Abstract
Objectives
To obtain an overview of recent evidence on efficacy and safety of pharmacological treatments in psoriatic arthritis (PsA).
Methods
This systematic literature research (SLR) investigated the efficacy and safety of conventional synthetic (cs), biological (b) and targeted synthetic (ts) disease-modifying antirheumatic drugs (DMARDs) in patients with PsA. A systematic database search using Medline, EMBASE, Cochrane CENTRAL was conducted to identify relevant articles published since the previous update in 2019 until 28 December 2022. Efficacy was assessed in trials while for safety observational data were also considered. Adverse events of special interest were infections (including herpes zoster, influenza and tuberculosis), malignancies, major adverse cardiovascular events, venous thromboembolisms, liver disease, laboratory changes and psychiatric adverse events. No meta-analyses were performed.
Results
For efficacy, of 3946 articles screened, 38 articles (30 trials) were analysed. The compounds investigated included csDMARDs (leflunomide, methotrexate), bDMARDs inhibiting IL17 (bimekizumab, brodalumab, ixekizumab, izokibep, secukinumab,), IL-23 (guselkumab, risankizumab, tildrakizumab), IL-12/23 (ustekinumab) as well as TNF (adalimumab, certolizumab-pegol, etanercept, infliximab, golimumab) and Janus Kinase inhibitors (JAKi) (brepocitinib, deucravacitinib, tofacitinib, upadacitinib). The compounds investigated were efficacious in improving signs and symptoms of PsA, improving physical functioning and quality of life. For safety, 2055 abstracts were screened, and 24 articles analysed: 15 observational studies and 9 long-term follow-ups of trials, assessing glucocorticoids, TNFi, IL-17i, JAKi, IL-12/23i and PDE4i (apremilast). Safety indicators were generally coherent with the previous SLR in 2019.
Conclusion
The results of this SLR informed the task force responsible for the 2023 update of the European Alliance of Associations for Rheumatology recommendations for pharmacological management of PsA.
Keywords: Psoriatic Arthritis, DMARDs (synthetic), Biological Therapy, Therapeutics
WHAT IS ALREADY KNOWN ON THIS TOPIC
Many drugs have become available over the last years in psoriatic arthritis (PsA); trials of new drugs have consistently been positive in terms of the primary outcomes, but it is difficult to obtain an overview of the efficacy and safety of systemic therapies for PsA, including conventional synthetic, biological and targeted synthetic disease-modifying antirheumatic drugs (DMARDs).
WHAT THIS STUDY ADDS
This systematic literature research (SLR) provides an overview of the efficacy and safety results of:
Established DMARDs, trials investigating efficacy of DMARDs on specific domains of PsA (axial, dactylitis, synovitis).
Novel biological DMARDs, including inhibitors of IL23-p19 (risankizumab, guselkumab, tildrakizumab), IL17A (izokibep), IL17A/F (bimekizumab).
Novel Janus kinase inhibitors, including brepocitinib (JAK1/TYK2), deucravacitinib (TYK2) and upadacitinib (JAK1/JAK2).
Studies on treatment tapering and stopping.
Safety analyses of observational data investigating established DMARDs on adverse events of special interest (infections, malignancies, cardiovascular and thrombotic events).
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This SLR informed the 2023 European Alliance of Associations for Rheumatology PsA management recommendations task force with the available evidence published since the previous update published in 2019.
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory disease occurring in patients with psoriasis, leading to the typical clinical hallmarks of oligoarthritis or polyarthritis, enthesitis and dactylitis; if left untreated, radiographic damage and, although rare, potentially mutilating joint destruction may occur.1 Axial disease in PsA was recognised already by Moll and Wright as an important musculoskeletal manifestation, however, many new insights were gained over the past decades,1 differentiating axial PsA from the manifestations observed in radiographic and non-radiographic axial spondyloarthritis, including genetics, pathological findings, clinical symptoms, subsequent radiographic changes and also potential implications for treatment.2
In 2012, the first European Alliance of Associations for Rheumatology (EULAR) management recommendations on pharmacological therapies for PsA were developed,3 with subsequent updates in 20154 and 2019 5. With an increasing depth of understanding immunological pathways of PsA, the body of evidence on the efficacy of new molecules targeting different modes of action (MOAs), such as inhibition of the p19 subunit of interleukin (IL)-236–8 and IL-17,9 but also Janus Kinase (JAK) including the tyrosine kinase 2 (TYK2) pathway, has significantly increased in recent years.10–12
Besides the mere development of new MOAs using established composite endpoints, recent studies also provided evidence on the efficacy of established disease-modifying antirheumatic drugs (DMARDs) on specific musculoskeletal manifestations, including synovitis,13 axial disease14 and dactylitis.15 Furthermore, head-to-head trials, comparing different MOAs,16–18 as well as conventional synthetic (cs) DMARD regimens19 and strategic trials, also on tapering strategies, were published.20 21 Increasing emphasis on adverse events of DMARD therapies gained attention over the past years due to safety signals identified in patients with rheumatoid arthritis and cardiovascular risk factors receiving a JAKi, namely tofacitinib (TOFA).22 This may also be important in PsA, given the high prevalence of comorbidities and risk factors, such as obesity, dyslipidaemia, metabolic syndrome, cardiovascular disease and smoking in patients with PsA.23
This systematic literature research (SLR) was conducted to summarise and update the evidence on pharmacological treatments in PsA since 2019,24 to inform the EULAR task force developing the 2023 update of the PsA management recommendations.25
Methods
This SLR was conducted according to the EULAR updated standard operating procedures and based on a protocol developed and approved by the task force.26
To update the evidence from the previous SLR with the data cut on 21 December 2018,24 articles published in English language between 1 January 2018 and 28 December 2022 were searched by an experienced librarian (JWS) using Medline (PubMed), Embase (OVID version), the Cochrane CENTRAL Register of Controlled Trials (Central) and the abstract archives of the EULAR and American College of Rheumatology (ACR) annual meetings. To prevent missing important publications, we included the year 2018 in the literature search, as manuscripts which carry the date of a specific year might be published with some delay. Like in previous SLRs, conference abstracts from the last 3 years (from 2020 to 2022) were eligible for inclusion. In the case of articles being published after the data cut of the literature search but before the EULAR task force meeting (17 April 2023), fully published manuscripts were eligible to be included only if they had been covered as a conference abstract in the initial systematic search. The search strategies are provided in online supplemental appendix (Text S1.1-S1.6).
ard-2024-225534supp001.pdf (1,018.1KB, pdf)
During the first steering committee meeting (21 November 2022), a research protocol was developed. In total, 10 research questions were defined. These research questions covered the areas of the efficacy and safety of already approved DMARDs, safety of JAK inhibitors (JAKi), the efficacy and safety of new molecules, drug–drug interactions, the efficacy of DMARDs on different disease manifestations of PsA, evidence on strategic trials as well as evidence on dose reduction and treatment discontinuation. Based on the research questions, detailed PICO definitions (population, intervention, control, outcome) were developed. Details on the research questions as well as the PICO definitions are shown in online supplemental table S1.10. For efficacy, only randomised controlled trials (RCTs) investigating non-steroidal anti-inflammatory drugs (NSAIDs), cs, biological (b) or targeted synthetic (ts) DMARDs in adult patients classified as having PsA were eligible for inclusion. Phase 2 studies were eligible for inclusion, if no phase 3 studies were available, as efficacy but also safety profiles of new molecules, targeting similar pathways as currently licensed drugs, might be informative. For safety, data of identified RCTs as well as long-term extensions studies of RCTs with adequately defined comparator arms (placebo (PBO) or active therapy) were eligible. In addition to RCT safety data (including long-term extensions with adequate comparator arms) also observational studies (including registry analyses, claims data, cohort studies and case–control studies) with adequately defined controls were eligible for inclusion.
Patient populations of interest were defined as patients classified as having PsA; patients could be either DMARD-naïve, or with intolerance and/or insufficient response (IR) to csDMARDs, patients who were bDMARD-IR and/or tsDMARD-IR or mixed populations with previous IR to cs-DMARDs or/and bDMARDs; in some studies patients with IR to NSAIDs were also eligible. All interventions of interest are listed in online supplemental table S1.7. Efficacy outcomes included composite measures of state (such as Disease Activity Index for Psoriatic Arthritis (DAPSA) or minimal disease activity (MDA)) and of improvement (such as ACR criteria), individual core-set measures of signs and symptoms, patient-reported outcomes (including fatigue, physical function). Safety outcomes included infections (serious infections, herpes zoster (HZ), opportunistic and fungal infections, tuberculosis (Tb)); malignancies; major adverse cardiovascular events (MACEs); venous thromboembolic events (VTE); liver disease; gastrointestinal side effects; incidence of inflammatory bowel disease (IBD), uveitis; depression, suicidal ideation and behaviour (online supplemental table S1.8 List S1.9).
10% of all titles and abstracts were screened by two researchers (AK and RJOF) with an agreement of 94%. All other titles and abstracts were screened and assessed by one researcher (AK). In the case of any uncertainties, these were discussed with the methodologist (LG).
The selected articles were then assessed in full detail for eligibility and data of finally eligible articles were extracted using standardised spreadsheet forms by one researcher (AK) and verified in detail for correctness by the co-methodologist (RJOF). Risk of bias (RoB) assessment was done using the Cochrane Collaborations RoB tool for RCTs (version 2), while the Newcastle-Ottawa scale was utilised for assessing RoB in cohort and case–control studies.27 Conference abstracts were not assessed for RoB.28 Due to the high heterogeneity of the finally selected studies, and for the purposes of recommendations, detailed information of each study rather than summary statistics was selected; thus, no meta-analyses were performed and results, including effect sizes derived from safety analyses were taken from the original articles and reported descriptively. Specific adverse events of special interest (AESI) occurring during the double-blind period are reported in detail, while summary estimates derived from long-term extension studies are reported directly following the respective sections on the double-blind period.
Results
Search results
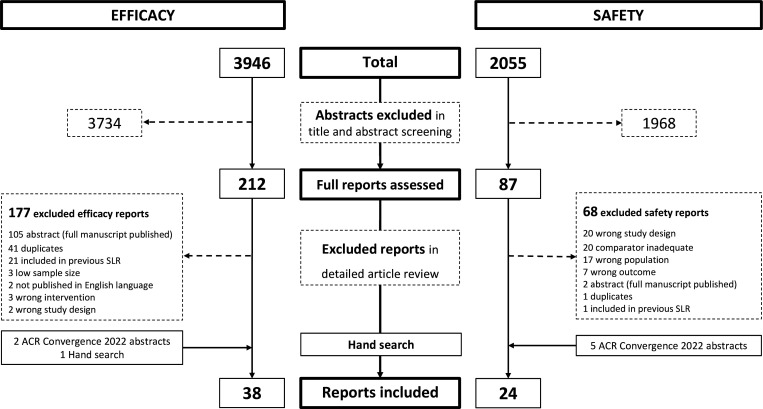
The efficacy search resulted in 3946 articles of which 212 references were selected to be assessed in the detailed article review, resulting in 38 articles describing 30 unique trials eligible for final inclusion in the SLR (figure 1). A detailed list of studies assessing the efficacy of DMARDs is shown in online supplemental table S2.1. Table 1 provides an overview of the drugs evaluated for efficacy and table 2 summarises the main reported efficacy outcomes of the studies included.
Figure 1.
PRISMA flow chart of the efficacy and safety search of studies in PsA published 2018–2022. ACR, American College of Rheumatology; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PsA, psoriatic arthritis; SLR, systematic literature research.
Table 1.
Summary of studies in psoriatic arthritis published 2018–2022 and included for evaluation of efficacy
| Intervention | Number of articles/ abstracts | Therapeutic compound | Target | References |
| csDMARDs, csDMARD combination versus other csDMARDs or placebo | 1 | Methotrexate (MTX) + Leflunomide MTX + Placebo |
Dihydrofolate reductase/adenosine metabolism (AICAR); dihydroorotate dehydrogenase | 19 |
| bDMARD ± csDMARDs versus placebo | 4 | Secukinumab | IL-17A | 13 14 29 30 |
| 3 | Izokibep | IL-17A | 43–45 | |
| 2 | Bimekizumab | IL-17A/F | 9 41 | |
| 1 | Brodalumab | IL-17A receptor | 42 | |
| 4 | Guselkumab | IL-23p19 | 7 35–37 | |
| 4 | Risankizumab | IL-23p19 | 8 38–40 | |
| 1 | Tildrakizumab | IL-23p19 | 6 | |
| bDMARD + csDMARD (combination therapy) versus bDMARD + placebo (monotherapy) | 1 | Ustekinumab+MTX vs ustekinumab+placebo | IL-12/23; dihydrofolate reductase+purine metabolism (AICAR) | 31 |
| bDMARD + csDMARD (combination therapy) versus csDMARD + placebo (monotherapy) | 1 | Golimumab+MTX vs placebo+MTX | TNF; dihydrofolate reductase+purine metabolism (AICAR) | 15 |
| tsDMARDs ± csDMARDs versus placebo | 1 | Brepocitinib | JAK1, TYK2 | 10 |
| 1 | Deucravacitinib | TYK2 | 11 | |
| 1 | Upadacitinib | JAK1, JAK2 | 12 | |
| bDMARDs versus other bDMARDs (head-to-head) | 2 | Secukinumab vs adalimumab | IL-17A vs TNF | 16 33 |
| 2 | Ixekizumab vs adalimumab | IL-17A vs TNF | 17 32 | |
| tsDMARDs versus bDMARDs (head-to-head) | 2 | Upadacitinib vs adalimumab | JAK1, JAK2 vs TNF | 18 34 |
| Strategic studies | 3 | 20 48 49 | ||
| csDMARD stopping | 1 | MTX | Dihydrofolate reductase+purine metabolim | 52 |
| bDMARD dose reduction and stopping | 2 | TNF | TNF | 21 50 |
| bDMARD stopping | 1 | Ixekizumab | IL-17A | 51 |
AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; IL, interleukin; JAK, Janus Kinase; TNF, tumour necrosis factor alpha; TNFi, tumour necrosis factor alpha inhibitor.
Table 2.
Summary of efficacy outcomes of studies in psoriatic arthritis published 2018–2022 and included for evaluation of efficacy
| Disease domain | Abbreviation | Full form |
| Composite scores | ACR response | American College of Rheumatology response definition |
| DAPSA | Disease Activity Index for Psoriatic Arthritis | |
| DAS28-ESR/CRP | Disease Activity Score-28 (with ESR/CRP) | |
| MDA/VLDA | Minimal Disease Activity / Very Low Disease Activity criteria | |
| PASDAS | Psoriatic Arthritis Disease Activity Score | |
| PsARC | Psoriatic Arthritis Response Criteria | |
| Peripheral arthritis | SJC66 | Swollen Joint Count 66 |
| TJC68 | Tender Joint Count 68 | |
| Patient-reported outcomes and Health-related quality of life |
EGA | Evaluator global assessment of disease activity |
| EQ5D | Euro Quality of Life five dimensions | |
| FACIT-F | Functional Assessment of Chronic Illness Therapy‐Fatigue | |
| Pain | Patient global assessment of pain | |
| PGA/PtGA | Patient global assessment of disease activity | |
| PSAID | Psoriatic Arthritis Impact of Disease | |
| SF36-MCS | Short Form 36 Mental Component Score | |
| WPAI | Work Productivity and Activity Impairment | |
| Physical function | HAQ-DI | Health Assessment Questionnaire Disability Index |
| SF36-PCS | Short Form 36 Physical Component Score | |
| Skin/nails | BSA | Body surface area |
| IGA | Investigator’s Global Assessment Scale | |
| NAPSI | Nail Psoriasis Severity Index | |
| PASI | Psoriasis Area and Severity Index | |
| Enthesitis | LEI | Leeds Enthesitis Index |
| MASES | Maastrich Ankylosing Spondylitis Enthesitis Score | |
| SPARCC | Spondyloarthritis Research Consortium of Canada Index | |
| – | Resolution of Enthesitis | |
| Dactylitis | DSS | Dactylitis Severity Score |
| LDI | Leeds Dactylitis Index | |
| – | Resolution of Dactylitis | |
| Axial disease | ASAS response | Assessment of SpondyloArthritis International Society response criteria |
| ASDAS | Ankylosing Spondylitis Disease Activity Score | |
| BASDAI | Bath Ankylosing Spondylitis Disease Activity Index | |
| BASFI | Bath Ankylosing Spondylitis Function Index | |
| BASMI | Bath Ankylosing Spondylitis Metrology Index | |
| Imaging | GLOESS | Global EULAR and OMERACT Synovitis Score |
| mSvDHS | modified Sharp van der Heijde Score | |
| – | Berlin MRI Score |
CRP, C reactive protein; ESR, erythrocyte sedimentation rate.
A total of 19 of the 30 trials (63.3%) were rated as having a low RoB; 7 of 30 trials (23.3%) received a rating of a high RoB—all due to an open-label or single-blinded trial design. Two trials (2/30, 6.6%) had an unclear RoB, both due to insufficient reporting of randomisation sequence generation and allocation concealment methods. One trial was published as a conference abstract only and was not assessed for RoB. All RoB assessments of efficacy studies are provided in online supplemental table S2.2, baseline characteristics are presented in online supplemental tables S2.3-S2.4, and detailed efficacy results are shown in online supplemental tables S2.5-S2.7.
For safety, beyond the adverse event profiles presented in the RCT publications already included in the efficacy search, further 2055 references were screened, and 87 articles assessed in detail with 24 studies finally included (figure 1). These 24 studies included 9 safety analyses of RCT data (long-term extensions and integrated safety analyses of AESI) as well as 15 observational studies: 8/24 (33.3%) with a low RoB, 1/24 (4.2%) with unclear and 10/24 (37.5%) with a high RoB (5 conference abstracts were not assessed for RoB). Investigations of AESI in RCTs were concerned with influenza, Tb, malignancies and thromboembolic events. Cohort studies investigated serious infections, Tb, solid and haematological malignancies, liver disease, MACEs as well as anxiety and depression (online supplemental table S3.1 provides a detailed list of safety studies included). RoB assessments of safety studies are shown in online supplemental table S3.2 and S3.3, population characteristics are presented in online supplemental table S3.4, with the main safety results shown in online supplemental tables S3.5.
Efficacy of csDMARDs
Only one RCT was available: the COMPLETE-PsA trial (low RoB).19 Patients with a diagnosis of PsA and without concomitant DMARD therapy were randomised to methotrexate (MTX) + leflunomide (LEF) combination therapy (n=39) or MTX+PBO (n=39). MTX+LEF combination therapy was superior to MTX+PBO in achieving the primary outcome (mean change in Psoriatic Arthritis Disease Activity Score; PASDAS) at week 16 (3.1±1.4 vs 3.7±1.3, treatment difference: –0.6, 90% CI –1.0 to −0.1; p=0.025). Nausea and vomiting were more frequent in the MTX+LEF arm (44% vs 28%), as was alanine aminotransferase elevation (31% vs 18%) and altered bowel habits (26% vs 8%).19
Efficacy of established bDMARDs
In total, six placebo-controlled trials investigating approved bDMARDs (one golimumab (GOL), four secukinumab (SEC), one ustekinumab (UST)) with or without concomitant csDMARDs were included (four with low, two with unclear RoB).13–15 29–31
In GO-DACT (low RoB), MTX monotherapy was compared with MTX+GOL (TNFi) combination therapy in patients naïve to MTX and bDMARD with active dactylitis. An interim analysis at 50% of the planned recruitment suggested favourable results, which led to stopping of patient recruitment. A significantly higher median reduction in the dactylitis severity score at week 24 (primary endpoint) was observed for the MTX+GOL arm (n=21) compared with the MTX+PBO (n=23) arm (−5 vs −2, p=0.026). Assessment of dactylitis via Leeds Dactylitis Index (LDI) also showed significant differences between the arms (−69.4 vs 31.1, p=0.042), while dactylitis remission at week 24 was achieved in 6/20 (30%) vs 4/22 (18%, p=0.477) of patients receiving MTX+GOL vs MTX+PBO, respectively. MRI assessment of dactylitis (secondary exploratory endpoint) showed a significant difference in favour of the MTX+GOL arm (p=0.017). Outcomes assessing other domains besides dactylitis (enthesitis, tender joints, swollen joints, skin, physical function) showed a trend towards better efficacy for the combination therapy arm, without significant differences between the arms.15
Four trials (two with low RoB, two with unclear RoB) investigated SEC (anti-IL-17A) treatment. CHOICE (unclear RoB) demonstrated superior efficacy of SEC 300 mg (but not SEC 150 mg) every 4 weeks vs PBO (ACR20 at week 16: 51.5% vs 36.9% vs 23.1% for SEC 300 mg (p<0.001), SEC 150 mg (p=0.10) and PBO respectively) in a biological naïve PsA population.29 The ULTIMATE trial investigated the change of ultrasound synovitis in biological naïve patients treated with SEC 300 mg every 4 weeks, 150 mg every 4 weeks or PBO. The primary endpoint (the ultrasound Global EULAR and OMERACT Synovitis Score mean change from baseline to week 12) was met with higher response rates in SEC (150 mg/300 mg combined group) treated patients vs PBO (−9±0.9 vs −6±0.9; difference: −3 (−6 to –1); one-sided p=0.004).13 Baraliakos et al. conducted the first RCT investigating DMARD therapy in an axial PsA population. MAXIMISE (low RoB) demonstrated superior efficacy of SEC 300 mg or 150 mg every 4 weeks when compared with PBO, meeting the study’s primary endpoint, ASAS20 response at week 12 (63% vs 66% vs 31%, respectively; p<0.001), as well as demonstrating improvement in MRI imaging (least squares mean difference vs PBO in total Berlin MRI score at week 12 for the entire spine: SEC 300 mg: −0.4±0.1, p<0.01; SEC 150 mg: −0.4±0.1, p<0.05; for the sacroiliac joints: SEC 300 mg −0.5±0.2, p<0.01; SEC 150 mg −0.5±0.2, p<0.01).14 FUTURE-4 compared SEC 150 mg every 4 weeks with and without loading (ie, SEC 150 mg at weeks 0, 1, 2 and 3) to PBO. Both regimens showed superior efficacy compared with PBO and no meaningful differences between the active treatment arms.30
The MUST trial (low RoB) was an investigator initiated, non-inferiority trial, comparing UST (IL-12/23i) + MTX combination therapy (n=88) to UST+PBO (n=85) in PsA patients naïve to UST. While patients received open-label UST treatment, concomitant therapy with either MTX or PBO was masked. At week 24, non-inferiority was observed for the primary outcome (mean DAS28-ESR), further no meaningful differences in secondary outcomes were observed between the groups.31
Efficacy of targeted synthetic DMARDs
Three trials investigating the efficacy of tsDMARDs were included (all low RoB).10–12
Inhibition of JAK 1 and 2 via upadacitinib (UPA; 15 mg or 30 mg once daily) was compared with PBO treatment in patients with prior IR to biological DMARDs in SELECT PsA 2 (low RoB). The trial met the primary endpoint, ACR20 at week 12, with significantly higher response rates in UPA-treated patients (UPA 15 mg once daily: 120/211, 56.9%, p<0.001); UPA 30 mg once daily: 139/218, 63.8%; PBO: 51/212, 24.1%, p<0.001). Further, all secondary endpoints were met.12
Deucravacitinib (DEUC), a selective, oral TYK2 inhibitor, was investigated in a phase 2 double-blind RCT (low RoB) in patients with IR to ≥1 previous NSAID or DMARD therapy. The ACR 20 response at week 16 (primary endpoint) was demonstrated to be significantly higher in DEUC 6 mg once daily (37/70, 52.9%, p=0.013) and DEUC 12 mg once daily (42/67, 62.7%, p<0.001) treated patients compared with PBO (21/66, 31.8%). Higher response rates in improvement of physical function, skin disease as well as enthesitis and dactylitis resolution were observed.11
Another phase 2 RCT (low RoB) investigated brepocitinib (BREP), an inhibitor of TYK2 and JAK1 with active disease despite previous NSAID or DMARD treatment. BREP 30 mg once daily as well as 60 mg once daily, but not BREP 10 mg once daily, met the primary endpoint (ACR20 at week 16) when compared with PBO treatment (PBO: 29/67, 43.3%; BREP 10 mg once daily: 20/31, 43.4%, p=not significant; BREP 30 mg once daily: 40/60, 66.7%, p=0.0197; BREP 60 mg once daily: 44/59, 74.6%, p=0.0006). Significant differences compared with placebo were also observed in PASI 75/90% responses for BREP 30 mg and 60 mg once daily. Exploratory analyses also showed benefit for BREP-treated patients in achieving MDA and improvement of enthesitis (only for BREP 60 mg once daily) and dactylitis outcomes (only for BREP 10 mg and 60 mg once daily), as well as physical function and fatigue.10
Head-to-head studies
Three head-to-head trials were included (RoB; table 3).16 18 32–34
Table 3.
Efficacy outcomes of head-to-head studies comparing JAK inhibitors to biological DMARDs
| Population | Study | RoB | Treatment | n | Primary endpoint | Result (%) | Head-to-head comparison | Endpoints at week 24 | |||||||
| ACR 20 (%) | ACR 50 (%) | ACR 70 (%) | PASI90 (%) | MDA (%) | LEI=0 n/N (%) | LDI-B=0 n/N (%) | ΔHAQ-DI | ||||||||
| csDMARD-IR; bDMARD naive | Mease 2020/Smolen 2020 (SPIRIT-H2H)17 32 | High (assessor blinded) | IXE 80 mg Q4W ± csDMARD | 283 | ACR50 + PASI100 at week 24 | 36.0 | S (met) | 68.9 | 50.5 | 31.8 | 71.7 | 47.7 | 95/159 (59.7) | 37/42 (88.1) | NR |
| ADA 40 mg Q2W ± csDMARD | 283 | 27.9 | Reference | 72.1 | 46.6 | 25.8 | 55.8 | 35.3 | 81/147 (55.1) | 54/58 (93.1) | NR | ||||
| csDMARD-IR; bDMARD naive | McInnes 2020 (EXCEED)16 | Low | SEC 300 mg Q4W (mono) | 426 | ACR20 at week 52 | 67 | S (not met) | 71 | 43 | NR | 63 | NR | 119/234 (51) | NR | −0.54 |
| ADA 40 mg Q2W (mono) | 427 | 62 | Reference | 64 | 40 | NR | 42 | NR | 116/264 (44) | NR | −0.51 | ||||
| csDMARD-IR; bDMARD naive | McInnes 2021 (SELECT PsA 1)18 | Low | UPA 15 mg OD±csDMARD | 429 | ACR 20 at week 12 | 70.6 | NI (met) S (not met) |
73.4 | 52.4 | 28.7 | 41.6 | 36.6 | 145/270 (53.7) | 104/136 (76.5) | −0.51 |
| UPA 30 mg OD ± csDMARD | 423 | 78.5 | NI (met) S (met) |
78.5 | 60.5 | 36.4 | 48.1 | 45.4 | 154/267 (57.7) | 101/127 (79.5) | −0.51 | ||||
| ADA 40 mg Q2W ± csDMARD | 429 | 65.0 | Reference | 67.1 | 44.3 | 22.6 | 45.0 | 33.3 | 125/265 (47.2) | 94/127 (74.0) | −0.39 | ||||
| PBO ± csDMARD | 423 | 36.2 | – | 45.2 | 18.9 | 5.2 | 16.6 | 12.3 | 78/241 (32.4) | 50/126 (39.7) | −0.19 | ||||
Results of secondary efficacy outcomes are shown at the timepoint of the primary endpoint.
ACR, American College of Rheumatology; ADA, adalimumab; bDMARD, biological disease-modifying antirheumatic drug; csDMARD, conventional synthetic DMARD; HAQ-DI, health assessment questionnaire-disability Index; IR, insufficient response; IXE, ixekizumab; JAK, Janus Kinase; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; NI, non-inferiority; NR, not reported; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; RoB, risk of bias; S, superiority; SEC, secukinumab; UPA, upadacitinib.
SPIRIT H2H (high RoB), an assessor-blinded, head-to-head trial compared open-label ixekizumab (IXE, IL17Ai) to adalimumab (ADA, TNFi) in bDMARD naïve patients with IR to csDMARDs. The primary endpoint was defined as simultaneous achievement of ACR50 and PASI100 response—assessed for superiority of IXE versus ADA at week 24. Significantly more patients achieved the primary outcome in the IXE group (102/283, 36%, p=0.036) vs ADA (79/283, 27.9%). No significant difference was found between the arms when comparing ACR (20/50/70) response rates, Health Assessment Questionnaire Disability (HAQ-DI) or dactylitis remission (LDI=0). However, comparing PASI75/90/100 results, these showed significantly higher response rates in IXE-treated patients compared with ADA. Other secondary endpoints such as DAPSA remission, MDA, VLDA were also in favour of IXE treatment.17 The results were maintained until week 54, with a significantly higher proportion of patients achieving a simultaneous ACR50+PASI 100 response (39% vs 26%, p<0.001).32
In the EXCEED trial (low RoB), bDMARD naïve patients were randomised to SEC 300 mg every 4 weeks or ADA 40 mg every 2 weeks monotherapy in a double-blind manner. The study was powered to show superiority of SEC over ADA in achieving an ACR20% response at week 52. The primary endpoint was not met, with an ACR20 response of 67% vs 62% (p=0.072) for SEC and ADA, respectively. Similar responses were observed between both treatment arms, with the exception of skin outcomes (PASI 75/100) showing higher responses in the SEC arm.16 33
Another double-blind head-to-head trial (SELECT-PsA 1, low RoB) compared UPA 15 mg once daily and 30 mg once daily to ADA 40 mg every 2 weeks and PBO treatment in patients with IR to csDMARDs. With ACR 20 response rates at week 12 of 303/429 (70.6%), 332/423 (78.5%), 279/429 (65.0%) and 153/423 (36.2%) for UPA 15 mg once daily, UPA 30 mg once daily, ADA 40 mg every 2 weeks and PBO, all active treatment arms showed superiority compared with placebo (p<0.001). Further, non-inferiority (margin 15%) of both UPA dosages was demonstrated when compared with ADA, while testing for superiority (vs ADA) was only shown for UPA 30 mg once daily (p<0.001) and not for UPA 15 mg once daily (hierarchical testing failed, no p value reported). Radiographic progression at week 24 was low in all active treatment arms.18 34
A summary of the trial results is shown in table 3.
Efficacy of novel biological DMARDs targeting the p19 subunit of IL23
In total six trials (five with low RoB, one with unclear RoB) were included.6–8 35–40
Three trials investigated guselkumab (GUS) an IL23-p19 inhibitor. DISCOVER-1 (low RoB) was a phase 3, double-blind RCT investigating patients with and without prior TNFi exposure. The ACR 20% response at week 24 (primary endpoint) was significantly higher in GUS-treated patients compared with placebo (GUS 100 mg every 4 weeks: 76/128, 59%; p<0.001; GUS 100 mg every 8 weeks: 66/127, 52%, p<0.001; PBO: 28/126, 22%). Results in favour of GUS versus PBO, with a trend towards better efficacy in the every 4 weeks arm, were observed also for more stringent outcomes (ACR70 response for GUS 100 mg every 4 weeks: 26/128, 20%; GUS 100 mg every 8 weeks: 15/127, 12%; PBO: 7/126, 6%), physical function, extra articular manifestations like dactylitis, enthesitis and skin disease. Consistent results (not multiplicity corrected) were also shown for patients with or without previous TNFi therapy.35 In DISCOVER-2 (low RoB) only patients who were naïve to bDMARDs were included. GUS treatment was superior to PBO for ACR20 (GUS 100 mg every 4 weeks: 156/245, 64%, p<0.001; GUS 100 mg every 8 weeks: 159/248, 64%, p<0.001; PBO: 81/246, 33%), however, no clear difference in other outcomes between the two GUS regimens was observed in this study except for radiographic damage progression, with only GUS 100 mg every 4 weeks but not GUS 100 mg every 8 weeks being significantly different versus PBO (Δmodified Sharp van der Heijde Score, mSvDHS: PBO: 0.95; 95% CI: 0.61 to 1.29; GUS 100 mg every 4 weeks: 0.29, −0.05 to 0.63, p=0.011; GUS 100 mg every 8 weeks: 0.52, 0.18 to 0.86, p=0.072).7 Assessing patients with axial involvement (as defined by the investigator) and evidence of sacroiliitis (MRI or pelvic radiograph as reviewed locally by the respective investigator) showed improvement in GUS-treated patients compared with PBO when assessing axial involvement with the Bath Ankylosing Spondylitis Disease Activity Index (GUS 100 mg every 4 weeks, n=95: −2.7, −3.2 to −2.2; GUS 100 mg every 8 weeks, n=83: −2.7, −3.2 to −2.2; PBO: −1.3, −1.8 to −0.9) and the Ankylosing Spondylitis Disease Activity Score (GUS 100 mg every 4 weeks: −1.4, −1.7 to −1.2; GUS 100 mg every 8 weeks: −1.4, −1.7 to −1.2; PBO: −0.7, −0.9 to −0.5) in a pooled post hoc analysis.36 COSMOS (unclear RoB) investigated GUS 100 mg every 8 weeks compared with PBO in patients who were TNFi-IR, demonstrating superior efficacy of GUS over PBO in achieving the primary endpoint (ACR 20 at week 24: GUS 100 mg every 8 weeks: 84/189, 44.4%, p<0.001; PBO: 19/96, 19.8%) as well as all other secondary endpoints (MDA, DAPSA, physical function, skin disease, enthesitis, dactylitis and fatigue).37
The efficacy and safety of risankizumab (RIS), another inhibitor of the IL23-p19 subunit, was investigated in two RCTs (both low RoB).38–40 In patients with IR to csDMARDs (KEEPsAKE 1), patients were randomised to RIS 150 mg (s.c. at weeks 0, 4 and 16). At week 24 the primary (ACR 20: RIS 150 mg: 277/482, 57.3%, p<0.001; PBO: 161/481, 33.5%) and most secondary endpoints (including MDA, PASI90, enthesitis, dactylitis, fatigue and physical function) except the secondary endpoint of radiographic damage progression (ΔmSvDHS at week 24: RIS 150 mg: 0.23, 0.02 to 0.44; PBO: 0.32, 0.11 to 0.53, p=0.50) were met.8 Half of the patients included in KEEPsAKE 2 (low RoB) had received bDMARD therapy before the study. Also, this study met its primary (ACR 20 at week 24: RZB 150 mg: 115/224, 51.3%, p<0.001; PBO: 58/219, 26.5%) and all secondary endpoints.38 Further, improvements in patient-reported outcomes, work productivity and health-related quality of life could be observed in patients treated with RIS.39 40 A phase 2b study (low RoB) investigated tildrakizumab (TIL; IL23-p19i) 200 mg every 4 weeks or every 12 weeks, 100 mg every 12 weeks and 20 mg every 12 weeks in patients with previous iR to NSAIDs or DMARDs. The primary endpoint was met by all TIL arms compared with PBO (ACR 20 at week 24: TIL response rates ranging from 71%–80%; PBO: 51%), with no clear dose response.6
Efficacy of novel bDMARDs targeting IL-17
Five trials on novel molecules targeting IL-17 were included (four with low RoB, one conference abstract).9 41–44
Bimekizumab (BKZ), a selective inhibitor of IL17-A and IL17-F was investigated in two placebo-controlled RCTs. BE OPTIMAL (low RoB) investigated patients naïve to bDMARDs against PBO and active control and showed superior efficacy of BKZ 160 mg every 4 weeks over PBO in achieving the primary endpoint (ACR50% response at week 16: BKZ: 189/431, 44%, p<0.001; PBO: 28/281, 10%, reference; ADA: 64/140, 46%, no formal comparison) as well as in reduction of signs and symptoms of other manifestations like skin disease, enthesitis, dactylitis and radiographic damage progression.9 Similarly, BE COMPLETE showed superior efficacy of BKZ versus PBO in patients with IR to TNFi.41
Brodalumab (BRO; IL-17-A receptor) was investigated in csDMARD IR patients in AMVISION 1 and 2. After randomisation of 962 patients, both trials were terminated early due to the sponsors’ decision based on observed events of suicidal ideation and behaviour. The primary endpoint (ACR 20 at week 16) was met in both studies, with significantly higher response rates in achieving ACR50/70, PASI responses and resolution of dactylitis/enthesitis.42
Izokibep (IZO) is an antibody mimetic, a protein with a small molecular size that can inhibit IL17A and is thus a biological DMARD, but not an antibody. It is administered every other week. A 16-week phase 2 study (conference abstract, RoB not assessed) comparing IZO and PBO showed significant results compared with placebo and a slight dose–response relationship (ACR50 at week 16: IZO 40mg every 2 weeks: 20/42, 48%, p<0.001; IZO 80mg every 2 weeks: 24/46, 52%, p<0.001; PBO: 6/43, 13%).43 44 Clinically relevant treatment benefits were also observed in enthesitis, dactylitis and nail disease.45
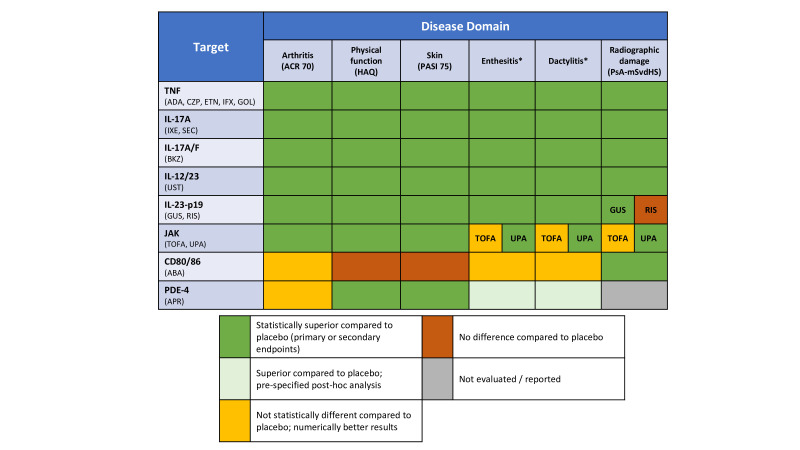
Figure 2 summarises and updates the evidence on established and new therapies based on the data presented and previous SLRs.24 46 47
Figure 2.
Efficacy results of randomised controlled trials stratified by mode of action and disease domain. Data from previous systematic literature research are also accounted for in this figure. *Different instruments used in studies. ABA, abatacept; ACR, American College of Rheumatology Response; ADA, adalimumab; APR, apremilast; BKZ, bimekizumab; CD, cluster of differentiation; CZP, certolizumab-pegol; ETN, etanercept; GOL, golimumab; GUS, guselkumab; HAQ, Health Assessment Questionnaire Disability Index; IL, interleukin; IFX, infliximab; IXE, ixekizumab; JAK, Janus kinases; PDE4, phosphodiesterase-4; PsA-mSvdHS, Psoriatic Arthritis modified Sharp van der Heijde Score; RIS, risankizumab; SEC, secukinumab; TNF, tumour necrosis factor; TOFA, tofacitinib; UPA, upadacitinib; UST, ustekinumab.
Strategic trials
Three strategic trials were included in this SLR (all with high RoB due to their open-label design).20 48 49
The NOR-DRUM trial investigated therapeutic infliximab (IFX) dosed based on therapeutic drug monitoring (TDM) versus IFX therapy without TDM. In total 411 patients with inflammatory immune-mediated diseases were included in the induction part of the trial, with 42 patients diagnosed with PsA. The primary outcome was the achievement of remission (defined as DAS28-ESR<2.6 for PsA) at week 30, with no difference comparing TDM to standard dosing in the overall analysis, and looking at the PsA subgroup, numerically but not statistically better results for the standard dosing arm (Disease Activity Score 28-ESR<2.6 at week 30: TDM: 5/20, 25%; standard IFX dosing: 12/22, 54.5%; adjusted difference 29.4%, −0.2% to 59.0%).49 The second part of this trial investigated the sustainment of response in patients who achieved DAS28<2.6 receiving IFX therapy, again comparing TDM to standard dosing. The PsA subgroup showed no clinically meaningful difference in the number of patients with sustained DAS28-ESR<2.6 from weeks 30 to 52 (TDM: 19/28, 67.9%; standard IFX dosing: 16/25, 64.0%; adjusted difference 6.2%, −19.5% to 31.9%).48
CONTROL was a strategic open-label study consisting of two parts: in part 1, patients with IR to MTX 15 mg/week were either randomised to dose escalation of MTX (20–25 mg/week) or additional treatment with adalimumab 40 mg every 2 weeks in combination with MTX 15 mg/week. The primary endpoint (MDA at week 16) was met, with significantly more patients in the ADA+MTX 15 mg/week arm (51/123, 41%) vs the MTX 20–25 mg/week dose escalation arm (16/122, 13%, p<0.001) achieving MDA. In part 2, patients who achieved MDA at week 16 received therapy modification (ADA 40 mg every 2 weeks+MTX 15 mg/week: ADA 40 mg every 2 weeks monotherapy) or continuation (MTX 20–25 mg/weekly) with most patients maintaining MDA at week 32 (41/51, 80% for ADA 40 mg every 2 weeks monotherapy vs 10/15, 67% for patients continuing MTX 20–25 mg/week monotherapy). Patients with IR were re-randomised at week 16 to treatment intensification: non-responders to ADA 40 mg every 2 weeks+MTX 15 mg/week were escalated to ADA 40 mg weekly+MTX 15 mg/week while those receiving MTX 20–25 mg/week received additional treatment with ADA 40 mg every 2 weeks. 17/57 (30%) of patients escalated from ADA 40 mg every 2 weeks (+MTX) to ADA 40 mg weekly+MTX 15 mg/week achieved MDA after 32 weeks, while 50/91 (55%) of patients with initial MTX non-response who received add-on ADA 40 mg every 2 weeks achieved MDA.20
Tapering and withdrawal
Four trials investigated treatment tapering and/or stopping (two with high, two with low RoB).21 50–52
Michielsens et al. conducted an open-label non-inferiority TNFi withdrawal trial (high RoB), using a treat-to-target (using PASDAS≤3.2 as target) dose-reduction and withdrawal strategy (n=42) compared with T2T treatment continuation without tapering (n=22) in patients with sustained PASDAS≤3.2. After 12 months, T2T tapering was shown to be non-inferior to a T2T strategy without tapering. Rates of patients in PASDAS LDA were comparable (71% vs 73%) with 24% (T2T with tapering) vs 77% (T2T without tapering) of patients receiving 100% of their daily defined dose and 36% (T2T tapering) vs 0% (no tapering) had stopped any DMARD use.21
Another open-label study (high RoB) investigated interval prolongation (ie, doubling the dosing interval) and subsequent stopping (after 6 months) of etanercept (ETN) treatment versus ETN continuation. After 6 months, 57% (vs 70%) of PsA patients could double their ETN interval while still remaining in MDA after 6 months.50
SPIRIT-P3 was a double-blind RCT, in which patients achieving MDA after receiving (open-label) ixekizumab for 36 weeks (158/394, 40%) were randomised to either undergo blinded withdrawal (receiving placebo) or continuation of ixekizumab treatment. The primary endpoint of time to relapse (ie, loss of MDA) until week 64 occurred more rapidly in patients who withdrew ixekizumab (median 22.3 weeks; 16.1 to 28.3, p<0.001). From week 24 to week 104, 67/79 patients (85%) experienced a relapse in the withdrawal group, compared with 30/79 (38%; p<0.001) in the continuation group. After re-treatment, almost all patients reachieved MDA (64/67, 95.5%) with a median time to re-achievement of MDA of 4.1 weeks (95% CI 4.1 to 4.3) in the ixekizumab withdrawal group.51
A withdrawal substudy (low RoB) of the long-term extension study of TOFA (OPAL Balance) investigated MTX withdrawal in patients receiving TOFA 5 mg BID (two times per day) for at least 24 months as well as MTX (7.5–20 mg/week) for at least 4 weeks. All patients received open-label TOFA and were randomised to either PBO (ie, MTX withdrawal; n=90) or continued MTX background therapy (n=89). After 6 months, no difference was observed in the co-primary endpoints PASDAS (difference 0.09, −0.13 to 0.31) and HAQ-DI (difference 0.03, −0.05 to 0.10).52
Table 4 summarises the main results of studies investigating dose reduction and withdrawal.
Table 4.
Primary outcomes of studies investigating DMARD dose reduction and withdrawal
| Study | RoB | Primary outcome(s) (unit) | Week | Treatment arm | n | Result | Difference/ 95% CI / p-value |
| bDMARD dose reduction and stopping | |||||||
| Michielsens 202221 | High (open-label) | PASDAS≤3.2 Stopped DMARD usage (n/%) |
52 | T2T TNFi with tapering | 42 | 30 (71%) 15 (36%)* |
Difference: 8% (−14% to 30%) |
| T2T TNFi without tapering | 22 | 16 (73%) 0 (0%)* |
|||||
| Ruwaard 202350 | High (open-label) | Sustained MDA (n/%) |
24 | ETN interval prolongation | 21 | 12 (57%) | Not reported |
| ETN continuation | 20 | 14 (70%) | |||||
| bDMARD stopping | |||||||
| Coates 2021 (SPIRIT-P3)51 | Low | Time to relapse (loss of MDA) (weeks) |
36 to 104 | PBO (IXE withdrawal) | 79 | 22.3 | 95% CI 16.1 to 28.3 <0.001 |
| IXE continuation | 79 | NE† | |||||
| csDMARD stopping | |||||||
| Nash 2021 (OPAL Balance)52 | Low | Change from baseline in PASDAS and HAQ-DI (LSM±SE) |
24 | PBO + TOFA 5 mg BID (MTX stopping) | 90 | 0.23±0.08 0.04±0.03 |
Difference PASDAS: 0.09 (95% CI –0.13 to 0.31) HAQ-DI: 0.03 (95% CI –0.05 to 0.10) |
| TOFA 5 mg BID + MTX (MTX continuation) | 89 | 0.14±0.08 0.02±0.03 |
|||||
*Secondary outcome.
†Not estimable, as <50% of patients experienced a relapse by the end of the study period.
BID, two times per day; DMARD, disease-modifying antirheumatic drug; ETN, etanercept; HAQ-DI, Health Assessment Questionnaire Disability Index; IXE, ixekizumab; LSM, least squares mean; MDA, minimal disease activity; MTX, methotrexate; PASDAS, Psoriatic Arthritis Disease Activity Score; PBO, placebo; RoB, risk of bias; SE, standard error; TNFi, tumour necrosis factor alpha inhibitor; TOFA, tofacitinib; T2T, treat-to-target.
Safety
Of the 24 additional reports included in the safety analysis (RoB: 8 low, 1 unclear, 10 high, 5 conference abstracts - not assessed), 9 were derived from long-term extension studies of RCTs, 12 were cohort studies including registry and claims analyses, 2 case–control studies and one prospective phase 4 observational study. Moreover, all trials included in the efficacy analysis were also assessed for safety. Detailed event rates of adverse events of interest are shown in online supplemental tables S3.4.1-S3.4.6. A summary of AESI of trials investigating tsDMARDs is shown in table 5.
Table 5.
Safety outcomes (adverse events of special interest) of RCTs investigating targeted synthetic DMARDs
| Population | Study | Risk of bias | Treatment | n | Safety endpoint (week) | Serious adverse event | Serious infectious event | Opportunistic infection | Herpes zoster | Malignancy (other than NMSC) | Major adverse cardiovascular event | Venous thromboembolism |
| csDMARD-IR; bDMARD naive | McInnes 2021 (SELECT PsA 1)18 | Low | UPA 15 mg OD ± csDMARD | 429 | 24 | 14 (3.3) | 5 (1.2) | 1 (0.2) | 4 (0.9) | 1 (0.2) | 0 | 0 |
| UPA 30 mg OD ± csDMARD | 423 | 13 (3.1) | 4 (0.9) | 0 | 3 (0.7) | 1 (0.2) | 0 | 1 (0.2) | ||||
| ADA 40 mg Q2W ± csDMARD | 429 | 16 (3.7) | 3 (0.7) | 0 | 0 | 3 (0.7) | 2 (0.5) | 2 (0.5) | ||||
| PBO ± csDMARD | 423 | 26 (6.1) | 11 (2.6) | 2 (0.5) | 5 (1.2) | 0 | 1 (0.2) | 1 (0.2) | ||||
| bDMARD-IR | Mease 2021 (SELECT PsA 2)12 | low | UPA 15 mg OD ± csDMARD | 211 | 24 | 12 (5.7) | 1 (0.5) | 0 | 3 (1.4) | 2 (0.9) | 1 (0.5) | 1 (0.5) |
| UPA 30 mg OD ± csDMARD | 218 | 18 (8.3) | 6 (2.8) | 2 (0.9) | 8 (3.7) | 2 (0.9) | 0 | 0 | ||||
| PBO ± csDMARD | 212 | 4 (1.9) | 1 (0.5) | 0 | 2 (0.9) | 0 | 0 | 0 | ||||
| Mixed (NSAID/csDMARD/bDMARD-IR) | Mease 2022 (DEUC Phase 2)11 | low | DEUC 6 mg OD ± csDMARD | 70 | 16 | 0 | 0 | 0 | 0 | 0 | NR | 0 |
| DEUC 12 mg OD ± csDMARD | 67 | 0 | 0 | 0 | 0 | 0 | NR | 0 | ||||
| PBO ± csDMARD | 66 | 1 (1.5) | 0 | 0 | 0 | 0 | NR | 1 (1.5) | ||||
| Mixed (NSAID/csDMARD/bDMARD-IR) | Mease 2023 (BREP Phase 2)10 | low | PBO ± csDMARD | 67 | 16 | 1 (1.5) | 0 | 0 | 0 | 0 | 0 | 0 |
| BREP 10 mg OD ± csDMARD | 31 | 0 | 0 | 0 | 1 (3.2) | 0 | 0 | 0 | ||||
| BREP 30 mg OD ± csDMARD | 60 | 3 (5.0) | 2 (3.3) | 0 | 1 (1.7) | 0 | 0 | 0 | ||||
| BREP 60 mg OD ± csDMARD | 59 | 1 (1.7) | 0 | 0 | 0 | 2 (3.3) | 0 | 0 |
ADA, adalimumab; bDMARD, biological disease-modifying antirheumatic drug; BREP, brepocitinib; csDMARD, conventional synthetic DMARD; DEUC, deucravacitinib; IR, insufficient response; NMSC, non-melanoma skin cancer; NR, not reported; NSAID, non-steroidal anti-inflammatory drug; OD, once daily; RCT, randomised controlled trial; UPA, upadacitinib.
Infections and infestations
Randomised controlled trials and long-term extension studies
In EXCEED comparison of SEC with ADA showed similar rates of serious infections (SEC: 7/426, 1,6%; ADA: 6/427, 1.4%), however, candida infections occurred numerically more frequently in SEC-treated patients (SEC 16/426, 4%; ADA: 7/427, 2%).16 Two cases of candida skin infection were observed in TIL-treated patients (compared with none in the PBO arm).6 Candida and other fungal infections were also more frequent in BKZ-treated patients compared with PBO (BE COMPLETE: BKZ: 7/267, 3%; PBO: 0, 0%; BE OPTIMAL: BKZ 20/431, 5%; PBO: 4/281, 1%; ADA: 1/140, <1%) with no systemic fungal infections observed.9 41 No serious infection, HZ or opportunistic infection was observed in the DEUC phase 2 trial until week 16.11 Also, in the GUS trials, no signal for increased rates of serious infections was observed.7 35 37
Rates of candida and fungal infections were very low in studies investigating IL23-p19 inhibitors, with few cases of local skin candidiasis.6–8 35 38
In integrated safety analyses of GUS, three cases of opportunistic infections were observed in the DISCOVER-2 trial (after week 52), with an otherwise consistent safety profile compared with the placebo-controlled period and no cases of active Tb.53
Long-term safety of PsA patients in the UPA trial programme (comparing UPA 15 mg once daily (n=907) to ADA 40 mg every 2 weeks (n=429) treated patients, data for UPA 30 mg once daily not reported; low RoB) showed higher rates for UPA for serious infections (exposure adjusted event rate, EAER, 95% CI: UPA 15mg once daily: 3.9, 3.1 to 4.9; ADA: 1.4, 0.8 to 2.5), opportunistic infections (UPA 15mg once daily: 0.5, 0.2 to 0.9; ADA: 0) and HZ rates (UPA 15mg once daily: 3.6, 2.8 to 4.6; ADA: 0.4, 0.1 to 1.1). Of note, 29 of 93 (31.2%) COVID-19 infections occurring in UPA-treated patients were serious (ADA: 4/37, 10.8%) with 6 (6.5%) fatal cases for UPA-treated versus none in ADA-treated patients. No cases of active Tb occurred in both groups.54
An analysis of influenza occurrence in the TOFA trial programme (low RoB) showed numerically increased rates of influenza infections in TOFA 5 mg BID (5/347, 196.2 patient years (PY), incidence rate, 95% CI: 2.51, 0.81 to 5.85) and TOFA 10 mg BID treated patients (5/344, 192.2 PY, incidence rate, 95% CI: 2.56, 0.83 to 5.97) compared with ADA 40 mg every other week (no event).55
Observational studies
Investigation of incidence of Tb (Tb activation, Tb development or Tb reactivation) in the secukinumab trial database (low RoB) on 2523 PsA patients with in total 4943 PY did not find cases of active Tb during the programme.56 A Slovenian cohort study (low RoB) found similar Tb incidence rates in PsA patients receiving TNF treatment compared with the general population of non-endemic countries with 2 cases occurring in 413 patients (1849 PY; standardised incidence rate, SIR, 95% CI: 5.8, 0.3 to 112).57
An observational study (low RoB) of PsA and SpA patients in four Nordic registers (DANBIO, ROB-FIN, NOR-DMARD and ARTIS/SRQ) compared TNFi to SEC-treated patients to assess the differences in risk of hospitalised infections. Although not powered to investigate the PsA subpopulation separately, a trend towards a higher risk for hospitalised infections in SEC-treated patients (incidence rate, 95% CI: 5.6, 4.1 to 7.5; reference) compared with TNFi (adjusted Hazard Ratio, aHR: ADA: 0.59, 0.34 to 1.03; ETN: 0.59, 0.28 to 1.20; GOL: 0.57, 0.28 to 1.20; IFX: 0.88, 0.54 to 1.42) was observed.58
A claims database study (high RoB) also compared the risk of hospitalised infections in Pso and PsA patients receiving DMARD therapy, comparing UST to TNFi and IL17i as well as apremilast (APR). Lower rates of hospitalisations due to infection were observed for UST (reference) treated patients compared with the other therapies in the overall comparison, with a similar trend found in the sensitivity analysis investigating PsA patients only (aHR, 95% CI: ADA: 1.67 (0.55 to 5.07); APR: 1.72 (0.68 to 4.30); CZP: 1.28 (0.74 to 2.20), ETN: 1.41 (0.40 to 4.98), GOL: 1.62 (0.18 to 14.41), IFX: 2.89 (1.26 to 6.63), IXE: 15.05 (4.27 to 53.04) and SEC: 1.93 (0.97 to 3.87)).59 Another claims database study (high RoB) compared the risk for infections requiring hospitalisation between TNF, IL17 or IL12/23 inhibition in PsA and Pso patients. No statistical difference was observed in serious infection for PsA patients between the different MOA (aHR (95% CI): IL-17 vs TNF: 0.73 (0.36 to 1.45); IL-12/23 vs TNF: 0.59 (0.38 to 0.92); IL-17 vs IL12/23: 1.01 (0.53 to 1.92)).60
A matched case–control study from the DANBIO registry (unclear RoB) compared the risk for serious infections in patients starting bDMARD treatment. Controls were randomly selected (matched by sex, age and postal code) from the general population without PsA. In 12 months 89/2429 (3.7%; HR, 95% CI: 3.4, 2.7 to 4.3) of PsA patients experienced a serious infection vs 262/24288 (1.1%) of matched controls (reference). Patients with comorbidities were at higher risk (HR, 95% CI: one comorbidity: 5.3, 3.4 to 8.2; two or more comorbidities: 4.9, 2.5 to 9.6; without comorbidities: 2.5, 1.8 to 3.5), as well as patients receiving glucocorticoid (GC) treatment (GC use: 5.3, 3.3 to 8.5; no GC use: 2.9, 2.2 to 3.9).61
The PsABio study was an observational postmarketing surveillance study (high RoB), investigating UST (n=494) and TNFi (n=557) treatment in patients receiving these treatments as first-line, second-line or third-line therapy. The choice of the bDMARD was made by the treating rheumatologist. UST users showed numerically lower numbers of serious adverse events (UST: 31/494, 6.3%, (4.3% to 8.8%); TNFi: 40/557, 7.2% (5.2% to 9.7%)). Exposure-adjusted incidence rates (EAIR) showed a higher risk for the occurrence of infections in TNFi versus UST-treated patients (EAIR not reported). Rates of serious or opportunistic infections were similar (UST: 6/494, 1.2% (0.4% to 2.6%); TNFi: 5/557, 0.9% (0.3% to 2.1%)).62
MACEs and arterial thrombotic events
Randomised controlled trials and long-term extension studies
During the placebo controlled period of the SELECT PsA trials, in SELECT PsA 1, no MACE occurred in the UPA treatment arms, one in the PBO and two in the ADA arm.18 In SELECT PsA 2, one MACE occurred in UPA 15 mg OD treated patients (compared with none in the PBO and UPA 30 mg OD arm).12 No MACE was seen in either the DEUC or the BREP phase 2 trial.10 11
During the long-term safety period comparing UPA 15 mg OD (n=907) to ADA 40 mg every 2 weeks (n=429) rates of (adjudicated) MACE were similar between both groups (EAER, 95% CI: UPA 15mg OD: 0.3, 0.1 to 0.6; ADA: 0.3, 0.1 to 1.0).54 63
Analysis of the long-term safety data of PsA patients treated with TOFA (high RoB) showed 1/347 cases of arterial thromboembolisms in the TOFA 5 mg BID arm (IR: 0.5, 95% CI: 0.01 to 2.78), and 2/106 in the ADA arm (IR 2.16, 95% CI: 0.26 to 7.82) and no event in the TOFA 10 mg BID arm.64
No signal for MACE was identified in KEEPsAKE 1 and 2, investigating RIS8 38 and in the integrated safety analyses of GUS.53
Observational studies
Two investigations of claims data (high RoB) found no increased risk of myocardial infarction or MACE in patients receiving bDMARD therapy compared with patients receiving csDMARDs, GC or APR.65 66 A cohort study using the French health insurance and hospital discharge database found an increased risk of MACE in bDMARD naïve patients without previous cardiovascular disease initiating IL12/23i therapy (aHR, 95% CI: 2.0, 1.3 to 3.0) or IL17i therapy (1.9, 1.2 to 3.0) compared with TNFi (reference). No increased risk was found when comparing TNFi to APR (1.3, 0.8 to 2.2), while JAKis were not studied.65 A study from Hong Kong (conference abstract, RoB not assessed) did not find bDMARD treatment to be associated with occurrence of MACE, but it was associated with elevated acute-phase reactants over time (aHR, 95% CI: 1.16, 1.11 to 1,21; p<0.001), as well as with GC usage (1.93, 1.04 to 3.57; p=0.036).66
In the UST arm of the PsABio study (see details above), two myocardial infarctions and one cardiac arrest occurred, compared with three myocardial infarctions in the TNFi arm.62
Venous thromboembolisms
Comparing UPA 15 mg OD (n=907) to ADA 40 mg every 2 weeks (n=429) rates of (adjudicated) VTE were similar for both groups (VTE: UPA 15mg OD: 0.2, 0.1 to 0.5); ADA: 0.2, 0 to 0.8) in patients receiving UPA.54 63 No VTE was observed in the DEUC and BREP phase 2 trial.10 11
Analysis of the long-term safety data of PsA patients treated with TOFA 5 mg BID or 10 mg BID (high RoB) showed low event rates, with one VTE (deep vein thrombosis, incidence rate, 95% CI: 0.51, 0.01 to 2.83) occurring in the TOFA 10 mg BID arm (1/344, 197.2 PY) vs none in the ADA arm (92.6 PY) and none in the TOFA 5 mg BID arm (201.1 PY) during the active-controlled period. VTEs were experienced by patients who had baseline cardiovascular or VTE risk factors 64
Malignancies
Randomised controlled trials and long-term extension studies
During the placebo-controlled period of SELECT-PsA 1, five malignancies (excluding non-melanoma skin cancer) were observed: one in the UPA 15 mg OD arm (neuroendocrine carcinoma), one in the UPA 30 mg OD arm (malignant lung neoplasm) and three in the ADA 40 mg every 2 weeks arm (colon cancer, ovarian cancer, uterine cancer), with none in the PBO arm.18 In the PBO controlled period of the SELECT-PsA 2 trial, malignancies observed in each of the UPA arms were: one prostate cancer, one rectal cancer in UPA 15 mg OD (n=2); one rectal adenocarcinoma and one ovarian/endometrial cancer in the UPA 30 mg OD arm (n=2), compared with none in the PBO arm.12 In the UPA long-term extension, rates of malignancies (excluding non-melanoma skin cancer) were similar (UPA 15 mg OD: 0.6, 0.3 to 1.1; ADA: 0.4, 0.1 to 1.1), with higher age (HR, 95% CI: 3.1, 1.7 to 5.8) and body mass index (1.1, 1.003 to 1.15), being significant predictors for development of malignancies in patients receiving UPA 15 mg OD.54 67 Rates of non-melanoma skin cancer were numerically higher (UPA: 0.8, 0.4 to 1.3; ADA: 0.2, 0 to 0.8) for UPA-treated patients. One basal cell carcinoma occurred in the BREP 60 mg once daily arm compared with no events in the other arms.10
In the phase 2 trial investigating BREP, one malignancy was observed (uterine leiomyoma) in the BREP 60 mg OD arm compared with no events in the other treatment arms.10 No malignancy occurred during the 16 weeks of the DEUC phase 2 trial.11
An analysis of malignancy occurrence during SEC treatment in the SEC trial database showed a cumulative incidence of 51/4902 malignancies in PsA patients (EAIR per 100 patient years, 95% CI: 1.04, 0.77 to 1.37), with non-melanoma skin cancer (basal cell carcinoma, n=15; squamous cell carcinoma, n=2) being the most prevalent. The incidence rate was in line with the expected rate of the general population (SEER database, standardised incidence rate, 95% CI: 1.16, 0.80 to 1.62).68 No signal for an increased risk for malignancies was found in the integrated safety analysis of GUS.53
Observational studies
No difference in the occurrence of malignancies between UST and TNFi-treated patients was observed in the PsABio study.62
In a British cohort study (high RoB) the incidence of cancer was found to be comparable in PsA patients receiving TNFi treatment compared with the general population (SIR, 95% CI: 0.94, 0.65 to 1.34), however, an increased risk for non-melanoma skin cancer was observed (2.12, 1.19 to 3.50). Also, increased all-cause mortality (standardised mortality rate, 95% CI: 1.56, 1.12 to 2.11) and increased risk of death from coronary heart disease (2.42, 1.11 to 4.59) were found.69 No increased risk for development of solid cancer was found in a large Nordic cohort study (unclear RoB) comparing PsA patients exposed to TNFi treatment with bDMARD naïve PsA patients (aHR, 95% CI: Nordic clinical rheumatology registers: 1.0, 0.9 to 1.2; and national patient registers: 0.8, 0.7 to 1.0).70 A similar analysis (low RoB) investigating haematological malignancies did not find an increased risk for PsA patients receiving TNFi therapy compared with bDMARD naïve patients (incidence rate ratio, 95% CI: TNFi: 0.96, 0.68 to 1.35; bDMARD naive: 0.84, 0.64 to 1.10). However, an increased risk of haematological malignancies compared with the general population was observed (incidence rate ratio, 95% CI: 1.35, 1.17 to 1.55).71
Adverse events of special interest
Randomised controlled trials and long-term extension studies
In EXCEED, new cases of IBD were more frequent in the SEC arm compared with ADA (two cases vs none).16
In the PBO-controlled period (until week 16) of the phase 2 trial investigating DEUC, acne was reported in 2/70 (2.9%) patients receiving DEUC 6 mg once daily and 1/67 (1.5%) in the 12 mg once daily arm—compared with none in the PBO group. Also, acneiform dermatitis was reported in 2/70 (2.9%), 2/67 (3%) in DEUC 6 mg/12 mg once daily treated patients compared with none in the PBO arm.11
In the DISCOVER-1 and DISCOVER-2 trials, similar rates of suicidal ideation in GUS-treated patients, compared with PBO were observed, with no events occurring in the studies investigating BKZ and TIL.6–9 35 37 38 41 In COSMOS, one patient in the GUS arm experienced two events of conversion disorder, which resolved after discontinuation of GUS. Another patient with a previous history of a suicidal attempt did report depressive symptoms after receiving GUS—the study drug was discontinued, and no further follow-up occurred.37 Trials investigating BRO (AMVISION 1 and 2) were terminated early following a sponsor’s decision due to events of suicidal ideation and behaviour observed in the study programme.42
In the integrated safety analyses of GUS, no cases of IBD or anaphylactic reactions were observed.53 No risk for MACE or malignancies was observed in the PBO and long-term extension period for APR-treated patients.72 Also, investigation of long-term safety from the UST PsA trial database did not reveal any new signal regarding the safety of MACE, malignancies or Tb.73
Observational studies
A US claims database analysis (high RoB, n=30 426, 60 497 PY) investigating the incidence of anxiety and depression disorder found especially users of GC (as an adjunctive therapy) to be at higher risk for depression (aHR, 95% CI: 1.5, 1.1 to 2.0) and a trend regarding anxiety (1.3, 0.9 to 1.9) with no signal for other DMARDs including bDMARDs and apremilast investigated.72
Another US claims database study (high RoB) investigated the impact of TNFi treatment on development of liver disease (cirrhosis or non-alcoholic fatty liver disease) and found a higher risk in the overall cohort (including patients with rheumatoid arthritis, ankylosing spondylitis and IBD), with a trend towards higher rates of liver disease also in the PsA subcohort (TNFi vs no TNFi use: aHR, 95% CI: 1.25, 0.88 to 1.76).74
Discussion
The aim of this SLR was to summarise the evidence regarding efficacy and safety of pharmacological therapies of PsA since the elaboration of the 2019 EULAR PsA management recommendations.
Similar to other SLRs, also this SLR has strengths and limitations. Data of trials included were not pooled through meta-analyses, due to high heterogeneity of the trials. Instead, the data were reported descriptively, avoiding (indirect) meta-analytical comparisons between drugs. On the other hand, the literature was fully searched in a systematic manner using predefined criteria. Though 10% of articles were screened in duplicate, each article was analysed for RoB and quality; and the data synthesis was descriptive which avoids overinterpretation.
Evidence on the efficacy of csDMARDs and especially MTX was confirmed with clinically meaningful improvements of disease activity observed in the COMPLETE-PsA and GO-DACT trials but is still based on comparisons to other treatment regimens and not to placebo. Combination of MTX with leflunomide provided significantly better efficacy results than MTX monotherapy, however, with substantially higher rates of adverse events.19
The efficacy of other meanwhile well-established therapies was confirmed and furthermore expanded with trials investigating the efficacy of IL-17 inhibition on specific musculoskeletal domains which showed significant differences compared with placebo treatment for axial disease (MAXIMISE),14 and synovitis (ULTIMATE),13 as well as the efficacy of golimumab in combination with MTX (vs MTX monotherapy) on dactylitis (GO-DACT).15 Several recent trials used more stringent and clinically meaningful efficacy outcomes than the commonly used ACR20% response as a (co)primary efficacy endpoint (ACR50% response at week 16/24).9 17 32 41 Non-inferiority of ustekinumab monotherapy compared with ustekinumab+MTX combination therapy was shown in the MUST study.31 Head-to-head comparisons of IL-17i (secukinumab (EXCEED), ixekizumab (SPIRIT-H2H)) compared with TNFi (adalimumab) showed comparable ACR response rates, but better skin responses in IL-17i-treated patients.16 32 Two observational studies found higher rates of hospitalised infections in IL-17i-treated patients (compared with TNFi)58 59, while another study did not show an increased risk.60 No benefit of therapeutic drug monitoring was seen in infliximab-treated PsA patients.48 49 Studies investigating molecules targeting the p19 subunit of IL-23 (guselkumab, risankizumab, tildrakizumab) as well as new molecules inhibiting IL-17A (izokibep) and IL-17A/F (bimekizumab),9 41 43–45 showed superior efficacy when compared with placebo across many disease domains (including arthritis, enthesitis, dactylitis, skin and nail disease) as well as improvement of physical function,6–8 37 39 40 Fungal infections occurred more frequently in patients receiving IL-17i compared with placebo and TNFi treatment. Only very few cases of fungal infections were observed in IL23-p19i-treated patients.6–8 35 38
RCTs on novel targeted synthetic DMARDs (brepocitinib (JAK1/TYK2) deucravacitinib (TYK2), upadacitinib (JAK1/2)) showed significantly higher response rates compared with placebo treatment,10–12 as well as superiority of UPA 30 mg (but not 15 mg) compared with adalimumab 40 mg every 2 weeks on the expense of more adverse events.18 34
In regard to safety, higher event rates in upadactinib-treated patients of serious infections (including serious and fatal COVID-19 infections), opportunistic infections, and HZ rates were observed, compared with adalimumab, while rates of MACE and VTEs were similar.54 Also, decreases of haemoglobin levels were higher in upadactinib-treated patients, while they increased on adalimumab.12 Increased rates of acne and acneiform rashes were reported in deucravacitinib-treated patients.11
No trial investigating the efficacy of glucocorticoids was identified, however, observational studies provided evidence of increased adverse event rates (MACE, serious infections, anxiety and depression) in patients treated with systemic glucocorticoids.66 75
Trials on strategic tapering (using a T2T approach) were reassuring, showing the potential of successful treatment withdrawal while maintaining disease control,21 but abrupt treatment cessation led to an elevated risk of disease flares.51
Results of the safety analysis also highlighted the importance of comorbidities in PsA patients, with higher risks of serious infections especially in patients with one or more comorbidities compared with patients without comorbidities,61 a higher risk for VTEs in patients with cardiovascular or VTE risk factors.64 While not associated with any DMARD therapy, an increased risk for non-melanoma skin cancer in PsA patients was observed, as well as an increased all-cause mortality and death from coronary heart disease.69
This SLR provided the task force with the evolved evidence since 2018 for the 2023 update of the EULAR recommendations on pharmacological management of PsA.
Footnotes
Handling editor: David S Pisetsky
@FerreiraRJO, @LGossec
Contributors: All authors contributed and finally approved the current manuscript. AK accepts full responsibility for the work and the conduct of the study, had access to the data, and controlled the decision to publish together with the senior author (LG) and the convenor of the taskforce (JSS).
Funding: The European Alliance of Associations for Rheumatology (EULAR) funded this literature review.
Competing interests: AK, Speakers bureau, Consultancy: AbbVie, Amgen, Galapagos, Janssen, Eli Lilly, MSD, Novartis, Pfizer and UCB. JSS: research grants from Abbvie, Astra-Zeneca, Lilly, Galapagos; Royalties from Elsevier (textbook); consulting fees from Abbvie, Galapagos/Gilead, Novartis-Sandoz, BMS, Samsung, Sanofi, Chugai, R-Pharma, Lilly; Honoraria from Samsung, Lilly, R-Pharma, Chugai, MSD Janssen, Novartis-Sandoz; participation in advisory board from Astra-Zeneca. RJOF, research grants: Medac, Lilly; Consulting fees: Sanofi. HB, none. XB: Scientific grants: Abbvie, MSD, Novartis. Consultancies, honoraria and advisory board member for Abbvie, Amgen, Celltrion, Chugai, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, Roche, Sandoz, UCB. Membership on an entity’s Board of Directors or advisory committees: ASAS President, EULAR President Elect. DA, research grants: Galapagos, Lilly; Consulting fees: Abbvie, Gilead, Janssen, Lilly, Merck, Novartis, Sanofi. DGM, research grants: Janssen, Abbvie, Lilly, Novartis. UCB, BMS, Moonlake; consulting fees: Janssen, Abbvie, Lilly, Novartis. UCB, BMS, Moonlake, Celgene; honoraria: Janssen, Abbvie, Lilly, Novartis. UCB, BMS, Moonlake; support to attending meetings: Novartis Janssen. DvdH: consulting fees AbbVie, Argenx, Bayer, BMS, Galapagos, Gilead, Glaxo-Smith-Kline, Janssen, Lilly, Novartis, Pfizer, Takeda, UCB Pharma. Director of Imaging Rheumatology bv. Associate editor Annals Rheumatic Diseases, editorial board member Journal of Rheumatology and RMD Open, Advisor Assessment Axial Spondyloarthritis international Society. IBM, Honoraria/consultation fees and grants/research supports: Abbvie, Amgen, BMS, Causeway Therapeutics, Cabaletta, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Sanofi, UCB, Evelo, Compugen, AstraZeneca, Moonlake; Non Exec Roles: NHS GGC Board Member, Evelo Board of Directors, Versus Arthritis Trustee Status; Stock or Stock Options: Evelo, Cabaletta, Compugen, Causeway Therapeutics, Dextera. BAE, none. KLW, Research: BMS, Pfizer; Consulting: Pfizer, AbbVie, Union Chimique Belge (UCB), Eli Lilly & Company, Galapagos, GlaxoSmithKline (GSK), Roche, Gilead, BMS, Regeneron, Sanofi, AstraZeneca, Novartis, W-HB, Honoraria: Abbvie, Almirall, BMS, Janssen, Leo, Eli Lilly, Novartis, UCB; Expert testimony: Novartis; Participation on a Data Safety Monitoring Board or Advisory Board: Abbvie, Almirall, BMS, Janssen, Leo, Eli Lilly, Novartis, UCB. JWS, none. LG reports grants from AbbVie, Biogen, Lilly, Novartis, UCB, personal fees from AbbVie, Amgen, BMS, Celltrion, Janssen, Lilly, MSD, Novartis, Pfizer, UCB, non-financial support from AbbVie, Amgen, Galapagos, Janssen, MSD, Novartis, Pfizer, UCB, outside the submitted work.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. All data relevant to the study are included in the article or uploaded as online supplemental information. Detailed data of all included studies are also available in the public domain.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55–78. 10.1016/0049-0172(73)90035-8 [DOI] [PubMed] [Google Scholar]
- 2. McGonagle D, David P, Macleod T, et al. Predominant ligament-centric soft-tissue involvement differentiates axial psoriatic arthritis from ankylosing spondylitis. Nat Rev Rheumatol 2023;19:818–27. 10.1038/s41584-023-01038-9 [DOI] [PubMed] [Google Scholar]
- 3. Gossec L, Smolen JS, Gaujoux-Viala C, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis 2012;71:4–12. 10.1136/annrheumdis-2011-200350 [DOI] [PubMed] [Google Scholar]
- 4. Gossec L, Smolen JS, Ramiro S, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. 10.1136/annrheumdis-2015-208337 [DOI] [PubMed] [Google Scholar]
- 5. Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. 10.1136/annrheumdis-2020-217159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mease PJ, Chohan S, Fructuoso FJG, et al. Efficacy and safety of tildrakizumab in patients with active psoriatic arthritis: results of a randomised, double-blind, placebo-controlled, multiple-dose, 52-week phase IIb study. Ann Rheum Dis 2021;80:1147–57. 10.1136/annrheumdis-2020-219014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mease PJ, Rahman P, Gottlieb AB, et al. Guselkumab in biologic-naive patients with active psoriatic arthritis (DISCOVER-2): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1126–36. 10.1016/S0140-6736(20)30263-4 [DOI] [PubMed] [Google Scholar]
- 8. Kristensen LE, Keiserman M, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 keepsake 1 trial. Ann Rheum Dis 2022;81:225–31. 10.1136/annrheumdis-2021-221019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McInnes IB, Asahina A, Coates LC, et al. Bimekizumab in patients with psoriatic arthritis, naive to biologic treatment: a randomised, double-blind, placebo-controlled. The Lancet 2023;401:25–37. 10.1016/S0140-6736(22)02302-9 [DOI] [PubMed] [Google Scholar]
- 10. Mease P, Helliwell P, Silwinska-Stanczyk P, et al. Efficacy and safety of the Tyk2/Jak1 inhibitor brepocitinib for active psoriatic arthritis: a phase IIb randomized controlled trial. Arthritis Rheumatol 2023;75:1370–80. 10.1002/art.42519 [DOI] [PubMed] [Google Scholar]
- 11. Mease PJ, Deodhar AA, van der Heijde D, et al. Efficacy and safety of selective Tyk2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis 2022;81:815–22. 10.1136/annrheumdis-2021-221664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mease PJ, Lertratanakul A, Anderson JK, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PSA 2. Ann Rheum Dis 2021;80:312–20. 10.1136/annrheumdis-2020-218870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Agostino MA, Schett G, López-Rdz A, et al. Response to secukinumab on synovitis using power doppler ultrasound in psoriatic arthritis: 12-week results from a phase III study, ULTIMATE. Rheumatology (Oxford) 2022;61:1867–76. 10.1093/rheumatology/keab628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis 2021;80:582–90. 10.1136/annrheumdis-2020-218808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vieira-Sousa E, Alves P, Rodrigues AM, et al. GO-DACT: a phase 3B randomised, double-blind, placebo-controlled trial of golimumab plus methotrexate (MTX) versus placebo plus MTX in improving dactylitis in MTX-naive patients with psoriatic arthritis. Ann Rheum Dis 2020;79:490–8. 10.1136/annrheumdis-2019-216500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McInnes IB, Behrens F, Mease PJ, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3B trial. Lancet 2020;395:1496–505. 10.1016/S0140-6736(20)30564-X [DOI] [PubMed] [Google Scholar]
- 17. Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of Ixekizumab and adalimumab in biological-naïve patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis 2020;79:123–31. 10.1136/annrheumdis-2019-215386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med 2021;384:1227–39. 10.1056/NEJMoa2022516 [DOI] [PubMed] [Google Scholar]
- 19. Mulder MLM, Vriezekolk JE, van Hal TW, et al. Comparing methotrexate monotherapy with methotrexate plus leflunomide combination therapy in psoriatic arthritis (COMPLETE-PSA): a double-blind, placebo-controlled, randomised, trial. The Lancet Rheumatology 2022;4:e252–61. 10.1016/S2665-9913(22)00028-5 [DOI] [PubMed] [Google Scholar]
- 20. Coates LC, Tillett W, D’Agostino M-A, et al. Comparison between adalimumab introduction and methotrexate dose escalation in patients with inadequately controlled psoriatic arthritis (CONTROL): a randomised, open-label, two-part, phase 4 study. The Lancet Rheumatology 2022;4:e262–73. 10.1016/S2665-9913(22)00008-X [DOI] [PubMed] [Google Scholar]
- 21. Michielsens CA, den Broeder N, van den Hoogen FH, et al. Treat-to-target dose reduction and withdrawal strategy of TNF inhibitors in psoriatic arthritis and axial spondyloarthritis: a randomised controlled non-inferiority trial. Ann Rheum Dis 2022;81:1392–9. 10.1136/annrheumdis-2022-222260 [DOI] [PubMed] [Google Scholar]
- 22. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 23. Ishchenko A, Pazmino S, Neerinckx B, et al. Comorbidities in early psoriatic arthritis: data from METAPSA cohort study. In: Arthritis care & research. 2023. 10.1002/acr.25230 [DOI] [PubMed] [Google Scholar]
- 24. Kerschbaumer A, Smolen JS, Dougados M, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature research for the 2019 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2020;79:778–86. 10.1136/annrheumdis-2020-217163 [DOI] [PubMed] [Google Scholar]
- 25. Gossec L, Kerschbaumer A, Ferreira RJO, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann Rheum Dis 2024;83:706–19. doi:ard-2024-225531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Project grant application | EULAR. Available: https://www.eular.org/project-grant-application [Accessed 8 Nov 2023].
- 27. Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wells GA. University of Ottawa, Department of Epidemiology and Commuunity Medicine. Available: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Accessed 12 Jul 2023].
- 29. Nguyen T, Churchill M, Levin R, et al. Secukinumab in United States biologic-naïve patients with psoriatic arthritis: results from the randomized, placebo-controlled CHOICE study. J Rheumatol 2022;49:894–902. 10.3899/jrheum.210912 [DOI] [PubMed] [Google Scholar]
- 30. Kivitz AJ, Nash P, Tahir H, et al. Efficacy and safety of subcutaneous secukinumab 150 mg with or without loading regimen in psoriatic arthritis: results from the FUTURE 4 study. Rheumatol Ther 2019;6:393–407. 10.1007/s40744-019-0163-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koehm M, Rossmanith T, Foldenauer AC, et al. Methotrexate plus ustekinumab versus Ustekinumab monotherapy in patients with active psoriatic arthritis (MUST): a randomised, multicentre, placebo-controlled, phase 3B, non-inferiority trial. Lancet Rheumatol 2023;5:e14–23. 10.1016/S2665-9913(22)00329-0 [DOI] [PubMed] [Google Scholar]
- 32. Smolen JS, Mease P, Tahir H, et al. Multicentre, randomised, open-label, parallel-group study evaluating the efficacy and safety of Ixekizumab versus adalimumab in patients with psoriatic arthritis naïve to biological disease-modifying antirheumatic drug: final results by week 52. Ann Rheum Dis 2020;79:1310–9. 10.1136/annrheumdis-2020-217372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goupille P, Behrens F, Coates LC, et al. Pos1044 effect of secukinumab versus adalimumab on ACR core components and health-related quality of life in patients with psoriatic arthritis: results from the exceed study. Ann Rheum Dis 2021;80(Suppl 1):797–8. 10.1136/annrheumdis-2021-eular.1562 [DOI] [Google Scholar]
- 34. Strand V, Mease PJ, Soriano ER, et al. Improvement in patient-reported outcomes in patients with Psoriatic arthritis treated with Upadacitinib versus placebo or Adalimumab: results from SELECT-PSA 1. Rheumatol Ther 2021;8:1789–808. 10.1007/s40744-021-00379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Deodhar A, Helliwell PS, Boehncke W-H, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet 2020;395:1115–25. 10.1016/S0140-6736(20)30265-8 [DOI] [PubMed] [Google Scholar]
- 36. Mease PJ, Helliwell PS, Gladman DD, et al. Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. The Lancet Rheumatology 2021;3:e715–23. 10.1016/S2665-9913(21)00105-3 [DOI] [PubMed] [Google Scholar]
- 37. Coates LC, Gossec L, Theander E, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: results through one year of a phase IIIB. Ann Rheum Dis 2022;81:359–69. 10.1136/annrheumdis-2021-220991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Östör A, Van den Bosch F, Papp K, et al. Efficacy and safety of risankizumab for active psoriatic arthritis: 24-week results from the randomised, double-blind, phase 3 keepsake 2 trial. Ann Rheum Dis 2022;81:351–8. 10.1136/annrheumdis-2021-221048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kristensen LE, Soliman AM, Papp K, et al. Risankizumab improved health-related quality of life, fatigue, pain and work productivity in psoriatic arthritis: results of keepsake 1. Rheumatology (Oxford) 2023;62:629–37. 10.1093/rheumatology/keac342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ostor AJK, Soliman AM, Papp KA, et al. Improved patient-reported outcomes in patients with psoriatic arthritis treated with risankizumab: analysis of the phase 3 trial keepsake 2. RMD Open 2022;8:e002286. 10.1136/rmdopen-2022-002286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Merola JF, Landewé R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-Α inhibitors: a randomised, double-blind. The Lancet 2023;401:38–48. 10.1016/S0140-6736(22)02303-0 [DOI] [PubMed] [Google Scholar]
- 42. Mease PJ, Helliwell PS, Hjuler KF, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis 2021;80:185–93. 10.1136/annrheumdis-2019-216835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Behrens F, Taylor PC, Wetzel D, et al. Op0258 Izokibep (Aby-035) in patients with active psoriatic arthritis – 16-week results from a phase 2 study. Ann Rheum Dis 2022;81(Suppl 1):170–1. 10.1136/annrheumdis-2022-eular.536 [DOI] [Google Scholar]
- 44. Achievement of different treatment targets with Izokibep demonstrates efficacy benefits in patients with active psoriatic arthritis: results from a 16-week randomized. Placebo-Controlled Phase 2 Clinical Trial - ACR Meeting Abstracts; Available: https://acrabstracts.org/abstract/achievement-of-different-treatment-targets-with-izokibep-demonstrates-efficacy-benefits-in-patients-with-active-psoriatic-arthritis-results-from-a-16-week-randomized-placebo-controlled-phase-2-clini/ [accessed 21 Aug 2023]. [Google Scholar]
- 45. Izokibep demonstrates clinically relevant efficacy benefits on enthesitis, dactylitis and nail outcomes in active PSA patients: A 16-week randomized. Placebo-controlled Trial - ACR Meeting Abstracts; Available: https://acrabstracts.org/abstract/izokibep-demonstrates-clinically-relevant-efficacy-benefits-on-enthesitis-dactylitis-and-nail-outcomes-in-active-psa-patients-a-16-week-randomized-placebo-controlled-trial/ [accessed 21 Aug 2023]. [Google Scholar]
- 46. Ramiro S, Smolen JS, Landewé R, et al. Pharmacological treatment of psoriatic arthritis: a systematic literature review for the 2015 update of the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2016;75:490–8. 10.1136/annrheumdis-2015-208466 [DOI] [PubMed] [Google Scholar]
- 47. Ash Z, Gaujoux-Viala C, Gossec L, et al. A systematic literature review of drug therapies for the treatment of psoriatic arthritis: current evidence and meta-analysis informing the EULAR recommendations for the management of psoriatic arthritis. Ann Rheum Dis 2012;71:319–26. 10.1136/ard.2011.150995 [DOI] [PubMed] [Google Scholar]
- 48. Syversen SW, Jørgensen KK, Goll GL, et al. Effect of therapeutic drug monitoring vs standard therapy during maintenance Infliximab therapy on disease control in patients with immune-mediated inflammatory diseases: a randomized clinical trial. JAMA 2021;326:2375–84. 10.1001/jama.2021.21316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Syversen SW, Goll GL, Jørgensen KK, et al. Effect of therapeutic drug monitoring vs standard therapy during Infliximab induction on disease remission in patients with chronic immune-mediated inflammatory diseases: a randomized clinical trial. JAMA 2021;325:1744–54. 10.1001/jama.2021.4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruwaard J, L’ Ami MJ, Kneepkens EL, et al. Interval prolongation of etanercept in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: a randomized controlled trial. Scandinavian Journal of Rheumatology 2023;52:129–36. 10.1080/03009742.2022.2028364 [DOI] [PubMed] [Google Scholar]
- 51. Coates LC, Pillai SG, Tahir H, et al. Withdrawing Ixekizumab in patients with psoriatic arthritis who achieved minimal disease activity: results from a randomized, double-blind withdrawal study. Arthritis Rheumatol 2021;73:1663–72. 10.1002/art.41716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nash P, Mease PJ, Fleishaker D, et al. Tofacitinib as monotherapy following methotrexate withdrawal in patients with psoriatic arthritis previously treated with open-label tofacitinib plus methotrexate: a randomised, placebo-controlled substudy of OPAL balance. Lancet Rheumatol 2021;3:e28–39. 10.1016/S2665-9913(20)30339-8 [DOI] [PubMed] [Google Scholar]
- 53. Safety of guselkumab in patients with psoriatic disease: an integrated analysis of 11 phase 2/3. Clinical Studies in Psoriasis and Psoriatic Arthritis - ACR Meeting Abstracts; Available: https://acrabstracts.org/abstract/safety-of-guselkumab-in-patients-with-psoriatic-disease-an-integrated-analysis-of-11-phase-2-3-clinical-studies-in-psoriasis-and-psoriatic-arthritis/ [accessed 2 Nov 2023]. [Google Scholar]
- 54. Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of Upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open 2023;9:e002735. 10.1136/rmdopen-2022-002735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Winthrop KL, Yndestad A, Henrohn D, et al. Influenza adverse events in patients with rheumatoid arthritis, ulcerative colitis, or psoriatic arthritis in the tofacitinib clinical development programs. Rheumatol Ther 2023;10:357–73. 10.1007/s40744-022-00507-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elewski BE, Baddley JW, Deodhar AA, et al. Association of secukinumab treatment with tuberculosis reactivation in patients with psoriasis, psoriatic arthritis, or ankylosing spondylitis. JAMA Dermatol 2021;157:43–51. 10.1001/jamadermatol.2020.3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rotar Z, Svetina P, Tomsic M, et al. Tuberculosis among patients treated with TNF inhibitors for rheumatoid arthritis, ankylosing spondylitis and psoriatic arthritis in Slovenia: a cohort study. BMJ Open 2020;10:e034356. 10.1136/bmjopen-2019-034356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Glintborg B, Di Giuseppe D, Wallman JK, et al. Is the risk of infection higher during treatment with secukinumab than with TNF inhibitors? an observational study from the nordic countries. Rheumatology (Oxford) 2023;62:647–58. 10.1093/rheumatology/keac358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jin Y, Lee H, Lee MP, et al. Risk of hospitalization for serious infection after initiation of ustekinumab or other biologics in patients with psoriasis or psoriatic arthritis. Arthritis Care Res (Hoboken) 2022;74:1792–805. 10.1002/acr.24630 [DOI] [PubMed] [Google Scholar]
- 60. Li X, Andersen KM, Chang H-Y, et al. Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann Rheum Dis 2020;79:285–91. 10.1136/annrheumdis-2019-216102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krabbe S, Grøn KL, Glintborg B, et al. Risk of serious infections in arthritis patients treated with biological drugs: a matched cohort study and development of prediction model. Rheumatology (Oxford) 2021;60:3834–44. 10.1093/rheumatology/keaa876 [DOI] [PubMed] [Google Scholar]
- 62. Gossec L, Siebert S, Bergmans P, et al. Long-term effectiveness and persistence of ustekinumab and TNF inhibitors in patients with psoriatic arthritis: final 3-year results from the Psabio real-world study. Ann Rheum Dis 2023;82:496–506. 10.1136/ard-2022-222879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. MACE and VTE across upadacitinib clinical trial programs in rheumatoid arthritis, psoriatic arthritis. and Ankylosing Spondylitis - ACR Meeting Abstracts; Available: https://acrabstracts.org/abstract/mace-and-vte-across-upadacitinib-clinical-trial-programs-in-rheumatoid-arthritis-psoriatic-arthritis-and-ankylosing-spondylitis/ [accessed 2 Nov 2023]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79:1400–13. 10.1136/annrheumdis-2019-216761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pina Vegas L, Le Corvoisier P, Penso L, et al. Risk of major adverse cardiovascular events in patients initiating biologics/apremilast for psoriatic arthritis: a nationwide cohort study. Rheumatology (Oxford) 2022;61:1589–99. 10.1093/rheumatology/keab522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Risk factors for major cardiovascular events (MACE) in inflammatory arthritis: a time-dependent analysis on the inflammatory burden, use of dmards. NSAIDs, and Steroid - ACR Meeting Abstracts; Available: https://acrabstracts.org/abstract/risk-factors-for-major-cardiovascular-events-mace-in-inflammatory-arthritis-a-time-dependent-analysis-on-the-inflammatory-burden-use-of-dmards-nsaids-and-steroid/ [accessed 5 Nov 2023]. [Google Scholar]
- 67. Malignancy in the Upadacitinib Clinical Trial Programs for Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis - ACR Meeting Abstracts, Available: https://acrabstracts.org/abstract/malignancy-in-the-upadacitinib-clinical-trial-programs-for-rheumatoid-arthritis-psoriatic-arthritis-and-ankylosing-spondylitis/ [Accessed 2 Nov 2023].
- 68. Lebwohl M, Deodhar A, Griffiths CEM, et al. The risk of malignancy in patients with secukinumab-treated psoriasis, psoriatic arthritis and ankylosing spondylitis: analysis of clinical trial and postmarketing surveillance data with up to five years of follow-up. Br J Dermatol 2021;185:935–44. 10.1111/bjd.20136 [DOI] [PubMed] [Google Scholar]
- 69. Fagerli KM, Kearsley-Fleet L, Mercer LK, et al. Malignancy and mortality rates in patients with severe psoriatic arthritis requiring tumour-necrosis factor alpha inhibition: results from the British Society for Rheumatology Biologics register. Rheumatology (Oxford) 2019;58:80–5. 10.1093/rheumatology/key241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hellgren K, Ballegaard C, Delcoigne B, et al. Risk of solid cancers overall and by subtypes in patients with psoriatic arthritis treated with TNF inhibitors - a nordic cohort study. Rheumatology (Oxford) 2021;60:3656–68. 10.1093/rheumatology/keaa828 [DOI] [PubMed] [Google Scholar]
- 71. Cordtz RL, Askling J, Delcoigne B, et al. Haematological malignancies in patients with psoriatic arthritis overall and treated with TNF inhibitors: a nordic cohort study. RMD Open 2022;8:e002776. 10.1136/rmdopen-2022-002776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Exposure-adjusted incidence rate for adverse events of special interest in patients with psoriatic arthritis treated with apremilast - ACR meeting abstracts. Available: https://acrabstracts.org/abstract/exposure-adjusted-incidence-rate-for-adverse-events-of-special-interest-in-patients-with-psoriatic-arthritis-treated-with-apremilast/ [Accessed 2 Nov 2023].
- 73. Ghosh S, Gensler LS, Yang Z, et al. Ustekinumab safety in psoriasis, psoriatic arthritis, and Crohn’s disease: an integrated analysis of phase II/III clinical development programs. Drug Saf 2019;42:751–68. 10.1007/s40264-019-00797-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tang K-T, Dufour J-F, Chen P-H, et al. Antitumour necrosis factor-Α agents and development of new-onset cirrhosis or non-alcoholic fatty liver disease: a retrospective cohort. BMJ Open Gastroenterol 2020;7:e000349. 10.1136/bmjgast-2019-000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vasilakis-Scaramozza C, Persson R, Hagberg KW, et al. The risk of treated anxiety and treated depression among patients with psoriasis and psoriatic arthritis treated with apremilast compared to biologics, dmards and corticosteroids: a cohort study in the United States Marketscan database. J Eur Acad Dermatol Venereol 2020;34:1755–63. 10.1111/jdv.16231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2024-225534supp001.pdf (1,018.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. All data relevant to the study are included in the article or uploaded as online supplemental information. Detailed data of all included studies are also available in the public domain.