Abstract
Objectives
This study systematically reviewed the literature on the effect of home-based supportive care (HbSC) programmes on the quality of life (QoL) of patients with advanced cancer.
Methods
The research question ‘Do home-based supportive care programmes for patients with advanced cancer improve their QoL?’ was addressed. After registering the plan with PROSPERO (CRD42022341237), literature published from 1 January 1990 to 30 May 2023 was searched on PubMed, Embase, Cochrane database, CINAHL and Web of Science, and reviewed for inclusion based on predefined criteria. This review only included trial studies published in English.
Results
Of 5,276 articles identified, 17 studies were judged suitable for inclusion in this review. The components of HbSC programmes included home visits, patient and caregiver education, home nursing, psychotherapy, exercise, telephone consultation, and multidisciplinary team meetings. Nine studies reported improvements in QoL, including social functioning, emotional functioning, and subjective QoL.
Conclusion
HbSC programmes appear to enable the improvement of the QoL of patients with advanced cancer. The area of QoL that shows improvement could vary depending on the HbSC components. More studies that address HbSC programmes are needed to select patients at the proper time and provide suitable programmes for patients to benefit most.
Keywords: Supportive care, Home Care Services, Quality of life, Cancer
WHAT IS ALREADY KNOWN ON THIS TOPIC
Patients with advanced cancer prefer to live at home, accepting the inevitable and preparing for their death.
Home-based supportive care (HbSC) programmes for patients receiving palliative cancer care have provided more satisfactory medical practices.
However, prior studies have not thoroughly investigated the effects of HbSC programmes on the quality of life (QoL).
WHAT THIS STUDY ADDS
HbSC programmes consisted of home visits, patient and caregiver education, home nursing, psychotherapy, exercise, telephone consultation and multidisciplinary team meetings.
HbSC programmes appear to be able to improve QoL in patients with advanced cancer.
Service components provided in HbSC programmes were related to various areas of QoL.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
ΗbSC programmes should encompass multidisciplinary service components and include team meetings to exchange opinions.
HbSC programmes are needed to select patients at the proper time and provide suitable programs for patients to benefit most.
Introduction
Improvements in the treatment and management of cancer have led to an increase in life years in patients with advanced cancer.1 2 As all patients could not be cured, several patients with advanced cancer are surviving with cancer. To manage their cancer-related symptoms and strive their daily lives, hospitalisation is frequently needed; even though that is an unwanted experience for them.3 Patients with advanced-stage cancer have a desire to live at home to accept the inevitable, and to prepare for their death.4 However, compared with the enormous interest and investment in cancer treatment, there is relatively insufficient interest and investment in care for the lives of patients with advanced cancer at home.
A literature review on the supportive care needs of patients with cancer suggests that supportive treatments, including the provision of information and spiritual support, are necessary.5 This is particularly relevant for elderly patients with cancer, who also require the need for extended support networks beyond immediate family members and assistance with financial issues. To enable patients to stay at home until the end of life, various factors come into play, including patient preferences, home healthcare provision, social support networks, diverse healthcare policies and the advancement of palliative care.6 In addition to various information and spiritual support, patients with advanced cancer may require daily medical care, and if there is restricted access to the necessary care and medical services, they may also need frequent hospitalisations and readmissions against their wishes.7 8 To facilitate their stay at home, appropriate services are crucial, such as home-based supportive care (HbSC) programmes. HbSC involves medical staff visiting patients to provide medical service, allowing patients to live in their preferred homes. Patients receiving palliative care expressed satisfaction with the medical interventions provided through HbSC.9 Through a systematic review, Higginson and Sen-Gupta verified that home care was the favoured choice among patients with advanced cancer.10 Therefore, to meet patient preferences and elevate their quality of life (QoL), HbSC for patients with advanced cancer is a valuable endeavour.
Healthcare systems have been moving towards a value-based healthcare system that emphasises value over volume of services in recent years.11 Value could be defined as outcomes achieved considering the individual patient rather than volume of services delivered by healthcare providers.12 In this aspect, the value of HbSC can be measured by the improvement in the QoL of patients with advanced cancer receiving HbSC. However, the effect of HbSC on patients’ QoL has not been thoroughly investigated before. A systematic review in 1998 concluded that the effectiveness of comprehensive home care programmes is still ambiguous, with only two out of five randomised studies noting positive effects on the physical aspects of patients’ QoL.13 In a systematic review from 2016, the level of QoL varied depending on the patient group included in this study, and a lack of controlled clinical trials for HbSC targeting patients with advanced cancer was highlighted.14 Nevertheless, there has been no investigation on studies after 2016, and no research on the impact of the provided programmes on QoL. Therefore, a comprehensive and systematic review is needed on the effects of HbSC intervention programmes on the QoL of patients with advanced cancer. This study performed a systematic review to assess the impact of HbSC programmes on the QoL in patients with advanced cancer.
Materials and methods
Search strategy and selection methods
The review question was as follows: ‘Do supportive home care programmes for patients with advanced cancer improve their QoL and reduce unplanned hospital visits?’ The protocol of this systematic review was registered in PROSPERO (CRD42022341237). We searched articles from PubMed, Embase, Cochrane database, CINAHL and Web of Science, published from 1 January 1990 to 30 May 2023. The search strategy was developed with an experienced librarian as table 1, box 1.
Table 1.
PICOs for the systematic review
| PICO elements | Keywords |
| P (Patient) | Patients with advanced cancer |
| I (Intervention) | Interventional home care programme for participants |
| C (Control) | Usual care |
| O (Outcome) | Quality of life |
Box 1. Search strings for the systematic review (OVID Medline).
Search terms
exp Neoplasms/
cancer*.ab,ti.
neoplasm*.ab,ti.
(tumor* or tumour*).ab,ti.
oncol*.ab,ti.
carcinoma*.ab,ti.
malignan*.ab,ti.
Malignanc*.ab,ti.
Neoplasia*.ab,ti.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
exp Home Care Services/
exp Home Care Agencies/
((home or domicil* or outreach or resident* or housing) adj3 (agencie* or team* or center* or centre* or treat* or care or interven* or therap* or management or model* or program* or service* or base* or nurs* or palliative* or health or visit*)).ab,ti.
((posthospital or communit* or mobile or ambulator*) adj3 (agencie* or team* or center* or centre* or treat* or care or interven* or therap* or management or model* or program* or service* or base* or nurs* or palliative* or health or visit*)).ab,ti.
(homecare or home care or homebased or home based or domiciliary care).ab,ti.
11 or 12 or 13 or 14 or 15
exp ‘Quality of Life’/
(Qualit* adj3 Life).ab,ti.
(well being or wellness or QoL or HRQoL).ab,ti.
17 or 18 or 19
exp ‘Randomized Controlled Trials as Topic’/
(rct or rcts).ab,ti.
randomi*.ab,ti.
(trial or trials).ab,ti.
Random-Allocat*.ab,ti.
((Double* or single* or treb* or tripl*) adj3 (Blind* or mask*)).ab,ti.
controlled trial*.ab,ti.
placebo*.ab,ti.
randomly*.ab,ti.
21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29
31 10 and 16 and 20 and 30
We investigated trial studies of home-based programmes. Trial studies included the management of medical, physical and psychological symptoms. We did not include individual components of palliative care, such as advanced care planning. The results of each search were downloaded into a reference management software program to identify duplicate articles and further review. Two authors (D-WL and IYH) screened the records and selected articles according to the predefined inclusion and exclusion criteria. The inclusion criteria were as follows: (1) a clinical trial study; (2) a study on patients with advanced cancer (incurable and/or palliative stage); (3) an intervention programme must be an HbSC programme; and (4) QoL must be reported as an outcome variable. The exclusion criteria were as follows: (1) presented outcomes in irrelevant forms; (2) not able to extract the size of the association; (3) letter, commentary, or review articles; (4) the study used an identical study population to other included study; (5) articles not written in English; and (6) non-human studies. If the two authors disagreed about the eligibility of a study, the authors agreed after discussion and deriving a mutual understanding with a third author (BC).
Data extraction
We extracted the following data from all articles using a data-extraction sheet: first author, year of publication, country, study design, number of participants, aim of the study, inclusion criteria for participants, percentage of primary cancer site of participants, exclusion criteria for participants, intervention programmes, details of the intervention programme, components of the intervention programme (home visiting, education, training, nursing, counselling, clinic visiting, tele healthcare, team meeting, period/number of visits, total programme duration), team members and their roles, provided programme for the control group, outcome measurement methods for QoL, timing of the outcome measurement, and QoL-related results including main results, effects measurement, effect size (point estimate, difference, standard deviation [SD], and 95% confidence interval).
Quality assessment
The quality assessment of each article was conducted according to the Methodology Checklist of Scottish Intercollegiate Guidelines Network (SIGN).15 After the assessment of the internal validity, the overall assessment was checked using three options: those designated as ++ (high quality; all or most of all standards are met. The results of the study will not be changed by the unmet standards); + (acceptable; some of the standards are met. It is assumed that the results will not be changed by the unmet standards); – (low quality; all or most of all standards are not met. It is assumed that the results of the study could be changed by the unmet standards).
Results
Search results
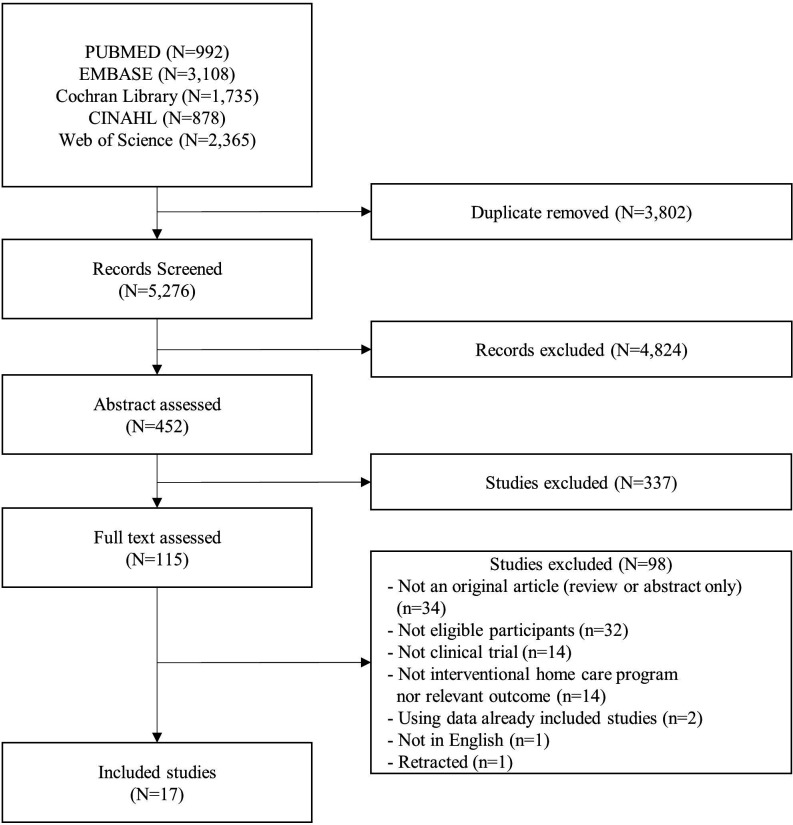
Figure 1 shows the process of selecting relevant studies for the systematic review. We screened 9078 records and removed 3802 duplicated records. Next, we excluded 4824 articles based on the title among 5,276 records screened. We assessed abstracts of 452 articles and excluded 337 irrelevant studies. Full texts of 115 studies were examined, and 98 studies were excluded. We manually checked the reference lists of the assessed full texts. Finally, 17 studies were included for the systematic review. Table 2 shows the results of the SIGN checklist for the included studies. We scored 4 studies as high-quality studies, 11 as acceptable studies and 2 as low-quality studies.
Figure 1.
Flow diagram of the study selection process.
Table 2.
SIGN checklist for randomised controlled trials
| First author (year) | 1.1. | 1.2. | 1.3. | 1.4. | 1.5. | 1.6. | 1.7. | 1.8. | 1.9. | 1.10. | 2.1. |
| Cheville et al (2019)16 | Y | Y | Y | N | Y | Y | Y | Intervention group 2=13.4% Intervention group 3=16.9%, Control group=12.8% |
Y | Y | + |
| Edbrooke et al (2019)26 | Y | Y | Y | N | Y | Y | Y | Intervention group=24.5%, Control group=23.5% | Y | Y | + |
| Nordly et al (2019)18 | Y | Y | N | N | Y | Y | Y | Intervention group=11.7%, Control group=21.17% | Y | D | + |
| Ammari et al (2018)28 | Y | Y | N | N | Y | Y | Y | Intervention group=43%, Control group=38% | Y | N | + |
| Kleijin et al (2018)19 | Y | Y | N | N | Y | Y | Y | Intervention group=30.9%, Control group=25.0% | Y | Y | + |
| Pilegaard et al (2018)29 | Y | Y | N | N | Y | Y | Y | Intervention group=18%, Control group=20% | Y | N | ++ |
| Steel et al (2016)21 | Y | Y | Y | y | Y | Y | Y | Intervention group=29.2%, Control group=35.0% | Y | Y | ++ |
| Lehto et al (2015)17 | Y | Y | Y | N | Y | Y | Y | Intervention group=80%, Control group=80% | Y | Y | + |
| Uitdehaag et al (2014)22 | Y | Y | N | N | Y | Y | Y | Intervention group=48.6%, Control group=55.9% | Y | D | ++ |
| Cheville et al (2013)27 | Y | Y | Y | Y | Y | Y | Y | Intervention group=18.2%, Control group=9.1% | Y | D | ++ |
| Northouse et al (2013)30 | Y | Y | N | N | N | Y | Y | Intervention group=38.8%, Control group=36.2% | Y | D | + |
| Xiao et al (2013)20 | Y | Y | N | N | Y | Y | N | Intervention group=22.5%, Control group=25% | Y | D | – |
| Hermann et al (2012)25 | Y | N | N | N | N | N | Y | Not provided. The total of 76% of patients answered completely | Y | N | – |
| Mills et al (2009)31 | Y | Y | N | N | Y | Y | Y | Intervention group=(2 mo) 36.8%, (4 mo) 47.4%, Control group=(2mo) 27.6%, (4mo) 53.4% | Y | D | + |
| Molassiotis et al (2009)23 | Y | Y | N | N | Y | Y | Y | Intervention group=49%, Control group=55% | Y | D | + |
| De Wit et al (2001)24 | Y | Y | N | N | Y | Y | Y | Intervention group=41%, Control group=20% | Y | D | + |
| Jordhøy et al (2001)32 | Y | Y | Y | N | N | N | Y | Questionnaires completed in 68%–78% at 6 months, but more than half the participants were dead in 6 months. | Y | D | + |
1.1 The study addresses an appropriate and clearly focused question.
1.2 The assignment of participants to treatment groups is randomised (excluded item in quasi-experimental study).
1.3 An adequate concealment method is used (excluded item in quasi-experimental study).
1.4 The design keeps participants and investigators ‘blind’ about treatment allocation.
1.5 The treatment and control groups are similar at the start of the trial.
1.6 The only difference between groups is the treatment under investigation.
1.7 All relevant outcomes are measured in a standard, valid, and reliable manner.
1.8 What percentage of the individuals or clusters recruited into each treatment arm of the study dropped out before the study was completed?
1.9 All participants are analysed in the groups to which they were randomly allocated (often referred to as intention-to-treat analysis).
1.10 Where the study is conducted at more than one site, results are comparable for all sites.
2.1. How well was the study conducted to minimise bias?
SIGN, Scottish Intercollegiate Guidelines Network.
Description of identified studies
Table 3 shows the included studies and their respective study design, country of origin, number of participants, inclusion criteria and primary cancer site of participants. All studies were controlled trial studies, including 16 RCT studies and 1 controlled study. The countries of study origin were the USA (n=5), Denmark (n=3), the UK (n=2), the Netherlands (n=3), Norway (n=1), China (n=1), Australia (n=1) and Germany (n=1). The number of participants ranged from 40 to 516.16 17 All studies were characterised by patients with advanced cancer on palliative care, including the following terms: palliative, unresectable, incurable, metastatic, inoperable and few months of life expectancy. Participants with various primary cancer sites were identified among the included studies.
Table 3.
Description of included studies
| Study | Design | Country | N | Aim | Inclusion criteria | Primary cancer site |
| Cheville et al (2019)16 | RCT | USA | 516 | To determine whether collaborative telerehabilitation and pharmacological pain management improve function, lessen pain and reduce requirements for inpatient care. | 1) ≥ 18 years 2) Stage IIIC or IV solid or hematologic cancer. 3) AM-PAC basic mobility score ranging from 53 to 66. 4) life expectancy of more than 6 months. 5) Fluency in English, sufficient auditory acuity for effective telephone conversation. |
Haematological (20.2%) Prostate (17.8%) Breast (14%) GI (10.1%) Gynaecological (7.4%) Lung (7.2%) Endocrine (5.4%) Melanoma (3.7%) Renal (3.1%) Other (11.2%) |
| Edbrooke et al (2019)26 | RCT | Australia | 92 | To assess the efficacy of home-based rehabilitation versus usual care in inoperable lung cancer. | 1) ≥ 18 years. 2) Able to read and write English. 3) ECOG-PS of ≤ 2. 4) Clinical Frailty Scale score of <7. 5) Physician-rated life expectancy of >6 months. |
Non-small cell lung cancer (100%) |
| Nordly et al (2019)18 | RCT | Denmark | 340 | To investigate whether a systematic fast-track transition from oncological treatment to specialised palliative care at home for patients with incurable cancer reinforced with a psychological dyadic intervention could result in more time spent at home and death at home. Secondary aims were to investigate effects on QoL, symptomatology and survival. | 1) ≥ 18 years 2) Incurable cancer with limited or no antineoplastic treatment options or resignation of antineoplastic treatment 3) A wish in agreement with their closest informal caregiver to spend most time possible at home |
Lung (23.2%) GI (19.8%) Female genitalia (13.2%) CNS (11.1%) Head and neck (5.7%) Breast (7.5%) Connective tissue (4.5%) Others (3.9%) |
| Ammari et al (2018)28 | RCT | Denmark | 57 | To test whether a family-and-coping-oriented basic palliative homecare intervention can enhance the QoL, decrease anxiety and depression for patients with advanced cancer and their closest relatives, and reduce patients’ acute hospital admissions. | 1) Palliative nature 2) Patients had to live in their homes in one of the two main municipalities of the capital. 3) Both patients and relatives had to be Danish speaking, ≥ 18 years |
GI (21%) Lung (24%) Breast (3.5%) Prostate (33.3%) Head and neck (8.7%) Gynaecological (5.2%) Neuroendocrine (3.5%) |
| Kleijin et al (2018)19 | RCT | Netherlands | 107 | To evaluate the efficacy of an intervention combining Life-Review Therapy and Memory Specificity Training (LRT-MST) to improve ego-integrity and despair among patients with cancer in palliative care. | 1) Adult > 18-years-old patients with all types of cancer and all cancer treatment modalities 2) Receiving palliative care 3) An expected prognosis of more than three months |
Lung (61.7%) Breast (4.7%) Haematology (21.5%) Head and neck(1.0%) Other(10.3%) |
| Pilegaard et al (2018)29 | RCT | Denmark | 242 | To evaluate the efficacy of the ‘Cancer Home Life-Intervention’ compared with usual care regarding patients’ performance of, and participation in, everyday activities, and their health-related QoL. | 1) Home-living adults (⩾18 years) diagnosed with advanced cancer 2) WHO Performance Status 1–2 |
Gastrointestinal (30.6%), Lung (19.8%) Breast (15.3%) Prostate (12.4%) Head and neck (7.0%) Bladder (6.2%) Gynaecological (5.8%) Other (2.5%) Missing (0.4%) |
| Steel et al
(2016)21 |
RCT | USA | 178 | To examine the efficacy of a collaborative care intervention in reducing depression, pain and fatigue and improve QoL. | 1) Patients diagnosed with hepatocellular, cholangiocarcinoma, gallbladder, neuroendocrine, and pancreatic carcinoma or other primary cancers that have metastasised to the liver (eg, ovarian, breast, colorectal). 2) biopsy and/or radiograph proven diagnosis of cancer 3) ≥ 21 years |
Hepatocellular carcinoma and cholangiocarcinoma (64%) Other primary cancers with liver metastases (36%) |
| Lehto et al
(2015)17 |
RCT | USA | 40 | To test acceptability, feasibility and preliminary efficacy of the mindfulness-based therapies protocol on symptom and HRQoL outcomes for patients receiving treatment for advanced lung cancer. | 1) English speaking 2) ≥ 21 years 3) Active treatment (radiation and/or chemotherapy) 4) Diagnosis of stage III/IV non-small cell lung cancer 5) Karnofsky functional status score ≥ 80 |
Non-small cell lung cancer (100%) |
| Uitdehaag et al (2014)22 | RCT | Netherlands | 66 | To compare nurse-led follow-up at home with conventional medical follow-up in the outpatient clinic for patients with incurable primary or recurrent oesophageal, pancreatic, or hepatobiliary cancer. | Patients with unresectable or recurrent upper GI cancer | Oesophagus/gastric (51.5%) Pancreatic/duodenum (22.7% Hepatic/common bile duct (25.8%) |
| Cheville et al (2013)27 | RCT | USA | 66 | To conduct an adequately powered trial of a home-based exercise intervention that can be facilely integrated into established delivery and reimbursement structures. | 1) Patients with stage IV lung and colorectal cancer 2) Ambulatory Post-Acute Care Computer Adaptive Test scores between 50 and 75 |
Colorectal (48.4%) Lung (51.6%) |
| Northouse et al (2013)30 | RCT | USA | 484 | To find out if specific interventions (brief or extensive) are more effective than usual care for patient–caregiver pairs and whether certain factors, like a patient’s risk for distress, make the interventions more or less effective. | 1) Diagnosed with advanced breast, colorectal, lung, or prostate cancer (stage III or IV), within diagnosed, progression, or change of treatment of cancer within 6 months 2) A life expectancy of ≥ 6 months 3) Aged ≥ 21 years 4) Living within 75 miles of participating cancer centres and having a family caregiver |
Breast (32.4%) Colorectal (25.4%) Lung (29.1%) Prostate (13%) |
| Xiao et al
(2013)20 |
RCT | China | 80 | To determine the effect of a life-review programme on QoL among Chinese patients with advanced cancer. | 1) ≥ 18 years, newly admitted home-base hospice patients 2) Advanced cancer awareness of their diagnosis, prognosis, and therapy 3) No communication impairments |
GI cancer (50%) Respiratory (28.7%) Gynaecologic (17.5%) Others (3.7%) |
| Hermann et al
(2012)25 |
Controlled trial | Germany | 87 | To evaluate whether a specific training in Germany (PAMINO) has any improving impact on the care of palliative patients and their health-related QoL. | 1) ≥ 18 years, Palliative situation with cancer, 2) Sufficient command of German to understand the study information and the questionnaires and |
Lung (13.5%) Colon (12.5%) Breast (11.5) Stomach (8.3%) Prostate (7.3%) Other (46.9%) |
| Mills et al
(2009)31 |
RCT | UK | 115 | To examine the effect of weekly completion of a patient-held QoL diary in routine oncology practice for palliative care patients. | 1) Patients with inoperable lung cancer | Lung cancer |
| Molassiotis et al (2009)23 | RCT | UK | 164 | To assess the effectiveness of a symptom-focused home care programme in patients with cancer who were receiving oral chemotherapy related to toxicity levels, anxiety, depression, QoL and service utilisation | 1) 18 years or older who had breast or colorectal cancer 2) Life expectancy longer than six months 3) Starting capecitabine, could self-care 4) could communicate in English |
Colorectal cancer (67.1%) Breast cancer (32.9%) |
| De Wit et al (2001)24 | RCT | Netherlands | 104 | To investigate the role of district nurses in the care of patients with cancer and chronic pain at home, as well as the effects of a Pain Education Programme for patients and their district nurses. | 1) In pain for ≥ 1 month 2) Experiencing pain related to cancer, cancer therapy, or illness 3) Expected to live for at least three months 4) Could read and speak Dutch 5) Accessible by telephone 6) Not residing in a nursing home or retirement home |
Genitourinary (26.7%) Breast (24.4%), Bone, connective tissue, and skin (22.2%) Digestive organs and peritoneum (7.4%) Lip, oral cavity, and pharynx (4.4%) Respiratory and intrathoracic organs (3.7%) Other (11.1%) |
| Jordhøy et al (2001)32 | RCT (Cluster randomised trial) |
Norway | 434 | To assess the impact of comprehensive palliative care on patients’ QoL. The intervention was based on cooperation between a palliative medicine unit and the community service and was compared with conventional care. | 1) Incurable, malignant disease 2) Life expectancy between 2 and 9 months 3) Aged > 18 years |
Gastrointestinal (41.7%) Lung (12%) Breast and female genitals (15.4%) Prostate and male genitals (9.4%) Kidney/vesical/ureter (6.7%), Lymphomas (3%) Skin (2.8%) Others (9%) |
AIDS, acquired immunodeficiency syndrome; AM-PAC, Activity Measure for Post-acute Care; CNS, central nervous system; ECOG-PS, ECOG Performance Status; GI, gastrointestinal; GP, general practitioner; PAMINO, Palliativmedizinische Initiative Nordbaden; QoL,quality of life RCT, randomised controlled trial; WHO, world health organization.
Study characteristics
The types of interventions were diverse, including not only simple symptom management but also emotional support, multidisciplinary team-based patient care, rehabilitation and exercise, among others. Nordly et al,18 Kleijin et al,19 Lehto et al 17 and Xiao et al 20 examined the impact of psychological support on patients’ QoL and symptoms. Steel et al 21 investigated the effects of multidisciplinary management on QoL, depression, pain, fatigue and other factors. Uitdehaag et al,22 Molassiotis et al,23 and De Wit et al 24 assessed the effectiveness of nurse-led symptom control, while Study Hermann et al 25 examined the impact of standard education for doctor. Cheville et al,16 Edbrooke et al 26 and Cheville et al 27 evaluated the effects of rehabilitation and exercise interventions, with Cheville et al 16 specifically focusing on the effects of telerehabilitation. Ammari et al, 28 Pilegaard et al 29and Northouse et al 30 provided interventions in the form of counselling and information for patients and their caregivers. In Mills et al 31 ’s study, patients periodically measured their own QoL.
Cancer types
Most of the studies did not have restrictions on the type of cancer under investigation, while some studies specifically targeted certain cancer types. Edbrooke et al,26 Lehto et al 17 and Mills et al 31 focused on patients with lung cancer, and Steel et al 21 ’s study included patients with primary or secondary liver cancer. Cheville et al 27 and Molassiotis et al 23 conducted research on lung cancer (51.6%) and colorectal cancer (48.4%) or colorectal cancer (67.1%) and breast cancer (32.9%).
Inclusion criteria
Most studies targeted adults aged 18 or 21 years and older. However, in the three studies, there was no clear age criterion.22 24 27 The expected life expectancy varied, with some studies having a minimum of 3 months,19 24 others requiring at least 6 months,16 23 26 30 and some falling within the range of 2–9 months.32 Depending on the study, participants were either in a palliative or hospice care setting19 20 25 28 or in an earlier stage.17 21 22 27 29–31 The studies targeted patients with preserved functionality, characterised by ECOG PS ≤2,26 WHO PS 1-2,29 Karnofsky functional status score ≥80,17 Activity Measure for Post-acute Care (AM-PAC) basic mobility score ranging from 53 to 66,16 or Ambulatory Post-Acute Care Computer Adaptive Test scores between 50 and 75.27
Characteristics of intervention
Table 4 shows the characteristics of home care programmes across the studies. We classified the characteristics of the interventions. Interventions across studies included home visit (n=12), education (n=13), nursing (n=6), psychological consultation (n=6), clinic visit (n=4), check-up via phone (n=11) and multidisciplinary team meeting (n=7). Many studies provided home visit and education, but the studies reported after 2010 additionally included psychological intervention, exercise or rehabilitation programmes.
Table 4.
Intervention characteristics in studies of home care programmes for patients with advanced cancer
| Study | Intervention characteristics | Healthcare professionals | Number of home visits | Total programme duration | Intervention description | ||||||
| Home visit | Education | Nursing | Psychological | Clinic visit | Check-up via phone | Team meeting | |||||
| Cheville et al (2019)16 | X | O | O | X | O | O | O | Physical therapist, nurse, doctor | Not applicable | 6 months | Telerehabilitation with/without pharmacological pain management |
| Edbrooke et al (2019)26 | O | O | X | X | X | O | O | Physical therapist, nurse | 1–3 visits | 6 months | Home-based rehabilitation |
| Nordly et al (2019)18 | O | X | O | O | X | X | O | Doctor, nurse psychologist | ≥ 2 visits | 6 months | Existential–phenomenological therapy |
| Ammari et al (2018)28 | O | O | O | O | X | X | O | Nurse | 6 visits, every 3 weeks | 24 weeks | The FamCope intervention; consulting services to cope with problems and needs |
| Kleijin et al (2018)19 | O | X | X | O | X | X | X | Psychologist | 4 visits, ≤ 4 weeks | 2 months | Life-review therapy and memory specificity training |
| Pilegaard et al (2018)29 | O | O | X | X | X | O | O | Occupational therapist | 1–3 visits | ≤ 3 weeks | Cancer home-life Intervention |
| Steel et al (2016)21 | X | X | X | X | O | O | X | Doctor, nurse | Not applicable | 6 months | Collaborative care intervention |
| Lehto et al (2015)17 | X | O | X | O | X | O | X | Nurse | Not applicable | 11 weeks | Mindfulness-based therapies |
| Uitdehaag et al (2014)22 | O | O | X | X | O | O | X | Nurse, doctor | Monthly | Up to 13 months | Nurse-led follow-up |
| Cheville et al (2013)27 | X | O | X | X | X | O | X | Physical therapist, nurse, doctor | Not applicable | 8 weeks | Home-based exercise program |
| Northouse et al (2013)30 | O | O | X | O | X | O | X | Nurse | 4 visits | 10 weeks | Home-based informative and supportive programme |
| Xiao et al (2013)20 | O | O | O | O | O | O | X | Nurse | 3 visits per 3 weeks | 6 weeks | Psychological support programme |
| Hermann et al (2012)25 | O | O (for GPs) | X | X | X | X | X | Doctor | Monthly | 6 months | (service provided by) PAMINO trained GP |
| Mills et al (2009)31 | X | O | X | X | X | X | X | Not described | Not applicable | 16 weeks | Regular recording of QoL data |
| Molassiotis et al (2009)23 | O | O | X | X | X | O | O | Nurse | 1 visit per each cycle of the chemotherapy | 4.5 months (six cycles of chemotherapy) | Home care nursing programme |
| De Wit et al (2001)24 | O | O | O | X | X | O | X | Nurse (in the hospital and district) | Average 7.4 visits | 8 weeks | Pain education programme |
| Jordhøy et al (2001)32 | O | X | O | X | X | X | O | (Community) GPs, nurse (hospital) nurse, physiotherapist, social workers, nutritionist, priest, physician | Not described | Not described | Palliative medicine unit |
GP, general practitioner; PAMINO, Palliativmedizinische Initiative Nordbaden; QoL, quality of life.
Interventions were provided by various healthcare professions: nurse, doctor, dietitian, health-technician, coordinator, social workers, physiotherapist, nutritionist, priest, psychologist and physical therapist. Most of the studies were driven by nurses; further, interventions were provided in addition to services other than nursing services. The most frequent home care service was provided through a home visit,18–20 22–26 28–31 and some services checking the status of patients via phone.16 17 20–24 26 27 29 30 There was a type of intervention in which the QoL of the patient was continuously written in a diary and only reported to the medical staff.31 The total programme duration ranged from 3 weeks29 up to 13 months,22 and the number of visits varied across the studies.
Uitdehaag et al reported the results of home-based nurse-led follow-up for patients with advanced cancer, an experienced specialist nurse visited the patient’s home once a month to conduct repeated assessments of the patient’s symptoms and issues.22 Regular communication occurred with the attending physician and the patient’s general practitioner. When necessary, patients had the option to contact the nurse by phone. Palliativmedizinische Initiative Nordbaden (PAMINO) is a multidisciplinary educational programme based on the curriculum of the German Medical Association (Bundesärztekammer) and the Association for Palliative Medicine, and the results was reported by Mills et al. 25 It covers topics such as pain psychology, legal aspects, clear communication with patients, ethics and attitudes, pain management, symptom control, specialised pain therapy, end-of-life care requirements, physician communication, burnout, palliative care in geriatrics and long-term care. Northouse et al reported the effects of home-based informative and supportive programme in 2012, a home-based dyadic intervention that provides information and support to patients with cancer and caregivers through nurses with home visits and contact via phone.30 Molassiotis et al reported that home care nursing programme is a multimodal programme and includes symptom assessment, patient education, and/or treatment of symptoms based on the agreed protocols.23 Home visits occur during the first week of the programme, and subsequent home visits or monitoring phone calls are performed per week during all cycles by a nurse. When multiple toxicities occurred, the home care nurses assessed patients further, asking whether they could be managed at home or required additional medical support, such as earlier consultations with their clinicians or emergency departments or cancer centres, and facilitated these visits. In the study of De Wit about the pain education programme, patients were called at home at 3 and 7 days postdischarge to determine whether the pain information was sufficient, and district nurses received patients’ pain complaints from the hospital and visited their homes.24 Mills et al tested the effects of recording QoL data. During the regular recording of QoL data intervention, patients completed their QoL diary at home regularly each week to share the information with any health professional involved in their care for 16 weeks.31 Jordhøy et al provided the programme by the palliative medicine unit (PMU), follow-up consultations by the GP and the community nurse at home.32 The PMU consultant team comprised the GP, the community nurse and a consultant nurse or physician from the PMU. With the patient and the informal caregiver, individual treatment plans were set up, and GP and the community nurse follow-up consultations at home were arranged according to patients’ needs and predefined minimum standards. The PMU also participated in the inpatient care. Pilegaard et al conducted that the cancer home-life intervention is a tailored, occupational therapy-based, and adaptive programme by occupational therapists.29 They participated in three home visits during the study period. This programme enabled patients to perform and participate in everyday activities at home that they prioritise but face difficulties performing.
Cheville et al reported in 2013 that the Home-Based Exercise Programme with a 90-min instructional sessions and a pedometer-based walking programme comprising two sets of five-exercise routines.27 At 1, 3, 5 and 7 weeks from the baseline, patients contact the physical trainers who had provided their initial instruction via calls for a short interview to screen for concerning signs or symptoms. In 2019, Chevillie et al reported telerehabilitation that home-based exercise programme, with or without pharmacological pain management.16 It involved the implementation of an individualised fitness programme delivered by physical therapist fitness care managers through telephone communication, and in some cases, nurse pain care manager-directed pharmacological pain management. Contrastingly, the control group underwent automated monitoring at intervals of 2 weeks or 1 month to assess pain and function. The experimental group, on the other hand, evaluated pain and function at baseline, 3 months, and 6 months through telephone interviews. Edbrooke et al also reported other home-based exercise programme.26 In the home-based rehabilitation programme, participants received an initial home visit, followed by weekly phone calls to review their exercise programme and receive symptom management support. The exercise programme consisted of aerobic exercise at least twice a week and resistance training for the lower limbs. To standardise the programme, the physical therapist scripted the content of each exercise session, including various aspects of the exercises. Similarly, the nurse scripted sessions during phone calls to address symptom management and the current management strategies. Assessments were conducted at baseline, 9 weeks and 6 months.
Nordly et al reported that the existential–phenomenological therapy combines specialised palliative care with psychological intervention, promoting QoL and relieving physical, mental, social and spiritual suffering.18 Patients and informal caregivers had two sessions with a psychologist within the first month, followed by needs-based interventions. The FamCope intervention study reported by Ammari et al, provided consulting services to cope with problems and needs of patients with advanced cancer.28 Families in the experimental arm received six home visits in a 3-week interval, in addition to usual care. Kleijin et al investigated the effects of the Life-Review Therapy and Memory Specificity Training, comprising an approximately 1-hour interview programme with 4 weekly sessions on a particular lifetime period (childhood, adolescence, adulthood and whole life span) conducted with a psychologist.19 Steel et al investigated the collaborative care intervention providing a psychoeducational website with self-management strategies, bulletin board and other resources to participants.21 Additionally, participants had face-to-face meetings with a care coordinator during physician appointments every 2 months, and telephone follow-up sessions occurred every 2 weeks. The assessment of the intervention’s effectiveness was conducted at baseline and after 6 months. Letho et al reported the effects of mindfulness-based therapies consisting of trained nurses visiting patients at their homes and conducting 45-min sessions, which included gentle yoga training, practices to expand awareness and relevant discussions. This intervention lasted for 6 weeks.17 The psychological support programme comprises three sessions to review patients’ lives and formulating a life-review booklet with Erikson’s theory and Confucian thoughts.20
Outcomes related to QoL
Table 5 shows the effect of home care programmes on patients’ QoL. QoL tools used in the studies included the following: Euro QoL-5 Dimension, 36-Item Short Form Survey European Organisation for Research and Treatment of Cancer (EORTC) QoL Questionnaire, Core 30 (EORTC QLQ-C30); a shortened version of the EORTC QLQ-C30 (EORTC QLQ-C15-PAL); Functional Assessment of Cancer Therapy—General (FACT-G) and Functional Assessment of Cancer Therapy—Lung (FACT-L); Hospital Anxiety and Depression Scale (HADS). Nine studies reported that interventions improved QoL.21 16–18 20 23 26 27 30 A study shows that psychological interventions improved social functioning (−12.7±5.1, p = 0.014), global QoL (−8.2±4.0, p=0.04) and emotional functioning (−9.1±3.5, p=0.007) of EROTC QLQ-C30 after 6 months.18 Another study shows the psychological support effects of the programme on overall QoL of patients with advanced cancer; between-group (p<0.001) and interaction effects (p<0.001) were significant.20 In another study of the home-based informative and supportive programme, the social domain of patients’ QoL was significantly different (p=0.002) between the intervention and control groups, measured by the interaction term in Multivariate Analysis of Covariance for the repeated measured data.30 Mean changes and SD of FACT-G subscale between the home-based exercise intervention and control groups in mobility (4.88±4.66 vs 0.23±5.22, p=0.002), fatigue (4.46±8.65 vs −0.79±9.11, p=0.03), and sleep quality (1.46±1.88 vs −0.11±1.71, p=0.002).27 Collaborative care intervention showed the improvement among patients with advanced cancer in overall QoL from baseline to 6 months follow-up.21 Patients with home care nursing programmes showed improved financial problem and decreased anxiety, compared to before its implementation.23 However, some interventions reduced QoL. In cases in which patients with lung cancer who could not operate were regularly recorded for QoL, and there was no provision of appropriate information, mean differences of FACT-L, FACT-G and Palliative Care QoL Index (PQLI) changes in score from 0 to 4 months between the intervention and control groups were −10.4 (p=0.04),–8.7 (p=0.04) and 0 (p=0.93), respectively.31
Table 5.
Results of home care programmes on QoL for patients with advanced cancer
| Study | Inclusion criteria | Intervention | QoL measurement | Measurement timing | Result |
| Cheville et al (2019)16 | 1) ≥ 18 years 2) Stage IIIC or IV solid or hematologic cancer. 3) Activity Measure for Post-acute Care (AM-PAC) basic mobility score ranging from 53 to 66. 4) Life expectancy of more than 6 months. 5) Fluency in English, sufficient auditory acuity for effective telephone conversation. |
Telerehabilitation with/with out pharmacological pain management | EQ-5D-3L | Baseline, 3,6 months by telephone interview. | Compared with the control group, the telerehabilitation arm 2 had improved QoL (0.04; 95% CI 0.004 to 0.071; p=0.01). |
| Edbrooke et al (2019)26 | 1) ≥ 18 years. 2) Able to read and write English. 3) ECOG-PS of ≤2. 4) Clinical Frailty Scale score of <7. 5) Physician-rated life expectancy of >6 months. |
Home-based rehabilitation | FACT-L | Baseline, 9 weeks, 6 month | At 6 months, it showed significant differences favouring the intervention group. (FACT-L total score mean 13.0 (3.9 to 22.1), p=0.005, FACT-L Lung Cancer Subscale 4.7 (1.6 to 7.7), p=0.003, FACT-L Trial Outcome Index 10.4 (4.0 to 16.9), p<0.001) |
| Nordly et al (2019)18 | 1) Aged ≥ 18 years 2) Incurable cancer with limited or no antineoplastic treatment options or resignation of antineoplastic treatment 3) A wish in agreement with their closest informal caregiver to spend most time possible at home |
Existential–phenomenological therapy+specialised palliative care | EORTC QLQ-C30 HADS |
Baseline, 2, 4, 8 weeks 6 months |
An improvement of positive effects on SF (−12.7 ± 5.1, p= .014), QoL (−8.2 ± 4.0, p= .040), and EF (−9.1 ± 3.5, p=0.007) after 6 months. No statistical differences between groups on HADS |
| Ammari et al (2018)28 | 1) Palliative nature 2) Patients had to live in their homes in one of the two main municipalities of the capital 3) Both patients and relatives had to be Danish speaking, ≥ 18 years |
The FamCope intervention; Consulting services to cope with problems and needs | EORTC QLQ-C30 HADS |
Baseline, 16 weeks and 24 weeks | No difference in changes in scores of outcomes for global quality-of-life, functional scales, or symptom scales on the EORTC QLQ-C30 tool. No statistical differences between groups on HADS |
| Kleijin et al (2018)19 | 1) Adult (> 18-years-old) patients with cancer all types of cancer and all cancer treatment modalities 2) Receiving palliative care 3) An expected prognosis of more than 3 months |
Life-review therapy and memory Specificity training (LRT-MST) | EORTC QLQ-C15-PAL HADS |
Baseline, 4 weeks and 8 weeks | No significant differences between the two groups were found regarding the course, distress (HADS-T: p=0.30), anxiety (HADS-A: p=0.44), depression (HADS-D: p=0.54), QoL (EORTC QLQ-PAL15, p=0.058) |
| Pilegaard et al (2018)29 | 1) Home-living adults diagnosed with advanced cancer 2) WHO Performance Status 1–2 |
Cancer home-life intervention | EORTC QLQ-C30 | Baseline, 6 weeks and 12 weeks | No significant mean change in EORTC QLQ-C30 between-group from baseline to 6-week follow-up (−0.21, 95% CI −5.97 to 5.54), and baseline to 12-week follow-up (−1.61, 95% CI −7.95 to 4.73) |
| Steel et al
(2016)21 |
1) Patients diagnosed with hepatocellular, cholangiocarcinoma, gallbladder, neuroendocrine, and pancreatic carcinoma or other primary cancers that have metastasised to the liver (eg, ovarian, breast, colorectal). 2) Biopsy and/or radiograph proven diagnosis of cancer 3) ≥ 21 years |
Collaborative care intervention | FACT-G | Baseline, 6 months | Statistically and clinically significant changes in overall QoL were observed with an effect size of 0.99 from baseline to 6 months follow-up (p=0.05, d=0.99) |
| Lehto et al
(2015)17 |
1) English speaking 2) ≥ 21 years 3) active treatment (radiation and/or chemotherapy) 4) diagnosis of stage III/IV non-small cell lung cancer 5) Karnofsky functional status score ≥ 80 |
Mindfulness-based therapies | SF-36 | Baseline, 8, 11 wks | Significant improvement and large effect sizes for physical function (p=0.01, d=0.96) and social function (p=0.01, d=0.82). |
| Uitdehaag et al (2014)22 | Patients with unresectable or recurrent upper GI cancer | Nurse-led follow-up | EuroQoL-5D and EORTC QLQ-C30 | 1.5, 4, 7, 10, 13 months | No significant effect on QoL. The difference between the median EuroQoL-5D index score in the nurse-led follow-up group and the conventional medical follow-up group was not significant at both time points (0.78, IQR 0.31 to 0.88 at one and a half months and 0.78, IQR 0.33 to 0.84 at 4 months vs 0.67, IQR 0.33 to 0.78 at one and a half months and 0.69, IQR 0.31 to 0.81 at 4 months, respectively) |
| Cheville et al (2013)27 | 1) Patients with stage IV lung and colorectal cancer Ambulatory Post-Acute Care (AM-PAC) Computer Adaptive Test (CAT) (described subsequently) scores between 50 and 75 | Home-Based Exercise Programme | FACT-G | Baseline, 8 weeks | Mean changes and SDs between the intervention and control groups in mobility (4.88±4.66 vs 0.23±5.22, p=0.002), fatigue (4.46±8.65 vs −0.79±9.11, p=0.03), and sleep quality (1.46±1.88 vs −0.109±1.71, p=0.002) |
| Northouse et al (2013)30 | 1) Diagnosed with advanced breast, colorectal, lung, or prostate cancer (stage III or IV), within diagnosed, progression, or change of treatment of cancer within 6 months 2) A life expectancy of ≥6 months 3) Aged ≥21 years 4) Living within 75 miles of participating cancer centres and having a family caregiver |
Home-based informative and supportive programme | FACT | Baseline, 3 months, and 6 months | Social domain of QoL of patients was significantly different (F = 4.28, p= 0.002) between the intervention and control groups, measured by the interaction term in MANCOVA for the repeated measured data |
| Xiao et al (2013)20 | 1) Adult (≥ 18 years), newly admitted home-base hospice patients 2) Advanced cancer awareness of their diagnosis, prognosis, and therapy 3) No communication impairments |
Psychological support programme | Self-report single-item scale (0–10) to assess overall QoL | Baseline, 3 weeks and 6 weeks | The effects of the programme on overall QoL were significant, between-group (p<0.001) and interaction effects (p<0.001) |
| Hermann et al
(2012)25 |
1) ≥ 18 years, Palliative situation with cancer, 2) Sufficient command of German to understand the study information and the questionnaires and |
PAMINO trained GP | QLQ-C15-PAL | The last available assessment from patients before either their death or the end of the 6 month observation period. | No significant difference in QLQ-C15-PAL results. Overall QoL was 37.7 (SD±25.5) in the intervention group and 39.4 (SD±26.3) in control group and the difference was not statistically significant. |
| Mills et al (2009)31 | 1) Patients with inoperable lung cancer | Regular recording of QoL data | FACT-L, FACT-G,PQLI | Baseline, 2 months and 4 months | The intervention group showed the lower QoL scores than the control group. Mean differences of FACT-L, FACT-G, and PQLI changes in score from 0 to 4 months between the intervention group and the control group were −10.4 (p=0.04), –8.7 (p=0.04), and 0.0 (p=0.93), respectively |
| Molassiotis et al (2009)23 | 1) 18 years or older who had breast or colorectal cancer 2) Life expectancy longer than 6 months 3) Starting capecitabine, could self-care 4) Could communicate in English |
Home care nursing programme (Cambridge hospital at home) |
EORTC QLQ-C30 HADS |
Baseline and every 6 weeks | QoL scores were similar between the two arms, except for financial problems. FI was significant improved in the homecare group (p=0.004, n=36 vs. p=0.248, n=30). The experimental group experienced less anxiety than did the control group (p=0.001; n=35 vs. p=0.023; n=34). There were no significant differences in depression |
| De Wit et al (2001)24 | 1) In pain for ≥ 1 month 2) Experiencing pain related to cancer, cancer therapy, or illness 3) Expected to live for at least 3 months 4) Can read and speak Dutch 5) Accessible by telephone 6) Not residing in a nursing home or retirement home |
Pain education programme | EORTC QLQ-C30 | Baseline, 2, 4, and 8 weeks post-discharge | When comparing the difference in functioning and symptom scales between pretest and 4 weeks post-discharge of control group and that of intervention group, interaction effects were not significant |
| Jordhøy et al (2001)32 | 1) Incurable, malignant disease 2) Life expectancy between 2 and 9 months 3) Aged > 18 years |
Palliative medicine unit | EORTC QLQ-C30 | Monthly, from baseline to 6 months after trial entry | There was no difference on any EORTC QLQ-C30; scale/item change during the study period was clinically significant |
CI, confidence interval; EF, emotional functioning; EORTC QLQ-C15-PAL, a shortened version of the European Organisation for Research and Treatment of Cancer QoL Questionnaire Core 30 for palliative cancer care patients; EQ-5D-3L, European Quality of Life 5 Dimensions 3 Level Version; EROTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire, Core 30; FACT, Functional Assessment of Cancer Therapy; FACT-G, Functional Assessment of Cancer Therapy—General; FACT-L, Functional Assessment of Cancer Therapy—Lung; FI, financial difficulties; FI, Financial problem; HADS, Hospital Anxiety and Depression Scale; IQR, Interquartile range; MANCOVA, Multivariate Analysis of Covariance; PF, physical functioning; PQLI, Palliative Care Quality of Life Index; QoL, Quality of Life; SD, standard deviation; SF, social functioning.
Discussion
Our review shows that the effectiveness of HbSC programmes, when compared with standard care for patients with advanced cancer, has positive effects on measurable and value-related outcomes like QoL. Seventeen studies investigated the effects of home care programmes on the QoL of patients with advanced cancer. Nine studies show the positive effects on QoL, including social functioning, emotional functioning and subjective QoL.16–18 20 23 26 27 30 However, inconsistent results were found according to the components of interventions. Owing to the diversity of the intervention and study population, studies failed to show a consistent pattern. Nevertheless, programmes such as team meetings, periodic management (home visits or check-up via phone), and nursing and psychological support affected the positive outcomes.
Home-based programmes can improve specific domains of the life of patients with advanced cancer. The domains of QoL associated with the intervention can differ by the specialised programme of home-based care. Home-based nursing care can improve financial difficulties and mood status.23 Providing information and supporting patients can empower patients’ social functioning,30 although the results were inconsistent across the studies.28 Psychological programmes can improve social and emotional functioning and the overall QoL of patients with advanced cancer.18 20 Further, exercise programmes can improve patients’ mobility, fatigue and sleep quality.27 Although the overall QoL rating was not related with the intervention programme as cancer progresses, various domains of life can be supported by home-based programmes. Further, this study implies that home-based care should comprise diverse programmes with multidisciplinary components, which target the individual specific domains of life.
This study showed that HbSC programmes improve patient’s QoL in some domains, but the analysis was limited owing to the variability of the sample included. Each study included patients with various cancer types and settings. Some included patients with an adjuvant setting, some included patients with incurable states, and some studies targeted patients with terminal conditions. Although most studies have yet to present or insufficiently mention information on care timing, among the studies that suggested survival information, the psychological programme improved social and emotional functioning in a study with a 6-month survival of 60%.18 Further, studies with a 12-month survival rate of 60% or more showed improvement in mobility, fatigue and sleep quality.27 Moreover, there was no difference in QoL in other studies with a 24-week survival rate of 70%, and no difference in QoL was identified in studies with a median overall survival of 2 months in the programme group.33 Palliative care is appropriate for patients with any stage of cancer, and the benefits of early palliative care on QoL improvements are well known.34–36 Although some studies in our analysis have confirmed the advantage of early palliative care, more RCTs for HbSC are necessary to prove this. However, the effectiveness of palliative care could be more mitigated in people with mild symptoms, good QoL and good performance, but this could not be confirmed owing to insufficient information in the analysed studies. A more controlled clinical trial is needed to find a subgroup benefiting more from palliative care.
This systematic review did not yield the quantitative size of the association between HbSC programme and QoL. However, the authors reviewed the literature and came to the following conclusions. Interventions provided in HbSC programme should be based on a multidisciplinary team and include the monitoring and management of pain and side effects of cancer treatment, and provide psychological support. Periodic home visits by medical staffs and direct online consultation systems are needed, whereby long-term low-intensity visits by non-professional medical staff seems ineffective. Finally, caregivers who care for the patients must also be the targets of HbSC programmes.
HbSC was studied in Denmark, the USA and the UK. As studies included in the systematic review focused on the effects of HbSC, there was no description or suggestion on policies or healthcare systems. According to the literature, these countries have supported HbSC. The Danish healthcare system is universal and based on the principles of free and equal access to healthcare.37 Denmark has a comprehensive home-based primary care system, from preventive services to rehabilitation services, cooperating with resources in the community.38 Home-visiting nursing services based on a doctor’s prescription are provided by the local government free of charge in Denmark.39 The primary healthcare team composed of GPs and community nurses is also involved in the palliative pathway when the terminally ill patient stays at home.40 The USA, which does not have a public health insurance system that covers all citizens, provides home healthcare through the Medicare system, a public health insurance system for the elderly and the disabled.41 This programme includes the management and evaluation of treatment plans, education and training of patients and caregivers, and management of drugs, including injection, tube replacement and rehabilitation. These services are provided under a contract with a doctor.42 The UK National Healthcare Service provides healthcare services by taxation under the responsibility and authority of the central government. In the UK, home nursing can be provided instead of the typical care given at hospitals or nursing homes for patients with terminal illnesses.43 Countries where more than three included studies were reported regarded HbSC as a public domain and supported HbSC by the government.
In general, considering the complex problems faced by patients with advanced cancer, palliative care is recommended to be provided by an integrative team of physicians, nurses, social workers, chaplains and pharmacists. Moreover, HbSC could produce better outcomes when performed by a multidisciplinary team. Care providers should offer a holistic evaluation and a detailed and tailored plan for each patient for high-quality palliative care for patients and caregivers through home care. This plan should include medical care, nutritional support, psychosocial care, pastoral care, management for caregivers and end-of-life care. Further, as physical activity can improve the QoL and relieve symptoms even in patients with advanced cancer, home care could be more effective by including individualised exercise therapy in the home care programme. HbSC also should provide strengthened control and management for medical needs for cancer-related symptoms including pain, physical activity for patients with advanced cancer, and psychological needs while staying at home or by predicting the place of care by anticipating the course of a patient’s disease. If symptom control is insufficient at home, patients with cancer tend to stay or die at a medical institution rather than at home.44 45 Early identification of proper patients and intervention are challenging but essential for patients to benefit sufficiently. For timely palliative care, regular screening of HbSC needs among inpatients and outpatients and establishing criteria for selecting patients are necessary. Additionally, as patient status and condition continuously change, periodic multidisciplinary evaluation and coordination of plans are crucial.
Moreover, most studies conducted the patient assessment and care through home visits, outpatient clinics and telephones. Recently, there have been attempts to expand access to palliative care through telemedicine despite barriers such as technical problems46 and participants’ digital literacy.47 Although there are some restrictions, telemedicine is expected to be settled in the medical field soon. Accordingly, it is assumed that telemedicine can be actively used in HbSC. Further, the adoption of digital health technologies, such as wearable devices and mobile healthcare programmes, will provide advantages such as anxiety relief and cognition of emergent situations.
One obstacle preventing patients’ use of HbSC is an economic problem. In the current situation in which the cost of inpatient hospice care is supported by health insurance, the payment of home care costs for each patient is a significant burden compared with inpatient care. However, if value and satisfaction for end-of-life patients are considered as the effectiveness, home-based palliative care is more cost-effective than inpatient hospice care. Therefore, it is essential to expand insurance support so that older patients with cancer can receive HbSC programmes free of the economic burden.
We systematically searched the literature on the effects of HbSC programmes on QoL of patients with advanced cancer. Unlike previous systematic reviews of the literature could not determine the efficiency of HbSC programmes on QoL,13 14 our study showed that HbSC programmes could be effective according to the content and aim of HbSC programme. However, this study has several limitations. First, as blinding and randomisation are difficult to accomplish in research on the current topic, there could be potential bias in the studies included in this systematic literature review. In all studies included, home care was provided to all patients enrolled in the intervention arm, and these patient populations were selected for vague inclusion criteria regarding the state of illness. Additionally, standard palliative care through outpatient clinics was provided to the control arm, which could have diluted the difference between the intervention and control groups. Second, there is also a possibility of information bias owing to language restriction. The current review only explored studies published in English. However, owing to varying cultural and medical backgrounds in different nations, studies conducted in various nations are likely to report different results. Third, there is a relative lack of quality studies evaluated highly according to SIGN criteria. As pain and QoL continually exacerbate, particularly in patients with advanced cancer, it is difficult to assess the impact of home care on QoL; a more sophisticated study design may be needed. It is needed that further research with delicately defined outcome indicators and more patients with identical cancer status.
Conclusion
HbSC programmes appear to improve QoL in patients with advanced cancer. Services provided in the programmes can influence various areas of QoL. More studies address HbSC programmes needed patients provide suitable programmes for patients to benefit most.
Acknowledgments
We thank PARK Eun-Sun, a medical librarian at Seoul National University College of Medicine in South Korea, for her contributions in developing the search strategy in MEDLINE database, Embase, Cochrane library, and Web of Science.
Footnotes
IYH and G-UW contributed equally.
D-WL and BC contributed equally.
Contributors: IYH: investigation, writing – original draft. G-UW: writing – original draft. SYL, SHY, and M-SK: conceptualisation, writing - review and editing. KHK and JS: writing - review and editing. HJJ and MSJ: investigation, writing - review & editing. SKB and EHJ: writing - review & editing. D-WL: conceptualisation, methodology, investigation, visualisation, writing – original draft, guarantor (responsible for the overall content of the manuscript). BC: supervision, project administration, investigation.
Funding: This research was supported by a grant from the Patient-Centered Clinical Research Coordinating Center (PACEN), funded by the Ministry of Health & Welfare, Republic of Korea (grant number HC21C0115).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Sun E, Lakdawalla D, Reyes C, et al. The determinants of recent gains in cancer survival: an analysis of the surveillance, epidemiology, and end results (SEER) database. JCO 2008;26(15_suppl):6616. 10.1200/jco.2008.26.15_suppl.6616 [DOI] [Google Scholar]
- 2. Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet 2002;360:1131–5. 10.1016/S0140-6736(02)11199-8 [DOI] [PubMed] [Google Scholar]
- 3. Earle CC, Park ER, Lai B, et al. Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol 2003;21:1133–8. 10.1200/JCO.2003.03.059 [DOI] [PubMed] [Google Scholar]
- 4. Pritchard RS, Fisher ES, Teno JM, et al. Influence of patient preferences and local health system characteristics on the place of death. J Am Geriatr Soc 1998;46:1242–50. 10.1111/j.1532-5415.1998.tb04540.x [DOI] [PubMed] [Google Scholar]
- 5. Smith A, Hyde YM, Stanford D. Supportive care needs of cancer patients: a literature review. Pall Supp Care 2015;13:1013–7. 10.1017/S1478951514000959 [DOI] [PubMed] [Google Scholar]
- 6. Gomes B, Higginson IJ. Factors influencing death at home in terminally ill patients with cancer: systematic review. BMJ 2006;332:515–21. 10.1136/bmj.38740.614954.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bekelman JE, Halpern SD, Blankart CR, et al. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA 2016;315:272–83. 10.1001/jama.2015.18603 [DOI] [PubMed] [Google Scholar]
- 8. Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004;22:315–21. 10.1200/JCO.2004.08.136 [DOI] [PubMed] [Google Scholar]
- 9. Finlay IG, Higginson IJ, Goodwin DM, et al. Palliative care in hospital, hospice, at home: results from a systematic review. Ann Oncol 2002;13(Suppl 4):257–64. 10.1093/annonc/mdf668 [DOI] [PubMed] [Google Scholar]
- 10. Higginson IJ, Sen-Gupta GJ. Place of care in advanced cancer: a qualitative systematic literature review of patient preferences. J Palliat Med 2000;3:287–300. 10.1089/jpm.2000.3.287 [DOI] [PubMed] [Google Scholar]
- 11. Tseng EK, Hicks LK. Value based care and patient-centered care: divergent or complementary Curr Hematol Malig Rep 2016;11:303–10. 10.1007/s11899-016-0333-2 [DOI] [PubMed] [Google Scholar]
- 12. Porter ME. What is value in health care N Engl J Med 2010;363:2477–81. 10.1056/NEJMp1011024 [DOI] [PubMed] [Google Scholar]
- 13. Smeenk FW, van Haastregt JC, de Witte LP, et al. Effectiveness of home care programmes for patients with incurable cancer on their quality of life and time spent in hospital: systematic review. BMJ 1998;316:1939–44. 10.1136/bmj.316.7149.1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nordly M, Vadstrup ES, Sjøgren P, et al. Home-based specialized palliative care in patients with advanced cancer: a systematic review. Pall Supp Care 2016;14:713–24. 10.1017/S147895151600050X [DOI] [PubMed] [Google Scholar]
- 15. Health Improvement Scotland . Scottish intercollegiate guideline network. SIGN methodology checklist: systematic review and meta-analyses; 2014.
- 16. Cheville AL, Moynihan T, Herrin J, et al. Effect of collaborative telerehabilitation on functional impairment and pain among patients with advanced-stage cancer: a randomized clinical trial. JAMA Oncol 2019;5:644–52. 10.1001/jamaoncol.2019.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lehto RH, Wyatt G, Sikorskii A, et al. Home‐based Mindfulness therapy for lung cancer symptom management: a randomized feasibility trial. Psychooncology 2015;24:1208–12. 10.1002/pon.3755 [DOI] [PubMed] [Google Scholar]
- 18. Nordly M, Skov Benthien K, Vadstrup ES, et al. Systematic fast-track transition from oncological treatment to dyadic specialized palliative home care: DOMUS–a randomized clinical trial. Palliat Med 2019;33:135–49. 10.1177/0269216318811269 [DOI] [PubMed] [Google Scholar]
- 19. Kleijn G, Lissenberg-Witte BI, Bohlmeijer ET, et al. The efficacy of life review therapy combined with memory specificity training (LRT-MST) targeting cancer patients in palliative care: a randomized controlled trial. PLoS One 2018;13:e0197277. 10.1371/journal.pone.0197277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao H, Kwong E, Pang S, et al. Effect of a life review program for Chinese patients with advanced cancer: a randomized controlled trial. Cancer Nurs 2013;36:274–83. 10.1097/NCC.0b013e318268f7ba [DOI] [PubMed] [Google Scholar]
- 21. Steel JL, Geller DA, Kim KH, et al. Web-based collaborative care intervention to manage cancer-related symptoms in the palliative care setting. Cancer 2016;122:1270–82. 10.1002/cncr.29906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uitdehaag MJ, van Putten PG, van Eijck CHJ, et al. Nurse-led follow-up at home vs. conventional medical outpatient clinic follow-up in patients with incurable upper gastrointestinal cancer: a randomized study. J Pain Symptom Manage 2014;47:518–30. 10.1016/j.jpainsymman.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 23. Molassiotis A, Brearley S, Saunders M, et al. Effectiveness of a home care nursing program in the symptom management of patients with colorectal and breast cancer receiving oral chemotherapy: a randomized. J Clin Oncol 2009;27:6191–8. 10.1200/JCO.2008.20.6755 [DOI] [PubMed] [Google Scholar]
- 24. de Wit R, van Dam F. From hospital to home care: a randomized controlled trial of a pain education programme for cancer patients with chronic pain. J Adv Nurs 2001;36:742–54. 10.1046/j.1365-2648.2001.02047.x [DOI] [PubMed] [Google Scholar]
- 25. Hermann K, Engeser P, Szecsenyi J, et al. Palliative patients cared for at home by PAMINO-trained and other Gps - health-related quality of life as measured by QLQ-C15-PAL and POS. BMC Palliat Care 2012;11:13. 10.1186/1472-684X-11-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edbrooke L, Aranda S, Granger CL, et al. Multidisciplinary home-based rehabilitation in inoperable lung cancer: a randomised controlled trial. Thorax 2019;74:787–96. 10.1136/thoraxjnl-2018-212996 [DOI] [PubMed] [Google Scholar]
- 27. Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage 2013;45:811–21. 10.1016/j.jpainsymman.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ammari ABH, Hendriksen C, Rydahl-Hansen S. Results from the family and coping oriented palliative homecare intervention study (Famcope)—a randomized controlled trial. J Psychosoc Oncol 2018;36:557–81. 10.1080/07347332.2018.1460003 [DOI] [PubMed] [Google Scholar]
- 29. Pilegaard MS, la Cour K, Gregersen Oestergaard L, et al. The ‘cancer home-life intervention’: a randomised controlled trial evaluating the efficacy of an occupational therapy–based intervention in people with advanced cancer. Palliat Med 2018;32:744–56. 10.1177/0269216317747199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a brief and extensive dyadic intervention for advanced cancer patients and their family Caregivers. Psychooncology 2013;22:555–63. 10.1002/pon.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mills ME, Murray LJ, Johnston BT, et al. Does a patient-held quality-of-life diary benefit patients with inoperable lung cancer J Clin Oncol 2009;27:70–7. 10.1200/JCO.2008.17.5687 [DOI] [PubMed] [Google Scholar]
- 32. Jordhøy MS, Fayers P, Loge JH, et al. Quality of life in palliative cancer care: results from a cluster randomized trial. J Clin Oncol 2001;19:3884–94. 10.1200/JCO.2001.19.18.3884 [DOI] [PubMed] [Google Scholar]
- 33. Ma C-J, Huang C-W, Yeh Y-S, et al. Supplemental home parenteral nutrition improved nutrition status with comparable quality of life in malnourished unresectable/metastatic gastric cancer receiving salvage chemotherapy. Support Care Cancer 2021;29:1977–88. 10.1007/s00520-020-05687-4 [DOI] [PubMed] [Google Scholar]
- 34. Kavalieratos D, Corbelli J, Zhang D, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA 2016;316:2104–14. 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vanbutsele G, Van Belle S, Surmont V, et al. The effect of early and systematic integration of palliative care in oncology on quality of life and health care use near the end of life: a randomised controlled trial. Eur J Cancer 2020;124:186–93. 10.1016/j.ejca.2019.11.009 [DOI] [PubMed] [Google Scholar]
- 36. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet 2014;383:1721–30. 10.1016/S0140-6736(13)62416-2 [DOI] [PubMed] [Google Scholar]
- 37. The Ministry of Health . Health in Denmark. An overview. København: Ministry of Health; 2017. [Google Scholar]
- 38. Kyu-Sik LJ, Min-Kyung H, Yu-Mi P. Community Care Theory and policy. Seoulo: Korea Institute for Health & Welfare Policy, 2019. [Google Scholar]
- 39. Ministry of Health Denmark . Healthcare in Denmark: An Overview. København: Ministry of Health Denmark, 2017. [Google Scholar]
- 40. Neergaard MA. Palliative home care for cancer patients in Denmark Aarhus University; 2009. [Google Scholar]
- 41. Stanhope M, Lancaster J. Public health nursing e-book: Population-centered health care in the community. Amsterdam: Elsevier Health Sciences, 2019: 1114. [Google Scholar]
- 42. Centers for Medicare and Medicaid Services . Medicare program – general information. 2019. Available: https://www.cms.gov/about-cms/what-we-do/medicare [Accessed 1 Nov 2023].
- 43. Chang J, Peysakhovich F, Wang W, et al. The UK health care system. United Kingdom 2011;30:2019. [Google Scholar]
- 44. Okamoto Y, Fukui S, Yoshiuchi K, et al. Do symptoms among home palliative care patients with advanced cancer decide the place of death? Focusing on the presence or absence of symptoms during home care. J Palliat Med 2016;19:488–95. 10.1089/jpm.2015.0184 [DOI] [PubMed] [Google Scholar]
- 45. Smith EL, Hann DM, Ahles TA, et al. Dyspnea, anxiety, body consciousness, and quality of life in patients with lung cancer. J Pain Symptom Manage 2001;21:323–9. 10.1016/s0885-3924(01)00255-x [DOI] [PubMed] [Google Scholar]
- 46. Finucane AM, O’Donnell H, Lugton J, et al. Digital health interventions in palliative care: a systematic meta-review. NPJ Digit Med 2021;4:64. 10.1038/s41746-021-00430-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Payne S, Tanner M, Hughes S. Digitisation and the patient–professional relationship in palliative care. Palliat Med 2020;34:441–3. 10.1177/0269216320911501 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available in a public, open access repository.