Abstract
Obesity is a primary risk factor for osteoarthritis. While previous work has addressed relationships between in vivo cartilage mechanics, composition, and obesity in the tibiofemoral joint, there is limited information on these relationships in the patellofemoral joint. The purpose of this study was to compare the patellofemoral cartilage mechanical response to walking in participants with normal and obese body mass indices (BMIs). Additionally, patellar cartilage T1rho relaxation times were measured before exercise to characterize the biochemical composition of the tissue. Fifteen participants (eight with normal BMI and seven with obese BMI) underwent baseline magnetic resonance imaging (MRI) of their right knee. They then walked on a treadmill for 20 min at a speed normalized to their leg length before a second MRI scan. Subsequently, three-dimensional models of the bones and articular surfaces of the patellofemoral joint were created via manual segmentation of the pre- and post-exercise MR images to compute cartilage thickness and strain. Strain was defined as the change in patellofemoral cartilage thickness normalized to the baseline thickness. Results showed that participants with an obese BMI exhibited significantly increased patellofemoral cartilage strain compared to those with a normal BMI (5.4 ± 4% vs. 1.7 ± 3%, respectively; p = 0.003). Furthermore, patellar cartilage T1rho values were significantly higher in participants with obese versus normal BMIs (95 ms vs. 83 ms, respectively; p = 0.049), indicative of decreased proteoglycan content in those with an obese BMI. In summary, the altered patellofemoral cartilage strain and composition observed in those with an obese BMI may be indicative of cartilage degeneration.
Keywords: BMI, Gait, Knee, MRI, Osteoarthritis
1. Introduction
Obesity, defined as a body mass index (BMI) greater than 30 kg/m2, continues to increase in the United States. Approximately 39% of adults are currently classified as obese (Hales et al., 2017). Obesity is a primary risk factor for a number of medical conditions, including the development of knee osteoarthritis (OA) (Felson, 2005; Runhaar et al., 2011). OA is a disabling joint disease that presents with articular cartilage degeneration, osteophyte formation, joint space narrowing, and pain (Altman et al., 1987; Jeffery, 1975; Kellgren and Lawrence, 1957). Given that there is no pharmacologic disease-modifying treatment for OA, medical intervention focuses on pain relief, and, in the case of symptomatic end-stage OA, joint replacement surgery is recommended (American College Of Rheumatology Subcommittee On Osteoarthritis Guidelines, 2000; Jordan et al., 2003). For individuals with overweight and obese BMIs, moderate weight loss accomplished through diet and exercise is encouraged in an effort to reduce pain, lessen inflammation, decrease knee joint loads, and improve health-related quality of life (Atukorala et al., 2016; Gersing et al., 2016; Messier et al., 2004; Messier et al., 2018; Serebrakian et al., 2015).
Obesity is particularly detrimental to the patellofemoral joint (PFJ). There is evidence indicating that an elevated BMI is associated with higher grades of cartilage degeneration in the PFJ in comparison to the medial and lateral compartments of the tibiofemoral joint (Du et al., 2021). Obesity is also a risk factor for patellofemoral joint pain (Hart et al., 2020). Further, radiographic PFJ OA has been shown to develop first with subsequent progression to the tibiofemoral joint (Duncan et al., 2011).
OA is associated with a disruption of the mechanical function of cartilage (Cutcliffe et al., 2021; Setton et al., 1999). To assess cartilage mechanical function in vivo, previous work has used a knee cartilage “stress test” consisting of magnetic resonance imaging (MRI) scans before and after a designated activity to quantify the cartilage response (Collins et al., 2020; Crook et al., 2021; Heckelman et al., 2020a; Paranjape et al., 2019). This “stress test” enables the quantification of cartilage strain, defined as the change in cartilage thickness post- exercise normalized to the pre-exercise thickness (Collins et al., 2020; Crook et al., 2021; Heckelman et al., 2020a; Paranjape et al., 2019). Furthermore, previous work has investigated quantitative MRI as a potential biomarker for cartilage health (Collins et al., 2018a; Collins et al., 2018b; Gersing et al., 2016; Heckelman et al., 2020b; Koff et al., 2007; Pedoia et al., 2016; Regatte et al., 2006; Serebrakian et al., 2015; Taylor et al., 2019; Wang et al., 2020). For example, T1rho relaxation times are inversely related to the proteoglycan concentration in cartilage (Collins et al., 2018a). The proteoglycan concentration in cartilage decreases during OA progression, suggesting that T1rho relaxation times are indicative of cartilage degeneration (Matzat et al., 2013).
We recently reported that tibiofemoral cartilage compressive strain and T1rho relaxation times were elevated in individuals with an obese BMI as compared to those with a normal BMI (Collins et al., 2018a; Collins et al., 2020; Widmyer et al., 2013), suggesting that both cartilage mechanical function and composition are disrupted with obesity. What has not been explored is how patellofemoral cartilage compressive strains and T1rho relaxation times compare in these groups. Therefore, the objective of this study was to test the hypothesis that in vivo patellofemoral cartilage compressive strain would be larger in those with an obese BMI as compared to those with a normal BMI. We also hypothesized that an obese BMI would be associated with increased patellar cartilage T1rho relaxation times at baseline (prior to exercise), indicative of possible cartilage degeneration.
2. Methods
2.1. Study participants
Following Institutional Review Board approval, eight participants with a normal BMI (18.5–24.9 kg/m2) and seven with an obese BMI (BMI ≥ 30 kg/m2) were recruited to participate in this study (Table 1) (Collins et al., 2018a; Collins et al., 2020). The participants in this study were the same as were used in Collins et al. (2018a) and Collins et al. (2020); however, the present study focused on the patellofemoral joint, which has not been previously analyzed. Our sample size was based on prior investigations from our lab that quantified significant changes in knee cartilage thickness following a bout of treadmill walking in eight asymptomatic individuals (Collins et al., 2020; Collins et al., 2018b; Lad et al., 2016). Written informed consent was obtained prior to participation. The individuals with a normal BMI and those with an obese BMI had significantly different BMIs, but they did not have significantly different ages, heights, or male to female ratios (Collins et al., 2018b). Participants with a history of knee injury, knee surgery, or any OA-related symptoms were excluded. All participants were tested first thing in the morning to minimize the effect of diurnal cartilage loading on thickness and T1rho measurements (Coleman et al., 2013; Taylor et al., 2019). Additionally, participants were asked to abstain from strenuous activities the day prior to and the morning of testing.
Table 1.
Participant demographics.
| BMI Status | BMI Range (kg/m2) | Male Participants | Female Participants | Mean Age (years) | Age Range (years) |
|---|---|---|---|---|---|
| Normal | 18–25 | 5 | 3 | 30 | 23–43 |
| Obese | 30–36 | 3 | 4 | 32 | 22–45 |
2.2. Image acquisition & walking protocol
Upon arrival, participants rested in a supine position for 45 min to allow their knee cartilage to relax to its baseline thickness (Heckelman et al., 2020b). The participants then underwent magnetic resonance (MR) imaging of their right knee. Sagittal MR images were obtained using a 3.0 T MR scanner (Trio Tim, Siemens; Malvern, PA) and an eight-channel knee coil (Invivo Corporation; Orlando, FL) with a double echo steady-state (DESS) and a T1rho mapping pulse sequence (Table 2) (Lad et al., 2016; Okafor et al., 2014; Sutter et al., 2015; Taylor et al., 2019). Following the pre-exercise MR scan, participants were transported in a wheelchair to an adjacent room where they walked on a level treadmill for 20 min (Paranjape et al., 2019). The walking speed (v) was normalized to each participant’s leg length (L) using a Froude number (Fr = v2/(L*g)) of 0.25 and g = 9.8 m/s2 (Alexander and Jayes, 1983), which corresponded to an average walking speed of 3.4 ± 0.1 mph. Subjects were prohibited from holding the handrail unless it was needed to recover balance. Each participant wore a digital pedometer (Zip; Fitbit, Inc.; San Francisco, CA) on their waistband to capture the number of loading cycles performed during the 20-minute walk. Immediately after walking, participants returned to the MRI scanner for a post-exercise DESS scan. T1rho images were not acquired post-exercise.
Table 2.
MRI Scan Parameters.
| Sequence | DESS | T1rho |
|---|---|---|
| Flip angle (degrees) | 25 | 15 |
| Repetition time (ms) | 17 | 3500 |
| Echo Time (ms) | 6 | 5.9 |
| Field of view (cm) | 16 × 16 | 14 × 14 |
| Matrix | 512 × 512 | 128 × 256, interpolated to 256 × 256 |
| Resolution (mm) | 0.3 × 0.3 × 1.0 |
1.1 × 0.5 × 3.0, interpolated to 0.5 × 0.5 × 3.0 |
| Spin-lock frequency (Hz) | – | 500 |
| Spin-lock times (ms) | – | 5, 10, 40, 80 |
| Acquisition Time (mm: ss) | 9:49 | 12:30 |
DESS = double echo steady-state.
2.3. Knee alignment
Knee joint alignment was assessed based on joint angles measured via the long axes of the femur and tibia on coronal MR images (Collins et al., 2020). All participants had neutral knee joint alignment (within 2–3 degrees of varus/valgus) (Cherian et al., 2014).
2.4. Strain analysis
The DESS images were imported into Rhinoceros (Robert McNeel & Associates; Seattle, WA), a solid modeling software. The patella, distal femur (trochlear region), and their articulating surfaces were manually outlined by a single investigator and were assessed for quality control by a fellowship-trained musculoskeletal radiologist with over 30 years of experience. This technique has been previously shown to measure changes in cartilage thickness within a resolution of 1% (Coleman et al., 2013; Cutcliffe et al., 2020). The tracings of each MR slice were then compiled to create 3D models of both the bone and cartilage. Afterwards, the pre- and post-exercise bone models were aligned using an iterative closest point algorithm to enable site-specific comparisons of cartilage thickness using Geomagic (3D Systems; Research Triangle Park, NC) and Mathematica (Wolfram Research; Champaign, IL) (Crook et al., 2021; Owusu-Akyaw et al., 2018). Cartilage thickness was defined as the distance between each cartilage surface mesh vertex and the closest vertex on the corresponding bone surface mesh (Fig. 1). Patellar and trochlear cartilage thickness measurements were averaged within evenly distributed circular regions (11 on the patellar surface and 15 on the trochlear surface) with a radius of 2.5 mm (Fig. 2) (Owusu-Akyaw et al., 2018). Approximately 15–20 pairs of cartilage and bone points were located within each 2.5 mm sampling radius. Cartilage strain within each sampling region was defined as the difference between mean values of post- and pre-exercise cartilage thickness values divided by the mean pre-exercise thickness value.
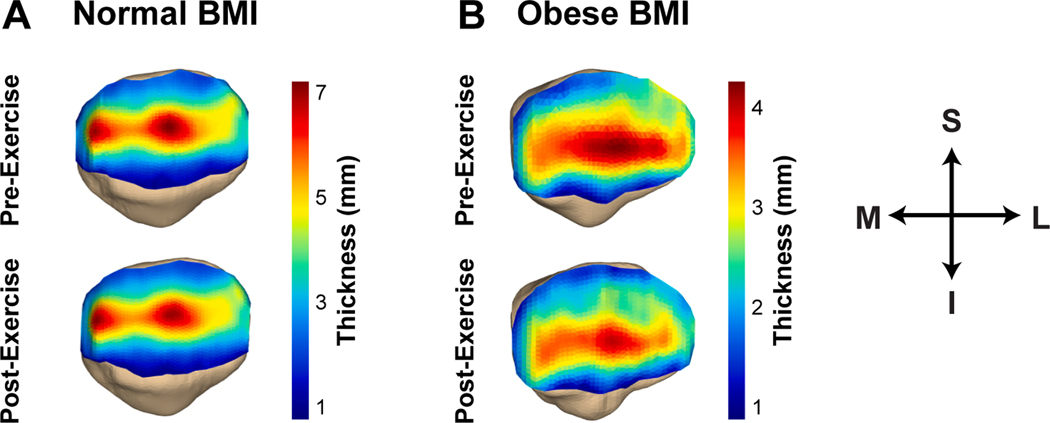
Fig. 1.
Representative patellar cartilage thickness maps from an individual with (A) a normal BMI and (B) an obese BMI. In this figure, blue represents areas with thin cartilage, whereas red represents areas with thick cartilage. (S: superior, I: inferior, M: medial, L: lateral). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
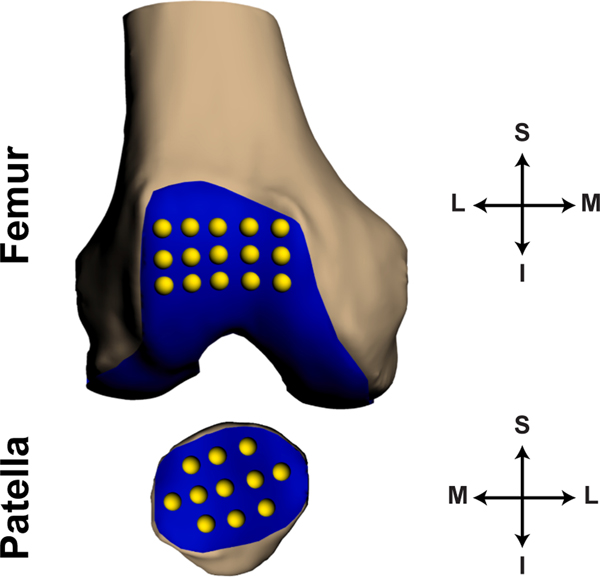
Fig. 2.
Placement of grid points on the trochlear (15 points) and patellar (11 points) articulating surfaces used to calculate strain. Here the patella is rotated 180 degrees about the coronal (S-I) axis to display the articular surface. (S: superior, I: inferior, M: medial, L: lateral).
2.5. T1rho analysis
The T1rho-weighted MR images were analyzed using custom image processing software (MATLAB; The MathWorks, Inc.; Natick, MA). All voxels containing patellar cartilage were manually selected in spin-lock time (TSL) = 5 ms images in order to calculate patellar cartilage T1rho relaxation times. These voxels were tracked across all spin-lock times, and the signal intensities were used in the following exponential decay model to compute the T1rho relaxation time within each voxel:
where S(TSL) is the signal intensity at each spin-lock time, S0 is the maximum signal intensity, and T1ρ is the T1rho relaxation time. Finally, T1rho relaxation times of all voxels within the segmented region of the patellar cartilage were averaged to yield a single value per participant (Fig. 3).
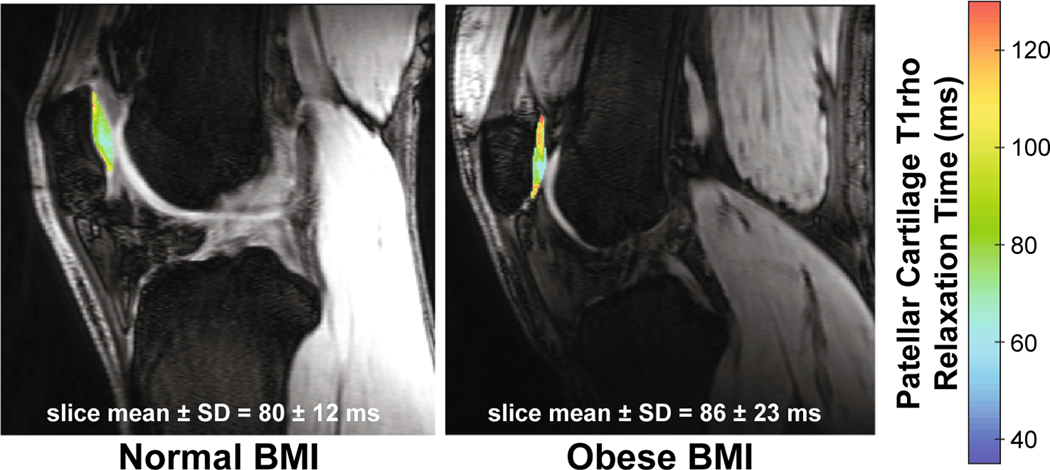
Fig. 3.
Colormaps of patellar cartilage T1rho relaxation times in one representative participant with a normal BMI and one representative participant with an obese BMI. Higher T1rho relaxation times (red), as illustrated in the individual with the obese BMI, are representative of a lower relative proteoglycan concentration in comparison to the individual with the normal BMI. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.6. Statistical analysis
The data were summarized using routine descriptive statistics. Each variable was assessed for normality using Shapiro-Wilk tests. Unpaired t-tests were used to compare baseline patellar and trochlear cartilage thicknesses between groups based on BMI. A two-way analysis of variance (ANOVA) was performed to determine the influence of BMI (normal versus obese) and location (patella versus trochlea) on cartilage strain. An unpaired t-test was used to compare mean patellar T1rho relaxation times between the two groups. All statistical analyses were performed using Statistica (StatSoft, Inc.; Tulsa, OK). Statistical significance was defined as a p-value of less than 0.05. All results are presented as the mean ± standard deviation.
3. Results
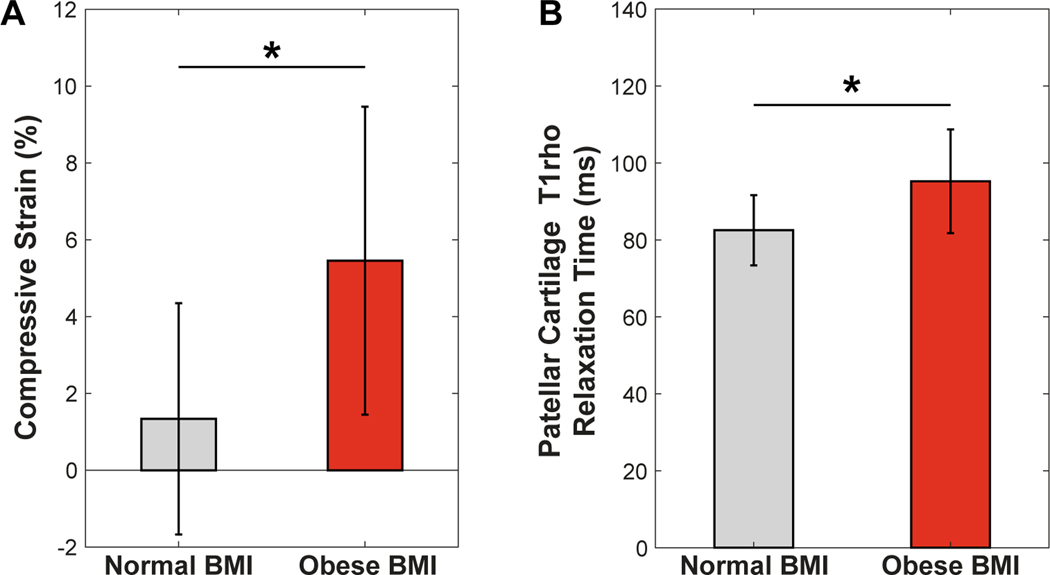
No differences in baseline patellar (p =0.058) or trochlear (p =0.14) cartilage thickness between the groups with a normal and obese BMI were observed. A significant main effect of BMI was found with respect to mean PFJ cartilage strains (normal BMI = 1.7 ± 3% vs. obese BMI = 5.4 ± 4%; p = 0.003; Fig. 4A). There was no significant main effect of location (p = 0.90) and no interaction effect (p = 0.12) on cartilage strains. Mean patellar cartilage T1rho relaxation times were significantly greater in obese compared to normal BMI participants (95 ± 13 ms vs. 83 ± 9 ms; p = 0.049; Fig. 4B).
Fig. 4.
(A) Individuals with an obese BMI (30–36 kg/m2) incurred a significantly larger mean (±standard deviation) patellofemoral cartilage compressive strain as compared with individuals with a normal BMI (18–25 kg/m2; *p = 0.003). (B) Similarly, individuals with an obese BMI had an elevated mean (±standard deviation) baseline patellar cartilage T1rho relaxation time as compared to their counterparts with a normal BMI (*p = 0.049). (BMI: body mass index).
4. Discussion
Obesity is a primary and modifiable risk factor of knee OA (Felson, 2005; Runhaar et al., 2011). Unfortunately, the precise mechanism by which obesity leads to OA is unclear. To better understand the biomechanical effects of obesity on the knee joint, we compared in vivo patellofemoral cartilage strains in response to a “stress test” of treadmill walking (Paranjape et al., 2019) in participants with a normal BMI versus an obese BMI. We also characterized the baseline biochemical state of patellar cartilage as measured by T1rho relaxation times. Our results indicated that treadmill walking significantly increased patellofemoral cartilage strain approximately three-fold in participants with an obese BMI compared to participants with a normal BMI. We also measured significantly higher patellar cartilage T1rho relaxation times in individuals with an obese BMI prior to exercise, indicative of a lower proteoglycan concentration in the cartilage of these participants than in the group of individuals with a normal BMI.
Previously, our lab investigated diurnal changes in cartilage thickness in individuals with an obese BMI and did not detect significant changes in patellofemoral cartilage thickness between the morning and evening measurements (Widmyer et al., 2013). However, since compressive strain depends on mechanical loading frequency, both the variety of daily activities chosen by each individual and each participant’s loading patterns throughout the day would likely affect changes in patellofemoral articular cartilage. For instance, some participants may have a sedentary day while others have a more physically demanding day. Additionally, due to the viscoelastic nature of cartilage, the loading rate of the tissue must also be considered (Li and Herzog, 2004). Thus, our study controlled for both load duration and walking speed by requiring our participants to complete a 20-minute walk at a speed normalized to each individual’s leg length. Controlling for walking speed across participants was important due to the tendency of individuals with an obese BMI to walk slower, presumably to limit high joint loading (Vakula et al., 2019). As previously noted, we found no significant differences in the number of steps taken during the walking task between the BMI groups, suggesting that a similar number of loading cycles was experienced by each group (Collins et al., 2020).
Previous work has reported a relationship between knee malalignment and the risk of developing OA, particularly in individuals with overweight and obese BMIs (Brouwer et al., 2007). Malalignment potentially disrupts the normal transmission of force across the knee, which could lead to abnormal peak pressures in the lateral or medial compartments and promote degeneration of articular cartilage (Tetsworth and Paley, 1994). In particular, both valgus and varus malalignment have been shown to increase the risk of development and progression of PFJ OA (Cahue et al., 2004). However, in the present study, there was no significant valgus or varus malalignment in our participants based on the joint geometry in the neutral position within the MR scanner (Cherian et al., 2014; Collins et al., 2020). As it is possible that knee alignment differs during weight-bearing, future studies may investigate the effect of knee alignment on PFJ strains.
A possible explanation for the increased patellofemoral strain in individuals with an obese BMI observed in this study may involve the considerable loads that the PFJ sustains during common physical activities (Kim et al., 2019; Reilly and Martens, 1972). For instance, peak PFJ contact forces can reach three times body weight while stair climbing and seven to eight times body weight during squatting activities (Reilly and Martens, 1972). Therefore, it is feasible that PFJ loads are exacerbated by increased BMI (Kim et al., 2019). Importantly, the PFJ strains reported here are likely underestimates of the maximal cartilage strains incurred in the PFJ joint. This is due to cartilage recovery post-exercise when the cartilage is in an unloaded position within the MR scanner (Cutcliffe et al., 2020). While every effort was made to minimize the time elapsed between the end of the walking exercise and the start of post-exercise imaging (less than four minutes), it is likely that some cartilage recovery occurred prior to and during the second DESS acquisition (Collins et al., 2018b). Nevertheless, we were still able to measure significant differences in PFJ cartilage strain between the normal BMI and obese participants. Finally, loads during overground walking may be different than those experienced on a treadmill (Van Hooren et al., 2020). However, we decided to use a treadmill to provide a more controlled environment for walking. It was also conveniently located adjacent to the MR scanner, minimizing cartilage recovery prior to post-exercise imaging.
In addition to the biomechanical effects of a high BMI on articular cartilage, obesity induces a state of low-grade systemic inflammation secondary to excess adiposity (Blüher, 2008; Cancello and Clément, 2006; Galic et al., 2010; Invitti, 2002; Tilg and Moschen, 2008). The production of pro-inflammatory cytokines, known as adipokines, may operate alongside other cytokines in the joint to promote cartilage degeneration (McNulty et al., 2011). Furthermore, pro-inflammatory cytokines have been shown to induce changes in cartilage composition, such as a decrease in proteoglycan content (McNulty et al., 2011). As a consequence, proteoglycan loss decreases cartilage stiffness, potentially resulting in increased deformations following loading (Setton et al., 1999). Our findings are consistent with this previous work; we demonstrated that participants with an obese BMI had lower proteoglycan content, as measured by elevated T1rho relaxation times, and exhibited greater patellofemoral cartilage compressive strains in response to load. Therefore, it is possible that obesity induces changes in the mechanical properties of cartilage by disrupting its extracellular matrix, making it more susceptible to loading (Collins et al., 2018a; Collins et al., 2021).
In the present investigation, patellofemoral cartilage strain and patellar cartilage T1rho relaxation times were quantified. While patellar cartilage T1rho relaxation times were only assessed at baseline, patellofemoral strain was measured in response to joint loading. Walking was chosen in this study as it is one of the most common activities of daily living and can easily be controlled. Future studies may investigate the effects of other activities on the patellofemoral joint—especially those that require greater knee flexion—as these may generate much higher PFJ forces (Lee et al., 2003).
5. Conclusion
In conclusion, this study examined the mechanical response of patellofemoral cartilage to acute loading in individuals with normal and obese BMIs. We found increased patellofemoral compressive strain in participants with an obese BMI as compared to those with a normal BMI following a 20-minute treadmill walk. Moreover, we found that patellar cartilage T1rho relaxation times were higher in individuals with an obese BMI prior to any prescribed load, indicating a decreased relative proteoglycan concentration in the cartilage. Consequently, it is possible that alterations in cartilage composition due to obesity result in increases in cartilage strain. Furthermore, increased strain induced by obesity may contribute to cartilage degeneration.
Acknowledgments
We would like to thank the National Institutes of Health (NIH) for their support of this project (AR074800, AR065527, AR079184). We would also like to thank the Center for Advanced Magnetic Resonance Development (CAMRD) at Duke University Hospital for their assistance with image acquisition and Brian J. Soher, PhD for his technical support related to this work. The authors also acknowledge Donald T. Kirkendall, PhD, ELS, a contracted medical editor, for his assistance in preparing this paper for submission.
Footnotes
CRediT authorship contribution statement
K.S. Tamayo: Writing - original draft, Visualization, Investigation, Formal analysis. L.N. Heckelman: Writing - review & editing, Visualization, Investigation, Validation, Formal analysis, Methodology, Supervision. C.E. Spritzer: Writing - review & editing, Conceptualization, Investigation, Methodology, Supervision. L.E. DeFrate: Conceptualization, Funding acquisition, Writing - review & editing, Methodology, Supervision, Resources. A.T. Collins: Conceptualization, Data curation, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alexander RM, Jayes AS, 1983. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J. Zool. 201, 135–152. [Google Scholar]
- Altman RD, Fries JF, Bloch DA, Carstens J, Cooke TD, Genant H, Gofton P, Groth H, McShane DJ, Murphy WA, et al. , 1987. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 30, 1214–1225. [DOI] [PubMed] [Google Scholar]
- American College Of Rheumatology Subcommittee On Osteoarthritis Guidelines, 2000. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 43, 2000, 1905–1915. [DOI] [PubMed] [Google Scholar]
- Atukorala I, Makovey J, Lawler L, Messier SP, Bennell K, Hunter DJ, 2016. Is There a Dose-Response Relationship Between Weight Loss and Symptom Improvement in Persons With Knee Osteoarthritis? Arthritis Care Res. (Hoboken) 68, 1106–1114. [DOI] [PubMed] [Google Scholar]
- Blüher M, 2008. The inflammatory process of adipose tissue. Pediatr. Endocrinol. Rev. 6, 24–31. [PubMed] [Google Scholar]
- Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, Pols HA, Bierma-Zeinstra SM, 2007. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 56, 1204–1211. [DOI] [PubMed] [Google Scholar]
- Cahue S, Dunlop D, Hayes K, Song J, Torres L, Sharma L, 2004. Varus-valgus alignment in the progression of patellofemoral osteoarthritis. Arthritis Rheum. 50, 2184–2190. [DOI] [PubMed] [Google Scholar]
- Cancello R, Clément K, 2006. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG 113, 1141–1147. [DOI] [PubMed] [Google Scholar]
- Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA, 2014. Mechanical, Anatomical, and Kinematic Axis in TKA: Concepts and Practical Applications. Curr. Rev. Musculoskelet Med. 7, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman JL, Widmyer MR, Leddy HA, Utturkar GM, Spritzer CE, Moorman CT 3rd, Guilak F, DeFrate LE, 2013. Diurnal variations in articular cartilage thickness and strain in the human knee. J. Biomech. 46, 541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Hatcher CC, Kim SY, Ziemian SN, Spritzer CE, Guilak F, DeFrate LE, McNulty AL, 2018a. Selective Enzymatic Digestion of Proteoglycans and Collagens Alters Cartilage T1rho and T2 Relaxation Times. Ann. Biomed. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Hu G, Newman H, Reinsvold MH, Goldsmith MR, Twomey-Kozak JN, Leddy HA, Sharma D, Shen L, DeFrate LE, Karner CM, 2021. Obesity alters the collagen organization and mechanical properties of murine cartilage. Sci. Rep. 11, 1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Kulvaranon M, Spritzer CE, McNulty AL, DeFrate LE, 2020. The Influence of Obesity and Meniscal Coverage on In Vivo Tibial Cartilage Thickness and Strain. Orthop. J. Sports. Med. 8, 2325967120964468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Kulvaranon ML, Cutcliffe HC, Utturkar GM, Smith WAR, Spritzer CE, Guilak F, DeFrate LE, 2018b. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Res. Ther. 20, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook BS, Collins AT, Lad NK, Spritzer CE, Wittstein JR, DeFrate LE, 2021. Effect of walking on in vivo tibiofemoral cartilage strain in ACL-deficient versus intact knees. J. Biomech. 116, 110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutcliffe HC, Davis KM, Spritzer CE, DeFrate L, 2020. The Characteristic Recovery Time as a Novel, Noninvasive Metric for Assessing In Vivo Cartilage Mechanical Function. Ann. Biomed. Eng. 48, 2901–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutcliffe HC, Kottamasu PK, McNulty AL, Goode AP, Spritzer CE, DeFrate LE, 2021. Mechanical metrics may show improved ability to predict osteoarthritis compared to T1rho mapping. J. Biomech. 129, 110771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du JY, Sivasundaram L, Trivedi NN, Voos JE, Victoroff BN, 2021. Obesity Is Preferentially Associated With Patellofemoral Compartment Wear: A Magnetic Resonance Imaging Assessment. J. Am. Acad. Orthop. Surg. 29, e722–e731. [DOI] [PubMed] [Google Scholar]
- Duncan R, Peat G, Thomas E, Hay EM, Croft P, 2011. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann. Rheum. Dis. 70, 1944–1948. [DOI] [PubMed] [Google Scholar]
- Felson DT, 2005. Relation of obesity and of vocational and avocational risk factors to osteoarthritis. J. Rheumatol. 32, 1133–1135. [PubMed] [Google Scholar]
- Galic S, Oakhill JS, Steinberg GR, 2010. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 316, 129–139. [DOI] [PubMed] [Google Scholar]
- Gersing AS, Solka M, Joseph GB, Schwaiger BJ, Heilmeier U, Feuerriegel G, Nevitt MC, McCulloch CE, Link TM, 2016. Progression of cartilage degeneration and clinical symptoms in obese and overweight individuals is dependent on the amount of weight loss: 48-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 24, 1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL, 2017. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- Hart HF, Van Middelkoop M, Stefanik JJ, Crossley KM, Bierma-Zeinstra S, 2020. Obesity is related to incidence of patellofemoral osteoarthritis: the Cohort Hip and Cohort Knee (CHECK) study. Rheumatol. Int. 40, 227–232. [DOI] [PubMed] [Google Scholar]
- Heckelman LN, Riofrio AD, Vinson EN, Collins AT, Gwynn OR, Utturkar GM, Goode AP, Spritzer CE, DeFrate LE, 2020a. Dose and Recovery Response of Patellofemoral Cartilage Deformations to Running. Orthop J Sports Med 8, 2325967120967512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckelman LN, Smith WA, Riofrio AD, Vinson EN, Collins AT, Gwynn OR, Utturkar GM, Goode AP, Spritzer CE, DeFrate LE, 2020b. Quantifying the biochemical state of knee cartilage in response to running using T1rho magnetic resonance imaging. Sci. Rep. 10, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invitti C, 2002. Obesity and low-grade systemic inflammation. Minerva Endocrinol. 27, 209–214. [PubMed] [Google Scholar]
- Jeffery AK, 1975. Osteophytes and the osteoarthritic femoral head. J. Bone Joint Surg. Br. 57, 314–324. [PubMed] [Google Scholar]
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, Gunther K, Hauselmann H, Herrero-Beaumont G, Kaklamanis P, Lohmander S, Leeb B, Lequesne M, Mazieres B, Martin-Mola E, Pavelka K, Pendleton A, Punzi L, Serni U, Swoboda B, Verbruggen G, Zimmerman-Gorska I, Dougados M, 2003. Standing Committee for International Clinical Studies Including Therapeutic Trials, E., 2003. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann. Rheum. Dis. 62, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellgren J, Lawrence J, 1957. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Browning RC, Lerner ZF, 2019. The effects of pediatric obesity on patellofemoral joint contact force during walking. Gait Post. 73, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff MF, Amrami KK, Kaufman KR, 2007. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis Cartilage 15, 198–204. [DOI] [PubMed] [Google Scholar]
- Lad NK, Liu B, Ganapathy PK, Utturkar GM, Sutter EG, Moorman Iii CT, Garrett WE, Spritzer CE, DeFrate LE, 2016. Effect of normal gait on in vivo tibiofemoral cartilage strains. J. Biomech. 49, 2870–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TQ, Morris G, Csintalan RP, 2003. The influence of tibial and femoral rotation on patellofemoral contact area and pressure. J. Orthop. Sports Phys. Ther. 33, 686–693. [DOI] [PubMed] [Google Scholar]
- Li LP, Herzog W, 2004. Strain-rate dependence of cartilage stiffness in unconfined compression: the role of fibril reinforcement versus tissue volume change in fluid pressurization. J. Biomech. 37, 375–382. [DOI] [PubMed] [Google Scholar]
- Matzat SJ, van Tiel J, Gold GE, Oei EH, 2013. Quantitative MRI techniques of cartilage composition. Quant. Imag. Med. Surg. 3, 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty AL, Miller MR, O’Connor SK, Guilak F, 2011. The effects of adipokines on cartilage and meniscus catabolism. Connect. Tissue Res. 52, 523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, Ettinger WH, Pahor M, Williamson JD, 2004. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: The arthritis, diet, and activity promotion trial. Arthritis Rheum. 50, 1501–1510. [DOI] [PubMed] [Google Scholar]
- Messier SP, Resnik AE, Beavers DP, Mihalko SL, Miller GD, Nicklas BJ, deVita P, Hunter DJ, Lyles MF, Eckstein F, Guermazi A, Loeser RF, 2018. Intentional Weight Loss in Overweight and Obese Patients With Knee Osteoarthritis: Is More Better? Arthritis Care Res (Hoboken) 70, 1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor EC, Utturkar GM, Widmyer MR, Abebe ES, Collins AT, Taylor DC, Spritzer CE, Moorman CT 3rd, Garrett WE, DeFrate LE, 2014. The effects of femoral graft placement on cartilage thickness after anterior cruciate ligament reconstruction. J. Biomech. 47, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Akyaw KA, Heckelman LN, Cutcliffe HC, Sutter EG, Englander ZA, Spritzer CE, Garrett WE, DeFrate LE, 2018. A comparison of patellofemoral cartilage morphology and deformation in anterior cruciate ligament deficient versus uninjured knees. J. Biomech. 67, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape CS, Cutcliffe HC, Grambow SC, Utturkar GM, Collins AT, Garrett WE, Spritzer CE, DeFrate LE, 2019. A new stress test for knee joint cartilage. Sci. Rep. 9, 2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedoia V, Li X, Su F, Calixto N, Majumdar S, 2016. Fully automatic analysis of the knee articular cartilage T1rho relaxation time using voxel-based relaxometry. J. Magn. Reson. Imaging 43, 970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R, 2006. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J. Magnetic Resonance Imag. JMRI 23, 547–553. [DOI] [PubMed] [Google Scholar]
- Reilly DT, Martens M, 1972. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop. Scand. 43, 126–137. [DOI] [PubMed] [Google Scholar]
- Runhaar J, Koes BW, Clockaerts S, Bierma-Zeinstra SMA, 2011. A systematic review on changed biomechanics of lower extremities in obese individuals: A possible role in development of osteoarthritis. Obes. Rev. 12, 1071–1082. [DOI] [PubMed] [Google Scholar]
- Serebrakian AT, Poulos T, Liebl H, Joseph GB, Lai A, Nevitt MC, Lynch JA, McCulloch CE, Link TM, 2015. Weight loss over 48 months is associated with reduced progression of cartilage T2 relaxation time values: data from the osteoarthritis initiative. J. Magnetic Resonance Imag. JMRI 41, 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton LA, Elliott DM, Mow VC, 1999. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage 7, 2–14. [DOI] [PubMed] [Google Scholar]
- Sutter EG, Widmyer MR, Utturkar GM, Spritzer CE, Garrett WE Jr., DeFrate LE, 2015. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. American J. Sports Med. 43, 370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Collins AT, Heckelman LN, Kim SY, Utturkar GM, Spritzer CE, Garrett WE, DeFrate LE, 2019. Activities of daily living influence tibial cartilage t1rho relaxation times. J. Biomech. 82, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsworth K, Paley D, 1994. Malalignment and degenerative arthropathy. Orthop. Clin. North Am. 25, 367–377. [PubMed] [Google Scholar]
- Tilg H, Moschen AR, 2008. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 14, 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakula MN, Fisher KL, Garcia SA, Holmes SC, Post BK, Costa PB, Pamukoff DN, 2019. Quadriceps Impairment Is Associated with Gait Mechanics in Young Adults with Obesity. Med. Sci. Sports Exerc. 51, 951–961. [DOI] [PubMed] [Google Scholar]
- Van Hooren B, Fuller JT, Buckley JD, Miller JR, Sewell K, Rao G, Barton C, Bishop C, Willy RW, 2020. Is Motorized Treadmill Running Biomechanically Comparable to Overground Running? A Systematic Review and Meta-Analysis of Cross-Over Studies. Sports Med. 50, 785–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ai S, Tian F, Liow MHL, Wang S, Zhao J, Tsai TY, 2020. Higher Body Mass Index Is Associated With Biochemical Changes in Knee Articular Cartilage After Marathon Running: A Quantitative T2-Relaxation MRI Study. Orthop. J. Sports Med. 8, 2325967120943874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmyer MR, Utturkar GM, Leddy HA, Coleman JL, Spritzer CE, Moorman CT 3rd, DeFrate LE, Guilak F, 2013. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum. 65, 2615–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]