Abstract
Aging-related cardiovascular disease is influenced by multiple factors, with oxidative stress being a key contributor. Aging-induced endoplasmic reticulum (ER) stress exacerbates oxidative stress by impairing mitochondrial function. Furthermore, a decline in antioxidants, including peroxiredoxins (PRDXs), augments the oxidative stress during aging. To explore if ER stress leads to PRDX degradation during aging, young adult (3 mo.) and aged (24 mo.) male mice were studied. Treatment with 4-phenylbutyrate (4-PBA) was used to alleviate ER stress in young adult and aged mice. Aged hearts showed elevated oxidative stress levels compared to young hearts. However, treatment with 4-PBA to attenuate ER stress reduced oxidative stress in aged hearts, indicating that ER stress contributes to increased oxidative stress in aging. Moreover, aging resulted in reduced levels of peroxiredoxin 3 (PRDX3) in mitochondria and peroxiredoxin 4 (PRDX4) in myocardium. While 4-PBA treatment improved PRDX3 content in aged hearts, it did not restore PRDX4 content in aged mice. These findings suggest that ER stress not only leads to mitochondrial dysfunction and increased oxidant stress but also impairs a vital antioxidant defense through decreased PRDX3 content. Additionally, the results suggest that PRDX4 may contribute an upstream role in inducing ER stress during aging.
Keywords: Oxidative stress, electron transport chain, reactive oxygen species, 4-phenylbutyric acid
1. Introduction
As humans continue to live longer, the number of elderly individuals in the population is growing (Foreman et al., 2018), resulting in a higher prevalence of cardiovascular diseases. Aged hearts are more vulnerable to stress conditions, such as chemotherapy and ischemia-reperfusion (Brandão et al., 2021; Lesnefsky et al., 2017; Rigacci et al., 2020), and are at a greater risk of injury. Dysfunctional mitochondria are a leading cause of the increased susceptibility to injury in aged hearts (Campbell et al., 2019; Chen et al., 2021; Lesnefsky et al., 2016; Lesnefsky et al., 2006; Whitson et al., 2021). While mitochondria are critical for generating energy to support cardiac function, they are also a key source of injury by producing reactive oxygen species (ROS) (Hofer et al., 2009; Karamanlidis et al., 2013; Lesnefsky et al., 2016; Lesnefsky et al., 2017). The mitochondrial electron transport chain (ETC) is the primary source of ROS in cardiac myocytes (Chen et al., 2003), and in aged hearts, the ETC is impaired, leading to increased ROS generation (Moghaddas et al., 2003). Studies have shown that an increase in ROS generation contributes to the aging process, as overexpression of mitochondria-targeted catalase in aged mice leads to an increase in lifespan (Dai et al., 2009).
Oxidative stress, which is the imbalance between the generation and inactivation of reactive oxygen species (ROS), is a crucial mechanism underlying mitochondrial damage during aging (Pagan et al., 2022). In the aged heart, ROS generation is increased due to impairments in complex I (Chen and Lesnefsky, 2021; Chen et al., 2020) and complex III (Moghaddas et al., 2003) of the electron transport chain (ETC). Another factor contributing to oxidative stress during aging is the alteration of mitochondrial antioxidant content, including glutathione peroxidases (GPX), superoxide dismutase (SOD), and peroxiredoxins (PRDXs) (Pagan et al., 2022). In aged hearts, the activities of GPX, SOD, catalase, and PRDX3 are decreased, suggesting that a deficiency of antioxidants contributes to oxidative stress during aging (Dai et al., 2008; Kayashima and Yamakawa-Kobayashi, 2012; Yu et al., 2020).
Apart from mitochondria, the endoplasmic reticulum (ER) is another source of ROS production (Pagan et al., 2022). The ER contributes a crucial role in regulating calcium homeostasis, lipid metabolism, and protein folding (Zhang and Ren, 2011). Protein folding is sensitive to the levels of calcium and redox status inside the ER lumen. Disruption of calcium homeostasis or redox status impairs protein folding, leading to ER stress (ER dysfunction) through the unfolded protein response (Chen et al., 2017; Zhang and Ren, 2011). H2O2, a byproduct generated during protein synthesis and folding in the ER, leads to ER stress (Groenendyk et al., 2013; Margittai et al., 2012). The ER stress causes disulfide bond formation during protein synthesis to become dysregulated, thereby increasing oxidative stress in the ER (Forrester et al., 2018). During aging, ER stress progressively increases (Chen and Lesnefsky, 2021; Chen et al., 2020), and chronic treatment with 4-phenylbutyric acid (4-PBA) decreases ER stress in aged mouse hearts (Chen et al., 2020). The 4-PBA treatment also attenuates the tunicamycin-induced ER stress in young rat hearts (He et al., 2021) and streptozotocin-diabetic hearts (Guo et al., 2017). 4-PBA is commonly used as a pharmacologic “ER stress inhibitor”. The 4-PBA decreases the ER stress by the interaction of hydrophobic regions of the compound with the exposed hydrophobic segments of unfolded proteins in the ER (Kolb et al., 2015; Pao et al., 2021).” This interaction protects the protein from aggregation, facilitates protein folding, and reduces ER stress (Pao et al., 2021). The occurrence of ER stress before mitochondrial dysfunction indicates that ER stress contributes to mitochondrial dysfunction during aging (Akande et al., 2022; Chen et al., 2020; Chen et al., 2021). Nonetheless, the mechanisms that lead to increased ER stress during aging are not yet fully understood.
PRDXs are a group of antioxidant enzymes that contribute a crucial role in the detoxification of H2O2 (Lee, 2020; Li et al., 2020). There are six types of PRDXs found in mammals, PRDX1–6 (Lee, 2020). PRDX1 is located in the cytosol and nucleus, whereas PRDX2, PRDX3, and PRDX6 are found in the cytosol and mitochondria. PRDX4 is present in the cytosol and endoplasmic reticulum (ER), and PRDX5 is located in the cytosol, peroxisomes, and mitochondria (Lee, 2020). A deficiency of PRDX4 has been linked to increased ER stress (Homma et al., 2018), but it is unclear if decreased PRDX4 content is responsible for the increased ER stress during aging. Similarly, PRDX3 deficiency has been associated with mitochondrial dysfunction and cellular senescence (Wu et al., 2016), and the content of PRDX3 is decreased in aged heart tissue (Dai et al., 2008). However, it is unclear if the decreased PRDX3 content is due to the increased ER stress in aged hearts. In this study, the researchers aimed to investigate the relationship between increased ER stress and potential PRDX defects present during aging in the heart. Three questions were addressed: (1) Does aging lead to decreased gene expression of PRDX1–6? (2) Does aging lead to decreased protein contents of PRDX1–6? (3) Does attenuation of the age-related ER stress alter the contents of PRDX1–6 in aged hearts? This study’s focus was to shed light on the potential defects of PRDXs during aging in the heart and how they relate to increased ER stress.
2. Methods
2.1. Aged animal model
The study followed the Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committees of Virginia Commonwealth University (AD20157) and the Richmond Department of Veterans Affairs Medical Center (02156). Male C57BL/6 mice, both young (3 months) and aged (24 months), were obtained from the National Institutes of Aging (NIA) colony Charles River Laboratories (Wilmington, MA). Female mice were studied to document that age-induced ER stress occurs in the female as well as in the male heart. Prior to tissue collection, mice were deeply anesthetized with pentobarbital (100 mg/kg, I.P.) to minimize any potential discomfort. Heart tissue was then collected for the isolation of RNA and mitochondria, as described previously (Chen et al., 2020).
2.2. PRDX gene expression and real time PCR
The miRNeasy kit (Qiagen, Germantown, MD, USA) was used to isolate total RNA in young and aged hearts. The isolated total RNA was assessed for quality and concentration using a Nanodrop-one spectrophotometer (ThermoFisher Scientific, Waltham, MA). The cDNA was synthesized from 2 μg of total RNA using a high capacity cDNA synthesis kit (Applied Biosystems, USA) with cycle conditions of 25°C for 10 min., 37°C for 120 min., and 85°C for 5 min. The expression of PRDX1–6 genes was quantified by real-time PCR in a CFX96 - C1000 Touch Thermal Cycler system (Bio-Rad, Hercules, CA, USA) using amplicon specific TaqMan assay probes (Applied Biosystems, USA). The primers were purchased from ThermoFisher Scientific (Waltham, MA) (see detail in Table 2). The real-time PCR conditions were 50°C for 2 min., 95°C for 5 min., and 39 cycles of 95°C for 3 sec. and 60°C for 1 min. Actin was used as the housekeeping control for gene expression analysis. The relative quantification of gene expression was determined using the 2-ΔΔCT method and normalized to their respective controls (Chen et al., 2020).
Table 2:
Primers used in the current study.
| Gene name | Catalog number | Assay ID |
|---|---|---|
| PRDX1 | 4453320 | Mm01621996_s1 |
| PRDX2 | 4448892 | Mm04208213_g1 |
| PRDX3 | 4448892 | Mm00545848_m1 |
| PRDX4 | 4448892 | Mm00450261_m1 |
| PRDX5 | 4448892 | Mm00465365_m1 |
| PRDX6 | 4448892 | Mm07306454_mH |
Primers were purchased from ThermoFisher Scientific (Waltham, MA).
2.3. Isolation of cytosol and mitochondria from young and aged hearts
SSM (subsarcolemmal mitochondria) and IFM (interfibrillar mitochondria) were isolated from a single mouse heart using the previously established protocol (Chen et al., 2020; Chen et al., 2021; Li et al., 2022). Briefly, the heart tissue was washed with isolation buffer A [100 mM KCl, 50 mM 3-(N-morpholino) propanesulfonic acid (MOPS), 1 mM EGTA, 5 mM MgSO4·7 H2O, 1 mM ATP, pH 7.4] at 4°C. The tissue was then minced and suspended in buffer B (buffer A + 0.2% bovine serum albumin) and homogenized with a polytron tissue processor (Brinkman Instruments, Westbury, NY) for 2.5 seconds at a speed setting of 10,000 rpm. The polytron homogenate was centrifuged at 500 x g for 10 minutes to separate the supernatant and pellet. The supernatant was further centrifuged at 3000 x g for 10 minutes to collect SSM. The remaining supernatant was subjected to a centrifugation step at 100,000 x g for 30 minutes to isolate particle-free cytosol. The pellet from the polytron homogenate was further utilized to isolate IFM. The pellet was resuspended in isolation buffer A with trypsin (5 mg/g, wet weight), homogenized, and incubated on ice for 10 minutes. The homogenate was centrifuged at 500 x g for 10 minutes to separate the supernatant and pellet, and the supernatant was further centrifuged at 3000 x g for 10 minutes to collect IFM. SSM and IFM were washed and suspended in KME (100 mM KCl, 50 mM MOPS, and 0.5 mM EGTA) before being used for the experimental study (Chen et al., 2020; Chen et al., 2021; Li et al., 2022). Protein content was measured by Lowry method (Lowry et al., 1951).
2.4. Measurement of oxidative stress in SSM and IFM
The Oxyblot technique is a reliable method for assessing oxidative stress levels by quantifying protein carbonylation (Wang and Powell, 2010). Oxidative stress in young and aged SSM, IFM, and heart homogenate was measured with a commercial Oxyblot™ kit (catalogue number, S7150) from Sigma-Aldrich (St. Louis, MO). The procedure involves derivatization of protein carbonyls with DNPH, followed by detection using the Oxyblot™ kit. In each experiment, 20 μg protein is derivatized and separated using standard SDS polyacrylamide electrophoresis under reducing conditions on a 4–15% pre-cast gel (BioRad, USA). The gels are then transferred to a PVDF membrane and probed with primary and secondary antibodies provided in the kit. The blots were developed using ECL Plus Western Blotting Detection Reagents (GE Healthcare Life Sciences, Piscataway, NJ). The membranes were then analyzed digitally using Image Lab 6.0 software (Bio-Rad). The intensity of each lane was quantified with same volume (area used to quatify the intensity of blots). Subunit 4 of cytochrome oxidase was uses as a loading control in oxyblot.
2.5. Attenuation of the ER stress in aged hearts
To decrease ER stress by stabilizing protein conformation, the chemical chaperone 4-phenylbutyrate (4-PBA) is frequently utilized (Basseri et al., 2009; Jain et al., 2016; Jian et al., 2016). For two weeks, 4-PBA (1 g/kg/day) (Basseri et al., 2009) was dissolved in drinking water and provided to 3-month-old and 24-month-old mice. Following that, mice were utilized for the isolation of cytosol and mitochondria (Chen et al., 2020).
2.6. Immunoblotting
Mitochondrial proteins were separated using Tris-glycine gels with either a 12% or 16.5% concentration, depending on the molecular weight of the targeted proteins. After separation, the proteins were transferred to a PVDF membrane using semi-dry transfer technology from Bio-Rad. The PVDF membrane was cut to facilitate blotting for both the targeted protein and the loading control. The blots were then incubated at room temperature in TBST buffer (10 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween20) containing 5% (w/v) non-fat dry milk for 1 hour, followed by an overnight incubation at 4°C with primary antibodies listed in Table 1. After three washes with TBST buffer to remove unattached primary antibodies, the membrane was incubated with a secondary antibody. Following another hour of incubation with a 1:10,000 dilution of HRP-conjugated anti-mouse or anti-rabbit IgG F(ab)2 (GE Healthcare Life Sciences, Piscataway, NJ) at room temperature, the blots were developed using ECL Plus Western Blotting Detection Reagents (GE Healthcare Life Sciences, Piscataway, NJ). The membranes were then analyzed digitally using Image Lab 6.0 software (Bio-Rad). No adjustments were made to the intensity of the immunoblotting images (Chen et al., 2020).
Table 1:
Antibodies used in the current study
| Antibody name | Company | Catalog number | Concentration |
|---|---|---|---|
| PRDX1 | ThermoFisher Scientific | PA3–750 | 1:1000 |
| PRDX2 | Cell Signaling | 46855 | 1:1000 |
| PRDX3 | Sigma-Aldrich | SAB5701418 | 1:500 |
| PRDX4 | Sigma-Aldrich | SAB4301759 | 1:1000 |
| PRDX5 | Sigma-Aldrich | SAB4501465 | 1:1000 |
| PRDX6 | Abcam | ab14715 | 1:500 |
| GAPDH | Cell Signaling | 5174 | 1:2500 |
| Subunit 4 of complex 4 | Cell Signaling | 4844 | 1:2500 |
| VDAC | Abcam | Ab15895 | 1:2000 |
2.7. Statistical analysis
The data are presented as mean ± standard error (Steel and Torrie, 1960). To compare differences between groups (≥ 3 groups), a one-way ANOVA was performed after normality and equal variance tests. When a significant F value was obtained, the means were compared using the Student-Newman-Keuls test for multiple comparisons. To compare differences between two groups, an unpaired Student t-test was conducted (SigmaStat 3.5, Systat, Richmond, CA). A p-value of less than 0.05 was considered statistically significant.
3. RESULTS
3.1. Aging increased oxidative stress in both SSM and IFM
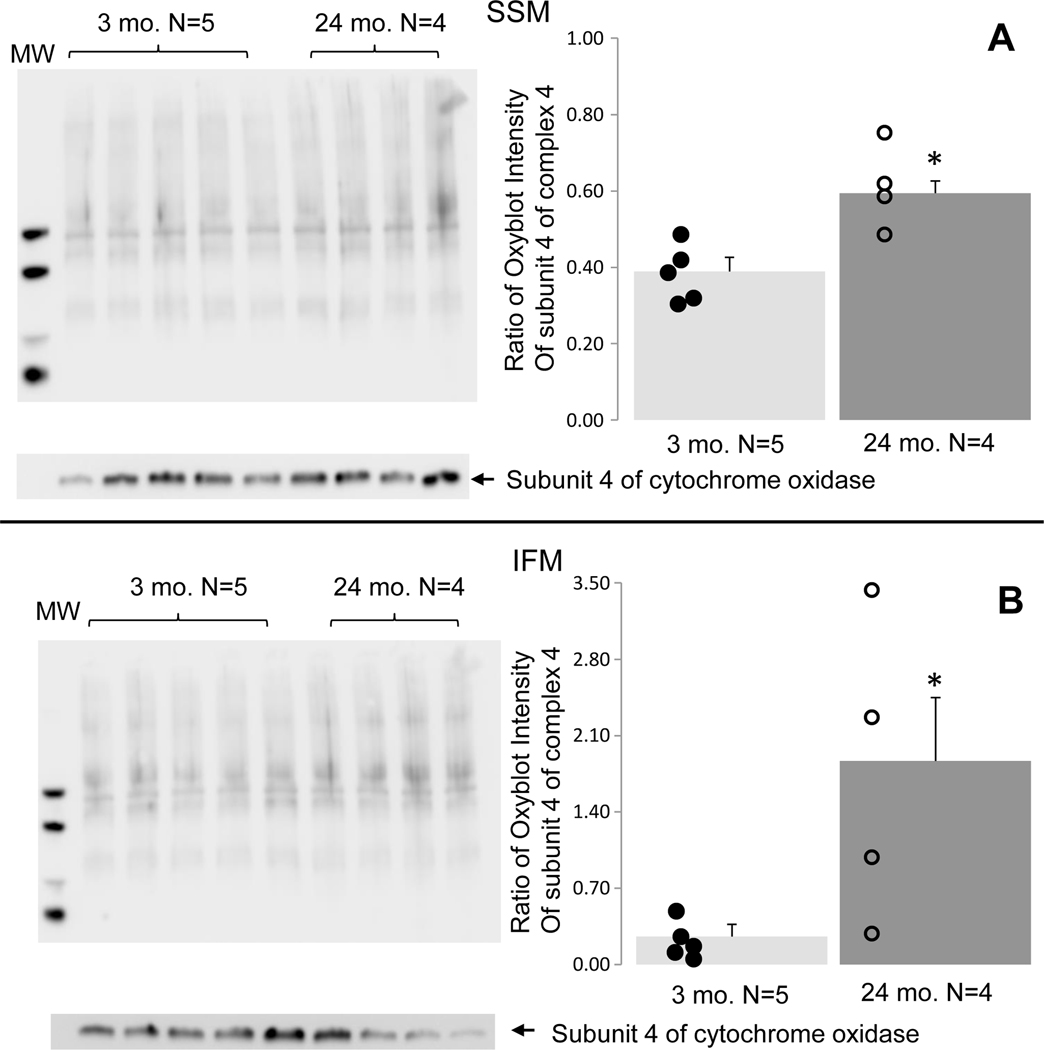
The Oxyblot™ assay was used to measure the level of oxidative stress by detecting carbonylated proteins. The results showed that compared to 3-month-old hearts, both the SSM (Figure 1A) and IFM (Figure 1B) isolated from 24-month-old hearts exhibited increased oxidative stress. These findings are in line with previous reports of increased ROS generation in mitochondria of aged hearts (Moghaddas et al., 2003).
Figure 1. Aging increased oxidative stress in both SSM and IFM.
Oxidative stress was assessed in young and aged mitochondria using an Oxyblot™ kit. The intensity of all bands in each lane was quantified, and subunit 4 of cytochrome oxidase was used as protein loading control. Compared to 3 mo., oxidative stress was increased in both aged SSM (Panel A) and IFM (Panel B). Mean ± SEM. *p <0.05 vs. 3 mo.
3.2. Aging had a minimal effect on PRDX1–6 gene expression
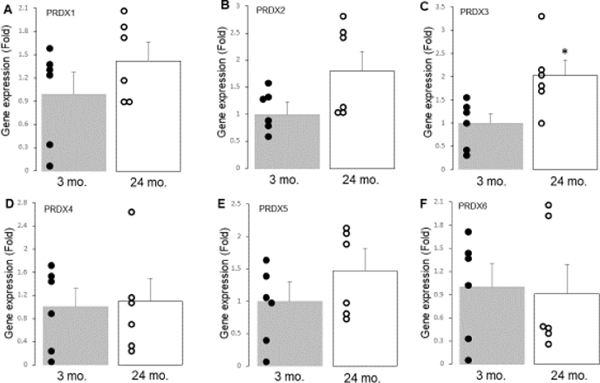
The gene expression of PRDX1–6 was measured using real-time PCR. The results showed that there were no significant differences in the gene expression of PRDX1 (Figure 2A), 2 (Figure 2B), 4(Figure 2D), 5(Figure 2E), and 6 (Figure 2F) between the 3-month-old and 24-month-old hearts. However, the expression of PRDX3 was increased in the 24-month-old hearts compared to the 3-month-old hearts (Figure 2C). These findings suggest that aging leads to increased PRDX3 gene expression but has a minimal effect on the expression of other PRDX genes.
Figure 2. The gene expression of PRDX1, PRDX2, PRDX3, PRDX4, PRDX5 and PRDX6 in 3 mo. and 24 mo. hearts is shown.
Real-time PCR was used to assess the gene expression of PRDX1–6 in young and aged hearts. There were no significant differences in PRDX1 (Panel A), PRDX2 (Panel B), PRDX4 (Panel D), PRDX5 (Panel E), and PRDX6 (Panel F) between 3 mo. and 24 mo. hearts. Aging led to increased PRDX3 expression vs. 3 mo. (Panel C). Mean ± SEM. p < 0.05 vs. 3 mo. n=6 in each group.
3.3. Aging led to decreased contents of PRDX3 and PRDX4
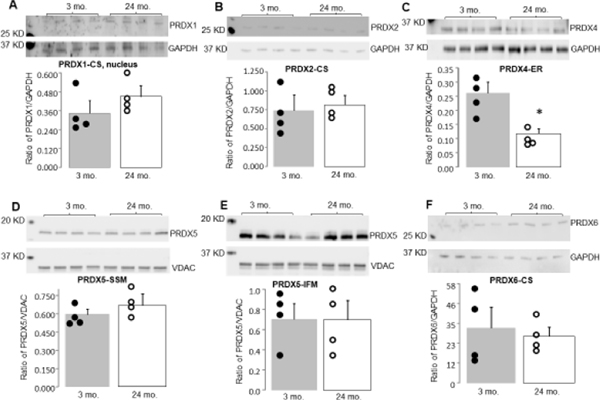
The quantity of PRDX1–6 proteins was measured using immunoblotting in tissue homogenates and isolated SSM and IFM. There were no significant differences in PRDX1 and 2 protein content in tissue homogenates between 3 mo. and 24 mo. (Figure 3A and B). While there was no significant difference in PRDX3 content in SSM between 3 mo. and 24 mo. (Figure 5A), the content of PRDX3 in IFM was decreased in 24 mo. compared to 3 mo. (Figure 5B). PRDX4 protein content was significantly decreased in the cytosol of 24 mo. compared to 3 mo. (Figure 3C). Aging did not alter the levels of PRDX5 in SSM and IFM isolated from 24 mo. compared to 3 mo. (Figure 3D and E). Aging did not affect PRDX6 protein content in the cytosol (Figure 3F).
Figure 3. The contents of PrDX1, PRDX2, PRDX4, PRDX5 and PRDX6 were measured.
Immunoblotting was used to assess the protein contents of PRDX enzyme contents in young adult and aged hearts. There were no significant differences in PRDX1 (Panel A) and PRDX2 (Panel B) between 3 mo. and 24 mo. hearts. Aging led to decreased PRDX4 content vs. 3 mo. (Panel C). Aging did not alter PRDX5 in SSM (Panel D), PRDX5 in IFM (Panel E), or PRDX6 contents (Panel F) compared to 3 mo. Mean ± SEM. p < 0.05 vs. 3 mo. n=4 in each group. CS, cytosol. Note that PRDX-3 levels are shown in Figure 5.
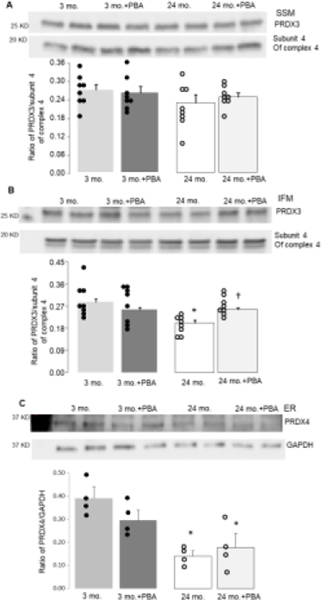
Figure 5. The 4-PBA treatment restored PRDX3 but not PRDX4 content in aged hearts.
4-PBA was used to decrease ER stress in aged hearts. Attenuation of the ER stress using 4-PBA did not improve the PRDX3 content in aged SSM (Panel A). The 4-PBA treatment did improve the PRDX3 content in aged IFM (Panel B), supporting that ER stress contributes to PRDX3 depletion during aging. However, the 4-PBA treatment did not alter PRDX4 content in aged hearts (Panel C), suggesting that PRDX4 is not a downstream target of the ER stress during aging. Mean ± SEM. *p <0.05 vs. 3 mo. † p<0.05 vs. 24 mo. vehicle.
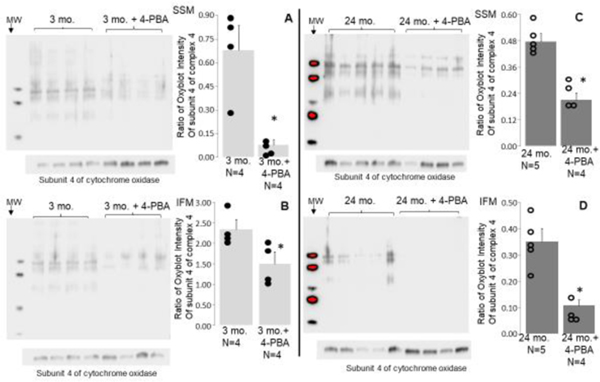
3.4. Attenuation of the ER stress decreased oxidative stress in aged SSM and IFM
Mice were treated with the chemical chaperone 4-PBA to reduce ER stress, which is associated with mitochondrial dysfunction during aging. The extent of oxidative stress was then assessed in SSM and IFM that were isolated from the treated hearts and compared to untreated hearts. The results showed that treatment with 4-PBA led to a reduction in oxidative stress in SSM isolated from both 3-month-old (Figure 4A) and 24-month-old (Figure 4C) hearts compared to untreated hearts. The 4-PBA treatment also decreased oxidative stress in IFM isolated from both 3-month-old (Figure 4B) and 24-month-old (Figure 4D) hearts compared to untreated hearts. This suggests that reducing ER stress is a potential mechanism for decreasing oxidative stress within mitochondria in both young and aged hearts.
Figure 4. Attenuation of the ER stress led to decreased oxidative stress in adult and aged hearts.
4-PBA treatment was used to decrease ER stress. The 4-PBA treatment decreased oxidative stress in 24 mo. SSM (Panel C) and IFM (Panel D). 4-PBA treatment also led to decreased oxidative stress in 3 mo. SSM (Panel A) and IFM (Panel B). These results indicate that ER stress contributes to increased oxidative stress in the mitochondria, especially during aging. The intensity of all bands in each lane was quantified, and subunit 4 of cytochrome oxidase was used as protein loading control. Mean ± SEM. *p <0.05 vs. vehicle treatment.
3.5. Attenuation of the ER stress improved PRDX3 content in aged IFM
To investigate the effect of 4-PBA treatment on PRDX3 content in aged SSM and IFM, PRDX3 content was compared in SSM and IFM isolated from 3-month-old and 24-month-old hearts with or without 4-PBA treatment. The 4-PBA treatment did not affect the content of PRDX3 in SSM isolated from 3-month-old nor 24-month-old hearts compared to corresponding vehicle treatment (Figure 5A). Although the 4-PBA treatment did not affect the content of PRDX3 in IFM from 3-month-old hearts, it improved the PRDX3 content in IFM from 24-month-old hearts compared to the vehicle (Figure 5B). These findings support that ER stress contributes to PRDX3 degradation during aging, and that attenuation of ER stress with 4-PBA treatment can improve PRDX3 content in aged IFM.
3.6. Attenuation of the ER stress did not affect PRDX4 content in aged heart
The effect of 4-PBA treatment on PRDX4 content was assessed in both 3 mo. and 24 mo. hearts. The results showed that compared to the vehicle, the 4-PBA treatment did not affect the PRDX4 content measured in either age group in total heart homogenate (Figure 5C). These findings suggest that ER stress is not a significant contributing factor to the decrease in PRDX4 content during aging.
4. Discussion
In this study, the researchers observed an increase in oxidative stress in aged heart mitochondria and found that attenuating ER stress using 4-PBA resulted in decreased oxidative stress in aged heart mitochondria. They also found that aging led to decreased PRDX3 content primarily in IFM and decreased PRDX4 content in aged hearts. The researchers discovered that 4-PBA treatment that decreased ER stress improved the PRDX3 content in aged IFM, indicating that PRDX3 is a downstream target of ER stress during aging. However, the 4-PBA treatment did not restore PRDX4 content, suggesting that a decrease in PRDX4 may be an upstream source to initiate the ER stress during aging. Based on these findings, targeting PRDX4 may be a potential strategy to mitigate ER stress and relieve mitochondrial dysfunction during aging. The summary is depicted in Figure 6.
Figure 6: A proposed schematic of the role of PRDX3 and PRDX4 in aged hearts.
A deficiency of PRDX4 is a potential mechanism of the increase in the ER stress that occurs during aging by augmenting oxidative stress. An increase in ER stress leads to mitochondrial dysfunction and PRDX3 defects during aging by activating mitochondrial calpain 1 (CPN1) (Chen et al., 2022b; Thompson et al., 2020). The 4-PBA treatment improves mitochondrial function and PRDX3 content by decreasing the ER stress that in turn attenuates the activation of mitochondrial calpain 1 (Chen et al., 2020; Chen et al., 2022b). Restoration of PRDX4 content is another potential strategy to decrease the ER stress during aging.
4.1. Oxidative stress and mitochondrial damage in aged hearts
The Mitochondrial Free Radical Theory of Aging suggests that an increase in ROS generation from the ETC contributes to mitochondrial dysfunction in aged hearts (Harman, 1956). ROS generation is increased in aged heart mitochondria (Chen et al., 2020; Moghaddas et al., 2003). Oxidative stress is also increased in aged human heart tissue (Quan et al., 2015). In the present study, oxidative stress was increased in aged SSM and IFM from mouse hearts. Excess ROS generation participates in cell toxicity and DNA damage. Mitochondrial DNA (mtDNA) is prone to oxidative damage due to its proximity to the ETC and relative lack of an endogenous repair system (Orogo et al., 2015). The mtDNA encodes 13 subunits of respiratory complexes in the ETC (Bratic and Trifunovic, 2010). Thus, the damaged mtDNA can increase oxidative stress by further impairing the ETC. The damaged ETC is a key source of increased ROS generation in cardiac myocytes (Chen et al., 2007). The ER is another source of ROS generation. H2O2 is produced in the ER during protein folding when a series of disulfide-exchange reactions form disulfide bonds (Ozgur et al., 2018; Tavender and Bulleid, 2010). H2O2 generated in the ER during protein folding increases oxidative stress if the H2O2 is not removed in a timely manner. In addition to the ETC, p66shc and monoamine oxidase (MAO) are other sources that generate ROS in mitochondria. The p66shc is a protein found in the mitochondrial intermembrane space that produces H2O2 by reducing molecular oxygen through a redox center coupled with the oxidation of cytochrome c. Removing p66shc increases resistance to oxidative stress and prolongs the lifespan of mice, indicating that ROS generated from p66shc contributes to aging (Giorgio et al., 2005; Migliaccio et al., 2013). MAO is a flavoenzyme located in the outer mitochondrial membrane that generates H2O2 when it breaks down neurotransmitters such as norepinephrine, epinephrine, and dopamine (Maggiorani et al., 2017). MAO includes two isoforms (MAO-A and MAO-B). Inhibition of MAO-A decreases H2O2 generation in aged hearts, indicating that MAO is a source of oxidative stress during aging (Maggiorani et al., 2017; Manzella et al., 2018). In the present study, the researchers found that attenuation of the ER stress using 4-PBA led to decreased oxidative stress in aged heart mitochondria (Figure 4C, 4D), indicating that ER stress-mediated mitochondrial defects augments oxidative stress during aging.
Interestingly, 4-PBA treatment also decreased oxidative stress in 3-month-old SSM and IFM (Figure 4A, 4B). Since there were no mitochondrial defects in 3-month-old heart mitochondria, the 4-PBA treatment is less likely to decrease oxidative stress in 3-month-old by improving mitochondrial function. The 4-PBA may decrease oxidative stress in 3-month-old by facilitating protein folding and assembly in the ER (Pao et al., 2021). NADPH oxidases are another source of ROS generation. NOX4 (NADPH oxidase 4) is mainly localized in the ER by interacting with calnexin (Prior et al., 2016). NOX4 is also found in mitochondria (Ozturk et al., 2017). Aging leads to increased ER stress, ROS generation, and NOX4 activity in ER isolated from aorta tissue (Lee et al., 2020). Knockout of NOX4 or 4-PBA treatment leads to a decrease in ER stress and ROS generation in the aged ER (Lee et al., 2020). Overexpression of NOX4 in mitochondria also increases the ROS generation accompanied by impaired mitochondrial function (Canugovi et al., 2019). Thus, the 4-PBA treatment may decrease oxidative stress by decreasing ROS generated from both mitochondria and the ER.
4.2. Aging and mitochondrial antioxidants
In addition to increased ROS generation, a decrease in antioxidants also contributes to oxidative stress during aging (Kaplán et al., 2019). The primary antioxidant enzymes are superoxide dismutase (SOD), catalase, and glutathione peroxidase. Superoxide is converted by SOD to H2O2 that is reduced to water and oxygen by catalase. Glutathione peroxidase converts peroxides and hydroxyl radicals into nontoxic forms by oxidizing reduced glutathione into glutathione disulfide (Kaplán et al., 2019). Aging leads to alterations in genes encoding for proteins implicated in ROS production and clearance pathways (Rizvi et al., 2021). SOD1 content is decreased in aged human hearts (Rizvi et al., 2021), and SOD2 content is only decreased in hearts from aged female patients (Barcena de Arellano et al., 2019). The activities of SOD1 and SOD2 are decreased in aged rat hearts (Kaplán et al., 2019). Overexpression of catalase within mitochondria extends lifespan, indicating the importance of H2O2 production during the progression of aging. The activity of glutathione peroxidase is decreased in aged rat hearts (Kaplán et al., 2019). Oxidative stress impairs sulfhydryl homeostasis by oxidizing protein sulfhydryl groups (Gallogly et al., 2009; Gorelenkova Miller and Mieyal, 2015; Sabens Liedhegner et al., 2012). Thioredoxins and glutaredoxins are essential to maintain sulfhydryl homeostasis (Azuma and Shearer, 2008; Gallogly et al., 2010; Gao et al., 2013; Sabens Liedhegner et al., 2012; Starke et al., 1997), but the contents of both are decreased in aged rat hearts (Kaplán et al., 2019). These results support the notion that the deficiency of antioxidants contributes to the increase in oxidative stress during aging.
Peroxiredoxins are important antioxidants that help neutralize peroxides by using electrons generated by thioredoxins. Although peroxiredoxins are abundant in the heart, there have been few studies on how they change with age. One study found that the content of PRDX2 was reduced in 26-month-old rat hearts (Kaplán et al., 2019)., but in the present study, we found that PRDX2 content was not significantly decreased in aged mouse hearts (24 months). This difference may be due to species and age variations. A previous study showed that PRDX3 content was decreased in aged left ventricle tissue. In the current study, aging led to decreased PRDX3 content mainly in IFM, supporting that aging leads to PRDX3 deficiency. Interestingly, the gene expression of PRDX3 is increased in aged hearts. An increase in PRDX3 gene expression should lead to an elevated PRDX3 protein content. However, PRDX3 content is decreased in aged mitochondria. These results suggest that the decreased PRDX3 content in aged hearts is less likely due to decreased protein synthesis. Post-translational modifications including the ubiquitin-proteasome pathway is a potential factor to decrease PRDX3 content in aged hearts (Huang et al., 2021). In addition, aging leads to decreased PRDX4 content in mouse hearts. However, aging has a limited effect on the contents of PRDX 1, 2, 5, and 6 in mouse heart. These results indicate that aging leads to the alteration of PRDX antioxidant capacity mainly reflected in the decrease in the contents of PRDX3 and PRDX4.
4.4. ER stress and PRDX3 degradation during aging
PRDX3 is a type of peroxiredoxin that contributes an important role in maintaining mitochondrial function and protecting against cellular damage (Wu et al., 2016). It is mainly located in the mitochondrial matrix and its deficiency has been associated with mitochondrial dysfunction and cellular senescence (Chen et al., 2014). In the heart, there are two populations of mitochondria: SSM and IFM, which have different properties including susceptibility to damage during aging (Fannin et al., 1999). IFM are more susceptible to damage due to increased ROS generation from complex III (Moghaddas et al., 2003). The study found that aging leads to decreased PRDX3 content in IFM, suggesting that a deficiency of PRDX3 may augment IFM damage during aging. Additionally, the study found that attenuation of ER stress, which is an upstream factor in inducing mitochondrial damage during aging, improves PRDX3 content in aged IFM, suggesting that PRDX3 is a downstream target of ER stress. Therefore, the deficiency of PRDX3 may contribute to the damage of IFM during aging, and targeting ER stress may be a potential therapeutic approach to prevent this damage.
In our previous study, we discovered that aging-induced ER stress activates mitochondrial calpain 1 (Chen et al., 2022b) which is a calcium-dependent protease that degrades various mitochondrial proteins, including apoptosis-inducing factor, subunits of complex I, and a subunit of pyruvate dehydrogenase in aged heart mitochondria (Chen et al., 2011; Chen et al., 2022a; Li et al., 2022). Attenuation of the ER stress leads to decreased calpain 1 activation in aged hearts (Chen et al., 2022a). In the present study, we found that treatment with 4-PBA, which attenuates ER stress, improves PRDX3 content in aged IFM. This suggests that aging may lead to the degradation of PRDX3 by activating mitochondrial calpain 1 through increased ER stress. Furthermore, PRDX3 content is also decreased in mouse heart mitochondria following ischemia-reperfusion, and genetic knockout of p53, a protein that accumulates in myocardium during aging, improves PRDX3 content in hearts following ischemia-reperfusion (Chen et al., 2019). Thus, an increase in p53 expression may also lead to decreased PRDX3 content during aging.
4.5. ER stress and PRDX4 defects during aging
PRDX4 is an important antioxidant enzyme mainly located in the endoplasmic reticulum (ER). It plays a crucial role in removing H2O2 generated during oxidative protein folding in the ER (Ozgur et al., 2018; Tavender and Bulleid, 2010). Thus, PRDX4 contributes an important role in the removal of H2O2 generated during protein folding. It is not surprising that genetic removal of PRDX4 increases the ER stress by enhancing oxidative stress (Homma et al., 2018; Lee, 2020). Elimination of PRDX4 also accelerates the aging process in ovarian tissue (Liang et al., 2020). ER stress is increased in the aged heart (Akande et al., 2022; Chen et al., 2020; Chen et al., 2021). We find that PRDX4 content is decreased in aged hearts. In addition, the attenuation of the ER stress using 4-PBA does not protect PRDX4 content in aged heats. The results suggest that PRDX4 is not a downstream target of the increase in ER stress with aging. Recent studies show that PRDX4 deficiency is a key factor to increase ER stress. Improvement of PRDX4 content leads to decreased ER stress (Guo et al., 2012). The decreased PRDX4 content in aged mouse hearts suggests that PRDX4 deficiency is a potential contributing factor to the increase in the ER stress observed during aging.
5. Limitations
While gene expression and protein content measurements provide important information about the expression and abundance of PRDX1–6, assessing their enzyme activities would provide more direct information regarding their functions in the heart. It would be interesting to investigate the activities of PRDX1–6 in young and aged hearts in future studies. Although 4-PBA is a commonly used compound to decrease ER stress (Basseri et al., 2009; Jain et al., 2016; Jian et al., 2016), the 4-PBA does have some off-target effects including effects on urea secretion and histone deacetylase activity. The 4-PBA has been used to treat urea cycle disorders by inhibiting histone deacetylase (Iannitti and Palmieri, 2011). Sirtuin 1 is one of histone deacetylases. Interestingly, genetic elimination of sirtuin 1 markedly impairs cardiac function in 12 mon. old mice (Hsu et al., 2017), supporting that inhibition of histone deacetylase does not provide benefits during aging. Our study shows that 4-PBA treatment leads to decreased ER stress in aged hearts (Chen et al., 2020). Therefore, we believe that the 4-PBA treatment improves the PRDX3 content by attenuating the ER stress. In the current study, we only used male mice. The effect of aging on PRDXs in female mice needs to be addressed in the future study. Since aging also leads to increased ER stress in the female heart (Supplementary Figure 1), we anticipate that similar results will be observed in aging female heart.
6. Conclusion
Our previous studies show that the attenuation of ER stress using 4-PBA or metformin improves mitochondrial function in aged mouse hearts (Chen et al., 2020; Chen et al., 2017; Chen et al., 2021). Here we show that attenuation of the ER stress also improves the content of a key mitochondria-targeted antioxidant, PRDX3, in aged heart mitochondria. In addition, aging leads to decreased PRDX4 content that may trigger the ER stress during aging. These results advance our understanding of the role of increased ER stress in the mitochondrial dysfunction that occurs during aging.
Supplementary Material
Highlights:
Aging decreased PRDX3 in cardiac mitochondria and PRDX4 in the ER
Attenuation of ER stress improved PRDX3 content in mitochondria in aged hearts
Attenuation of ER stress did not improve PRDX4 content in aged hearts
PRDX3 is a downstream target of age-induced ER stress in the heart
Acknowledgments:
This work was supported by R21 AG049461 (QC) from the National Institute on Aging, the Department of Veterans Affairs Office of Research and Development, Medical Research Service Merit Review Award (2IO1BX001355-01A2) (QC, EJL), and a Pauley Heart Center Pilot Project from Virginia Commonwealth University (Q.C.).
Footnotes
Conflicts of Interest:
The authors state they have no conflict of interest or financial interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Akande O, Chen Q, Cholyway R, Toldo S, Lesnefsky EJ and Quader M, 2022. Modulation of Mitochondrial Respiration During Early Reperfusion Reduces Cardiac Injury in Donation After Circulatory Death Hearts. J Cardiovasc Pharmacol. 80, 148–157. DOI: 10.1097/fjc.0000000000001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma M. and Shearer TR, 2008. The role of calcium-activated protease calpain in experimental retinal pathology. Surv Ophthalmol. 53, 150–63. DOI: 10.1016/j.survophthal.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcena de Arellano ML, Pozdniakova S, Kühl AA, Baczko I, Ladilov Y. and Regitz-Zagrosek V, 2019. Sex differences in the aging human heart: decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging (Albany NY). 11, 1918–1933. DOI: 10.18632/aging.101881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basseri S, Lhotak S, Sharma AM and Austin RC, 2009. The chemical chaperone 4-phenylbutyrate inhibits adipogenesis by modulating the unfolded protein response. J Lipid Res. 50, 2486–501. DOI: 10.1194/jlr.M900216-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão SR, Reis-Mendes A, Domingues P, Duarte JA, Bastos ML, Carvalho F, Ferreira R. and Costa VM, 2021. Exploring the aging effect of the anticancer drugs doxorubicin and mitoxantrone on cardiac mitochondrial proteome using a murine model. Toxicology. 459, 152852. DOI: 10.1016/j.tox.2021.152852. [DOI] [PubMed] [Google Scholar]
- Bratic I. and Trifunovic A, 2010. Mitochondrial energy metabolism and ageing. Biochim Biophys Acta. 1797, 961–7. DOI: 10.1016/j.bbabio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Campbell MD, Duan J, Samuelson AT, Gaffrey MJ, Merrihew GE, Egertson JD, Wang L, Bammler TK, Moore RJ, White CC, Kavanagh TJ, Voss JG, Szeto HH, Rabinovitch PS, MacCoss MJ, Qian WJ and Marcinek DJ, 2019. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic Biol Med. 134, 268–281. DOI: 10.1016/j.freeradbiomed.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canugovi C, Stevenson MD, Vendrov AE, Hayami T, Robidoux J, Xiao H, Zhang YY, Eitzman DT, Runge MS and Madamanchi NR, 2019. Increased mitochondrial NADPH oxidase 4 (NOX4) expression in aging is a causative factor in aortic stiffening. Redox Biol. 26, 101288. DOI: 10.1016/j.redox.2019.101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Na R. and Ran Q, 2014. Enhanced defense against mitochondrial hydrogen peroxide attenuates age-associated cognition decline. Neurobiol Aging. 35, 2552–2561. DOI: 10.1016/j.neurobiolaging.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Chen Q, Camara AK, Stowe DF, Hoppel CL and Lesnefsky EJ, 2007. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 292, C137–47. [DOI] [PubMed] [Google Scholar]
- Chen Q. and Lesnefsky EJ, 2021. Metformin and myocardial ischemia and reperfusion injury: Moving toward “prime time” human use? Transl Res. 229, 1–4. DOI: 10.1016/j.trsl.2020.10.006. [DOI] [PubMed] [Google Scholar]
- Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A. and Lesnefsky EJ, 2011. Activation of mitochondrial mu-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun. 415, 533–8. DOI: 10.1016/j.bbrc.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Samidurai A, Thompson J, Hu Y, Das A, Willard B. and Lesnefsky EJ, 2020. Endoplasmic reticulum stress-mediated mitochondrial dysfunction in aged hearts. Biochim Biophys Acta Mol Basis Dis. 1866, 165899. DOI: 10.1016/j.bbadis.2020.165899. [DOI] [PubMed] [Google Scholar]
- Chen Q, Thompson J, Hu Y, Das A. and Lesnefsky EJ, 2017. Metformin attenuates ER stress-induced mitochondrial dysfunction. Transl Res. 190, 40–50. DOI: 10.1016/j.trsl.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Thompson J, Hu Y. and Lesnefsky EJ, 2021. Chronic metformin treatment decreases cardiac injury during ischemia-reperfusion by attenuating endoplasmic reticulum stress with improved mitochondrial function. Aging (Albany NY). 13, 7828–7845. DOI: 10.18632/aging.202858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Thompson J, Hu Y. and Lesnefsky EJ, 2022a. The mitochondrial electron transport chain contributes to calpain 1 activation during ischemia-reperfusion. Biochem Biophys Res Commun. 613, 127–132. DOI: 10.1016/j.bbrc.2022.04.117. [DOI] [PubMed] [Google Scholar]
- Chen Q, Thompson J, Hu Y. and Lesnefsky EJ, 2022b. Reversing mitochondrial defects in aged hearts: role of mitochondrial calpain activation. Am J Physiol Cell Physiol. 322, C296–310. DOI: 10.1152/ajpcell.00279.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL and Lesnefsky EJ, 2003. Production of reactive oxygen species by mitochondria: Central role of complex III. J Biol Chem. 278, 36027–36031. [DOI] [PubMed] [Google Scholar]
- Chen Z, Boor PJ, Finnerty CC, Herndon DN and Albrecht T, 2019. Calpain-mediated cleavage of p53 in human cytomegalovirus-infected lung fibroblasts. FASEB Bioadv. 1, 151–166. DOI: 10.1096/fba.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC and Rabinovitch PS, 2009. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 119, 2789–97. DOI: 10.1161/circulationaha.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Escobar GP, Hakala KW, Lambert JM, Weintraub ST and Lindsey ML, 2008. The left ventricle proteome differentiates middle-aged and old left ventricles in mice. J Proteome Res. 7, 756–65. DOI: 10.1021/pr700685e. [DOI] [PubMed] [Google Scholar]
- Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO and Hoppel CL, 1999. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 372, 399–407. [DOI] [PubMed] [Google Scholar]
- Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, Brown JC, Friedman J, He J, Heuton KR, Holmberg M, Patel DJ, Reidy P, Carter A, Cercy K, Chapin A, Douwes-Schultz D, Frank T, Goettsch F, Liu PY, Nandakumar V, Reitsma MB, Reuter V, Sadat N, Sorensen RJD, Srinivasan V, Updike RL, York H, Lopez AD, Lozano R, Lim SS, Mokdad AH, Vollset SE and Murray CJL, 2018. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 392, 2052–2090. DOI: 10.1016/s0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q. and Griendling KK, 2018. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ Res. 122, 877–902. DOI: 10.1161/circresaha.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallogly MM, Shelton MD, Qanungo S, Pai HV, Starke DW, Hoppel CL, Lesnefsky EJ and Mieyal JJ, 2010. Glutaredoxin regulates apoptosis in cardiomyocytes via NFkappaB targets Bcl-2 and Bcl-xL: implications for cardiac aging. Antioxid Redox Signal. 12, 1339–53. DOI: 10.1089/ars.2009.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallogly MM, Starke DW and Mieyal JJ, 2009. Mechanistic and kinetic details of catalysis of thiol-disulfide exchange by glutaredoxins and potential mechanisms of regulation. Antioxid Redox Signal. 11, 1059–81. DOI: 10.1089/ars.2008.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XH, Qanungo S, Pai HV, Starke DW, Steller KM, Fujioka H, Lesnefsky EJ, Kerner J, Rosca MG, Hoppel CL and Mieyal JJ, 2013. Aging-dependent changes in rat heart mitochondrial glutaredoxins--Implications for redox regulation. Redox Biol. 1, 586–98. DOI: 10.1016/j.redox.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F. and Pelicci PG, 2005. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 122, 221–33. [DOI] [PubMed] [Google Scholar]
- Gorelenkova Miller O. and Mieyal JJ, 2015. Sulfhydryl-mediated redox signaling in inflammation: role in neurodegenerative diseases. Arch Toxicol. 89, 1439–67. DOI: 10.1007/s00204-015-1496-7. [DOI] [PubMed] [Google Scholar]
- Groenendyk J, Agellon LB and Michalak M, 2013. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol. 75, 49–67. DOI: 10.1146/annurev-physiol-030212-183707. [DOI] [PubMed] [Google Scholar]
- Guo R, Wu Z, Jiang J, Liu C, Wu B, Li X, Li T, Mo H, He S, Li S, Yan H, Huang R, You Q. and Wu K, 2017. New mechanism of lipotoxicity in diabetic cardiomyopathy: Deficiency of Endogenous H(2)S Production and ER stress. Mech Ageing Dev. 162, 46–52. DOI: 10.1016/j.mad.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Guo X, Yamada S, Tanimoto A, Ding Y, Wang KY, Shimajiri S, Murata Y, Kimura S, Tasaki T, Nabeshima A, Watanabe T, Kohno K. and Sasaguri Y, 2012. Overexpression of peroxiredoxin 4 attenuates atherosclerosis in apolipoprotein E knockout mice. Antioxid Redox Signal. 17, 1362–75. DOI: 10.1089/ars.2012.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D, 1956. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 11, 298–300. DOI: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- He J, Gong M, Wang Z, Liu D, Xie B, Luo C, Li G, Tse G. and Liu T, 2021. Cardiac abnormalities after induction of endoplasmic reticulum stress are associated with mitochondrial dysfunction and connexin43 expression. Clin Exp Pharmacol Physiol. 48, 1371–1381. DOI: 10.1111/1440-1681.13541. [DOI] [PubMed] [Google Scholar]
- Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, Wohlgemuth SE and Leeuwenburgh C, 2009. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 130, 297–307. DOI: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma T, Kurahashi T, Lee J, Nabeshima A, Yamada S. and Fujii J, 2018. Double Knockout of Peroxiredoxin 4 (Prdx4) and Superoxide Dismutase 1 (Sod1) in Mice Results in Severe Liver Failure. Oxid Med Cell Longev. 2018, 2812904. DOI: 10.1155/2018/2812904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YJ, Hsu SC, Hsu CP, Chen YH, Chang YL, Sadoshima J, Huang SM, Tsai CS and Lin CY, 2017. Sirtuin 1 protects the aging heart from contractile dysfunction mediated through the inhibition of endoplasmic reticulum stress-mediated apoptosis in cardiac-specific Sirtuin 1 knockout mouse model. Int J Cardiol. 228, 543–552. DOI: 10.1016/j.ijcard.2016.11.247. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang S, Li Y, Liu Z, Mi L, Cai Y, Wang X, Chen L, Ran H, Xiao D, Li F, Wu J, Li T, Han Q, Chen L, Pan X, Li H, Li T, He K, Li A, Zhang X, Zhou T, Xia Q. and Man J, 2021. Suppression of mitochondrial ROS by prohibitin drives glioblastoma progression and therapeutic resistance. Nat Commun. 12, 3720. DOI: 10.1038/s41467-021-24108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannitti T. and Palmieri B, 2011. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D. 11, 227–49. DOI: 10.2165/11591280-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain K, Suryakumar G, Ganju L. and Singh SB, 2016. Amelioration of ER stress by 4-phenylbutyric acid reduces chronic hypoxia induced cardiac damage and improves hypoxic tolerance through upregulation of HIF-1α. Vascul Pharmacol. 83, 36–46. DOI: 10.1016/j.vph.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Jian L, Lu Y, Lu S. and Lu C, 2016. Chemical Chaperone 4-Phenylbutyric Acid Reduces Cardiac Ischemia/Reperfusion Injury by Alleviating Endoplasmic Reticulum Stress and Oxidative Stress. Med Sci Monit. 22, 5218–5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplán P, Tatarková Z, Lichardusová L, Kmeťová Sivoňová M, Tomašcová A, Račay P. and Lehotský J, 2019. Age-Associated Changes in Antioxidants and Redox Proteins of Rat Heart. Physiol Res. 68, 883–892. DOI: 10.33549/physiolres.934170. [DOI] [PubMed] [Google Scholar]
- Karamanlidis G, Lee CF, Garcia-Menendez L, Kolwicz SC Jr., Suthammarak W, Gong G, Sedensky MM, Morgan PG, Wang W. and Tian R, 2013. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 18, 239–50. DOI: 10.1016/j.cmet.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayashima Y. and Yamakawa-Kobayashi K, 2012. Involvement of Prx3, a Drosophila ortholog of the thiol-dependent peroxidase PRDX3, in age-dependent oxidative stress resistance. Biomed Res. 33, 319–22. DOI: 10.2220/biomedres.33.319. [DOI] [PubMed] [Google Scholar]
- Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG and Ask K, 2015. The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Biochem Cell Biol. 61, 45–52. DOI: 10.1016/j.biocel.2015.01.015. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kim HK, Hoang TH, Yang S, Kim HR and Chae HJ, 2020. The correlation of IRE1α oxidation with Nox4 activation in aging-associated vascular dysfunction. Redox Biol. 37, 101727. DOI: 10.1016/j.redox.2020.101727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, 2020. Knockout Mouse Models for Peroxiredoxins. Antioxidants (Basel). 9. DOI: 10.3390/antiox9020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Chen Q. and Hoppel CL, 2016. Mitochondrial Metabolism in Aging Heart. Circ Res. 118, 1593–611. DOI: 10.1161/circresaha.116.307505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Chen Q, Tandler B. and Hoppel CL, 2017. Mitochondrial Dysfunction and Myocardial Ischemia-Reperfusion: Implications for Novel Therapies. Annu Rev Pharmacol Toxicol. 57, 535–565. DOI: 10.1146/annurev-pharmtox-010715-103335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, He D, Moghaddas S. and Hoppel CL, 2006. Reversal of mitochondrial defects before ischemia protects the aged heart. Faseb J. 20, 1543–5. [DOI] [PubMed] [Google Scholar]
- Li L, Thompson J, Hu Y, Lesnefsky EJ, Willard B. and Chen Q, 2022. Calpain-mediated protein targets in cardiac mitochondria following ischemia-reperfusion. Sci Rep. 12, 138. DOI: 10.1038/s41598-021-03947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YR, Zhu H. and Danelisen I, 2020. Role of Peroxiredoxins in Protecting Against Cardiovascular and Related Disorders. Cardiovasc Toxicol. 20, 448–453. DOI: 10.1007/s12012-020-09588-0. [DOI] [PubMed] [Google Scholar]
- Liang X, Yan Z, Ma W, Qian Y, Zou X, Cui Y, Liu J. and Meng Y, 2020. Peroxiredoxin 4 protects against ovarian ageing by ameliorating D-galactose-induced oxidative damage in mice. Cell Death Dis. 11, 1053. DOI: 10.1038/s41419-020-03253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL and Randall RJ, 1951. Protein measurement with the Folin phenol reagent. J Biol Chem. 193, 265–75. [PubMed] [Google Scholar]
- Maggiorani D, Manzella N, Edmondson DE, Mattevi A, Parini A, Binda C. and Mialet-Perez J, 2017. Monoamine Oxidases, Oxidative Stress, and Altered Mitochondrial Dynamics in Cardiac Ageing. Oxid Med Cell Longev. 2017, 3017947. DOI: 10.1155/2017/3017947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzella N, Santin Y, Maggiorani D, Martini H, Douin-Echinard V, Passos JF, Lezoualc’h F, Binda C, Parini A. and Mialet-Perez J, 2018. Monoamine oxidase-A is a novel driver of stress-induced premature senescence through inhibition of parkin-mediated mitophagy. Aging Cell. 17, e12811. DOI: 10.1111/acel.12811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margittai É, Löw P, Stiller I, Greco A, Garcia-Manteiga JM, Pengo N, Benedetti A, Sitia R. and Bánhegyi G, 2012. Production of H2O2 in the endoplasmic reticulum promotes in vivo disulfide bond formation. Antioxid Redox Signal. 16, 1088–99. DOI: 10.1089/ars.2011.4221. [DOI] [PubMed] [Google Scholar]
- Migliaccio E, Giorgio M. and Pelicci PG, 2013. p53 and aging: role of p66Shc. Aging (Albany NY). 5, 488–9. DOI: 10.18632/aging.100583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddas S, Hoppel CL and Lesnefsky EJ, 2003. Aging defect at the Qo site of complex III augments oxyradical production in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 414, 59–66. [DOI] [PubMed] [Google Scholar]
- Orogo AM, Gonzalez ER, Kubli DA, Baptista IL, Ong SB, Prolla TA, Sussman MA, Murphy AN and Gustafsson Å B, 2015. Accumulation of Mitochondrial DNA Mutations Disrupts Cardiac Progenitor Cell Function and Reduces Survival. J Biol Chem. 290, 22061–75. DOI: 10.1074/jbc.M115.649657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgur R, Uzilday B, Iwata Y, Koizumi N. and Turkan I, 2018. Interplay between the unfolded protein response and reactive oxygen species: a dynamic duo. J Exp Bot. 69, 3333–3345. DOI: 10.1093/jxb/ery040. [DOI] [PubMed] [Google Scholar]
- Ozturk N, Olgar Y, Er H, Kucuk M. and Ozdemir S, 2017. Swimming exercise reverses aging-related contractile abnormalities of female heart by improving structural alterations. Cardiol J. 24, 85–93. DOI: 10.5603/CJ.a2016.0069. [DOI] [PubMed] [Google Scholar]
- Pagan LU, Gomes MJ, Gatto M, Mota GAF, Okoshi K. and Okoshi MP, 2022. The Role of Oxidative Stress in the Aging Heart. Antioxidants (Basel). 11. DOI: 10.3390/antiox11020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao HP, Liao WI, Tang SE, Wu SY, Huang KL and Chu SJ, 2021. Suppression of Endoplasmic Reticulum Stress by 4-PBA Protects Against Hyperoxia-Induced Acute Lung Injury via Up-Regulating Claudin-4 Expression. Front Immunol. 12, 674316. DOI: 10.3389/fimmu.2021.674316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior KK, Wittig I, Leisegang MS, Groenendyk J, Weissmann N, Michalak M, Jansen-Dürr P, Shah AM and Brandes RP, 2016. The Endoplasmic Reticulum Chaperone Calnexin Is a NADPH Oxidase NOX4 Interacting Protein. J Biol Chem. 291, 7045–59. DOI: 10.1074/jbc.M115.710772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan C, Cho MK, Perry D. and Quan T, 2015. Age-associated reduction of cell spreading induces mitochondrial DNA common deletion by oxidative stress in human skin dermal fibroblasts: implication for human skin connective tissue aging. J Biomed Sci. 22, 62. DOI: 10.1186/s12929-015-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigacci L, Annibali O, Kovalchuk S, Bonifacio E, Pregnolato F, Angrilli F, Vitolo U, Pozzi S, Broggi S, Luminari S, Merli F, Spina M, Bolis S, Margiotta-Casaluci G, Scalzulli R, Cox C, Mamusa AM, Santoro A, Zinzani PL, Ferrari S, Gini G, Vigliotti ML, Mulè A. and Flenghi L, 2020. Nonpeghylated liposomal doxorubicin combination regimen (R-COMP) for the treatment of lymphoma patients with advanced age or cardiac comorbidity. Hematol Oncol. 38, 478–486. DOI: 10.1002/hon.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi F, Preston CC, Emelyanova L, Yousufuddin M, Viqar M, Dakwar O, Ross GR,Faustino RS, Holmuhamedov EL and Jahangir A, 2021. Effects of Aging on Cardiac Oxidative Stress and Transcriptional Changes in Pathways of Reactive Oxygen Species Generation and Clearance. J Am Heart Assoc. 10, e019948. DOI: 10.1161/jaha.120.019948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabens Liedhegner EA, Gao XH and Mieyal JJ, 2012. Mechanisms of altered redox regulation in neurodegenerative diseases--focus on S--glutathionylation. Antioxid Redox Signal. 16, 543–66. DOI: 10.1089/ars.2011.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke DW, Chen Y, Bapna CP, Lesnefsky EJ and Mieyal JJ, 1997. Sensitivity of protein sulfhydryl repair enzymes to oxidative stress. Free Radic Biol Med. 23, 373–84. [DOI] [PubMed] [Google Scholar]
- Steel R. and Torrie J, 1960. Principles and procedures of statistics, Mc Graw-Hill, New York. [Google Scholar]
- Tavender TJ and Bulleid NJ, 2010. Peroxiredoxin IV protects cells from oxidative stress by removing H2O2 produced during disulphide formation. J Cell Sci. 123, 2672–9. DOI: 10.1242/jcs.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Maceyka M. and Chen Q, 2020. Targeting ER stress and calpain activation to reverse age-dependent mitochondrial damage in the heart. Mech Ageing Dev. 192, 111380. DOI: 10.1016/j.mad.2020.111380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. and Powell SR, 2010. Decreased sensitivity associated with an altered formulation of a commercially available kit for detection of protein carbonyls. Free Radic Biol Med. 49, 119–21. DOI: 10.1016/j.freeradbiomed.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson JA, Martín-Pérez M, Zhang T, Gaffrey MJ, Merrihew GE, Huang E, White CC, Kavanagh TJ, Qian WJ, Campbell MD, MacCoss MJ, Marcinek DJ, Villén J. and Rabinovitch PS, 2021. Elamipretide (SS-31) treatment attenuates age-associated post-translational modifications of heart proteins. Geroscience. 43, 2395–2412. DOI: 10.1007/s11357-021-00447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WB, Menon R, Xu YY, Zhao JR, Wang YL, Liu Y. and Zhang HJ, 2016. Downregulation of peroxiredoxin-3 by hydrophobic bile acid induces mitochondrial dysfunction and cellular senescence in human trophoblasts. Sci Rep. 6, 38946. DOI: 10.1038/srep38946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Wang F, Wang J, Zhang D. and Zhao X, 2020. Ketogenic diet attenuates aging-associated myocardial remodeling and dysfunction in mice. Exp Gerontol. 140, 111058. DOI: 10.1016/j.exger.2020.111058. [DOI] [PubMed] [Google Scholar]
- Zhang Y. and Ren J, 2011. Thapsigargin triggers cardiac contractile dysfunction via NADPH oxidase-mediated mitochondrial dysfunction: Role of Akt dephosphorylation. Free Radic Biol Med. 51, 2172–84. DOI: 10.1016/j.freeradbiomed.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.