Abstract
Objective
New modes of action and more data on the efficacy and safety of existing drugs in psoriatic arthritis (PsA) required an update of the EULAR 2019 recommendations for the pharmacological treatment of PsA.
Methods
Following EULAR standardised operating procedures, the process included a systematic literature review and a consensus meeting of 36 international experts in April 2023. Levels of evidence and grades of recommendations were determined.
Results
The updated recommendations comprise 7 overarching principles and 11 recommendations, and provide a treatment strategy for pharmacological therapies. Non-steroidal anti-inflammatory drugs should be used in monotherapy only for mild PsA and in the short term; oral glucocorticoids are not recommended. In patients with peripheral arthritis, rapid initiation of conventional synthetic disease-modifying antirheumatic drugs is recommended and methotrexate preferred. If the treatment target is not achieved with this strategy, a biological disease-modifying antirheumatic drug (bDMARD) should be initiated, without preference among modes of action. Relevant skin psoriasis should orient towards bDMARDs targeting interleukin (IL)-23p40, IL-23p19, IL-17A and IL-17A/F inhibitors. In case of predominant axial or entheseal disease, an algorithm is also proposed. Use of Janus kinase inhibitors is proposed primarily after bDMARD failure, taking relevant risk factors into account, or in case bDMARDs are not an appropriate choice. Inflammatory bowel disease and uveitis, if present, should influence drug choices, with monoclonal tumour necrosis factor inhibitors proposed. Drug switches and tapering in sustained remission are also addressed.
Conclusion
These updated recommendations integrate all currently available drugs in a practical and progressive approach, which will be helpful in the pharmacological management of PsA.
Keywords: Psoriatic Arthritis, Treatment, Biological Therapy, Biosimilar Pharmaceuticals
Introduction
Psoriatic arthritis (PsA) is a disease which has benefited from notable progress over recent years. Concepts have evolved, such as very early diagnosis and pre-PsA, as well as defining treatment targets and applying a holistic approach to comorbidity management.1–4 Pharmacological options have extended, with the approval of new agents targeting various modes of action for PsA (as well as skin psoriasis). Drugs licensed for PsA now include (1) conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX), sulfasalazine and leflunomide; (2) biological (b) DMARDs targeting tumour necrosis factor (TNF), the interleukin (IL)-12/23 or IL-23 pathway, and the IL-17A and IL-17A/F pathway; and (3) targeted synthetic (ts) DMARDs that inhibit Janus kinases (JAKs) or phosphodiesterase 4 (PDE4) (table 1).5 New safety data have emerged in inflammatory arthritis, particularly a worldwide cautionary comment regarding JAK inhibitors (JAKis), following a large randomised controlled trial (RCT) of tofacitinib in rheumatoid arthritis (RA).6–8 Since the last EULAR recommendations for the pharmacological management of PsA in 2019, the field has changed significantly.9–12 An update of the EULAR PsA management recommendations was therefore timely.9
Table 1.
Disease-modifying treatment options for psoriatic arthritis in 2023
| Type of DMARD | Target | Name of drug |
| csDMARD |
|
|
| bDMARD | TNF |
|
| IL-12/23 |
|
|
| IL-17A |
|
|
| IL-17A/F |
|
|
| IL-23-p19 |
|
|
| CTLA4 |
|
|
| tsDMARD | PDE4 |
|
| JAK |
|
Drugs currently authorised as of December 2023 for use in psoriatic arthritis.
bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DMARD, disease-modifying antirheumatic drug; IL, interleukin; JAK, Janus kinase; PDE4, phosphodiesterase 4; TNF, tumour necrosis factor; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
This update addresses the non-topical, pharmacological management of PsA, with a specific focus on musculoskeletal (MSK) manifestations, while also addressing the spectrum of PsA, including how skin psoriasis, extra-MSK manifestations and comorbidities should influence treatment choices.
Methods
In accordance with the EULAR updated standardised operating procedures,13 the process leading to this update included a data-driven approach and expert opinion.
After approval for an update by the EULAR Council in September 2022, taskforce members were selected by the convenor (JSS) and the methodologist (LG), to include more than one-third of new members, as well as country and gender representation. For the first time, experts from Australia, Japan and North America participated. Representatives from the health professionals in rheumatology (HPR) committee, patient research partners from PARE (People with Arthritis/Rheumatism) and young colleagues from the EMEUNET (EMerging EUlar NETwork) were included. Five members were recruited through an open call to EULAR countries via a competitive application process.
In October 2022, the steering group had its first meeting. The steering group consisted of seven rheumatologists (including the convenor, the methodologist and the fellow: JSS, LG, AK, DA, XB, IBM and DGM), a dermatologist (W-HB), an infectious disease specialist (KLW), an experienced fellow rheumatologist (AK), a patient research partner (HB) and two health professionals (BAE and RJOF, the latter acting in the capacity of a junior methodologist). Questions were then defined and addressed through a systematic literature review (SLR), performed by the fellow (AK) between November 2022 and April 2023, for the literature pertaining to pharmacological treatments of PsA and published since the previous SLR (ie, since the end of 2018).5
The taskforce comprised the steering group and 23 other experts; members came from 19 different countries (of which 15 were EULAR countries), and included 27 rheumatology specialists, 2 dermatologists, 1 infectious disease specialist, 2 people affected with PsA acting as patient research partners, 2 HPRs and 3 rheumatology/epidemiology fellows/trainees. Overall, 47% of the taskforce members had not participated in the previous update in 2019. In April 2023, the taskforce met for a physical meeting to develop the updated bullet points. Each point was discussed in detail both in smaller (breakout) groups and in plenary sessions until consensus was reached. Group approval was sought through votes (by raised hands) for each bullet point; the limit for acceptance of individual recommendations was set at ≥75% majority among the taskforce for the first voting round; then (after discussions and potential reformulations) at ≥67% majority; and finally, if required, the last round of votes was accepted with >50% acceptance or else a proposal was rejected.13
Although the SLR was a strong component of the discussions, the process was not only evidence-based but also experience-based and consensus-based, and included consideration of safety, efficacy, cost and long-term data. The levels of evidence (LoE) and grades of recommendation (GoR) were determined for each recommendation based on the Oxford Evidence Based System.13 14 In May 2023, an anonymised email-based voting on the level of agreement (LoA) among the taskforce members was performed on a 0–10 scale (with 10 meaning full agreement) allowing calculation of mean LoA.
Results
These recommendations address non-topical pharmacological treatments with a main focus on MSK manifestations. These recommendations concern stakeholders, such as experts involved in the care of patients with PsA, particularly rheumatologists and other health professionals (such as rheumatology nurses), general practitioners, dermatologists and other specialists; and also people with PsA as well as other stakeholders, for example, government and hospital officials, patient organisations, regulatory agencies and reimbursement institutions.
The overarching principles (OAPs) and recommendations are shown in table 2, with LoE, GoR and LoA. The updated recommendations include 7 OAPs (vs 6 in 2019) and 11 recommendations (vs 12 in 2019, due to merges). Of the 11 recommendations, only 4 are unchanged compared with 2019 (the modifications compared with the 2019 recommendations are represented in table 3).
Table 2.
2023 updated EULAR recommendations for the pharmacological management of psoriatic arthritis
| Overarching principles | Level of agreement, mean (SD) | |||
| A | Psoriatic arthritis is a heterogeneous and potentially severe disease, which may require multidisciplinary treatment. | 10.0 (0.1) | ||
| B | Treatment of psoriatic arthritis patients should aim at the best care and must be based on a shared decision between the patient and the rheumatologist, considering efficacy, safety, patient preferences and costs. | 9.7 (0.6) | ||
| C | Rheumatologists are the specialists who should primarily care for the musculoskeletal manifestations of patients with psoriatic arthritis; in the presence of clinically relevant skin involvement, a rheumatologist and a dermatologist should collaborate in diagnosis and management. | 9.7 (0.5) | ||
| D | The primary goal of treating patients with psoriatic arthritis is to maximise health-related quality of life, through control of symptoms, prevention of structural damage, normalisation of function and social participation; abrogation of inflammation is an important component to achieve these goals. | 9.9 (0.3) | ||
| E | In managing patients with psoriatic arthritis, consideration should be given to each musculoskeletal manifestation and treatment decisions made accordingly. | 9.8 (0.4) | ||
| F | When managing patients with psoriatic arthritis, non-musculoskeletal manifestations (particularly skin, eye and gastrointestinal tract) should be taken into account; comorbidities such as obesity, metabolic syndrome, cardiovascular disease or depression should also be considered. | 9.7 (0.7) | ||
| G | The choice of treatment should take account of safety considerations regarding individual modes of action to optimise the benefit–risk profile. | 9.9 (0.4) | ||
| Recommendations | Level of evidence | Grade of recommendation | Level of agreement, mean (SD) | |
| 1 | Treatment should be aimed at reaching the target of remission or, alternatively, low disease activity, by regular disease activity assessment and appropriate adjustment of therapy. | 1b | A | 9.5 (1.0) |
| 2 | Non-steroidal anti-inflammatory drugs may be used to relieve musculoskeletal signs and symptomsa; local injections of glucocorticoids may be considered as adjunctive therapyb. | 1ba, 3bb | Aa, Cb | 9.5 (0.7) |
| 3 | In patients with polyarthritis, or those with monoarthritis/oligoarthritis and poor prognostic factorsa (eg, structural damage, elevated acute phase reactants, dactylitis or nail involvement), a csDMARD should be initiated rapidly, with methotrexate preferred in those with clinically relevant skin involvement. | 1b, 4a | B, Ca | 9.3 (0.8) |
| 4 | In patients with peripheral arthritis and an inadequate response to at least one csDMARD, therapy with a bDMARD should be commenced. | 1a | A | 9.5 (1.3) |
| 5 | In patients with peripheral arthritis and an inadequate response to at least one bDMARD, or when a bDMARD is not appropriatea, a JAKi may be considered, taking safety considerations* into account. | 1b, 4a | B, Da | 9.1 (1.5) |
| 6 | In patients with mild disease and an inadequate response to at least one csDMARD, in whom neither a bDMARD nor a JAKi* is appropriate, a PDE4 inhibitor may be considered. | 1b | B | 8.7 (1.1) |
| 7 | In patients with unequivocal enthesitis and an insufficient response to NSAIDs or local glucocorticoid injections, therapy with a bDMARD should be considered. | 1b | B | 9.5 (0.9) |
| 8 | In patients with clinically relevant axial disease with an insufficient response to NSAIDs, therapy with an IL-17A inhibitor, a TNF inhibitor, an IL-17 A/F inhibitor or a JAKi* should be considered. | 1b | B | 9.4 (1.3) |
| 9 | The choice of the mode of action should reflect non-musculoskeletal manifestations related to psoriatic arthritis; with clinically relevant skin involvement, preference should be given to an IL-17A or IL-17A/F or IL-23 or IL-12/23 inhibitor; with uveitis to an anti-TNF monoclonal antibody; and with IBD to an anti-TNF monoclonal antibody or an IL-23 inhibitor or IL-12/23 inhibitor or a JAKi*. | 1b | B | 9.6 (0.7) |
| 10 | In patients with an inadequate response or intolerance to a bDMARD or a JAKi, switching to another bDMARD or JAKi* should be considereda, including one switch within a classb. | 1ba, 4b | C | 9.5 (0.7) |
| 11 | In patients in sustained remission, tapering of DMARDs may be considered. | 2b | B | 9.4 (1.2) |
‘Mild disease’ is defined as oligoarticular or entheseal disease without poor prognostic factors and limited skin involvement.
csDMARDs (conventional synthetic DMARDs) include methotrexate, sulfasalazine or leflunomide. bDMARDs (biologic DMARDs) here include TNF inhibitors (both original and biosimilars), drugs targeting the IL-17 and IL-12–23/IL-23-p19 pathways, and in the context of recommendation 10 also CTLA4 (cytotoxic T-lymphocyte–associated antigen 4) inhibition. JAKis (Januse kinase inhibitors) include tofacitinib and upadacitinib.
The superscript letters ‘a’ and ‘b’ are used to link a part of the recommendation to a level of evidence.
The table shows the level of evidence, grade of recommendation and level of agreement among taskforce members (0–10 scale).
*For JAKis, caution is needed for patients aged 65 years or above, those who are current or past long-time smokers, with a history of atherosclerotic cardiovascular disease or other cardiovascular risk factors or with other malignancy risk factors, and with known risk factors for venous thromboembolism.
bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; CTLA4, cytotoxic T-lymphocyte–associated antigen 4; DMARDs, disease-modifying antirheumatic drugs; IBD, inflammatory bowel disease; IL, interleukin; JAKi, Janus kinase inhibitor; NSAIDs, non-steroidal anti-inflammatory drugs; PDE4, phosphodiesterase 4; TNF, tumour necrosis factor.
Table 3.
Comparison of the 2019 and 2023 EULAR recommendations for the management of psoriatic arthritis
| 2019 version | Changes performed | 2023 version | ||
| Overarching principles | ||||
| A | Psoriatic arthritis is a heterogeneous and potentially severe disease, which may require multidisciplinary treatment. | Unchanged | A | Psoriatic arthritis is a heterogeneous and potentially severe disease, which may require multidisciplinary treatment. |
| B | Treatment of psoriatic arthritis patients should aim at the best care and must be based on a shared decision between the patient and the rheumatologist, considering efficacy, safety and costs. | Reformulated | B | Treatment of psoriatic arthritis patients should aim at the best care and must be based on a shared decision between the patient and the rheumatologist, considering efficacy, safety, patient preferences and costs. |
| C | Rheumatologists are the specialists who should primarily care for the musculoskeletal manifestations of patients with psoriatic arthritis; in the presence of clinically significant skin involvement, a rheumatologist and a dermatologist should collaborate in diagnosis and management. | Reformulated | C | Rheumatologists are the specialists who should primarily care for the musculoskeletal manifestations of patients with psoriatic arthritis; in the presence of clinically relevant skin involvement, a rheumatologist and a dermatologist should collaborate in diagnosis and management. |
| D | The primary goal of treating patients with psoriatic arthritis is to maximise health-related quality of life, through control of symptoms, prevention of structural damage, normalisation of function and social participation; abrogation of inflammation is an important component to achieve these goals. | Unchanged | D | The primary goal of treating patients with psoriatic arthritis is to maximise health-related quality of life, through control of symptoms, prevention of structural damage, normalisation of function and social participation; abrogation of inflammation is an important component to achieve these goals. |
| E | In managing patients with psoriatic arthritis, consideration should be given to each musculoskeletal manifestation and treatment decisions made accordingly. | Unchanged | E | In managing patients with psoriatic arthritis, consideration should be given to each musculoskeletal manifestation and treatment decisions made accordingly. |
| F | When managing patients with psoriatic arthritis, non-musculoskeletal manifestations (skin, eye and gastrointestinal tract) should be taken into account; comorbidities such as metabolic syndrome, cardiovascular disease or depression should also be considered. | Reformulated | F | When managing patients with psoriatic arthritis, non-musculoskeletal manifestations (particularly skin, eye and gastrointestinal tract) should be taken into account; comorbidities such as obesity, metabolic syndrome, cardiovascular disease or depression should also be considered. |
| New | G | The choice of treatment should take account of safety considerations regarding individual modes of action to optimise the benefit–risk profile. | ||
| Recommendations | ||||
| 1 | Treatment should be aimed at reaching the target of remission or, alternatively, low disease activity, by regular disease activity assessment and appropriate adjustment of therapy. | Unchanged | 1 | Treatment should be aimed at reaching the target of remission or, alternatively, minimal/low disease activity, by regular monitoring and appropriate adjustment of therapy. |
| 2 and 3 |
|
Merged and modified | 2 | Non-steroidal anti-inflammatory drugs may be used to relieve musculoskeletal signs and symptoms; local injections of glucocorticoids may be considered as adjunctive therapy. |
| 4 and 5 |
|
Merged and modified | 3 | In patients with polyarthritis, or those with monoarthritis/oligoarthritis and poor prognostic factors (eg, structural damage, elevated acute phase reactants, dactylitis or nail involvement), a csDMARD should be initiated rapidly, with methotrexate preferred in those with clinically relevant skin involvement. |
| 6 | In patients with peripheral arthritis and an inadequate response to at least one csDMARD, therapy with a bDMARD should be commenced; when there is relevant skin involvement, an IL-17 inhibitor or IL-12/23 inhibitor may be preferred. | Split into two recommendations | 4 | In patients with peripheral arthritis and an inadequate response to at least one csDMARD, therapy with a bDMARD should be commenced. |
| 7 | In patients with peripheral arthritis and an inadequate response to at least one csDMARD and at least one bDMARD, or when a bDMARD is not appropriate, a JAK inhibitor may be considered. | Modified | 5 | In patients with peripheral arthritis and an inadequate response to at least one bDMARD, or when a bDMARD is not appropriate, a JAKi may be considered, taking safety considerations into account. |
| 8 | In patients with mild disease and an inadequate response to at least one csDMARD, in whom neither a bDMARD nor a JAK inhibitor is appropriate, a PDE4 inhibitor may be considered. | Unchanged | 6 | In patients with mild disease and an inadequate response to at least one csDMARD, in whom neither a bDMARD nor a JAKi is appropriate, a PDE4 inhibitor may be considered. |
| 9 | In patients with unequivocal enthesitis and insufficient response to NSAIDs or local glucocorticoid injections, therapy with a bDMARD should be considered. | Unchanged | 7 | In patients with unequivocal enthesitis and an insufficient response to NSAIDs or local glucocorticoid injections, therapy with a bDMARD should be considered. |
| 10 | In patients with predominantly axial disease which is active and has insufficient response to NSAIDs, therapy with a bDMARD should be considered, which according to current practice is a TNF inhibitor; when there is relevant skin involvement, IL-17 inhibitor may be preferred. | Modified | 8 | In patients with clinically relevant axial disease with an insufficient response to NSAIDs, therapy with an IL-17Ai, a TNFi, an IL-17 A/Fi or a JAKi should be considered. |
| New | 9 | The choice of the mode of action should reflect non-musculoskeletal manifestations related to psoriatic arthritis; with clinically relevant skin involvement, preference should be given to an IL-17A or IL-17A/F or IL-23 or IL-12/23 inhibitor; with uveitis to an anti-TNF monoclonal antibody; and with IBD to an anti-TNF monoclonal antibody or an IL-23i or IL-12/23i or a JAKi. | ||
| 11 | In patients who fail to respond adequately to or are intolerant of a bDMARD, switching to another bDMARD or tsDMARD should be considered, including one switch within a class. | Modified | 10 | In patients with an inadequate response or intolerance to a bDMARD or a JAKi, switching to another bDMARD or JAKi should be considered, including one switch within a class. |
| 12 | In patients in sustained remission, cautious tapering of DMARDs may be considered. | Reformulated | 11 | In patients in sustained remission, tapering of DMARDs may be considered. |
bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; DMARD, disease-modifying antirheumatic drug; IBD, inflammatory bowel disease; IL, interleukin; JAK, Janus kinase; JAKi, Janus kinase inhibitor; NSAID, non-steroidal anti-inflammatory drug; PDE4, phosphodiesterase 4; TNF, tumour necrosis factor; TNFi, tumour necrosis factor inhibitor; tsDMARD, targeted synthetic disease-modifying antirheumatic drug.
Overarching principles
Of the seven OAPs, three remain unchanged, three were reworded and one has been added (overarching principle G). For more information on the thought process leading to the OAPs (unchanged or slightly changed), please refer to the 2015 and 2019 recommendations manuscripts.9 15 Key points from the discussion of the OAPs are addressed in the following:
A. Psoriatic arthritis is a heterogeneous and potentially severe disease, which may require multidisciplinary treatment (unchanged).
Although PsA is potentially severe, not all patients will develop severe forms.16 17 Multidisciplinary management is helpful for many patients, through collaboration between physicians of different specialties and HPRs with the appropriate expertise.18 19
B. Treatment of psoriatic arthritis patients should aim at the best care and must be based on a shared decision between the patient and the rheumatologist, considering efficacy, safety, patient preferences and costs.
This OAP was modified from 2019 to add patient preferences as an element to be considered and emphasise the importance of shared decision-making to maximise treatment adherence and efficacy while at the same time minimise complications driven by uncontrolled (active) disease as well as potential side effects of pharmacological drugs.20 21
C. Rheumatologists are the specialists who should primarily care for the musculoskeletal manifestations of patients with psoriatic arthritis; in the presence of clinically relevant skin involvement, a rheumatologist and a dermatologist should collaborate in diagnosis and management.
We consider that rheumatology experts provide the best care for patients with PsA, given their experience with the many drugs used to treat these and other rheumatic and musculoskeletal diseases (RMDs), including the important aspects of safety and comorbidities. Consultation with dermatologists and sometimes other specialists may be helpful in individual clinical scenarios (see also overarching principles F and G). A very slight rewording was performed to discuss skin involvement as ‘clinically relevant’ rather than ‘clinically significant’ for more homogeneity with other bullet points. This bullet point does not address the role of HPRs, who are usually not prescribers in EULAR countries.
D. The primary goal of treating patients with psoriatic arthritis is to maximise health-related quality of life, through control of symptoms, prevention of structural damage, normalisation of function and social participation; abrogation of inflammation is an important component to achieve these goals (unchanged).
For more details, please see the 2019 update of these recommendations.9
E. In managing patients with psoriatic arthritis, consideration should be given to each musculoskeletal manifestation and treatment decisions made accordingly (unchanged).
For more details, please refer to the 2019 update.9
F. When managing patients with psoriatic arthritis, non-musculoskeletal manifestations (skin, eye and gastrointestinal tract) should be taken into account; comorbidities such as obesity, metabolic syndrome, cardiovascular disease or depression should also be considered.
The wording ‘such as obesity’ was added, since obesity is frequent in PsA and can influence outcomes.22 23 Obesity concerns excess body fat, while metabolic syndrome is a collection of risk factors that increase the likelihood of developing cardiovascular disease and type 2 diabetes. Obesity is a significant contributor to the development of metabolic syndrome. The taskforce members discussed if other comorbidities should be added, but it was felt that the term ‘such as’ entails that comorbidities overall should be considered, without a need to list them. Depression and potentially other mental health issues may influence treatment choice. Central sensitisation to pain perception is frequent in PsA and also influences outcomes; this may lead to difficulties in disease management.24 25 Bone health and malignancies were also specifically highlighted. The management of comorbidities poses specific issues, in particular as to who is responsible for managing distinct disease domains. Solutions need to be applied according to the individual patient, each country’s specific setting and healthcare system organisation.
G. The choice of treatment should take account of safety considerations regarding individual modes of action to optimise the benefit–risk profile (new).
Given new data on the safety of different modes of action, the taskforce proposed this new OAP to emphasise the importance of taking into account safety considerations for each patient.6 The taskforce was aware that this item is somewhat redundant with overarching principle B but wished to emphasise the importance of benefit–risk assessment when considering the use of specific agents.
Recommendations
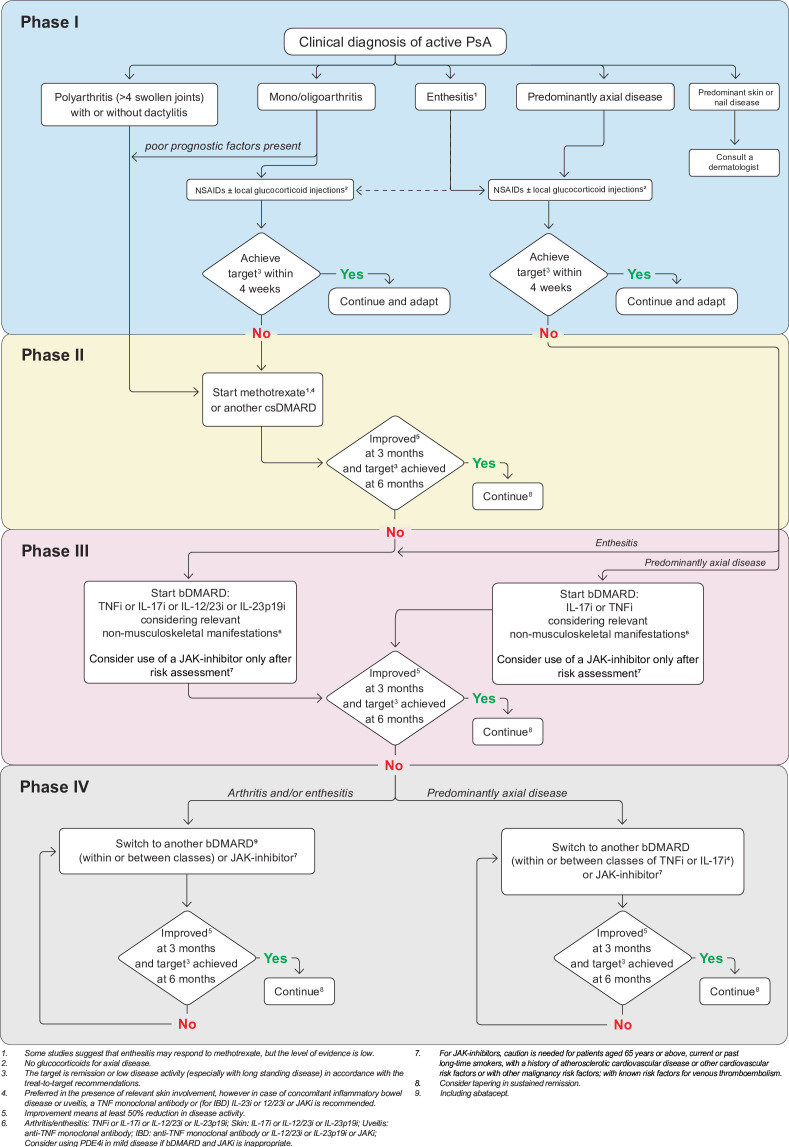
Of note, these recommendations are centred on non-topical pharmacological treatments; topical and non-pharmacological treatments are also important in PsA but are outside our scope. Figure 1 shows a summarised algorithm of the treatment proposals.
Figure 1.
2023 EULAR recommendations algorithm for the management of PsA. bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic disease-modifying antirheumatic drug; IBD, inflammatory bowel disease; L, interleukin; JAK, Janus kinase inhibitor; JAKi, Janus kinase inhibitor; NSAID, non-steroidal anti-inflammatory drugs; TNF, tumour necrosis factor; TNFI, tumour necrosis factor inhibitor.
Some safety issues will be briefly addressed, but for a full picture of the adverse event profile of different drugs the package inserts should be consulted.
Recommendation 1
Treatment should be aimed at reaching the target of remission or, alternatively, low disease activity, by regular disease activity assessment and appropriate adjustment of therapy.
This (unchanged) recommendation is in keeping with the principles of treating-to-target.26 27 Given the lack of new data to support treat-to-target in PsA, the LoE and GoR are also unchanged. The use of instruments to assess disease activity has been addressed in the treat-to-target recommendations.26 The definition of remission in PsA remains a subject of debate.28–30 For the context of these recommendations, remission should be seen as an abrogation of inflammation.
The taskforce members emphasised that disease activity should be regularly assessed across individual involved manifestations (eg, joints, skin, enthesitis, dactylitis, axial disease), and that treatment adjustments will depend on the predominant manifestation of the disease at a given moment.31
Recommendation 2
Non-steroidal anti-inflammatory drugs may be used to relieve musculoskeletal signs and symptoms; local injections of glucocorticoids may be considered as adjunctive therapy.
This recommendation deals with the short-term use of symptomatic treatment. It was developed by merging the two previous recommendations 2 and 3, which dealt separately with non-steroidal anti-inflammatory drugs (NSAIDs) and glucocorticoids, as both only serve to relieve symptoms in the short term. It was decided to no longer allude to systemic glucocorticoids in a bullet point, since the data underlying the prescription of systemic glucocorticoids in PsA are scarce. Moreover, glucocorticoids harbour many potential safety issues, in particular when taking into account the high prevalence of comorbidities and cardiovascular risk factors in PsA.3 32 However, the taskforce members agreed that, in some selected cases, systemic glucocorticoid therapy may be helpful for some patients, especially for polyarticular forms and/or as bridging therapy.
NSAIDs offer symptomatic relief to patients with MSK involvement, but have not shown any efficacy in psoriasis. NSAIDs and local glucocorticoid injections are useful to relieve symptoms and local inflammation temporarily, and may be used combined with DMARDs as needed (please see recommendation 3). However, the safety aspects of (potentially long-term) NSAID use have to be taken into account.
The taskforce emphasised that the vast majority of patients should not be treated with NSAIDs alone (without DMARDs), in keeping with a proactive treat-to-target approach to PsA. Only patients with very mild peripheral disease, or with predominant entheseal or axial disease, may sufficiently benefit from NSAIDs as monotherapy. Even in these cases, it is proposed that the use of symptomatic treatments alone should usually be short term, for example, limited to 4 weeks or so. In peripheral arthritis, this duration is based on the opinion of the group; in predominant axial disease, it is in keeping with the Assesment of Spondyloarthritis International Society (ASAS)/EULAR recommendations for axial spondyloarthritis (axSpA) whereby persistent disease after 4 weeks of treatment is considered a failure of NSAIDs.33 On the other hand, for patients with predominant axial disease who experience significant improvement in clinical symptoms, continuous NSAID use may be proposed if needed to control symptoms, always taking the risks and benefits into account. Of note, data regarding the efficacy of NSAIDs in enthesitis are limited.
Recommendation 3
In patients with polyarthritis or those with monoarthritis/oligoarthritis and poor prognostic factors (eg, structural damage, elevated acute phase reactants, dactylitis or nail involvement), a csDMARD should be initiated rapidly, with methotrexate preferred in those with clinically relevant skin involvement.
Among patients with peripheral arthritis,34 35 a distinction is made according to the number of swollen joints and according to prognostic factors.36 In 2019, polyarthritis and monoarthritis/oligoarthritis with poor prognostic markers were addressed in separate bullet points, which were merged for clarity in this update (table 3). Oligoarticular disease is defined as arthritis (swollen joints) of up to four (included) joints.9 This definition applies to clinical detection (rather than imaging). The prognostic factors have also been previously defined9 17 and are unchanged.
We recommend rapid csDMARD start, concomitant (or close) with the initiation of symptomatic therapy, for both patients with polyarticular disease and patients with oligoarticular disease and poor prognostic factors. Patients with oligoarticular disease and lack of poor prognostic factors should also receive a csDMARD, but there is less urgency for these patients given the more favourable long-term prognosis. The latter may receive csDMARDs after a longer delay, and potentially a period of symptomatic treatment alone (see recommendation 2). Since there is a lack of strong evidence to support this approach of rapid treatment introduction, this recommendation was mainly based on expert opinion.
Of note, there is no specific recommendation for dactylitis. We consider dactylitis as an association of (oligo)synovitis, tenosynovitis and enthesitis. Patients with isolated dactylitis should be treated similarly to patients with oligoarthritis; this includes the use of joint glucocorticoid injections and csDMARDs, which have shown efficacy in relieving dactylitis.37
The first DMARD should be a csDMARD (meaning MTX, leflunomide or sulfasalazine). The decision concerning the first-line DMARD is important and led to much taskforce discussion, and has been put as an element for further research in the research agenda (table 4). The continued prioritisation of csDMARDs reflects consensual expert opinion within the taskforce that favoured the benefit–risk–cost balance of csDMARDs and in particular MTX over targeted drugs. The absence of new data indicating the superiority of a b/tsDMARD as first-line, and in the presence of new data on MTX, was seen as confirming the efficacy of this drug in PsA.5 37–39
Table 4.
Research agenda indicating priorities for future research in PsA
| Theme | Question |
| Responsibility |
|
| Pathogenesis |
|
| Very early PsA |
|
| Drug ordering/response prediction and biomarkers |
|
| Prognosis |
|
| First DMARD choices |
|
| Outcomes in PsA |
|
| Treatments |
|
| Contextual factors in PsA |
|
| Safety |
|
| Axial PsA |
|
| Comorbidities |
|
| Switches |
|
axSpA, axial spondyloarthritis; bDMARDs, biological disease-modifying antirheumatic drugs; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; DMARDs, disease-modifying antirheumatic drugs; JAKi, Janus kinase inhibitor; NSAIDs, non-steroidal anti-inflammatory drugs; PsA, psoriatic arthritis; tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs.
Since the EULAR recommendations adhere to a treat-to-target (T2T) approach which implies a reduction of disease activity by at least 50% within 3 months and reaching the treatment target within 6 months, a csDMARD should not be continued if these therapeutic goals are not attained. On csDMARD inefficacy, another DMARD, such as a bDMARD (see recommendation 4), can be rapidly instituted. Generally speaking, we recommend assessing the efficacy of the csDMARD and deciding if it should be pursued as monotherapy or not, after 12 weeks, in line with the T2T recommendations.26 Although MTX use in PsA has typically been founded on evidence from other immune-mediated diseases such as RA and psoriasis,40 there is also evidence for its efficacy in PsA, with recent confirmatory data both from observational data sources and from a randomised trial indicating that a proportion of patients will respond to escalation of doses of MTX.39 41–43 The efficacy–safety balance of MTX should be assessed regularly, given the general metabolic profile of patients with PsA which can put them at a higher risk for adverse events such as hepatotoxicity.42–44 The MTX dose should be sufficient, that is, usually between 20 mg and 25 mg weekly (about 0.3 mg/kg), and use of folate supplementation is recommended to reduce the adverse effects of MTX.45
Other csDMARDs (ie, leflunomide and sulfasalazine) are potential treatment options and have demonstrated efficacy in PsA peripheral arthritis.15 A recent trial of the combination of MTX with leflunomide indicated a low efficacy to safety ratio; thus, this association is not recommended.38
Recommendation 4
In patients with peripheral arthritis and an inadequate response to at least one csDMARD, therapy with a bDMARD should be commenced.
This recommendation is relevant to patients with peripheral arthritis and therefore is meant to include both those with monoarticular/oligoarticular and those with polyarticular disease. However, where peripheral involvement is limited and without poor prognostic factors, it is not unreasonable to apply a second csDMARD course before initiating a bDMARD/tsDMARD, when this decision is agreed by the prescriber and the patient.
After failure of at least one csDMARD, the taskforce proposed as next step one of the many available bDMARDs (table 1).5
JAKi is efficacious in PsA, but the taskforce decided that at present the efficacy–safety balance, costs and long-term experience with many bDMARDs clearly favour their recommendation over JAKi. Relevant comorbidities in many patients with PsA also favour bDMARD selection.
Regarding bDMARDs, no order of preference is given since no bDMARD has demonstrated superiority for joint involvement over other bDMARDs (table 1).46–48 Herein they are listed in numeric order of the targeted cytokine, and not in order of preference. However, in the context of the present recommendation, CTLA4 (cytotoxic T-lymphocyte–associated antigen 4) inhibition is not considered a good option due to its limited efficacy in clinical trials.49 The GoR is high for this bullet point, reflecting robust accrued data.50
Unlike MSK manifestations, non-MSK domains of PsA allow differential order of bDMARD recommendation (se recommendation 9).5 Two head-to-head trials of bDMARDs in PsA, both comparing an IL-17A inhibitor with adalimumab, showed similar efficacy for IL-17A inhibition and TNF inhibition, as regards efficacy on the joints, while skin responses are better with the former.46 47 We also note that there is evidence on the better efficacy of a bDMARD compared with MTX in skin psoriasis (and evidence for differences between bDMARDs, please see recommendation 9).51 52
All bDMARDs and JAKi showed efficacy regarding inhibition of radiographic progression; such data are lacking for apremilast.
The safety of the different available categories of bDMARDs appears acceptable in our SLR.5 All bDMARDs increase the risk of infections.5 The risks of TNF inhibitors (TNFis) are well known. Candidiasis (usually mucocutaneous) is more frequent with IL-17A and IL-17A/F inhibition, particularly the latter.53 54 While IL-23-p19i is a more recent addition to the armament, its safety appears satisfactory, in line with ustekinumab which also interferes with IL-23 (p40 chain) whose adverse event profile is well known and appears satisfactory.5
As a general rule, safety and comorbidities need to be taken into account when a decision to start a new drug is taken. More complete information regarding the safety aspects of bDMARDs is provided in the individual drug’s product information. Costs should also be taken into account, but these may vary at the country level; cost savings will occur in many countries due to the availability of biosimilar TNF blockers and potentially other biosimilars in due course. Personalised medicine, to facilitate an optimal choice of the first bDMARD, is currently difficult due to the lack of individualised predictors of response to treatment.55 As previously discussed, it is of key importance to take into account the patient phenotype and potential extra-MSK features (figure 1). Comorbidities are also to be considered.23 56 More research is needed on the predictors of drug response, including the effect of sex.57 58
Combination of a bDMARD with a csDMARD
First-line bDMARDs are often given in combination with csDMARDs, such as MTX.41 59 However, there are conflicting data regarding the added benefit of concomitant MTX with targeted DMARDs in patients with peripheral disease and no evidence of a benefit of MTX in patients with axial symptoms.33 60 61
MTX combination with bDMARDs has been explored mainly for TNFi; studies have generally found similar efficacy with or without concomitant MTX, although with increased drug survival when using MTX, in some studies.41 59 62 A recent large study reported increased remission rates with TNFi plus MTX combination therapy.59 With other modes of action, there is a lack of data to support comedication. Overall, the taskforce proposed to combine a first bDMARD with the previously prescribed csDMARD, in all cases where such a treatment has already been tolerated by the patient and in particular when the first bDMARD is a TNFi. For other modes of action, given the lack of data, we cannot recommend comedication, although the usual practice would be to continue a csDMARD when initiating a bDMARD (doses of the csDMARD can be diminished if needed).
Recommendation 5
In patients with peripheral arthritis and an inadequate response to at least one bDMARD, or when a bDMARD is not appropriate, a JAKi may be considered, taking safety considerations into account.
This recommendation elicited much debate. On the one hand, since 2019, new data have accrued on JAKis in terms of efficacy, such as the publication of positive trials on upadacitinib in PsA.63 On the other hand, there is currently a worldwide cautionary statement issued by both the Food and Drug Administration and the European Medicine Agency restricting the use of JAKis in all diseases including PsA, based on an increased risk of cardiovascular and malignancy events observed with tofacitinib in older patients with RA with cardiovascular risk factors.6–8 JAKis lead to increased general infection rates of similar magnitude to bDMARDs, but higher for herpes zoster infections.5 Drug safety for the JAKis tofacitinib and upadacitinib in the specific context of PsA was recently reported and appeared reassuring; however, follow-up was short and further data are warranted.64 65 While currently long-term extension data do not show increased cardiovascular/cancer risk related to JAKi use in PsA, there are no RCTs similar to the ORAL-Surveillance trial available at present in PsA. Therefore, the taskforce felt that the precautions related to RA also have to be taken for PsA, especially since various comorbidities important for the JAKi risk profile may be more prevalent in PsA than in RA (eg, obesity and cardiovascular risk factors). On the other hand, controlling inflammation is important to decrease cardiovascular risk.
Safety of JAKis should be carefully considered66; we propose in table 2 and figure 1 a shortened version of the EMA warning/limitation to use, which includes age, smoking status and other cardiovascular/venous/cancer risk factors.7 8
After much discussion, we considered that the efficacy–safety balance of JAKis did not justify putting JAKis on the same level as bDMARDs for order of choice (ie, proposing JAKis as usual treatment after insufficient response and/or intolerance to csDMARD treatment).
Therefore, JAKis are proposed usually as second-line targeted therapies (or third-line DMARDs). Of note, we recognise that, for some patients, JAKis may be a relevant option after a csDMARD; this is reflected in the wording of the bullet point (‘when a bDMARD is not appropriate’). This ‘non-appropriateness’ may include contraindications to bDMARDs, practical issues leading to a strong preference for oral administrations (eg, lack of proper conservation at regulated temperatures) and patient preferences, including risk of non-adherence to injections (in accordance with the first OAP concerning shared decision-making). Nevertheless, patients will have to weigh their preferences against potential risks.
The GoR was low for this recommendation, in particular regarding safety considerations, since the data are sparse in PsA and we had to rely on data taken from RA. The taskforce suggests using JAKi after bDMARDs have failed because several new bDMARDs with excellent effects on skin involvement and relatively good safety data are now available (IL-23, IL-17 inhibitors) and more long-term data on JAKi efficacy and safety are needed in PsA. The efficacy to safety ratio of JAKis was also put into the research agenda (table 4).
Currently, drugs from the tyrosine kinase 2 (TYK2) pathway inhibition are being assessed in PsA5; they are not currently licensed for use, and indeed the data are at this point limited in particular for safety (including in psoriasis where such therapy is licensed). Thus, we did not include TYK2 inhibition in the current recommendations.
Recommendation 6
In patients with mild disease and an inadequate response to at least one csDMARD, in whom neither a bDMARD nor a JAKi is appropriate, a PDE4 inhibitor may be considered.
This recommendation is unchanged from 2019, with unchanged LoE. ‘Mild disease’ is defined as oligoarticular or entheseal disease without poor prognostic factors and limited skin involvement.9 67 The FOREMOST trial recently confirmed the efficacy of apremilast compared with placebo in oligoarticular PsA.67 Nevertheless, the reason to place apremilast differently from bDMARDs or other tsDMARDs is not only based on its consistently relatively low efficacy, but also on the lack of structural efficacy data (thus putting the term ‘DMARD’ at risk since there are no data on inhibition of damage progression).
This recommendation received the lowest LoA within the taskforce, reflecting that more than a quarter of the taskforce participants were in favour of only discussing apremilast in the text without a specific bullet point.
The use of apremilast in combination with TNFi is off-label, and is a more costly drug combination with no supporting data and cannot be recommended.
Recommendation 7
In patients with unequivocal enthesitis and an insufficient response to NSAIDs or local glucocorticoid injections, therapy with a bDMARD should be considered.
This bullet point remains unchanged. Unequivocal enthesitis refers (as in 2019) to definite entheseal inflammation (which might need additional diagnostic imaging) to avoid overtreatment of entheseal pain not related to PsA (eg, in the context of widespread pain syndrome or repetitive mechanical stress).68 69 In terms of treatment options, the taskforce discussed the recent data indicating indirectly some efficacy for MTX in enthesitis.5 38 39 However, it was felt that the data for MTX were not sufficiently strong to propose MTX in the bullet point. We do acknowledge that, for some patients with enthesitis, MTX may be an option (figure 1).
For unequivocal predominant enthesitis, the proposal is to introduce a bDMARD (without a preference for a specific mode of action) since all currently approved bDMARDs have demonstrated efficacy on enthesitis, with similar magnitudes of response, although head-to-head trials are missing (figure 1).5 Here, costs may be important, but other manifestations will also have to be taken into account (see recommendations 8 and 9). Of note, although tsDMARDs are not mentioned specifically in the bullet point, they are an option in some cases of enthesitis (always considering benefit to risk ratios, in particular for JAKis).7 8
Recommendation 8
In patients with clinically relevant axial disease with an insufficient response to NSAIDs, therapy with an IL-17Ai, a TNFi, an IL-17 A/Fi or a JAKi should be considered.
The formulation for axial disease was modified from predominant to clinically relevant. For axial disease, in agreement also with the recently updated ASAS/EULAR axSpA recommendations,33 we continue to judge csDMARDs as not relevant. bDMARDs targeting TNF and IL-17A and IL-17A/F as well as tsDMARDs targeting JAK are recommended. For JAKis, safety issues should be considered. Of note, we propose a choice between the drugs, not a combination of the drugs.
For this recommendation, the order of the drugs listed is of relevance, meaning that IL-17A inhibition has been put first due to the availability of currently only one trial specifically investigating axial PsA and using secukinumab (the MAXIMISE trial),70 with the other drugs listed thereafter. Thus, the LoE is stronger for IL-17A inhibition than for the other drugs, where the data are derived from axial SpA.33
The other drugs are listed with TNF inhibition first due to long-term safety data, then IL-17 A/F inhibition which has been recently licensed for axial SpA and JAK inhibition as an option taking into account safety. JAKis are here proposed in the same recommendation as bDMARDs, also reflecting that comorbidity profiles of patients with predominant or isolated axial PsA may be more comparable to patients with axial SpA and therefore may have a more favourable safety profile with respect to cardiovascular and cancer risks than many patients with predominant peripheral arthritis. The taskforce discussed the circumstantial evidence that IL-23 inhibition may be efficacious for axial PsA; however, given negative trials for IL-12/23 inhibition in axSpA, the IL-23 pathway is not recommended here.33 71–73 Axial PsA remains a challenging form of PsA in terms of definition and differences with axial SpA; thus, this phenotype is part of the research agenda (table 4).
Recommendation 9
The choice of the mode of action should reflect non-musculoskeletal manifestations related to PsA; with clinically relevant skin involvement, preference should be given to an IL-17A or IL-17A/F or IL-23 or IL-12/23 inhibitor; with uveitis to an anti-TNF monoclonal antibody; and with IBD to an anti-TNF monoclonal antibody or an IL-23 inhibitor or IL-12/23 inhibitor or a JAKi.
This is a new recommendation to clarify more visibly than in 2019 (table 3) that the choice of drug should take into account not only the MSK PsA phenotype but also extra-MSK manifestations.
The first extra-MSK manifestation of interest in PsA is skin psoriasis. Although most patients with PsA present with skin psoriasis or have a personal history of skin psoriasis, registry data indicate that many patients with PsA have mild skin involvement.74 However, even limited skin psoriasis can be troublesome, since relevant skin involvement is defined as either extensive (body surface area involvement >10%), or as important to the patient, that is, impacting negatively their quality of life (such as is the case with face or genital involvement).9 For these patients, we recommend preferentially considering drugs targeting the IL-17A, IL-17A/F or IL-23 pathway (here, the order between drugs is cited in order of numbered cytokine, not preference). There are strong data, including head-to-head trials, in the field of skin psoriasis showing that drugs targeting the IL-23 and IL-17 pathways are superior to TNFis and to JAKis for skin psoriasis.51 52 75–78 This justified proposing these modes of action preferentially in case of relevant skin involvement. This is in keeping with psoriasis recommendations.79
Uveitis is not as frequent in PsA as it is in axial SpA; the prevalence is reported around 5%.80 However, uveitis can be severe and should influence treatment decisions. Currently, the only mode of action with direct proof of efficacy on uveitis is TNF inhibition through monoclonal antibodies (ie, adalimumab and infliximab). Thus, for patients with uveitis, an anti-TNF monoclonal antibody is preferred.
Inflammatory bowel disease (IBD) concerns 2%–4% of patients with PsA.80 The armamentarium for IBD has widened recently, and this recommendation reflects this fact, proposing that one of the modes of action currently licensed for IBD should be prescribed when it coexists with PsA. No order of preference is given here and prescribers are urged to adhere to EMA authorisations for IBD and take into account safety. For informative purposes, as of mid-2023, drugs authorised for IBD include anti-TNF monoclonal antibodies (ie, adalimumab and infliximab), the IL-12/23i ustekinumab, the IL-23i risankizumab (for Crohn’s disease) and two JAKis (one of which, tofacitinib, only for Crohn’s disease).81–85 IL-17is (both A and A/F) are not recommended in case of active IBD, given indications of a heightened risk of flares.86–88
Decisions for patients presenting with major skin involvement, with uveitis or with IBD should be discussed with the relevant specialist colleagues, as needed.
In all cases, the prescriber must refer to current drug authorisations and take into account safety and comorbidities.
To present an order for choosing drugs, we propose that the first element to take into account is the PsA subtype, then as a second element extra-MSK manifestations (always considering safety and comorbidities).
Recommendation 10
In patients with an inadequate response or intolerance to a bDMARD or a JAKi, switching to another bDMARD or JAKi should be considered, including one switch within a class.
This recommendation is unchanged from 2019, with unchanged LoE.9 After failing one targeted drug, it is logical to switch to another targeted drug; there are currently no strong data to prefer a switch with a change in mode of action to a switch within the same mode of action. Of note, this recommendation does not limit the total number of switches for a given patient. It also does not necessarily mean that more switches within a class could not be done, but the taskforce felt that a switch should not necessarily be done after one drug of a class has failed. Switches can be made, as appropriate, between bDMARDs, or between bDMARDs and JAKis. We include abatacept as a treatment option (table 1),49 but note that it demonstrated modest efficacy and hence this is an option to be used only after failing one or more other targeted drugs. The efficacy of bimekizumab, the dual IL-17 A/F inhibitor, appeared similar in TNF-naïve and TNF-experienced populations; this will warrant confirmation.53 54 Finally, a combination of bDMARDs is being explored, but cannot be recommended at this time.
Recommendation 11
In patients in sustained remission, tapering of DMARDs may be considered.
This bullet point is unchanged. However, more data have accrued on tapering, leading to a higher grade of recommendation.89–91 By tapering we mean ‘dose reduction’ not drug discontinuation since the latter usually leads to flares. Drug tapering is a logical step when patients are doing well over time, from a safety and a cost perspective (tapering is often performed by the patient himself/herself alone). On the other hand, long-term data are missing and currently drug tapering is off-label. For all of these reasons, the taskforce kept the tentative wording of ‘may be considered’ (to ensure it is not made mandatory) and of course in the context of a shared decision with the patient (as is the case also for the other treatment decisions).
Research agenda
The taskforce felt that many issues needed more data, and an extensive research agenda was developed (table 4).
Discussion
This paper presents updated recommendations for the management of PsA, a treatment algorithm and a research agenda. This update addresses all currently available drugs and modes of action, and recommends an order to their use, taking into account the phenotype of the MSK and the non-MSK manifestations.
These elements should be helpful in the management of individual patients, but also in the advocacy for better access to care and for research.
This 2023 update is a major update since most of the recommendations were modified substantially. The EULAR standardised operating procedures propose a voting system for updates which discourages minor modifications for rewordings.13 Since 2019, many new drugs have become available in PsA; the choice of which drug to prescribe to which patients rests on data related to efficacy, clinical phenotype, adverse event risk profile, tolerance, long-term data, cost and access. While laboratory biomarkers for stratified treatment approaches are lacking, the taskforce used clinical markers to develop clinical phenotypic preferences for specific drugs. In these updated recommendations, the taskforce applied expert opinion to the available data, to propose a pragmatic, logical order of a step-up approach to targeted treatments of PsA. The taskforce felt that proposing an order is helpful both for clinicians and to advocate for access to drugs for patients with PsA.
The drug options considered in these recommendations are currently licensed for PsA. We are aware that other drugs are being tested, or are available in other related conditions, especially skin psoriasis; however, these drugs are considered out of the scope of the present recommendations. Brodalumab was at the time of these recommendations only approved for psoriasis; TYK2 inhibitors such as deucravacitinib and brepocitinib have also been developed or in development for skin psoriasis and PsA; izokibep is a novel antibody mimetic, a small IL-17i currently undergoing testing; and an oral IL-23i is also in development.5
The taskforce had extensive discussions on the positioning of JAKi in the recommendations.63 92 We as a group feel that it is important to make haste slowly, and to uphold high safety standards when promoting drugs with only short-to-medium-term experience and for which long-term data are lacking—not least in PsA. In fact, this cautious attitude was also adhered to in the 2019 recommendations, and further safety developments have later confirmed that this attitude was appropriate.7 8 It is of key importance to continue monitoring the drugs and, ideally, perform controlled trials, as only hard and high-level data can be reassuring.
Costs are also an important aspect in patient management, and it is generally recommended to prescribe the cheaper drug if two agents have similar efficacy and safety. Of note, even if one mode of action may have somewhat better efficacy on certain manifestations, a less expensive agent could still be preferred as long as it does not bear much lesser efficacy in that disease domain. Biosimilars are available for several TNFis and have led to significant reduction in expenditure and more use in many countries, while their price is not much lower than that of originators in many other ones. Tofacitinib will soon become generic, and the same is true for apremilast, which should also lower the costs for these agents and allow wider application especially in less affluent countries. Thus, overall, the taskforce felt that the prescription of drugs would account for the relationships between efficacy, safety and cost, in line with the OAPs and the 11 recommendations which are summarised in the algorithm (figure 1). Many points are still to be confirmed in the management of PsA, leading to an extensive research agenda.93
In conclusion, the updated 2023 recommendations should be helpful to clinicians but also to health professionals and patients when discussing treatment options. They can also be helpful to promote access to optimal care. As new data become available and new drugs are authorised in PsA, these recommendations should be again updated.
Footnotes
Handling editor: Dimitrios T Boumpas
@LGossec, @FerreiraRJO, @lihi_eder, @dranielmar, @drpnash, @sshoopworrall
Contributors: All authors have contributed to this work and approved the final version.
Funding: Supported by EULAR (QoC016).
Competing interests: No support to any author for the present work. Outside the submitted work: LG: research grants: AbbVie, Biogen, Lilly, Novartis, UCB; consulting fees: AbbVie, Amgen, BMS, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, UCB; non-financial support: AbbVie, Amgen, Galapagos, Janssen, MSD, Novartis, Pfizer, UCB; membership on an entity’s Board of Directors or advisory committees: EULAR Treasurer. AK: speakers bureau, consultancy: AbbVie, Amgen, Galapagos, Janssen, Eli Lilly, MSD, Novartis, Pfizer, UCB. RJOF: research grants: Medac, Lilly; consulting fees: Sanofi. DA: research grants: Galapagos, Lilly; consulting fees: AbbVie, Gilead, Janssen, Lilly, Merck, Novartis, Sanofi. XB: research grants: AbbVie, MSD, Novartis; consultancies: AbbVie, Amgen, Celltrion, Chugai, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, Roche, Sandoz, UCB; membership on an entity’s Board of Directors or advisory committees: ASAS President, EULAR President Elect. W-HB: honoraria: AbbVie, Almirall, BMS, Janssen, Leo, Eli Lilly, Novartis, UCB; expert testimony: Novartis; participation on a Data Safety Monitoring Board or Advisory Board: AbbVie, Almirall, BMS, Janssen, Leo, Eli Lilly, Novartis, UCB. IBM: honoraria/consultation fees non-exec roles: NHS GGC Board Member, Evelo Board of Directors, Versus Arthritis Trustee Status; stock or stock options: Evelo, Cabaletta, Compugen, Causeway Therapeutics, Dextera. DGM: research grants: Janssen, AbbVie, Lilly, Novartis, UCB, BMS, Moonlake; consulting fees: Janssen, AbbVie, Lilly, Novartis, UCB, BMS, Moonlake, Celgene; honoraria: Janssen, AbbVie, Lilly, Novartis, UCB, BMS, Moonlake. KLW: research grants: BMS, Pfizer; consulting: Pfizer, AbbVie, AstraZeneca, BMS, Eli Lilly, Galapagos, GlaxoSmithKline (GSK), Gilead, Novartis, Moderna, Regeneron, Roche, Sanofi, UCB Pharma. AB: speakers fees: AbbVie, Amgen, AlphaSigma, AstraZeneca, Angelini, Biogen, BMS, Berlin-Chemie, Boehringer Ingelheim, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Teva, UCB, Zentiva; consultancies: Akros, AbbVie, Amgen, AlphaSigma, Biogen, Boehringer Ingelheim, Lilly, Mylan, MSD, Novartis, Pfizer, Roche, Sandoz, Sobi, UCB. PVB: consulting fees: AbbVie, Janssen-Cilag, Pfizer; honoraria: AbbVie, Bausch Health, Celltrion Healthcare, Eli Lilly, Gedeon Richter, IBSA Pharma, Infomed, Janssen-Cilag, Novartis, Pfizer, Sandoz; payment for expert testimony: Gedeon Richter; other: President, Hungarian Association of Rheumatologists. GRB: honoraria and/or speaker fees: AbbVie, BMS, Janssen, Lilly, Novartis, Pfizer. JDC: honoraria: UCB. PC: research grants: AbbVie, Amgen, Biogen, Jansen, Lilly, Novartis, UCB; consulting fees: AbbVie, Amgen, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, UCB. LE: consultation fee/advisory board: AbbVie, Novartis, Janssen, UCB, BMS, Eli Lilly; research/educational grants: AbbVie, Fresenius Kabi, Janssen, Amgen, UCB, Novartis, Eli Lilly, Sandoz, Pfizer. MLH: grant support: AbbVie, Biogen, BMS, Celltrion, Eli Lilly, Janssen Biologics BV, Lundbeck Foundation, MSD, Pfizer, Roche, Samsung Bioepis, Sandoz, Novartis, Nordforsk; honoraria: Pfizer, Medac, Sandoz; advisory board: AbbVie; past-chair of the steering committee of the Danish Rheumatology Quality Registry (DANBIO, DRQ), which receives public funding from the hospital owners and funding from pharmaceutical companies; cochair of EuroSpA, partly funded by Novartis. AI: research grants from AbbVie, Pfizer, Novartis; honoraria for lectures, presentations, speakers bureaus from AbbVie, Alfasigma, BMS, Celgene, Celltrion, Eli Lilly, Galapagos, Gilead, Janssen, MSD, Novartis, Pfizer, Sanofi Genzyme, Sobi; EULAR Board Member; EULAR Congress Committee, Education Committee and Advocacy Committee Advisor; EULAR Past President. LEK: consultancies: AbbVie, Amgen, Biogen, BMS, Celgene, Eli Lilly, Pfizer, UCB, Sanofi, GSK, Galapagos, Forward Pharma, MSD, Novartis, Janssen; has been representing rheumatology FOREUM scientific chair. RQ: consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Amgen-Celgene, Eli Lilly, Novartis, Janssen, Pfizer, MSD, UCB. DM: honoraria: UCB, Janssen, GSK, AstraZeneca, AbbVie; support to meetings: Janssen. HM-O: grant support: Janssen, Novartis, UCB; honoraria and/or speaker fees: AbbVie, Biogen, Eli Lilly, Janssen, Moonlake, Novartis, Pfizer, Takeda, UCB. PJM: grant support: AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Genascence, Janssen, Novartis, Pfizer, UCB; consulting fees: AbbVie, Acelyrin, Aclaris, Alumis, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Genascence, Inmagene, Janssen, Moonlake, Novartis, Pfizer, Takeda, UCB, Ventyx, Xinthera; honoraria: AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, UCB. PN: consulting fees and honoraria: AbbVie, Amgen, BMS, Lilly, Janssen, GSK, Novartis, UCB, Servatus; boards: Amgen, BMS, Janssen, GSK, Novartis, UCB; GRAPPA Steering Committee, Chair ASMPOC, ARA. LS: consulting fees: AbbVie, Almirall, Novartis, Janssen, Lilly, UCB, Pfizer, Bristol Myers Squibb, Boehringer Ingelheim; honoraria: AbbVie, Almirall, Novartis, Janssen, UCB, Pfizer, Takeda, Galderma, Biogen, Celgene, Celltrion, Lilly, Sanofi, Bristol Myers Squibb, Boehringer Ingelheim; support to attending meetings: AbbVie, Janssen, Lilly, Novartis, UCB, Galderma, Bristol Myers Squibb, Boehringer Ingelheim; participation in boards: AbbVie, Almirall, Novartis, Janssen, UCB, Pfizer, Galderma, Biogen, Lilly, Sanofi, Bristol Myers Squibb, Boehringer Ingelheim; GRAPPA Executive Board (elected), British Society for Medical Dermatology (BSMD) Committee. GS: honoraria: Novartis, Janssen. SJWS-W: grant support: Medical Research Council (MR/W027151/1). YT: research grants from Mitsubishi Tanabe, Eisai, Chugai, Taisho; speaking fees and/or honoraria from Eli Lilly, AstraZeneca, AbbVie, Gilead, Chugai, Boehringer Ingelheim, GlaxoSmithKline, Eisai, Taisho, Bristol Myers, Pfizer, Taiho. FEVdB: consultancy honoraria from AbbVie, Amgen, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB. AZ: speakers bureau: AbbVie, Novartis, Janssen, Lilly, UCB, Amgen; paid instructor for AbbVie, Novartis, UCB. DvdH: consulting fees AbbVie, Argenx, Bayer, BMS, Galapagos, Gilead, GlaxoSmithKline, Janssen, Lilly, Novartis, Pfizer, Takeda, UCB Pharma; Director of Imaging Rheumatology bv; Associate Editor for Annals of the Rheumatic Diseases; Editorial Board Member for Journal of Rheumatology and RMD Open; Advisor Assessment Axial Spondyloarthritis International Society. JSS: research grants from AbbVie, AstraZeneca, Lilly, Galapagos; royalties from Elsevier (textbook); consulting fees from AbbVie, Galapagos/Gilead, Novartis-Sandoz, BMS, Samsung, Sanofi, Chugai, R-Pharma, Lilly; honoraria from Samsung, Lilly, R-Pharma, Chugai, MSD, Janssen, Novartis-Sandoz; participation in advisory board from AstraZeneca.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Zabotti A, De Marco G, Gossec L, et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann Rheum Dis 2023;82:1162–70. 10.1136/ard-2023-224148 [DOI] [PubMed] [Google Scholar]
- 2. Alharbi S, Ye JY, Lee K-A, et al. Remission in psoriatic arthritis: definition and predictors. Semin Arthritis Rheum 2020;50:1494–9. 10.1016/j.semarthrit.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 3. Ferguson LD, Siebert S, McInnes IB, et al. Cardiometabolic comorbidities in RA and PSA: lessons learned and future directions. Nat Rev Rheumatol 2019;15:461–74. 10.1038/s41584-019-0256-0 [DOI] [PubMed] [Google Scholar]
- 4. Lubrano E, Scriffignano S, de Vlam K, et al. Triple jump for the optimal management of psoriatic arthritis: diet, sleep and exercise - a review. RMD Open 2023;9:e003339. 10.1136/rmdopen-2023-003339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerschbaumer A, Smolen JSS, Ferreira JO, et al. Efficacy and safety of pharmacological treatment of psoriatic arthritis: a systematic literature research informing the 2023 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2024:1–15. doi:ard-2024-225534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 7. European Medicine Agency statement. Available: https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki [Accessed 7 Nov 2023].
- 8. US food and Drug Administration. Available: https://www.fda.gov/safety/medical-product-safety-information/janus-kinase-jak-inhibitors-drug-safety-communication-fda-requires-warnings-about-increased-risk [Accessed 7 Nov 2023].
- 9. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of Psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coates LC, Soriano ER, Corp N, et al. Group for research and assessment of psoriasis and Psoriatic arthritis (GRAPPA): updated treatment recommendations for Psoriatic arthritis 2021. Nat Rev Rheumatol 2022;18:465–79. 10.1038/s41584-022-00798-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coates L, Gossec L. The updated GRAPPA and EULAR recommendations for the management of Psoriatic arthritis: similarities and differences. Joint Bone Spine 2023;90:105469. 10.1016/j.jbspin.2022.105469 [DOI] [PubMed] [Google Scholar]
- 12. Singh JA, Guyatt G, Ogdie A, et al. American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019;71:5–32. 10.1002/art.40726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Available: https://www.eular.org/web/static/lib/pdfjs/web/viewer.html?file=https://www.eular.org/document/download/680/b9eb08d0-faca-4606-8ed9-d0539b3f312a/660 [Accessed 1 Mar 2023].
- 14. Chalmers I, Glasziou P, Greenhalgh T, et al. The Oxford levels of evidence 2. In: Oxford Centre for Evidence-Based Medicine. Available: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence [accessed 1 Mar 2023]. [Google Scholar]
- 15. Gossec L, Smolen JS, Ramiro S, et al. European League against rheumatism (EULAR) recommendations for the management of Psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. 10.1136/annrheumdis-2015-208337 [DOI] [PubMed] [Google Scholar]
- 16. FitzGerald O, Ogdie A, Chandran V, et al. Psoriatic arthritis. Nat Rev Dis Primers 2021;7:59. 10.1038/s41572-021-00293-y [DOI] [PubMed] [Google Scholar]
- 17. Kerola AM, Kazemi A, Rollefstad S, et al. All-cause and cause-specific mortality in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: a nationwide registry study. Rheumatology (Oxford) 2022;61:4656–66. 10.1093/rheumatology/keac210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. So H, Tam L-S. Cardiovascular disease and depression in Psoriatic arthritis: multidimensional Comorbidities requiring Multidisciplinary management. Best Pract Res Clin Rheumatol 2021;35:101689. 10.1016/j.berh.2021.101689 [DOI] [PubMed] [Google Scholar]
- 19. Wendling D, Hecquet S, Fogel O, et al. French society for rheumatology (SFR) recommendations on the everyday management of patients with spondyloarthritis, including psoriatic arthritis. Joint Bone Spine 2022;89:105344. 10.1016/j.jbspin.2022.105344 [DOI] [PubMed] [Google Scholar]
- 20. Smolen JS, Gladman D, McNeil HP, et al. Predicting adherence to therapy in rheumatoid arthritis, psoriatic arthritis or ankylosing spondylitis: a large cross-sectional study. RMD Open 2019;5:e000585. 10.1136/rmdopen-2017-000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caso F, Chimenti MS, Navarini L, et al. Metabolic syndrome and psoriatic arthritis: considerations for the clinician. Expert Rev Clin Immunol 2020;16:409–20. 10.1080/1744666X.2020.1740593 [DOI] [PubMed] [Google Scholar]
- 22. Porta S, Otero-Losada M, Kölliker Frers RA, et al. Cardiovascular risk, and therapeutic management in obesity and Psoriatic arthritis. Front Immunol 2020;11:590749. 10.3389/fimmu.2020.590749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leung YY, Eder L, Orbai A-M, et al. Association between obesity and likelihood of remission or low disease activity status in psoriatic arthritis applying index-based and patient-based definitions of remission: a cross-sectional study. RMD Open 2023;9:e003157. 10.1136/rmdopen-2023-003157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Trouvin AP, Attal N, Perrot S. Assessing central sensitization with quantitative sensory testing in inflammatory rheumatic diseases: a systematic review. Joint Bone Spine 2022;89:105399. 10.1016/j.jbspin.2022.105399 [DOI] [PubMed] [Google Scholar]
- 25. Ballegaard C, Skougaard M, Guldberg-Møller J, et al. Comorbidities, pain and fatigue in psoriatic arthritis, psoriasis and healthy controls: a clinical cohort study. Rheumatology 2021;60:3289–300. 10.1093/rheumatology/keaa780 [DOI] [PubMed] [Google Scholar]
- 26. Smolen JS, Schöls M, Braun J, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis 2018;77:3–17. 10.1136/annrheumdis-2017-211734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet 2015;386:2489–98. 10.1016/S0140-6736(15)00347-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mease PJ, Coates LC. Considerations for the definition of remission criteria in psoriatic arthritis. Semin Arthritis Rheum 2018;47:786–96. 10.1016/j.semarthrit.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 29. Hagège B, Tan E, Gayraud M, et al. Remission and low disease activity in Psoriatic arthritis publications: a systematic literature review with meta-analysis. Rheumatology (Oxford) 2020;59:1818–25. 10.1093/rheumatology/keaa030 [DOI] [PubMed] [Google Scholar]
- 30. Landewé RBM, van der Heijde D. Use of multidimensional composite scores in rheumatology: parsimony versus subtlety. Ann Rheum Dis 2021;80:280–5. 10.1136/annrheumdis-2020-216999 [DOI] [PubMed] [Google Scholar]
- 31. Orbai A-M, de Wit M, Mease P, et al. International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis 2017;76:673–80. 10.1136/annrheumdis-2016-210242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vincken NLA, Balak DMW, Knulst AC, et al. Systemic glucocorticoid use and the occurrence of flares in psoriatic arthritis and psoriasis: a systematic review. Rheumatology 2022;61:4232–44. 10.1093/rheumatology/keac129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis 2023;82:19–34. 10.1136/ard-2022-223296 [DOI] [PubMed] [Google Scholar]
- 34. Zabotti A, Fagni F, Gossec L, et al. n.d. Subclinical psoriatic arthritis and new onset psoriatic arthritis in subjects transitioning from psoriasis: an analysis of 2 longitudinal cohorts. In Review, RMD Open [Google Scholar]
- 35. de Vlam K, Steinfeld S, Toukap AN, et al. The burden of psoriatic arthritis in the biologics era: data from the Belgian epidemiological psoriatic arthritis study. Rheumatology 2021;60:5677–85. 10.1093/rheumatology/keab233 [DOI] [PubMed] [Google Scholar]
- 36. Kishimoto M, Deshpande GA, Fukuoka K, et al. Clinical features of psoriatic arthritis. Best Pract Res Clin Rheumatol 2021;35:101670. 10.1016/j.berh.2021.101670 [DOI] [PubMed] [Google Scholar]
- 37. Vieira-Sousa E, Alves P, Rodrigues AM, et al. GO-DACT: a phase 3B randomised, double-blind, placebo-controlled trial of golimumab plus methotrexate (MTX) versus placebo plus MTX in improving dactylitis in MTX-naive patients with psoriatic arthritis. Ann Rheum Dis 2020;79:490–8. 10.1136/annrheumdis-2019-216500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mulder MLM, Vriezekolk JE, van Hal TW, et al. Comparing methotrexate monotherapy with methotrexate plus leflunomide combination therapy in psoriatic arthritis (COMPLETE-PSA): a double-blind, placebo-controlled, randomised, trial. Lancet Rheumatol 2022;4:e252–61. 10.1016/S2665-9913(22)00028-5 [DOI] [PubMed] [Google Scholar]
- 39. Coates LC, Tillett W, D’Agostino M-A, et al. Comparison between adalimumab introduction and methotrexate dose escalation in patients with inadequately controlled psoriatic arthritis (CONTROL): a randomised, open-label, two-part, phase 4 study. Lancet Rheumatol 2022;4:e262–73. 10.1016/S2665-9913(22)00008-X [DOI] [PubMed] [Google Scholar]
- 40. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying Antirheumatic drugs: 2022 update. Ann Rheum Dis 2023;82:3–18. 10.1136/ard-2022-223356 [DOI] [PubMed] [Google Scholar]
- 41. Lindström U, di Giuseppe D, Exarchou S, et al. Methotrexate treatment in early Psoriatic arthritis in comparison to rheumatoid arthritis: an observational nationwide study. RMD Open 2023;9:e002883. 10.1136/rmdopen-2022-002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev 2019;1:CD012722. 10.1002/14651858.CD012722.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Felten R, Lambert De Cursay G, Lespessailles E. Is there still a place for methotrexate in severe psoriatic arthritis. Ther Adv Musculoskelet Dis 2022;14. 10.1177/1759720X221092376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Curtis JR, Beukelman T, Onofrei A, et al. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann Rheum Dis 2010;69:43–7. 10.1136/ard.2008.101378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu L, Liu S, Wang C, et al. Folate supplementation for methotrexate therapy in patients with rheumatoid arthritis: a systematic review. J Clin Rheumatol 2019;25:197–202. 10.1097/RHU.0000000000000810 [DOI] [PubMed] [Google Scholar]
- 46. Mease PJ, Smolen JS, Behrens F, et al. SPIRIT H2H study group. A head-to-head comparison of the efficacy and safety of Ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020;79:123–31. 10.1136/annrheumdis-2019-215386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3B trial. Lancet 2020;395:1496–505. 10.1016/S0140-6736(20)30564-X [DOI] [PubMed] [Google Scholar]
- 48. Gossec L, Siebert S, Bergmans P, et al. Persistence and effectiveness of the IL-12/23 pathway inhibitor ustekinumab or tumour necrosis factor inhibitor treatment in patients with psoriatic arthritis: 1-year results from the real-world Psabio study. Ann Rheum Dis 2022;81:823–30. 10.1136/annrheumdis-2021-221640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mease PJ, Gottlieb AB, van der Heijde D, et al. Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis. Ann Rheum Dis 2017;76:1550–8. 10.1136/annrheumdis-2016-210724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McInnes IB, Sawyer LM, Markus K, et al. Targeted systemic therapies for psoriatic arthritis: a systematic review and comparative synthesis of short-term articular, dermatological, enthesitis and dactylitis outcomes. RMD Open 2022;8:e002074. 10.1136/rmdopen-2021-002074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 2022;5:CD011535. 10.1002/14651858.CD011535.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sbidian E, Chaimani A, Guelimi R, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 2023;7:CD011535. 10.1002/14651858.CD011535.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled, phase 3 trial (BE OPTIMAL). Lancet 2023;401:25–37. 10.1016/S0140-6736(22)02302-9 [DOI] [PubMed] [Google Scholar]
- 54. Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-Α inhibitors: a randomised. The Lancet 2023;401:38–48. 10.1016/S0140-6736(22)02303-0 [DOI] [PubMed] [Google Scholar]
- 55. Miyagawa I, Nakayamada S, Nakano K, et al. Precision medicine using different biological Dmards based on characteristic phenotypes of peripheral T helper cells in psoriatic arthritis. Rheumatology (Oxford) 2019;58:336–44. 10.1093/rheumatology/key069 [DOI] [PubMed] [Google Scholar]
- 56. Drosos GC, Vedder D, Houben E, et al. EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus and antiphospholipid syndrome. Ann Rheum Dis 2022;81:768–79. 10.1136/annrheumdis-2021-221733 [DOI] [PubMed] [Google Scholar]
- 57. Tarannum S, Leung Y-Y, Johnson SR, et al. Sex- and gender-related differences in Psoriatic arthritis. Nat Rev Rheumatol 2022;18:513–26. 10.1038/s41584-022-00810-7 [DOI] [PubMed] [Google Scholar]
- 58. Orbai A-M, Perin J, Gorlier C, et al. Determinants of patient-reported psoriatic arthritis impact of disease: an analysis of the association with sex in 458 patients from fourteen countries. Arthritis Care Res (Hoboken) 2020;72:1772–9. 10.1002/acr.24090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lindström U, Di Giuseppe D, Delcoigne B, et al. Effectiveness and treatment retention of TNF inhibitors when used as monotherapy versus comedication with csDMARDs in 15 332 patients with psoriatic arthritis. Ann Rheum Dis 2021;80:1410–8. 10.1136/annrheumdis-2021-220097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Behrens F, Cañete JD, Olivieri I, et al. Tumour necrosis factor inhibitor monotherapy vs combination with MTX in the treatment of PSA: a systematic review of the literature. Rheumatology (Oxford) 2015;54:915–26. 10.1093/rheumatology/keu415 [DOI] [PubMed] [Google Scholar]
- 61. Koehm M, Rossmanith T, Foldenauer AC, et al. Methotrexate plus ustekinumab versus ustekinumab monotherapy in patients with active psoriatic arthritis (MUST): a randomised, Multicentre, placebo-controlled, phase 3B, non-inferiority trial. Lancet Rheumatol 2023;5:e14–23. 10.1016/S2665-9913(22)00329-0 [DOI] [PubMed] [Google Scholar]
- 62. Fagerli KM, Lie E, van der Heijde D, et al. The role of methotrexate co-medication in TNF-inhibitor treatment in patients with psoriatic arthritis: results from 440 patients included in the NOR-DMARD study. Ann Rheum Dis 2014;73:132–7. 10.1136/annrheumdis-2012-202347 [DOI] [PubMed] [Google Scholar]
- 63. McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med 2021;384:1227–39. 10.1056/NEJMoa2022516 [DOI] [PubMed] [Google Scholar]
- 64. Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open 2023;9:e002735. 10.1136/rmdopen-2022-002735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79:1400–13. 10.1136/annrheumdis-2019-216761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kristensen LE, Danese S, Yndestad A, et al. Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL surveillance. Ann Rheum Dis 2023;82:901–10. 10.1136/ard-2022-223715 Available: 10.1136/ard-2022-223715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mease P, Gladman D, Coates L, et al. 16-week results from FOREMOST, a placebo-controlled study involving oligoarticular psoriatic arthritis treated with apremilast. Arthritis Rheumatol 2023;75. [Google Scholar]
- 68. Schett G, Lories RJ, D’Agostino M-A, et al. Enthesitis: from pathophysiology to treatment. Nat Rev Rheumatol 2017;13:731–41. 10.1038/nrrheum.2017.188 [DOI] [PubMed] [Google Scholar]
- 69. Marchesoni A, De Marco G, Merashli M, et al. The problem in differentiation between psoriatic-related polyenthesitis and fibromyalgia. Rheumatology 2018;57:32–40. 10.1093/rheumatology/kex079 [DOI] [PubMed] [Google Scholar]
- 70. Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis 2021;80:582–90. 10.1136/annrheumdis-2020-218808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Helliwell PS, Gladman DD, Chakravarty SD, et al. Effects of ustekinumab on spondylitis-associated endpoints in Tnfi-naïve active psoriatic arthritis patients with physician-reported spondylitis: pooled results from two phase 3, randomised, controlled trials. RMD Open 2020;6:e001149. 10.1136/rmdopen-2019-001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of ustekinumab in axial spondyloarthritis. Arthritis Rheumatol 2019;71:258–70. 10.1002/art.40728 [DOI] [PubMed] [Google Scholar]
- 73. Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. The Lancet Rheumatology 2021;3:e715–23. 10.1016/S2665-9913(21)00105-3 [DOI] [PubMed] [Google Scholar]
- 74. Ogdie A, Shin DB, Love TJ, et al. Body surface area affected by psoriasis and the risk for psoriatic arthritis: a prospective population-based cohort study. Rheumatology (Oxford) 2022;61:1877–84. 10.1093/rheumatology/keab622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bachelez H, van de Kerkhof PCM, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015;386:552–61. 10.1016/S0140-6736(14)62113-9 [DOI] [PubMed] [Google Scholar]
- 76. Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med 2017;376:1551–60. 10.1056/NEJMoa1607017 [DOI] [PubMed] [Google Scholar]
- 77. Gottlieb AB, Merola JF, Reich K, et al. Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized, double-blind head-to-head monotherapy study. Br J Dermatol 2021;185:1124–34. 10.1111/bjd.20413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of Ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol 2021;184:1047–58. 10.1111/bjd.19509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. Journal of the American Academy of Dermatology 2019;80:1029–72. 10.1016/j.jaad.2018.11.057 [DOI] [PubMed] [Google Scholar]
- 80. Pittam B, Gupta S, Harrison NL, et al. Prevalence of extra-articular manifestations in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology 2020;59:2199–206. 10.1093/rheumatology/keaa062 [DOI] [PubMed] [Google Scholar]
- 81. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. 10.1056/NEJMoa1602773 [DOI] [PubMed] [Google Scholar]
- 82. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. 10.1056/NEJMoa1606910 [DOI] [PubMed] [Google Scholar]
- 83. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. 10.1056/NEJMoa1900750 [DOI] [PubMed] [Google Scholar]
- 84. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022;399:2113–28. 10.1016/S0140-6736(22)00581-5 [DOI] [PubMed] [Google Scholar]
- 85. Loftus EV, Panés J, Lacerda AP, et al. Upadacitinib induction and maintenance therapy for Crohn’s disease. N Engl J Med 2023;388:1966–80. 10.1056/NEJMoa2212728 [DOI] [PubMed] [Google Scholar]
- 86. Letarouilly J-G, Pham T, Pierache A, et al. New-onset inflammatory bowel diseases among IL-17 inhibitor-treated patients: results from the case-control MISSIL study. Rheumatology 2022;61:2848–55. 10.1093/rheumatology/keab819 [DOI] [PubMed] [Google Scholar]
- 87. Emond B, Ellis LA, Chakravarty SD, et al. Real-world incidence of inflammatory bowel disease among patients with other chronic inflammatory diseases treated with Interleukin-17A or phosphodiesterase 4 inhibitors. Curr Med Res Opin 2019;35:1751–9. 10.1080/03007995.2019.1620713 [DOI] [PubMed] [Google Scholar]
- 88. Yamada A, Wang J, Komaki Y, et al. Systematic review with meta-analysis: risk of new onset IBD with the use of anti-Interleukin-17 agents. Aliment Pharmacol Ther 2019;50:373–85. 10.1111/apt.15397 [DOI] [PubMed] [Google Scholar]
- 89. Ye W, Tucker LJ, Coates LC. Tapering and discontinuation of biologics in patients with psoriatic arthritis with low disease activity. Drugs 2018;78:1705–15. 10.1007/s40265-018-0994-3 [DOI] [PubMed] [Google Scholar]
- 90. Coates LC, Pillai SG, Tahir H, et al. Withdrawing Ixekizumab in patients with psoriatic arthritis who achieved minimal disease activity: results from a randomized, double-blind withdrawal study. Arthritis Rheumatol 2021;73:1663–72. 10.1002/art.41716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ruwaard J, L’ Ami MJ, Kneepkens EL, et al. Interval prolongation of etanercept in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a randomized controlled trial. Scandinavian Journal of Rheumatology 2023;52:129–36. 10.1080/03009742.2022.2028364 [DOI] [PubMed] [Google Scholar]
- 92. Nash P, Mease PJ, Fleishaker D, et al. Tofacitinib as monotherapy following methotrexate withdrawal in patients with psoriatic arthritis previously treated with open-label tofacitinib plus methotrexate: a randomised, placebo-controlled substudy of OPAL balance. Lancet Rheumatol 2021;3:e28–39. 10.1016/S2665-9913(20)30339-8 [DOI] [PubMed] [Google Scholar]
- 93. Tarannum S, Widdifield J, Wu CF, et al. Understanding sex-related differences in Healthcare utilisation among patients with inflammatory arthritis: a population-based study. Ann Rheum Dis 2023;82:283–91. 10.1136/ard-2022-222779 [DOI] [PMC free article] [PubMed] [Google Scholar]