortex. For DBS, the most promising therapeutic targets are the anterior and centromedian nuclei of the thalamus, with anterior nucleus DBS being used for treating focal and secondarily generalized forms of DRE and centromedian nucleus DBS being applied for treating generalized epilepsies such as Lennox-Gastaut syndrome. Here, we discuss the indications, advantages and limitations of VNS, DBS and RNS in treating DRE and summarize the spatial distribution of neuroimaging observations related to epilepsy and stimulation using NeuroQuery and NeuroSynth.
Keywords: Epilepsy, Deep brain stimulation (DBS), Responsive neurostimulation, Vagus nerve stimulation (VNS), Neurostimulation, Neuroimaging meta analysis
Graphical abstract
Introduction
Seizures occur due to abnormal synchronous neuronal activity in the brain, causing transient clinical symptoms [1,2]. Epilepsy is diagnosed when two or more unprovoked or reflex seizures occur at least 24 h apart when a single unprovoked or reflex seizure occurs in a person with >60 % risk of having another seizure over the next ten years, or in individuals with a diagnosis of an epilepsy syndrome [3]. It is a common neurological disease with a global incidence of 7.6 per 1000 people, affecting approximately 50 million people worldwide [4]. Differences in the etiology of epilepsy are observed according to life stage, with children having genetic, perinatal insults, and malformations as the most common etiologies [5], and adults without a genetic predisposition, having brain injuries (e.g., trauma, tumours), infections and neuronal degeneration as the most common etiologies [6,7].
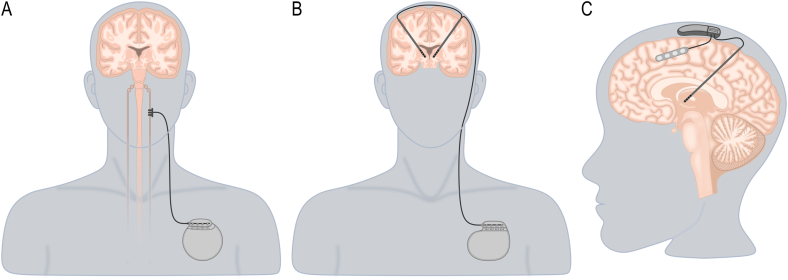
The treatment of epilepsy is mainly based on anti-seizure drugs, with about two-thirds of patients achieving seizure freedom 8. The remaining third of patients are considered to have drug-resistant epilepsy (DRE) and are eligible for alternative treatments, such as epilepsy surgery (i.e. surgical resection, disconnection, and neurostimulation) and non-pharmacological therapies (e.g. ketogenic diet) [[8], [9], [10]]. Neurostimulation is an option for patients with DRE who are not eligible for resective surgery, such as those with epileptic foci within eloquent cortex, or when no localization can be found on non-invasive multimodal assessment or invasive monitoring [8,11]. Vagus nerve stimulation (VNS, Fig. 1A), deep brain stimulation (DBS, Fig. 1B), and responsive neurostimulation (RNS, Fig. 1C) are the most widely utilized neurostimulation therapies and are based on open-loop or closed-loop stimulation paradigms that deliver stimulation when specific physiological parameters are detected [8,10,11]. Here, we provide an overview of the indications, advantages and limitations of VNS, DBS and RNS and summarize the spatial distribution of neuroimaging observations related to epilepsy and stimulation using two meta-analytic tools.
Fig. 1.
Illustration of the neurostimulation therapies used for treating drug-resistant epilepsy. A. The vagus nerve stimulation device comprises an electrode with three helical contacts connected to a subclavicular implantable pulse generator. B. The deep brain stimulation system comprises depth electrodes connected to a subclavicular implantable pulse generator. C. The responsive neurostimulation device comprises depth electrodes, cortical strips and a cranial implantable pulse generator.
Vagus nerve stimulation
VNS was first investigated in 1938 when Bailey and Bremner identified electrographic changes secondary to vagus nerve stimulation in cats [12]. In the years that followed, a multitude of studies delved deeper into the effects of VNS. This series of investigations reached a significant milestone in 1985 when Zabra et al. demonstrated that VNS could effectively terminate seizures in dogs [13]. These findings set the stage for the first human VNS implant, carried out by Penry and team in 1988 [14]. VNS was subsequently approved by the FDA in 1997 as an adjuvant therapy for adults with focal epilepsy [15]. Since then, VNS has been approved for use in children as young as four years old, and off label it has been used in children younger than one year of age with comparable outcomes [16]. In the modern context, it has emerged as a critical treatment option for children with DRE (Table 1).
Table 1.
Comparison of neurostimulation techniques.
| Vagus Nerve Stimulation | Deep Brain Stimulation | Responsive Neurostimulation | |
|---|---|---|---|
| Indication | Partial-onset seizures (Adults and children >4y, off-label in younger children) | Focal and secondary generalized seizures (ANT) or generalized epilepsy (CM) (Adults, off-label in children) | Focal epilepsy arising from 1 to 2 foci (Adults, off-label in children) |
| Advantages | Approved for pediatric use. MRI-compatible | Multiple targets allow for better patient selection. MRI-compatible | Can be applied to up to 2 eloquent foci. MRI-compatible |
| Limitations | Up to 50 % of patients remain refractory to treatment | Lack of significant pediatric data | Limited data on pediatric populations |
Abbreviations: ANT: Anterior nucleus of the thalamus; CM: Centromedial nucleus of the thalamus; MRI: Magnetic resonance imaging.
The VNS device is comprised of two components: the first is the electrode with three helical contacts, and the second is the implantable pulse generator (IPG; Fig. 1A). Two of the contacts on the electrode serve as a bipolar stimulation source, while the third contact functions as an anchor [17]. The implantation technique can be summarised as follows [18]. An incision is made on the neck to expose the left vagus nerve. A secondary incision is created in the left chest wall to house the IPG. The electrode is then routed between the two incisions, connecting the vagus nerve electrodes and IPG. The left vagus nerve is the typical site of implantation as the right vagus nerve controls the sinoatrial node, and stimulating it carries an elevated risk of causing bradycardia and additional cardiac side effects. However, there are instances where implantation on the left side is unfeasible (for instance, following an infection or neck irradiation), in which case, right-sided VNS has been carried out safely in pediatric patients [19].
The precise mechanisms of action for VNS remain incompletely clarified; however, it is believed that a complex network of communication between the brainstem and cerebral structures, referred to as the vagus afferent network, facilitates VNS's therapeutic effects [20]. Comprised of nearly 80 % afferent fibres, the vagus nerve first projects to the nucleus tractus solitarius in the brainstem. These fibres subsequently project to multiple brainstem centres, including the locus coeruleus, dorsal raphe nucleus, and parabrachial nucleus. Higher-order fibres project diffusely to cortical regions such as the thalamus, somatosensory cortex, anterior cingulate, and prefrontal cortex [20]. Through these pathways, VNS leads to the desynchronization of epileptiform activity [21], especially in the gamma band [22], and is thought to result in the modulation of thalamocortical circuits [23]. Support for this theory comes from previous works that have identified increased thalamic activation and higher preoperative thalamocortical connectivity as factors associated with better response to therapy [24,25].
While the efficacy of VNS has been studied extensively, most studies have focused on patients older than 12 years of age. E03 and E05 are two of the largest multicenter, randomized studies on VNS outcomes [26,27]. Both demonstrate the efficacy of high-intensity VNS in reducing seizure frequency compared to low-intensity stimulation. The E03 study randomly assigned 114 patients to high-intensity or low-intensity stimulation for 14 weeks. Those in the high-stimulation group experienced an average seizure reduction of 24.5 %, compared to 6.1 % in the low-stimulation group. Moreover, 31 % of high-stimulation patients saw their seizures reduced by more than 50 %, whereas only 14 % in the low-stimulation group achieved this. The subsequent E05 study split 196 patients into similar groups and assessed outcomes after three months. The results showed that the high-stimulation group experienced significantly greater seizure reductions than the low-stimulation group (28 % vs. 15 %). A 2011 meta-analysis by Englot and colleagues synthesized the results for 3321 patients across 74 clinical studies [28] and found that the fraction of patients achieving over 50 % response varies from 21 to 57 %. In 2021, a similar meta-analysis by Jain et al. examined VNS outcomes in pediatric patients from 99 articles and 3474 patients [29]. As in adults, the authors found that 56.4 % of patients achieved a seizure reduction greater than or equal to 50 %. Additionally, it has been shown that patients treated with VNS show increased quality of life at long-term follow-up [[30], [31], [32]]. However, it is uncertain if dichotomizing treatment outcomes (i.e., 50 % seizure reduction representing the patient to be a responder) is optimal and able to capture other domains of improvement that could be present independent of seizure outcome (e.g., quality of life, effects on mood, cognitive performance), and further studies are needed to address the question if focusing on patient-centred measures, such as quality of life and seizure severity, may be more relevant than absolute seizure reduction [33].
Nevertheless, the positive findings to date underscore the effectiveness of VNS, demonstrating that over half of the patients experienced a substantial reduction in seizure frequency, affirming the potential of this therapy in managing seizures in adults and children. Nevertheless, considering that half of the patients will fail to present satisfactory responses to VNS treatment, there is a growing need to identify biomarkers of treatment success that could be used to inform clinical practice. Patient characteristics such as young age and shorter duration of epilepsy prior to VNS have been suggested as factors associated with improved outcomes to VNS treatment [34,35]. Similarly, seizure characteristics, such as temporal region seizure onset, generalized epilepsy and tuberous sclerosis, are associated with greater response to VNS [28,36,37]. Connectomics studies have identified structural and functional connectivity patterns within the vagus afferent network that are associated with better outcomes to VNS treatment [20,25,38]. Electrical recordings, specifically the P300 component of scalp-recorded event-related potentials, have been shown to be distinct in patients who are considered to be responders to VNS and those deemed non-responders to treatment [39].
VNS serves as a viable palliative treatment choice for pediatric patients with DRE who either haven't responded to or aren't suitable for resective surgery. Meta-analyses of thousands of patients, both adults and children, have shown considerable advantages, with half of the patients undergoing VNS observing significant benefits following treatment [28,29]. However, the risks associated with VNS implants should be considered, as complications such as infection, hematoma, vocal cord paralysis, weakness in the lower face, and pain have been reported in these patients [40]. Hence, more research is necessary to improve treatment outcomes, which could be accomplished by determining optimal programming strategies and identifying biomarkers that can predict response to treatment [41].
Deep brain stimulation
DBS, primarily employed for the management of movement disorders such as Parkinson's disease, is being used as a treatment for patients with DRE [42] (Fig. 1B). Although several targets have been reported (i.e., subthalamic nucleus, pulvinar nucleus) with positive outcomes [43], the most investigated and promising DBS targets for the treatment of DRE include the anterior (ANT) and centromedian (CM) nuclei of the thalamus (Table 1) [44].
The ANT is a nuclear complex located in the anterior region of the thalamus [44,45] with extensive connections to the hippocampal formation, cingulate cortices and inferior parietal lobule, and has been considered to be a part of the ‘extended hippocampal formation’ playing a pivotal role in limbic and temporal lobe epilepsies [44,46]. ANT-DBS has demonstrated effectiveness in managing focal and secondarily generalized forms of DRE [47]. The most substantial evidence supporting its efficacy results from the Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy (SANTE) trial, a large, double-blind, randomized control trial, where a majority of adult patients with DRE experienced >50 % seizure reduction following ANT-DBS [48,49]. Several clinical studies have also reported encouraging, albeit variable, responses following ANT-DBS, with seizure reductions ranging from 11.5 % to 76 % [47]. This variability in outcome could be in parts associated with the stimulation settings chosen, as ANT-DBS has been shown to be more effective when using high-frequency stimulation between 90 and 200Hz with 90–160μs pulse width and 1.5–10V intensity. Although the current understanding of the mechanisms underlying ANT-DBS is limited, it is thought that the modulation of limbic and thalamocortical networks and reduced functional connectivity between the ANT and hippocampus are associated with a positive response to treatment [[50], [51], [52]]. ANT-DBS has also been reported to exert a neuroprotective effect by decreasing neuronal cell loss, inhibiting local immune response, inducing molecular changes in hippocampal neurons, and modulating neuronal glucose metabolism [47].
The CM is located within the caudal intralaminar group of thalamic nuclei with a widespread connectivity pattern, including projections to the striatum, thalamic nuclei, reticular formation, and somatomotor cortices, being considered prototypical of the cortical-thalamic-cortical system [44,53]. CM-DBS is particularly effective in treating generalized epilepsies and patients with Lennox-Gastaut syndrome (LGS) and is used for treating primary generalized DRE [44]. The treatment potential of CM-DBS should motivate studies in generalized seizures and developmental epileptic encephalopathies of childhood. The DBS of the Thalamic Centromedian Nucleus for Lennox-Gastaut Syndrome (ESTEL) trial demonstrated a significant reduction of electrographic seizures and a 50 % reduction of diary-reported seizures in half of the participants [54]. Several additional clinical studies and small-scale RCTs support the efficacy of CM-DBS in generalized forms of DRE, including patients exhibiting electroclinical features of LGS [[55], [56], [57]]. CM-DBS has been reported to be effective at both medium and high frequencies (60–145Hz) with 90–450μs pulse width and 1–10V intensity [44,54,57,58].
Similarly to ANT-DBS, the mechanisms underlying CM-DBS are not well understood. It has been postulated that CM-DBS produces anti-seizure effects by desynchronizing thalamocortical circuits and modulating reticular formation and somatomotor cortices [59,60]. These findings might explain the efficacy of CM-DBS in treating LGS patients, as these brain regions are associated with the spread of electroclinical features of LGS [61]. In LGS specifically, distinct brain areas are active during generalized paroxysmal fast activity (GPFA) [62]; connectivity of the volume of activated tissue by CM-DBS to GPFA-related regions, such as the prefrontal and premotor cortices, putamen, and pontine brainstem, is positively associated with seizure reduction following treatment [63].
While these studies highlight the potential of using thalamic DBS for treating DRE in adults, pediatric studies remain limited. A recent systematic review of DBS in children with DRE provided evidence to support thalamic DBS in pediatric populations. This study identified 52 children who received ANT or CM-DBS and found that >50 % seizure frequency reduction was observed in 9/14 children following ANT-DBS and 31/38 children following CM-DBS [64]. DBS electrodes were well-tolerated by participants, with infection being the only complication occurring in 10 % of patients [58,[65], [66], [67], [68], [69], [70], [71]]. Overall, thalamic DBS is a safe and well-tolerated treatment for DRE in patients ineligible for surgical resection, with ANT-DBS being considered to be effective in treating focal and secondarily generalized DRE, and CM-DBS being mainly used for treating primary generalized DRE and patients with LGS. However, the great variability in treatment response following thalamic DBS indicates the need for further clinical and preclinical studies to understand the neurobiological mechanism of treatment success and determine new biomarkers for treatment selection and predictors of response to treatment [47,72].
Responsive neurostimulation
RNS is a neuromodulatory treatment approved by the FDA for treating DRE [73]. The capacity for adaptive (closed-loop) neurostimulation is central to RNS, and this feature distinguishes it from existing continuous stimulation (open-loop) paradigms, including DBS and VNS (Fig. 1) [73]. The adaptive capabilities of RNS are particularly well-suited to seizures arising within eloquent cortex, whereby stimulation that might otherwise disrupt normal function activates solely during seizures [73,74]. In potential candidates for RNS, up to two seizure foci can be identified using intracranial EEG for subsequent simultaneous recording and stimulation by the implanted device (Table 1) [73]. The primary mechanism of action of RNS is through the desynchronization of epileptiform activity at its source, which is required for the propagation and continuation of ictal activity [74,75]. This is thought to be achieved acutely via suppression of intracellular activity due to membrane hyperpolarization and/or axonal conduction blockade and chronically via changes in gene expression that lead to synaptic plasticity, neurogenesis and cortical reorganization [[76], [77], [78], [79]].
The NeuroPace RNS System is the only closed-loop device with FDA approval for use in adult patients (18 and older) with focal epilepsy who failed to achieve seizure freedom after treatment with at least two anti-seizure medications [80,81]. As a relatively newer technology, few studies have evaluated the efficacy of RNS compared to other neuromodulatory treatments for DRE, particularly VNS and DBS. In adult patients with DRE, Morell et al. provided Class I evidence for the efficacy of RNS [73]. In this study, the RNS device was programmed to record abnormal electrocorticographic activity for one month following implantation. Individuals were subsequently randomized to either responsive or sham stimulation and studied for a 12-week blinded period followed by an 84-week open-label period. At the conclusion of the blinded period, the stimulation group experienced a significantly greater reduction in seizures (37.9 % vs 17.3 %), and this effect persisted throughout the open-label period. Seizures in the sham group significantly improved once stimulation was activated following the blinded period. The authors followed the patients for a total of two years and demonstrated a progressive and significant improvement over time, with a mean seizure reduction of 44 % at one year and 53 % at two years [73].
Pediatric data regarding RNS efficacy are more limited. Panov et al. followed 27 children after RNS implantation 82. Twenty-two of the 27 patients had seizure outcome data at one year, and 15 out of 22 children (68.2 %) achieved a greater than 50 % reduction in seizure activity, while 11 of the 15 achieved a greater than 75 % reduction. RNS has also been trialled targeting the CM in pediatric and adult populations with LGS and generalized seizures, showing reduced seizure frequency, duration and severity, improved post-ictal state and increased quality of life at long-term follow-up [80,81,83]. Currently, an FDA-approved trial of RNS (RNS System Responsive Thalamic Stimulation for Primary Generalized Seizures - NAUTILUS) is being conducted to investigate RNS efficacy in treating idiopathic generalized epilepsy, where patients experience primary generalized tonic-clonic seizures, with or without myoclonic or absence seizures, in individuals 12 years and older who failed treatment with a minimum of two antiseizure medications [35,84].
Although these data are potentially promising, systematic reviews and meta-analysis studies have shown comparable long-term results between the three techniques, with approximately 50 % seizure reduction in about 50 % of patients [35,37,74]. Additionally, it has been shown that stimulation during brain states with less epileptiform activity is associated with better outcomes, possibly via a shift in the inhibitory-excitatory balance that produces slower, long-lasting changes in brain dynamics [85]. These findings suggest the difference between DBS and RNS to be less meaningful than previously hypothesized. Thus, further studies regarding the neurobiological mechanisms of RNS, its applications in children, and a comparative analysis between its efficacy and that of other neuromodulatory treatments for DRE are needed.
Ethical concerns
Ethical concerns related to vulnerable pediatric populations and the risk of procedural complications must be addressed in high-quality randomized controlled trials to determine the benefits and long-term efficacy of neuromodulation therapies for pediatric patients [86]. It is crucial to carefully assess and communicate the potential risks and benefits of therapy, determine the optimal timing for offering the treatment, and consider the long-term consequences of brain stimulation for this population [87]. In addition to obtaining informed consent from the parent/guardian, it is also essential to discuss the role of informed assent given by the child [87]. A framework for advancing ethical discussions regarding pediatric DBS has been proposed, which prioritizes the protection of the child's best interests, considers the developmental context, implements strategies for mitigating known and unknown risks, critically appraises adult literature, and fosters communication and collaboration among practitioners [86]. In addition, there are concerns related to the capability of specific devices to detect neuronal activity and the secure storage of this data [88]. With the advancement of neurostimulation devices and therapies, new policies must be developed to maximize benefits and minimize patient harm.
Meta-analytic maps of neuroimaging findings
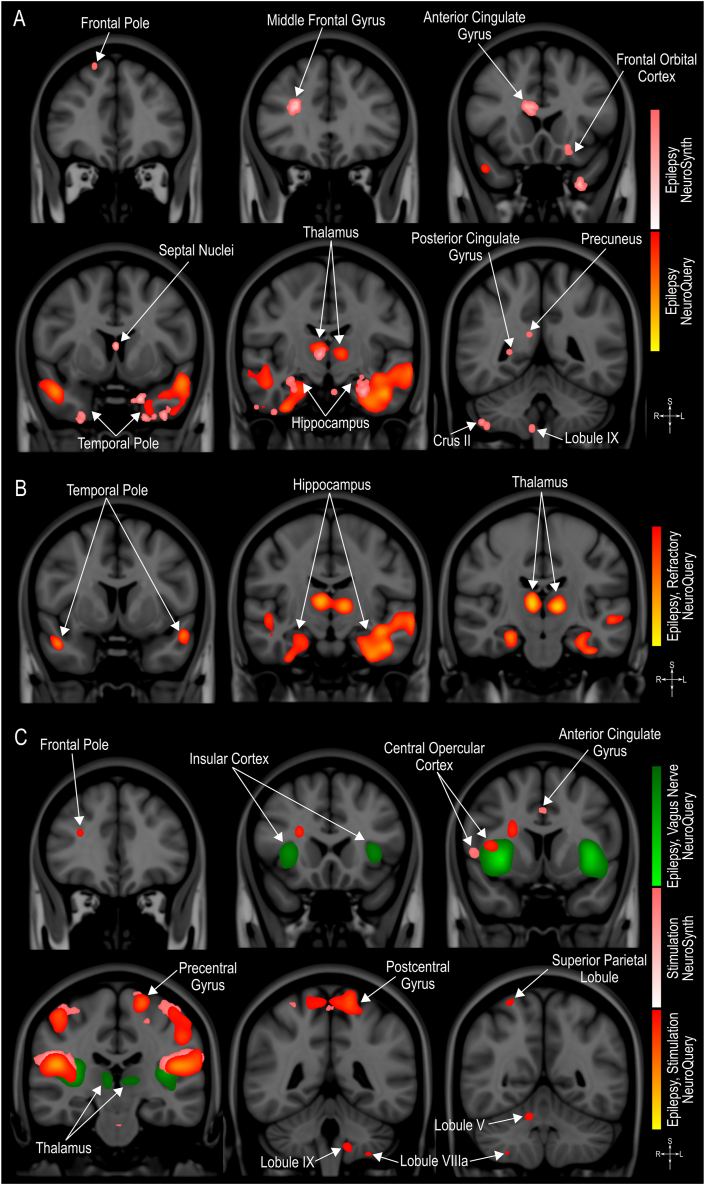
Long-term neuromodulation of seizure network activity is thought to induce beneficial brain plasticity that leads to a significant reduction in seizure frequency [11]. Indeed, several studies have reported on neuroimaging-detected morphological and functional brain changes in patients with epilepsy and those undergoing neurostimulation treatments [[89], [90], [91]]. However, the imaging findings vary greatly based on the etiology of epilepsy, patients' characteristics and the neuroimaging technique employed [[89], [90], [91]]. To summarize the spatial distribution of neuroimaging observations related to epilepsy and stimulation (e.g., MRI, fMRI), we used the NeuroQuery [92] and NeuroSynth [93] meta-analytic tools. These tools use text-mining and machine-learning techniques to automatically conduct large-scale, high-quality meta-analyses that will either predict the anatomical locations associated with the terms (i.e. NeuroQuery) or test, on a voxel-level statistical analysis, the consistency of the observations reported in the literature (i.e. NeuroSynth) [92,93]. The detailed methods, codes, validation, and quality control procedures used by these tools have previously been published [92,93], and the tools can be accessed at https://neuroquery.org/ and https://neurosynth.org/. Here, we generated maps using the terms epilepsy, stimulation, refractory and vagus nerve (Fig. 2).
Fig. 2.
Meta-analytic maps of neuroimaging findings. A. Maps generated using the term “Epilepsy”. B. Map generated using the terms “Epilepsy” and “Refractory”. C. Maps generated using the terms “Epilepsy”, “Stimulation” and “Vagus Nerve”. Maps were created on NeuroQuery [92,94] and NeuroSynth [93,95] and are shown on the MNI152 brain on the coronal plane [96].
When using the term epilepsy, we observed an overlap between maps generated with NeuroQuery and NeuroSynth in the temporal poles, thalamus and hippocampus (Fig. 2A), indicating that changes in these regions are involved in epilepsy and have a high potential to be observed in future studies. These areas are also the only ones detected when associating the term epilepsy with refractory (Fig. 2B). Other brain areas associated with epilepsy were also highlighted in these maps, such as frontal and cerebellar areas, septal nuclei, cingulate cortex and precuneus. Maps generated with the terms epilepsy and stimulation show a large overlap in the insular cortex, central opercular cortex and precentral and postcentral gyri (Fig. 2C). The insular cortex and the central opercular cortex also overlapped with the map generated with the terms epilepsy and vagus nerve. Additionally, frontal and cerebellar areas, thalamus and cingulate gyrus are also shown in these maps, and although it is not restricted to a single type of neurostimulation technique, it indicates a previous association and/or high potential to be detected in future studies and could help develop new hypothesis and guide research.
Epilepsy is a debilitating neurological disorder, with approximately one-third of patients failing to attain seizure freedom despite appropriate trials of anti-seizure medications. Patients with DRE who are not eligible for resective surgery can benefit from a long-term reduction in seizure frequency following neurostimulation approaches. VNS is the most widely available technology and is the only neurostimulation with FDA approval for use in children, thus being a standard initial choice for treating pediatric epilepsy. DBS and RNS present encouraging results in adults but require high levels of expertise for the implantation and programming of these devices, rendering their use only to select clinical centres. Nevertheless, considering that approximately 50 % of patients with VNS will remain refractory to treatment, DBS and RNS are promising additional therapeutic options, although outcomes to date appear similar with these more invasive techniques, and closed-loop stimulation has thus far proven to be no more effective than open-loop stimulation. Several studies have shown brain changes in patients with epilepsy and those being treated with neurostimulation; however, no biomarkers are available for predicting treatment response or selecting patients who are most likely to respond to therapy and, therefore, for whom these therapies should be offered. Further studies are needed to provide high-quality clinical evidence supporting the clinical utility of these technologies in both pediatric and adult populations and develop personalized therapies.
Authors' contributions
Flavia Venetucci Gouveia: Acquisition, analysis and interpretation of data, drafting and revising the manuscript; Nebras M. Warsi: Drafting and revising the manuscript;. Hrishikesh Suresh: Drafting and revising the manuscript; Rafi Matin: Drafting and revising the manuscript; George M. Ibrahim: Critically revising the manuscript and providing intellectual content. All authors approved the final version to be published
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by funds from Canadian Institutes of Health Research (CIHR) Postdoctoral Fellowship #472484 (FVG), The Rising Star - Shireen and Edna Marcus Excellence Award has been made possible by Brain Canada Foundation and the Shireen and Edna Marcus Foundation (FVG).
References
- 1.Fisher R.S., van Emde Boas W., Blume W., Elger C., Genton P., Lee P., et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005 Apr;46(4):470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.Falco-Walter J. Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology. Semin Neurol. 2020 Dec;40(6):617–623. doi: 10.1055/s-0040-1718719. [DOI] [PubMed] [Google Scholar]
- 3.Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014 Apr;55(4):475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 4.Epilepsy: a public health imperative. World Health Organization; 2019. https://www.who.int/publications/i/item/epilepsy-a-public-health-imperative Available from. [Google Scholar]
- 5.Aaberg K.M., Surén P., Søraas C.L., Bakken I.J., Lossius M.I., Stoltenberg C., et al. Seizures, syndromes, and etiologies in childhood epilepsy: The International League Against Epilepsy 1981, 1989, and 2017 classifications used in a population-based cohort. Epilepsia. 2017 Nov;58(11):1880–1891. doi: 10.1111/epi.13913. [DOI] [PubMed] [Google Scholar]
- 6.Bosak M., Słowik A., Kacorzyk R., Turaj W. Implementation of the new ILAE classification of epilepsies into clinical practice - A cohort study. Epilepsy Behav. 2019 Jul;96:28–32. doi: 10.1016/j.yebeh.2019.03.045. [DOI] [PubMed] [Google Scholar]
- 7.Liu S., Yu W., Lü Y. The causes of new-onset epilepsy and seizures in the elderly. Neuropsychiatr Dis Treat. 2016 Jun 17;12:1425–1434. doi: 10.2147/NDT.S107905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perucca P., Scheffer I.E., Kiley M. The management of epilepsy in children and adults. Med J Aust. 2018 Mar 19;208(5):226–233. doi: 10.5694/mja17.00951. [DOI] [PubMed] [Google Scholar]
- 9.Kwan P., Arzimanoglou A., Berg A.T., Brodie M.J., Allen Hauser W., Mathern G., et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010 Jun;51(6):1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Giraldo E., Sullivan J.E. Advances in the Treatment of Drug-Resistant Pediatric Epilepsy. Semin Neurol. 2020 Mar 17;40(2):257–262. doi: 10.1055/s-0040-1702941. [DOI] [PubMed] [Google Scholar]
- 11.Ryvlin P., Rheims S., Hirsch L.J., Sokolov A., Jehi L. Neuromodulation in epilepsy: state-of-the-art approved therapies. Lancet Neurol. 2021 Oct 25;20(12):1038–1047. doi: 10.1016/S1474-4422(21)00300-8. [DOI] [PubMed] [Google Scholar]
- 12.Bailey P., Bremer F. A sensory cortical representation of the vagus nerve: With a note on the effects of low blood pressure on the cortical electrogram. J Neurophysiol. 1938 Sep 1;1(5):405–412. [Google Scholar]
- 13.Zabara J. Peripheral control of hypersynchronous discharge in epilepsy. Electroencephalogr Clin Neurophysiol. 1985 Sep;61(3):S162. [Google Scholar]
- 14.Penry J.K., Dean J.C. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31(Suppl 2):S40–S43. doi: 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogbonnaya S., Kaliaperumal C. Vagal nerve stimulator: Evolving trends. J Nat Sci Biol Med. 2013 Jan;4(1):8–13. doi: 10.4103/0976-9668.107254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muthiah N., Joseph B., Varga G., Vodovotz L., Sharma N., Abel T.J. Investigation of the effectiveness of vagus nerve stimulation for pediatric drug-resistant epilepsies secondary to nonaccidental trauma. Childs Nerv Syst. 2023 May;39(5):1201–1206. doi: 10.1007/s00381-022-05817-9. [DOI] [PubMed] [Google Scholar]
- 17.Terry R.S., Tarver W.B., Zabara J. The implantable neurocybernetic prosthesis system. Pacing Clin Electrophysiol. 1991 Jan;14(1):86–93. doi: 10.1111/j.1540-8159.1991.tb04052.x. [DOI] [PubMed] [Google Scholar]
- 18.Reid S.A. Surgical technique for implantation of the neurocybernetic prosthesis. Epilepsia. 1990;31(Suppl 2) doi: 10.1111/j.1528-1157.1990.tb05847.x. S38-9. [DOI] [PubMed] [Google Scholar]
- 19.McGregor A., Wheless J., Baumgartner J., Bettis D. Right-sided vagus nerve stimulation as a treatment for refractory epilepsy in humans. Epilepsia. 2005 Jan;46(1):91–96. doi: 10.1111/j.0013-9580.2005.16404.x. [DOI] [PubMed] [Google Scholar]
- 20.Hachem L.D., Wong S.M., Ibrahim G.M. The vagus afferent network: emerging role in translational connectomics. Neurosurg Focus. 2018 Sep;45(3):E2. doi: 10.3171/2018.6.FOCUS18216. [DOI] [PubMed] [Google Scholar]
- 21.Jaseja H. EEG-desynchronization as the major mechanism of anti-epileptic action of vagal nerve stimulation in patients with intractable seizures: clinical neurophysiological evidence. Med Hypotheses. 2010 May;74(5):855–856. doi: 10.1016/j.mehy.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Fraschini M., Puligheddu M., Demuru M., Polizzi L., Maleci A., Tamburini G., et al. VNS induced desynchronization in gamma bands correlates with positive clinical outcome in temporal lobe pharmacoresistant epilepsy. Neurosci Lett. 2013 Mar 1;536:14–18. doi: 10.1016/j.neulet.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 23.Warsi N.M., Yan H., Wong S.M., Yau I., Breitbart S., Go C., et al. Vagus Nerve Stimulation Modulates Phase-Amplitude Coupling in Thalamic Local Field Potentials. Neuromodulation. 2023 Apr;26(3):601–606. doi: 10.1016/j.neurom.2022.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Liu W.C., Mosier K., Kalnin A.J., Marks D. BOLD fMRI activation induced by vagus nerve stimulation in seizure patients. J Neurol Neurosurg Psychiatry. 2003 Jun;74(6):811–813. doi: 10.1136/jnnp.74.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibrahim G.M., Sharma P., Hyslop A., Guillen M.R., Morgan B.R., Wong S., et al. Presurgical thalamocortical connectivity is associated with response to vagus nerve stimulation in children with intractable epilepsy. Neuroimage Clin. 2017 Sep 22;16:634–642. doi: 10.1016/j.nicl.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handforth A., DeGiorgio C.M., Schachter S.C., Uthman B.M., Naritoku D.K., Tecoma E.S., et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998 Jul;51(1):48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 27.A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995 Feb;45(2):224–230. doi: 10.1212/wnl.45.2.224. [DOI] [PubMed] [Google Scholar]
- 28.Englot D.J., Chang E.F., Auguste K.I. Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg. 2011 Dec;115(6):1248–1255. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 29.Jain P., Arya R. Vagus Nerve Stimulation and Seizure Outcomes in Pediatric Refractory Epilepsy: Systematic Review and Meta-Analysis. Neurology. 2021 Jun;96:22. doi: 10.1212/WNL.0000000000012030. [DOI] [PubMed] [Google Scholar]
- 30.Englot D.J., Hassnain K.H., Rolston J.D., Harward S.C., Sinha S.R., Haglund M.M. Quality-of-life metrics with vagus nerve stimulation for epilepsy from provider survey data. Epilepsy Behav. 2017 Jan;66:4–9. doi: 10.1016/j.yebeh.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryvlin P., Gilliam F.G., Nguyen D.K., Colicchio G., Iudice A., Tinuper P., et al. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: the PuLsE (Open Prospective Randomized Long-term Effectiveness) trial. Epilepsia. 2014 Jun;55(6):893–900. doi: 10.1111/epi.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markert M.S., Fisher R.S. Neuromodulation - Science and Practice in Epilepsy: Vagus Nerve Stimulation, Thalamic Deep Brain Stimulation, and Responsive NeuroStimulation. Expert Rev Neurother. 2018 Dec 11;19(1):17–29. doi: 10.1080/14737175.2019.1554433. [DOI] [PubMed] [Google Scholar]
- 33.Piper R.J., Ibrahim G.M., Tisdall M.M. Deep Brain Stimulation for Children with Generalized Epilepsy. Neurosurg Clin N Am. 2024 Jan;35(1):17–25. doi: 10.1016/j.nec.2023.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Colicchio G., Policicchio D., Barbati G., Cesaroni E., Fuggetta F., Meglio M., et al. Vagal nerve stimulation for drug-resistant epilepsies in different age, aetiology and duration. Childs Nerv Syst. 2010 Jun;26(6):811–819. doi: 10.1007/s00381-009-1069-2. [DOI] [PubMed] [Google Scholar]
- 35.Haneef Z., Skrehot H.C. Neurostimulation in generalized epilepsy: A systematic review and meta-analysis. Epilepsia. 2023 Apr;64(4):811–820. doi: 10.1111/epi.17524. [DOI] [PubMed] [Google Scholar]
- 36.Casazza M., Avanzini G., Ferroli P., Villani F., Broggi G. Vagal nerve stimulation: relationship between outcome and electroclinical seizure pattern. Seizure. 2006 Apr;15(3):198–207. doi: 10.1016/j.seizure.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Skrehot H.C., Englot D.J., Haneef Z. Neuro-stimulation in focal epilepsy: A systematic review and meta-analysis. Epilepsy Behav. 2023 May;142 doi: 10.1016/j.yebeh.2023.109182. [DOI] [PubMed] [Google Scholar]
- 38.Suresh H., Mithani K., Brar K., Yan H., Strantzas S., Vandenberk M., et al. Brainstem Associated Somatosensory Evoked Potentials and Response to Vagus Nerve Stimulation: An Investigation of the Vagus Afferent Network. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.768539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Taeye L., Vonck K., van Bochove M., Boon P., Van Roost D., Mollet L., et al. The P3 event-related potential is a biomarker for the efficacy of vagus nerve stimulation in patients with epilepsy. Neurotherapeutics. 2014 Jul;11(3):612–622. doi: 10.1007/s13311-014-0272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Révész D., Rydenhag B., Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr. 2016 Jul;18(1):97–104. doi: 10.3171/2016.1.PEDS15534. [DOI] [PubMed] [Google Scholar]
- 41.Workewych A.M., Arski O.N., Mithani K., Ibrahim G.M. Biomarkers of seizure response to vagus nerve stimulation: A scoping review. Epilepsia. 2020 Oct;61(10):2069–2085. doi: 10.1111/epi.16661. [DOI] [PubMed] [Google Scholar]
- 42.Frey J., Cagle J., Johnson K.A., Wong J.K., Hilliard J.D., Butson C.R., et al. Past, Present, and Future of Deep Brain Stimulation: Hardware, Software, Imaging, Physiology and Novel Approaches. Front Neurol. 2022 Mar 9;13 doi: 10.3389/fneur.2022.825178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan H., Wang X., Zhang X., Qiao L., Gao R., Ni D., et al. Deep brain stimulation for patients with refractory epilepsy: nuclei selection and surgical outcome. Front Neurol. 2023 May 12;14 doi: 10.3389/fneur.2023.1169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warsi N.M., Yan H., Suresh H., Wong S.M., Arski O.N., Gorodetsky C., et al. The anterior and centromedian thalamus: Anatomy, function, and dysfunction in epilepsy. Epilepsy Res. 2022 May;182 doi: 10.1016/j.eplepsyres.2022.106913. [DOI] [PubMed] [Google Scholar]
- 45.Grodd W., Kumar V.J., Schüz A., Lindig T., Scheffler K. The anterior and medial thalamic nuclei and the human limbic system: tracing the structural connectivity using diffusion-weighted imaging. Sci Rep. 2020 Jul 2;10(1) doi: 10.1038/s41598-020-67770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aggleton J.P., O’Mara S.M., Vann S.D., Wright N.F., Tsanov M., Erichsen J.T. Hippocampal-anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 2010 Jun;31(12):2292–2307. doi: 10.1111/j.1460-9568.2010.07251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bouwens van der Vlis T.A.M., Schijns O.E.M.G., Schaper F.L.W.V.J., Hoogland G., Kubben P., Wagner L., et al. Deep brain stimulation of the anterior nucleus of the thalamus for drug-resistant epilepsy. Neurosurg Rev. 2018 Jan 6;42(2):287–296. doi: 10.1007/s10143-017-0941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salanova V., Witt T., Worth R., Henry T.R., Gross R.E., Nazzaro J.M., et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015 Feb 6;84(10):1017–1025. doi: 10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salanova V., Sperling M.R., Gross R.E., Irwin C.P., Vollhaber J.A., Giftakis J.E., et al. The SANTÉ study at 10 years of follow-up: Effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 2021 Apr 8;62(6):1306–1317. doi: 10.1111/epi.16895. [DOI] [PubMed] [Google Scholar]
- 50.Laxpati N.G., Kasoff W.S., Gross R.E. Deep brain stimulation for the treatment of epilepsy: circuits, targets, and trials. Neurotherapeutics. 2014 Jul;11(3):508–526. doi: 10.1007/s13311-014-0279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Middlebrooks E.H., Grewal S.S., Stead M., Lundstrom B.N., Worrell G.A., Van Gompel J.J. Differences in functional connectivity profiles as a predictor of response to anterior thalamic nucleus deep brain stimulation for epilepsy: a hypothesis for the mechanism of action and a potential biomarker for outcomes. Neurosurg Focus. 2018 Aug;45(2):E7. doi: 10.3171/2018.5.FOCUS18151. [DOI] [PubMed] [Google Scholar]
- 52.Schaper F.L.W.V.J., Plantinga B.R., Colon A.J., Wagner G.L., Boon P., Blom N., et al. Deep Brain Stimulation in Epilepsy: A Role for Modulation of the Mammillothalamic Tract in Seizure Control? Neurosurgery. 2020 Sep 1;87(3):602–610. doi: 10.1093/neuros/nyaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ilyas A., Pizarro D., Romeo A.K., Riley K.O., Pati S. The centromedian nucleus: Anatomy, physiology, and clinical implications. J Clin Neurosci. 2019 May;63:1–7. doi: 10.1016/j.jocn.2019.01.050. [DOI] [PubMed] [Google Scholar]
- 54.Dalic L.J., Warren A.E.L., Bulluss K.J., Thevathasan W., Roten A., Churilov L., et al. DBS of Thalamic Centromedian Nucleus for Lennox-Gastaut Syndrome (ESTEL Trial) Ann Neurol. 2021 Dec 28;91(2):253–267. doi: 10.1002/ana.26280. [DOI] [PubMed] [Google Scholar]
- 55.Fisher R.S., Uematsu S., Krauss G.L., Cysyk B.J., McPherson R., Lesser R.P., et al. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992 Sep;33(5):841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 56.Velasco F., Velasco M., Jiménez F., Velasco A.L., Brito F., Rise M., et al. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. 2000 Aug;47(2):295–304. doi: 10.1097/00006123-200008000-00007. discussion 304-5. [DOI] [PubMed] [Google Scholar]
- 57.Agashe S., Burkholder D., Starnes K., Van Gompel J.J., Lundstrom B.N., Worrell G.A., et al. Centromedian Nucleus of the Thalamus Deep Brain Stimulation for Genetic Generalized Epilepsy: A Case Report and Review of Literature. Front Hum Neurosci. 2022 May 20;16 doi: 10.3389/fnhum.2022.858413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan H., Toyota E., Anderson M., Abel T.J., Donner E., Kalia S.K., et al. A systematic review of deep brain stimulation for the treatment of drug-resistant epilepsy in childhood. J Neurosurg Pediatr. 2018 Nov 30;23(3):274–284. doi: 10.3171/2018.9.PEDS18417. [DOI] [PubMed] [Google Scholar]
- 59.Klinger N.V., Mittal S. Clinical efficacy of deep brain stimulation for the treatment of medically refractory epilepsy. Clin Neurol Neurosurg. 2016 Jan;140:11–25. doi: 10.1016/j.clineuro.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 60.Torres Diaz C.V., González-Escamilla G., Ciolac D., Navas García M., Pulido Rivas P., Sola R.G., et al. Network Substrates of Centromedian Nucleus Deep Brain Stimulation in Generalized Pharmacoresistant Epilepsy. Neurotherapeutics. 2021 Apr 26;18(3):1665–1677. doi: 10.1007/s13311-021-01057-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dalic L.J., Warren A.E.L., Young J.C., Thevathasan W., Roten A., Bulluss K.J., et al. Cortex leads the thalamic centromedian nucleus in generalized epileptic discharges in Lennox-Gastaut syndrome. Epilepsia. 2020 Sep 18;61(10):2214–2223. doi: 10.1111/epi.16657. [DOI] [PubMed] [Google Scholar]
- 62.Arzimanoglou A., French J., Blume W.T., Cross J.H., Ernst J.P., Feucht M., et al. Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol. 2009 Jan;8(1):82–93. doi: 10.1016/S1474-4422(08)70292-8. [DOI] [PubMed] [Google Scholar]
- 63.Warren A.E.L., Dalic L.J., Bulluss K.J., BAppSci A.R., Thevathasan W., Archer J.S. The Optimal Target and Connectivity for Deep Brain Stimulation in Lennox-Gastaut Syndrome. Ann Neurol. 2022 Apr 28;92(1):61–74. doi: 10.1002/ana.26368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan M., Paktiawal J., Piper R.J., Chari A., Tisdall M.M. Intracranial neuromodulation with deep brain stimulation and responsive neurostimulation in children with drug-resistant epilepsy: a systematic review. J Neurosurg Pediatr. 2021 Oct 22:1–10. doi: 10.3171/2021.8.PEDS21201. [DOI] [PubMed] [Google Scholar]
- 65.Lim S.N., Lee S.T., Tsai Y.T., Chen I.A., Tu P.H., Chen J.L., et al. Electrical stimulation of the anterior nucleus of the thalamus for intractable epilepsy: a long-term follow-up study. Epilepsia. 2007 Feb;48(2):342–347. doi: 10.1111/j.1528-1167.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- 66.Lee K.J., Shon Y.M., Cho C.B. Long-term outcome of anterior thalamic nucleus stimulation for intractable epilepsy. Stereotact Funct Neurosurg. 2012 Aug 23;90(6):379–385. doi: 10.1159/000339991. [DOI] [PubMed] [Google Scholar]
- 67.Lee C.Y., Lim S.N., Wu T., Lee S.T. Successful Treatment of Refractory Status Epilepticus Using Anterior Thalamic Nuclei Deep Brain Stimulation. World Neurosurg. 2016 Nov 25;99:14–18. doi: 10.1016/j.wneu.2016.11.097. [DOI] [PubMed] [Google Scholar]
- 68.Kokoszka M.A., Panov F., La Vega-Talbott M., McGoldrick P.E., Wolf S.M., Ghatan S. Treatment of medically refractory seizures with responsive neurostimulation: 2 pediatric cases. J Neurosurg Pediatr. 2018 Feb 2;21(4):421–427. doi: 10.3171/2017.10.PEDS17353. [DOI] [PubMed] [Google Scholar]
- 69.Valentín A., Selway R.P., Amarouche M., Mundil N., Ughratdar I., Ayoubian L., et al. Intracranial stimulation for children with epilepsy. Eur J Paediatr Neurol. 2016 Nov 1;21(1):223–231. doi: 10.1016/j.ejpn.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 70.Kim S.H., Lim S.C., Yang D.W., Cho J.H., Son B.C., Kim J., et al. Thalamo-cortical network underlying deep brain stimulation of centromedian thalamic nuclei in intractable epilepsy: a multimodal imaging analysis. Neuropsychiatr Dis Treat. 2017 Oct 17;13:2607–2619. doi: 10.2147/NDT.S148617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Velasco A.L., Velasco F., Jiménez F., Velasco M., Castro G., Carrillo-Ruiz J.D., et al. Neuromodulation of the centromedian thalamic nuclei in the treatment of generalized seizures and the improvement of the quality of life in patients with Lennox-Gastaut syndrome. Epilepsia. 2006 Jul;47(7):1203–1212. doi: 10.1111/j.1528-1167.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 72.Hachem L.D., Yan H., Ibrahim G.M. Invasive Neuromodulation for the Treatment of Pediatric Epilepsy. Neurotherapeutics. 2019 Jan;16(1):128–133. doi: 10.1007/s13311-018-00685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morrell M.J., RNS System in Epilepsy Study Group Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011 Sep 27;77(13):1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- 74.Wong S., Mani R., Danish S. Comparison and Selection of Current Implantable Anti-Epileptic Devices. Neurotherapeutics. 2019 Apr;16(2):369–380. doi: 10.1007/s13311-019-00727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma B.B., Rao V.R. Responsive neurostimulation: Candidates and considerations. Epilepsy Behav. 2018 Oct 22;88:388–395. doi: 10.1016/j.yebeh.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 76.Warren R.J., Durand D.M. Effects of applied currents on spontaneous epileptiform activity induced by low calcium in the rat hippocampus. Brain Res. 1998 Sep 28;806(2):186–195. doi: 10.1016/s0006-8993(98)00723-9. [DOI] [PubMed] [Google Scholar]
- 77.Jensen A.L., Durand D.M. High frequency stimulation can block axonal conduction. Exp Neurol. 2009 Nov;220(1):57–70. doi: 10.1016/j.expneurol.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stone S.S.D., Teixeira C.M., Devito L.M., Zaslavsky K., Josselyn S.A., Lozano A.M., et al. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011 Sep 21;31(38):13469–13484. doi: 10.1523/JNEUROSCI.3100-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stavrinou L.C., Boviatsis E.J., Stathis P., Leonardos A., Panourias I.G., Sakas D.E. Sustained relief after discontinuation of DBS for dystonia: implications for the possible role of synaptic plasticity and cortical reorganization. J Neurol Surg A Cent Eur Neurosurg. 2012 May;73(3):175–178. doi: 10.1055/s-0032-1313590. discussion 178-9. [DOI] [PubMed] [Google Scholar]
- 80.Kwon C.S., Schupper A.J., Fields M.C., Marcuse L.V., La Vega-Talbott M., Panov F., et al. Centromedian thalamic responsive neurostimulation for Lennox-Gastaut epilepsy and autism. Ann Clin Transl Neurol. 2020 Oct;7(10):2035–2040. doi: 10.1002/acn3.51173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sisterson N.D., Kokkinos V., Urban A., Li N., Richardson R.M. Responsive neurostimulation of the thalamus improves seizure control in idiopathic generalised epilepsy: initial case series. J Neurol Neurosurg Psychiatry. 2022 Feb 25;93(5):491–498. doi: 10.1136/jnnp-2021-327512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Panov F., Ganaha S., Haskell J., Fields M., La Vega-Talbott M., Wolf S., et al. Safety of responsive neurostimulation in pediatric patients with medically refractory epilepsy. J Neurosurg Pediatr. 2020 Jul 31;26(5):525–532. doi: 10.3171/2020.5.PEDS20118. [DOI] [PubMed] [Google Scholar]
- 83.Roa J.A., Abramova M., Fields M., Vega-Talbott M.L., Yoo J., Marcuse L., et al. Responsive Neurostimulation of the Thalamus for the Treatment of Refractory Epilepsy. Front Hum Neurosci. 2022 Jul 15;16 doi: 10.3389/fnhum.2022.926337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.NeuroPace R.N.S.®. system responsive thalamic stimulation for primary generalized seizures (NAUTILUS) STUDY. 2022. https://clinicaltrials.gov/ct2/show/NCT05147571 Report No.: NCT05147571. Available from:
- 85.Anderson D.N., Charlebois C.M., Smith E.H., Davis T.S., Peters A.Y., Newman B.J., et al. Closed-loop stimulation in periods with less epileptiform activity drives improved epilepsy outcomes. Brain. 2023 Oct 5 doi: 10.1093/brain/awad343. awad343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davidson B., Elkaim L.M., Lipsman N., Ibrahim G.M. Editorial. An ethical framework for deep brain stimulation in children. Neurosurg Focus. 2018 Sep;45(3):E11. doi: 10.3171/2018.7.FOCUS18219. [DOI] [PubMed] [Google Scholar]
- 87.Behmer Hansen R.T., Dubey A., Smith C., Henry P.J., Mammis A. Paediatric deep brain stimulation: ethical considerations in malignant Tourette syndrome. J Med Ethics. 2020 Oct;46(10):668–673. doi: 10.1136/medethics-2020-106074. [DOI] [PubMed] [Google Scholar]
- 88.Muñoz K.A., Kostick K., Sanchez C., Kalwani L., Torgerson L., Hsu R., et al. Researcher Perspectives on Ethical Considerations in Adaptive Deep Brain Stimulation Trials. Front Hum Neurosci. 2020 Nov 12;14 doi: 10.3389/fnhum.2020.578695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Middlebrooks E.H., He X., Grewal S.S., Keller S.S. Neuroimaging and thalamic connectomics in epilepsy neuromodulation. Epilepsy Res. 2022 Mar 30;182 doi: 10.1016/j.eplepsyres.2022.106916. [DOI] [PubMed] [Google Scholar]
- 90.Cendes F., Theodore W.H., Brinkmann B.H., Sulc V., Cascino G.D. Neuroimaging of epilepsy. Handb Clin Neurol. 2016;136:985–1014. doi: 10.1016/B978-0-444-53486-6.00051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Middlebrooks E.H., Ver Hoef L., Szaflarski J.P. Neuroimaging in Epilepsy. Curr Neurol Neurosci Rep. 2017 Apr;17(4):32. doi: 10.1007/s11910-017-0746-x. [DOI] [PubMed] [Google Scholar]
- 92.Dockès J., Poldrack R.A., Primet R., Gözükan H., Yarkoni T., Suchanek F., et al. NeuroQuery, comprehensive meta-analysis of human brain mapping. Elife. 2020 Mar 4;9 doi: 10.7554/eLife.53385. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011 Jun 26;8(8):665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.NeuroQuery: brain maps to answer all your neuroscience queries. https://neuroquery.org/ Available from:
- 95.Neurosynth. https://neurosynth.org/ Available from:
- 96.McConnell Brain Imaging Centre BIC - The McConnell Brain Imaging Centre: ICBM 152 N Lin 2009. https://www.bic.mni.mcgill.ca/ServicesAtlases/ICBM152NLin2009 Available from: