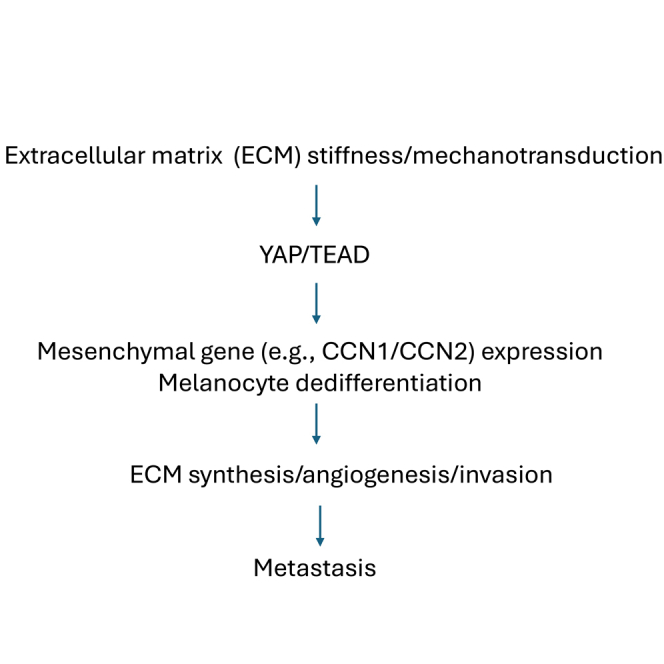
Summary
Hippo was first identified in a genetic screen as a protein that suppressed proliferation and cell growth. Subsequently, it was shown that hippo acted in a so-called canonical cascade to suppress Yorkie, the Drosophila equivalent of Yes-activated protein (YAP), a mechanosensitive transcriptional cofactor that enhances the activity of the TEAD family of transcription factors. YAP promotes fibrosis, activation of cancer-associated fibroblasts, angiogenesis and cancer cell invasion. YAP activates the expression of the matricellular proteins CCN1 (cyr61) and CCN2 (ctgf), themselves mediators of fibrogenesis and oncogenesis, and coordination of matrix deposition and angiogenesis. This review discusses how therapeutically targeting YAP through YAP inhibitors verteporfin and celastrol and its downstream mediators CCN1 and CCN2 might be useful in treating melanoma.
Subject areas: Molecular biology, Cell biology, Cancer
Graphical abstract
Molecular biology; Cell biology; Cancer
Introduction
The protein hippo was initially identified in Drosophila melanogaster using genetic screens that selected for mutations causing a cancer-like eye phenotype that was characterized by increased cell number and size, but decreased apoptosis (i.e., a hippopotamus-like phenotype.1,2 Subsequent analyses identified a so-called hippo kinase cassette, consisting of four proteins: Hippo (Hpo), Salvador (Sav), Warts (Wts), and Mob-as-tumor-suppressor (Mats).3 Hpo and Wts are serine/threonine kinases that are activated by phosphorylation, and act sequentially within the pathway. Biochemical studies of the Drosophila, and the related mammalian proteins, concluded that Hpo activation involves its autophosphorylation and the subsequent downstream phosphorylation of Sav, Wts, and Mats.2,4,5,6,7,8 Sav acts as a scaffold to coordinate binding of Hpo and Wts into a protein complex.2 Mats is an essential co-factor for Wts.7 Wts activity is also regulated by Hpo-dependent phosphorylation, and its autophosphorylation.6 Although multiple substrates for Hpo and Wts kinases have been identified, the pertinent target of Hpo, in the canonical hippo pathway, is Wts, and the pertinent downstream effector of Wts is the transcriptional co-activator Yorkie (Yki).8 yki encodes the Drosophila ortholog of the mammalian protein yes-associated protein (YAP) (Figure 1).9,10 Yki is required for normal tissue growth and is phosphorylated and inactivated by Wts. Human homologs of wts (i.e., lats), hpo (mst2), mats (mats1) and yki (yap) exist, and overexpression of these rescue the respective Drosophila mutants.2,4,5,6,7,8 That is, the components of the hippo cascade are evolutionarily conserved.
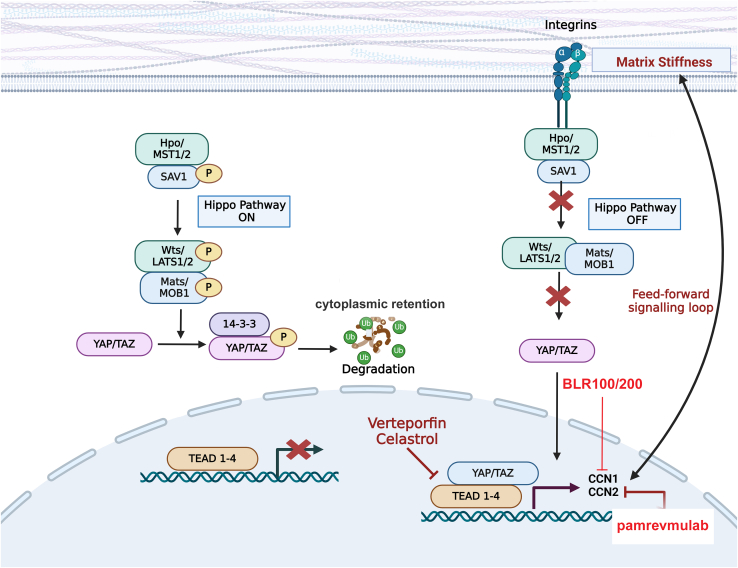
Figure 1.
Summary schematic of the canonical mechanosensitive YAP pathway
Note that when the hippo pathway is active (left hand side) YAP is phosphorylated and targeted for degradation. Conversely, when the hippo pathway is overridden (right hand side) in response to increases in ECM stiffness and mechanical tension, YAP and its downstream targets CCN1 and CCN2 act in a feedforward pathway to promote cell adhesion to ECM and therefore drive oncogenesis and fibrosis. For details, see text. The drugs verteporfin and celastrol act by antagonizing the interaction between YAP and TEAD. The CCN3-derived peptides BLR-100 and BLR-200 antagonize CCN1 and CCN2 activity. Red X refers to processed blocked (i.e., transcription of target genes or phosphorylation of YAP).
Large tumor suppressor (Lats) is the mammalian equivalent of wts; Lats1/2 kinase mediated phosphorylation of YAP at ser127 promotes the binding of 14-3-3 protein to YAP causing its cytoplasmic retention and its subsequent ubiquitination and degradation11,12 Prior to it being demonstrated as a mediator of the hippo pathway, mammalian YAP was identified as a protein that bound the proto-oncogene Yes,13 and as a transcriptional cofactor for the transcriptional enhanced associate domain (TEAD) family of transcription factors. Specifically, YAP had been found to bind to the carboxyl terminus of all TEAD family members and to promote the sequence-specific DNA binding of TEAD to target promoters.10 YAP/TEAD activate the promoters leading to the transcription of genes associated with fibrosis and cancers, such as the matricellular proteins CCN2 and CCN1.14,15,16,17 These features led to the conclusion that YAP, TEAD, CCN1 and CCN2 are key downstream effector molecules in the canonical mammalian hippo pathway (Figure 1). It should be pointed out, however, that YAP itself can interact with additional transcription factors, including p73;10 i.e., although this feature is not a focus of this current review, YAP can act as a transcriptional cofactor outside of the canonical hippo pathway.
Mechanotransduction and extracellular matrix (ECM) stiffness has long been understood to facilitate and prolong fibrogenesis (for reviews summarizing the data leading to this concept, please see18,19). This concept combined with the demonstrated link between YAP and the pro-adhesive and profibrotic gene CCN220 suggested a priori that YAP may be activated in response to increased matrix stiffness and therefore would be sensitive to mechanotransduction in cancers. Indeed, this hypothesis proved to be accurate.21,22,23 Specifically, YAP is activated in cancer-associated fibroblasts (CAF)s, and the use of siRNAs directed toward YAP indicated that YAP, in a fashion requiring the actomyosin cytoskeleton, was required for CAFs to promote matrix stiffening/remodeling, cancer cell invasion and angiogenesis.23 Matrix stiffening was further shown to enhance YAP activation, resulting in a feedforward signaling loop that helps maintain the CAF (myofibroblast) phenotype, resembling the situation observed with fibroblasts in fibrosis.17,23,24,25 The ability of mechanotransduction to activate YAP requires an intact actin cytoskeleton, and appears to act by overriding hippo-induced YAP phosphorylation.26 The exact mechanism underlying this switch remains to be clarified.
The consequence of these observations is that targeting the hippo/YAP pathway in fibrosis and cancers has become a topic of much interest (for representative reviews, please see27,28,29,30,31,32,33). In this review, we focus on the role of the canonical hippo/YAP pathway in controlling ECM/tumor/melanocyte interactions in melanoma.
The role of YAP/hippo in melanocyte biology
In skin, melanocytes are located on the basement membrane, rich in laminins and collagen type IV, at the dermal/epidermal junction. Skin melanocytes produce and secrete melanin, in melanosomes, which translocate into keratinocytes and cover their nuclei, thereby providing protection against ultraviolet light (UV)-induced damage.34
In development, melanocytes originate from the neural crest, a multipotent cell population which will specify multiple cells and tissues, including craniofacial cartilage and bones, melanocytes, and the peripheral nervous system (for reviews see35,36). The neural crest generates these numerous cell and tissue types in a process called delamination (division into multiple tissue populations), which involves cell migration from the neural tube (for reviews see35,36). In the mouse embryo, the binding to promoters of YAP/Taz and paired box 3 (Pax3), in a TEAD-independent fashion, is essential for expression of genes that promote melanocyte differentiation.37 Specifically, YAP and Taz are induced in a subset of neural crest cells and activate the expression of the Pax3 target gene microphthalmia-associated transcription factor (Mitf), also known as melanocyte-inducing transcription factor, a gene essential for melanogenesis.38 This process is impeded by Mst1 and Lats2.37 YAP also activates the wnt pathway; this activation is required for YAP to induce the melanocyte lineage.37 Specifically, the Mitf promoter is activated by wnt/β-catenin binding to its LEF1/TEF binding element.39
Melanocytes are normally in a quiescent state but can be activated in response to cell loss or injury. Quiescent epidermal melanocytes are polarized and bind to the basement membrane through the laminin-binding integrins α3β1 and α6β1, and to keratinocytes through dendrites involving the matricellular protein CCN3 and the collagen IV receptor discoidin domain receptor 1.40,41 Under these conditions, it has been hypothesized by Kim and colleagues that, in the resting state, melanocytes are not under mechanical stress, and therefore hippo/LATS1/2 are activated.42 Kim and colleagues further propose that, as with connective tissue fibroblasts, cell loss or injury results in loss of melanocyte contacts with the basement membrane and epithelial cells, resulting in increased mechanical load, thereby resulting in inhibition of LATS1/2 and activation of YAP/TAZ, resulting in increased proliferation and suppression of apoptosis.42 Although intriguing, this hypothesis has yet to be tested experimentally. A similar situation was suggested to operate in melanoma.
Melanoma is stimulated by a phenotypic switch caused by microenvironment-promoted YAP activation
Melanoma, which develops from melanocytes, is the most dangerous form of skin cancer. Melanoma may also rarely develop in the mouth, intestines or eye (a form called uveal melanoma).43,44 Epidemiological evidence supports the notion that the primary cause of melanoma is DNA damage caused by solar UV irradiation and a history of childhood sunburn that result in genetic lesions that produce cancerous cells,45,46,47 for reviews, see48,49.
Although melanocytes have stem cell-like properties (for an extensive discussion, please see Hoek and Goding50) melanoma progression appears to be driven via the transformation of melanocytes to cancer cells by a so-called phenotypic switch that involves microenvironmental signals acting on melanoma cells. This model posits that genetic lesions yielding constitutive activation of signal transduction pathways (such as ERK) together with microenvironment-mediated changes (for example, from a fibrotic ECM or hypoxia) results in a continuous switching between a differentiated, “proliferative” phenotype (characterized by high expression of neural crest and melanocyte markers), and a dedifferentiated, “invasive” phenotype characterized by low expression of melanocyte markers and high expression of mesenchymal cell markers.50,51,52,53 This mesenchymal phenotype is correlated with the development of drug resistance.54,55,56,57
In this regard, a priori, given their ability to modulate cell/microenvironment/ECM changes, including epithelial-mesenchymal transition, it would be anticipated that wnt, TGFβ and YAP would be involved in this switching.58,59,60,61,62 Indeed, recent experiments using human cancer cell lines suggest that TGFβ/SMAD and YAP/TAZ promote an invasive, whereas canonical Wnt signaling promotes a proliferative, phenotype switch.63 This invasive phenotype includes increased expression of a YAP/TGFβ signature including elevated CCN1 and CCN2 production and reduced expression of MITF63 (Figure 2). Thus, the YAP/hippo pathway, in concert with TGFβ production is highly implicated in initiating the invasive melanoma phenotype.
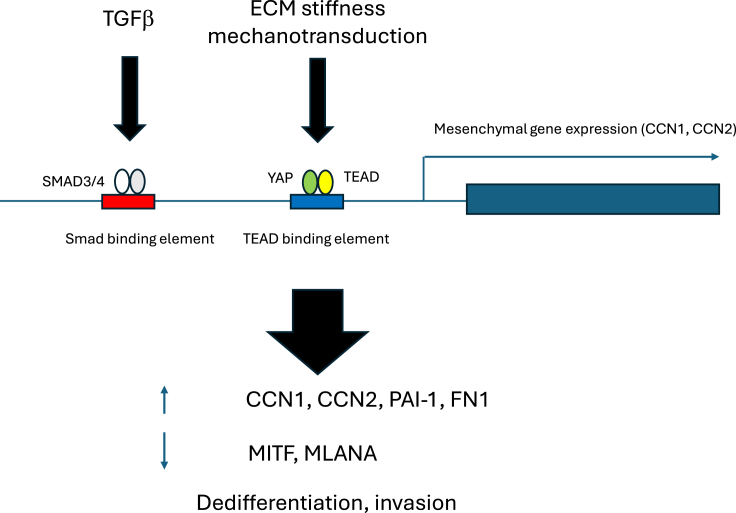
Figure 2.
TGFβ/SMAD and YAP/TAZ cooperate to induce a mesenchymal gene expression program in melanoma cells
TGFβ and mechanotransduction, through the canonical Smad and YAP/TEAD pathways, respectively, activate expression of a mesenchymal gene expression program (involving the induction of CCN1, CCN2, plasminogen activator inhibitor protein-1 (PAI-1) and fibronectin-1 (FN-1)), yet suppress differentiation markers such as MITF and melanoma antigen recognized by T-cells 1/melan-a (MLANA).
The hippo/YAP pathway in melanoma
Indeed, the YAP/hippo pathway is key coordinator of ECM production and angiogenesis in melanoma (for summaries, see26,27,28,29,30,31,32). For example, in a key study, the Mauviel group64 examined Hippo pathway component expression in a panel of human melanoma cell lines and melanocytic lesions. YAP and TAZ were found in both the nuclei and cytoplasm of benign nevi and superficial spreading melanoma. Moreover, siRNA knockdown of either YAP or TAZ in cancer cells dramatically reduced anchorage-independent growth, capacity to invade Matrigel in vitro, and ability to form lung metastases in mice following tail-vein injection. Conversely, the authors found that YAP overexpression increased melanoma cell invasiveness. These phenotypic alterations were paralleled by altered expression of the YAP target CCN2.64 In another study YAP facilitated melanoma cell migration via actin-related protein 2/3 complex subunit (ARPC5).65 YAP expression also contributed to actin remodeling-dependent proto-oncogene B-Raf (BRAF) inhibitor resistance in BRAF V600E mutant melanoma cells.34 Indeed, BRAF-inhibitor resistant melanoma cells display nuclear localization of both YAP and TAZ concomitant with persistent upregulation of a YAP gene expression signature, as visualized, for example, by significant upregulation of the YAP targets CCN1 and CCN2.64 Moreover, YAP, via its TEAD interaction domain, promotes proliferation, migration, invasion and transformation of melanoma cells.59 Indeed, the metastatic potential correlated with TEAD transcriptional activity.59 Similarly, expression of constitutively active YAP in zebrafish potently induces melanogenesis.66
The degree of YAP staining in primary human melanoma samples positively correlates with reduced patient survival.67,68,69 Similarly, in a bioinformatics study, down-regulation of latent-transforming growth factor beta-binding protein 4 (LTBP4) expression was seen in human melanoma tissues and cells, and predicted a poor clinical prognosis.70 LTBP4 overexpression in melanoma cells enhanced phosphorylation (inactivation) of YAP and inhibited CCN1 and CCN2 expression and reduced invasion, metastasis, and proliferation of melanoma cells.70 Overexpression of YAP rescued this effect of LTBP4.
A recent and extremely intriguing study found that a subset of melanoma patients possessed a mutated version of YAP that was hyperactive; this was the first indication of such a mutation in human cancers.67 These mutations are serine to alanine mutations at known targets of the hippo pathway.67 Similarly, ∼83% of uveal melanoma patients possess activating mutations in Gαq family members that ultimately result in the rac/cytoskeleton-dependent stimulation of YAP.71 These observations indicate that. in some melanoma patients, mutations can result in the activation of YAP.
Collectively, the above observations suggest that antagonizing YAP, or its downstream effectors CCN2 or CCN1, may have therapeutic value in treating melanoma.
YAP inhibitors
In this section, the focus will be on inhibitors that directly target YAP by antagonizing the interaction between YAP and TEAD.
Verteporfin
Verteporfin (VP), trade name Visudyne, is a benzoporphyrin derivative clinically used in photodynamic therapy for neovascular macular degeneration.72 Soon after its initial discovery, VP was found to have anti-adhesive properties in cultured human dermal fibroblasts.65,73 Further experiments revealed that VP, without light activation, acted as a small molecule inhibitor to impede the association between TEAD and YAP74,75 (Table 1). Thus, VP, as a YAP inhibitor, has gained interest as a possible anti-cancer drug.76,77 Indeed, not long after VP was identified, cell culture-based experiments suggested that VP may have therapeutic potential in treating cancers.78
Table 1.
Druggable targets in the YAP pathway
| Target | Drug | Phase in clinical development |
|---|---|---|
| YAP/TEAD | ||
| verteporfin | Approved for macular degeneration | |
| Pre-clinical for cancer | ||
| celastrol | Pre-clinical | |
| CCN2 | ||
| pamrevmulab | Phase III for pancreatic cancer | |
| CCN1/2 (using CCN3 mimics) | ||
| BLR100, BLR200 | Pre-clinical |
Several lines of evidence suggest that VP could be effective in treating melanoma. For example, in vitro and in vivo experiments indicate that VP, both with and without photodynamic therapy, has effects against melanoma cells.79,80,81,82,83,84,85,86 For example, in vitro, VP inhibited proliferation of and induced apoptosis in several uveal melanoma cell lines (specifically 92.1, Mel 270, Omm 1 and Omm 2.3).85 In addition, VP impaired stem cell properties of these cell lines, including their ability to form melanospheres.84 Similarly, VP, but not BRAF inhibitors, suppressed the increased ERK1/2 activity and elevated YAP1, TAZ and TEAD expression in BRAF inhibitor-resistant melanoma cancer stem cells.85 The same study provided an in vivo context for their results by using xenografts to show that treating BRAF inhibitor-resistant tumors with VP not only reduced YAP/TAZ expression and restored BRAF inhibitor suppression of ERK1/2 signaling, but also significantly impaired tumor growth.85 In an a more recent study, VP was shown in vitro, using Matrigel and transwell assays, to reduce A375 melanoma cell migration and invasion, accompanied by decreased CCN1 expression.86 Moreover, the same authors reported that, in uveal melanoma patients, TAZ levels negatively correlated with overall survival.86
Additional evidence supporting the concept that VP could be useful in the treatment of melanoma emerged from a recent study examining sirtuin 5 (SIRT5), itself a potential therapeutic target. SIRT5, a predominantly mitochondrial enzyme, promotes metabolic reprogramming, including activation of the electron transport chain and Krebs cycle.87 A recent report revealed that SIRT5 deacetylated TAZ, causing TAZ nuclear retention culminating in increased binding to the CCN2 promoter and elevated CCN2 expression in A375 cells.88 The same study reported that SIRT5 overexpression, in a VP-sensitive fashion, significantly increased metastasis of tail vein-injected B16F10 melanocytes to the lung.88
Collectively, these studies highlight the potential importance of the YAP/TAZ/CCN axis for treating metastatic melanoma.
Celastrol
Celastrol, a pentacyclic triterpenoid extracted from the roots of traditional Chinese medicine Tripterygium wilfordii, was identified, in a high-throughput screen, as a novel inhibitor of the interaction between YAP/TAZ and TEAD89 (Table 1). Celastrol acts as an antifibrotic agent and exhibits significant broad-spectrum anticancer activities.90,91,92 Celastrol blocks proliferation of melanoma cells in vitro and in vivo and metastasis in mouse models, associated with impairment of adhesive signaling.93,94,95,96 Celastrol also has antitumor activity in C57BL/6 mice bearing B16F10 tumors by both inhibiting tumor growth and increasing CD8+ T cells tumor penetration.97 The activity of celastrol is also associated with reduced CCN1 and CCN2 expression.91,98 Although poor water solubility and toxicity have limited the clinical potential of celastrol, much effort is being expended to circumvent these issues; for example, through the development of nanoparticle delivery systems.99
The YAP targets CCN1/CCN2
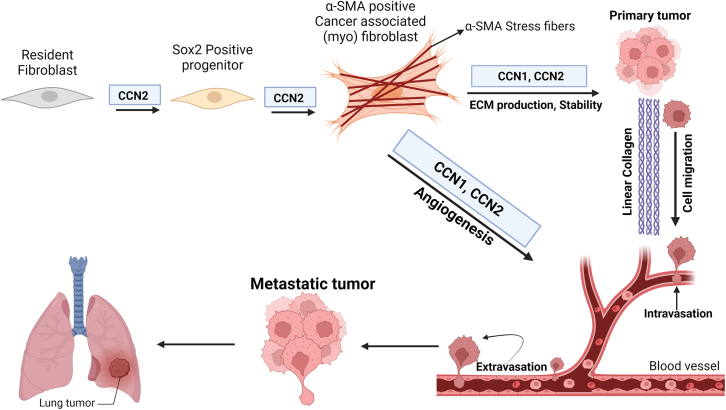
The CCN family of matricellular proteins have attracted interest due to their roles in modulating fibrosis and cancer progression, for reviews see.100,101 Including in melanoma cells, CCN1 and CCN2 expression is induced by TGFβ, RAS/MEK/ERK, hypoxia and mechanotransduction; indeed, they are considered prototypical YAP targets.13,14,102,103,104,105,106,107 CCN1 and CCN2 are pro-adhesive and signal through a variety of integrin heterodimers to promote angiogenic and ECM programs in vivo and in vitro.108,109,110,111,112 Mice postnatally deleted for either CCN1 or CCN2 in collagen-lineage fibroblasts are resistant to the syngeneic B16F10 model of melanoma metastasis.113,114,115,116,117 Specifically, loss of CCN1 or CCN2 from fibroblasts expressing a cre recombinase controlled by a promoter/enhancer derived from the human COL1A2 gene, later found to be expressed in so-called universal fibroblasts, resulted in reduced metastasis of subcutaneously injected B16F10 cells to the lung114,115 This reduced metastasis was accompanied by impaired angiogenesis (including vasculogenic mimicry) and ECM deposition.114,115,116,117 In patients, CAF-specific expression of CCN1 and CCN2 negatively associated with clinical outcome, and positively with checkpoint inhibitor resistance.114,115,116,117 Intriguingly, CCN2 appears to be involved with CAF plasticity, notably of universal fibroblasts, and myofibroblast differentiation,117 whereas CCN1 appears to be principally involved with ECM stability114 (Figure 3). Both CCN1 and CCN2 are associated with ECM/microenvironment-induced drug resistance in melanoma, and in other cancers.114,2,118,119,120
Figure 3.
Overlapping and complementary roles of CCN1 and CCN2 in melanoma metastasis
CCN2 expression by universal fibroblasts appears to promote fibroblast plasticity and myofibroblast differentiation; whereas both CCN1 and CCN2 expression by universal fibroblasts appears to promote ECM production and stability. CCN1 and CCN2 are both involved with neovascularization/angiogenesis. For details, see text.
Since CCN1 and CCN2 have overlapping and complementary functions in vivo (Figure 3), it may be blocking either of these proteins alone, for example with the anti-CCN2 antibody pamrevlumab (aka FG-3019)121,122,123 (Table 1), may be insufficient to achieve significant effects in patients. An alternative approach may be to impair their action through the application of an inhibitory protein, CCN3, or small peptides derived from the inhibitory CCN family member CCN3, such as BLR-100 or BLR-200 currently in development. Although these two peptide drugs have not yet been tested in melanoma, they have been shown to be effective in impairing progression in an animal model of human of pancreatic ductal adenocarcinoma (PDAC), where they greatly reduce the formation of the desmoplastic stroma, retard angiogenesis, suppress the formation of ascites (a biomarker of poor prognosis in humans), and the overall growth of the tumor124 (Table 1). Nonetheless, these findings are consistent with early in vitro data showing that YAP expression in CAFs coordinated ECM production and angiogenesis23 and imply that the functional mediators of YAP action in vivo may be CCN1 and CCN2, making them suitable targets for drug intervention. Indeed, it is tempting to speculate that CCN1 and CCN2 may deserve consideration as therapeutic targets for most, if not all, solid tumors.
Conclusion
Since the discovery that the hippo pathway plays a role in cancers by suppressing the proto-oncogene YAP, much interest has been expended on developing drugs that target YAP. Of note, the small molecule inhibitors verteporfin and celastrol have been identified based on their ability to block the interaction of the transcriptional cofactor YAP with its transcription factor target TEAD. Both these molecules appear to be effective in preclinical models of melanoma. Similarly, the downstream YAP targets CCN1 and CCN2, which could be targeted using CCN3-derived peptides, are required for angiogenesis, matrix remodeling and metastasis in a mouse melanoma model. These data strongly suggest that the YAP/CCN axis deserves consideration as a therapeutic target in melanoma.
Acknowledgments
Figures were compiled using Biorender. The APC was funded by the Canadian Institutes of Health Research (MOP-183830).
Author contributions
A.L. wrote the first draft of the manuscript. P.C. and A.N. designed the figures. J.N., A.N., P.C., and B.L.R. edited the manuscript.
Declaration of interests
B.L.R. is founder and CEO of BLR Bio and holds patents regarding the development of BLR100 and BLR200 as anti-fibrotic agents.
References
- 1.Harvey K.F., Pfleger C.M., Hariharan I.K. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 2.Wu S., Huang J., Dong J., Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 3.Harvey K., Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat. Rev. Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 4.Glantschnig H., Rodan G.A., Reszka A.A. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J. Biol. Chem. 2002;277:42987–42996. doi: 10.1074/jbc.M208538200. [DOI] [PubMed] [Google Scholar]
- 5.Lee K.K., Yonehara S. Phosphorylation and dimerization regulate nucleocytoplasmic shuttling of mammalian STE20-like kinase (MST) J. Biol. Chem. 2002;277:12351–12358. doi: 10.1074/jbc.M108138200. [DOI] [PubMed] [Google Scholar]
- 6.Wei X., Shimizu T., Lai Z.C. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26:1772–1781. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao W., Zhang S., Turenchalk G.S., Stewart R.A., St John M.A., Chen W., Xu T. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat. Genet. 1999;21:177–181. doi: 10.1038/5960. [DOI] [PubMed] [Google Scholar]
- 8.Huang J., Wu S., Barrera J., Matthews K., Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Strano S., Munarriz E., Rossi M., Castagnoli L., Shaul Y., Sacchi A., Oren M., Sudol M., Cesareni G., Blandino G. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J. Biol. Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 10.Vassilev A., Kaneko K.J., Shu H., Zhao Y., DePamphilis M.L. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B., Li L., Tumaneng K., Wang C.Y., Guan K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudol M., Bork P., Einbond A., Kastury K., Druck T., Negrini M., Huebner K., Lehman D. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J. Biol. Chem. 1995;270:14733–14741. doi: 10.1074/jbc.270.24.14733. [DOI] [PubMed] [Google Scholar]
- 14.Leask A., Holmes A., Black C.M., Abraham D.J. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J. Biol. Chem. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Pasolli H.A., Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl. Acad. Sci. USA. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quan T., Xu Y., Qin Z., Robichaud P., Betcher S., Calderone K., He T., Johnson T.M., Voorhees J.J., Fisher G.J. Elevated YAP and its downstream targets CCN1 and CCN2 in basal cell carcinoma: impact on keratinocyte proliferation and stromal cell activation. Am. J. Pathol. 2014;184:937–943. doi: 10.1016/j.ajpath.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B., Ye X., Yu J., Li L., Li W., Li S., Yu J., Lin J.D., Wang C.Y., Chinnaiyan A.M., et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 19.Leask A., Naik A., Stratton R.J. Back to the future: targeting the extracellular matrix to treat systemic sclerosis. Nat. Rev. Rheumatol. 2023;19:713–723. doi: 10.1038/s41584-023-01032-1. [DOI] [PubMed] [Google Scholar]
- 20.Chen C.C., Chen N., Lau L.F. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J. Biol. Chem. 2001;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- 21.Raghunathan V.K., Morgan J.T., Dreier B., Reilly C.M., Thomasy S.M., Wood J.A., Ly I., Tuyen B.C., Hughbanks M., Murphy C.J., Russell P. Role of substratum stiffness in modulating genes associated with extracellular matrix and mechanotransducers YAP and TAZ. Invest. Ophthalmol. Vis. Sci. 2013;54:378–386. doi: 10.1167/iovs.12-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 23.Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., Harrington K., Williamson P., Moeendarbary E., Charras G., Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F., Lagares D., Choi K.M., Stopfer L., Marinković A., Vrbanac V., Probst C.K., Hiemer S.E., Sisson T.H., Horowitz J.C., et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Link P.A., Choi K.M., Diaz Espinosa A.M., Jones D.L., Gao A.Y., Haak A.J., Tschumperlin D.J. Combined control of the fibroblast contractile program by YAP and TAZ. Am. J. Physiol. Lung Cell Mol. Physiol. 2022;322:L23–L32. doi: 10.1152/ajplung.00210.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 27.Park J., Hansen C.G. Cellular feedback dynamics and multilevel regulation driven by the hippo pathway. Biochem. Soc. Trans. 2021;49:1515–1527. doi: 10.1042/BST20200253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard A., Bojko J., Flynn B., Bowen S., Jungwirth U., Walko G. Targeting the Hippo/YAP/TAZ signalling pathway: Novel opportunities for therapeutic interventions into skin cancers. Exp. Dermatol. 2022;31:1477–1499. doi: 10.1111/exd.14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bednarski I.A., Ciążyńska M., Wódz K., Dróżdż I., Skibińska M., Narbutt J., Lesiak A. Hippo Signaling Pathway as a New Potential Target in Non-Melanoma Skin Cancers: A Narrative Review. Life. 2021;11:680. doi: 10.3390/life11070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi S., Cox A.G., Harvey K.F., Hogan B.M. Vasculature is getting Hip(po): Hippo signaling in vascular development and disease. Dev. Cell. 2023;58:2627–2640. doi: 10.1016/j.devcel.2023.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Sudol M., Shields D.C., Farooq A. Structures of YAP protein domains reveal promising targets for development of new cancer drugs. Semin. Cell Dev. Biol. 2012;23:827–833. doi: 10.1016/j.semcdb.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang Z., Meng Q., Xu J., Wang W., Zhang B., Liu J., Liang C., Hua J., Zhao Y., Yu X., Shi S. Signaling pathways in cancer-associated fibroblasts: recent advances and future perspectives. Cancer Commun. 2023;43:3–41. doi: 10.1002/cac2.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson B.J., Sahai E. MST kinases in development and disease. J. Cell Biol. 2015;210:871–882. doi: 10.1083/jcb.201507005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilchrest B.A., Blog F.B., Szabo G. Effects of aging and chronic sun exposure on melanocytes in human skin. J. Invest. Dermatol. 1979;73:141–143. doi: 10.1111/1523-1747.ep12581580. [DOI] [PubMed] [Google Scholar]
- 35.Thomas A.J., Erickson C.A. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- 36.Mort R.L., Jackson I.J., Patton E.E. The melanocyte lineage in development and disease. Development. 2015;142:1387–1632. doi: 10.1242/dev.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manderfield L.J., Engleka K.A., Aghajanian H., Gupta M., Yang S., Li L., Baggs J.E., Hogenesch J.B., Olson E.N., Epstein J.A. Pax3 and hippo signaling coordinate melanocyte gene expression in neural crest. Cell Rep. 2014;9:1885–1895. doi: 10.1016/j.celrep.2014.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goding C.R., Arnheiter H. MITF-the first 25 years. Genes Dev. 2019;33:983–1007. doi: 10.1101/gad.324657.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda K., Yasumoto K., Takada R., Takada S., Watanabe K., Udono T., Saito H., Takahashi K., Shibahara S. Induction of melanocyte-specific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 2000;275:14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- 40.Hara M., Yaar M., Tang A., Eller M.S., Reenstra W., Gilchrest B.A. Role of integrins in melanocyte attachment and dendricity. J. Cell Sci. 1994;107:2739–2748. doi: 10.1242/jcs.107.10.2739. [DOI] [PubMed] [Google Scholar]
- 41.Fukunaga-Kalabis M., Martinez G., Liu Z.J., Kalabis J., Mrass P., Weninger W., Firth S.M., Planque N., Perbal B., Herlyn M. CCN3 controls 3D spatial localization of melanocytes in the human skin through DDR1. J. Cell Biol. 2006;175:563–569. doi: 10.1083/jcb.200602132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.E., Finlay G.J., Baguley B.C. The role of the hippo pathway in melanocytes and melanoma. Front. Oncol. 2013;3:123. doi: 10.3389/fonc.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.https://www.cancer.gov/types/skin/hp/melanoma-treatment-pdq.
- 44.Amaro A., Gangemi R., Piaggio F., Angelini G., Barisione G., Ferrini S., Pfeffer U. The biology of uveal melanoma. Cancer Metastasis Rev. 2017;36:109–140. doi: 10.1007/s10555-017-9663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siskind V., Aitken J., Green A., Martin N. Sun exposure and interaction with family history in risk of melanoma, Queensland, Australia. Int. J. Cancer. 2002;97:90–95. doi: 10.1002/ijc.1563. [DOI] [PubMed] [Google Scholar]
- 46.Berking C., Takemoto R., Satyamoorthy K., Shirakawa T., Eskandarpour M., Hansson J., VanBelle P.A., Elder D.E., Herlyn M. Induction of melanoma phenotypes in human skin by growth factors and ultraviolet B. Cancer Res. 2004;64:807–811. doi: 10.1158/0008-5472.can-03-3438. [DOI] [PubMed] [Google Scholar]
- 47.Moan J., Dahlback A., Setlow R.B. Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation. Photochem. Photobiol. 1999;70:243–247. [PubMed] [Google Scholar]
- 48.Bennett D.C. How to make a melanoma: what do we know of the primary clonal events? Pigment Cell Melanoma Res. 2008;21:27–38. doi: 10.1111/j.1755-148X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 49.Bennett D.C. Ultraviolet wavebands and melanoma initiation. Pigment Cell Melanoma Res. 2008;21:520–524. doi: 10.1111/j.1755-148X.2008.00500.x. [DOI] [PubMed] [Google Scholar]
- 50.Hoek K.S., Goding C.R. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res. 2010;23:746–759. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- 51.Widmer D.S., Cheng P.F., Eichhoff O.M., Belloni B.C., Zipser M.C., Schlegel N.C., Javelaud D., Mauviel A., Dummer R., Hoek K.S. Systematic classification of melanoma cells by phenotype-specific gene expression mapping. Pigment Cell Melanoma Res. 2012;25:343–353. doi: 10.1111/j.1755-148X.2012.00986.x. [DOI] [PubMed] [Google Scholar]
- 52.Widmer D.S., Hoek K.S., Cheng P.F., Eichhoff O.M., Biedermann T., Raaijmakers M.I.G., Hemmi S., Dummer R., Levesque M.P. Hypoxia contributes to melanoma heterogeneity by triggering HIF1α-dependent phenotype switching. J. Invest. Dermatol. 2013;133:2436–2443. doi: 10.1038/jid.2013.115. [DOI] [PubMed] [Google Scholar]
- 53.Hoek K.S., Eichhoff O.M., Schlegel N.C., Döbbeling U., Kobert N., Schaerer L., Hemmi S., Dummer R. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 54.Boshuizen J., Koopman L.A., Krijgsman O., Shahrabi A., van den Heuvel E.G., Ligtenberg M.A., Vredevoogd D.W., Kemper K., Kuilman T., Song J.Y., et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat. Med. 2018;24:203–212. doi: 10.1038/nm.4472. [DOI] [PubMed] [Google Scholar]
- 55.Tirosh I., Izar B., Prakadan S.M., Wadsworth M.H., 2nd, Treacy D., Trombetta J.J., Rotem A., Rodman C., Lian C., Murphy G., et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diazzi S., Baeri A., Fassy J., Lecacheur M., Marin-Bejar O., Girard C.A., Lefevre L., Lacoux C., Irondelle M., Mounier C., et al. Blockade of the pro-fibrotic reaction mediated by the miR-143/-145 cluster enhances the responses to targeted therapy in melanoma. EMBO Mol. Med. 2022;14:e15295. doi: 10.15252/emmm.202115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferretti L.P., Böhi F., Leslie Pedrioli D.M., Cheng P.F., Ferrari E., Baumgaertner P., Alvarado-Diaz A., Sella F., Cereghetti A., Turko P., et al. Combinatorial Treatment with PARP and MAPK Inhibitors Overcomes Phenotype Switch-Driven Drug Resistance in Advanced Melanoma. Cancer Res. 2023;83:3974–3988. doi: 10.1158/0008-5472.CAN-23-0485. [DOI] [PubMed] [Google Scholar]
- 58.Chen S., McLean S., Carter D.E., Leask A. The gene expression profile induced by Wnt 3a in NIH 3T3 fibroblasts. J. Cell Commun. Signal. 2007;1:175–183. doi: 10.1007/s12079-007-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamar J.M., Stern P., Liu H., Schindler J.W., Jiang Z.G., Hynes R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc. Natl. Acad. Sci. USA. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Massagué J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katoh M., Katoh M. Molecular genetics and targeted therapy of WNT-related human diseases (Review) Int. J. Mol. Med. 2017;40:587–606. doi: 10.3892/ijmm.2017.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lüönd F., Pirkl M., Hisano M., Prestigiacomo V., Kalathur R.K., Beerenwinkel N., Christofori G. Hierarchy of TGFβ/SMAD, Hippo/YAP/TAZ, and Wnt/β-catenin signaling in melanoma phenotype switching. Life Sci. Alliance. 2021;5 doi: 10.26508/lsa.202101010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nallet-Staub F., Marsaud V., Li L., Gilbert C., Dodier S., Bataille V., Sudol M., Herlyn M., Mauviel A. Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. J. Invest. Dermatol. 2014;134:123–132. doi: 10.1038/jid.2013.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lui J.W., Moore S.P.G., Huang L., Ogomori K., Li Y., Lang D. YAP facilitates melanoma migration through regulation of actin-related protein 2/3 complex subunit 5 (ARPC5) Pigment Cell Melanoma Res. 2022;35:52–65. doi: 10.1111/pcmr.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vittoria M.A., Kingston N., Kotynkova K., Xia E., Hong R., Huang L., McDonald S., Tilston-Lunel A., Darp R., Campbell J.D., et al. Inactivation of the Hippo tumor suppressor pathway promotes melanoma. Nat. Commun. 2022;13:3732. doi: 10.1038/s41467-022-31399-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X., Tang J.Z., Vergara I.A., Zhang Y., Szeto P., Yang L., Mintoff C., Colebatch A., McIntosh L., Mitchell K.A., et al. Somatic Hypermutation of the YAP Oncogene in a Human Cutaneous Melanoma. Mol. Cancer Res. 2019;17:1435–1449. doi: 10.1158/1541-7786.MCR-18-0407. [DOI] [PubMed] [Google Scholar]
- 68.Menzel M., Meckbach D., Weide B., Toussaint N.C., Schilbach K., Noor S., Eigentler T., Ikenberg K., Busch C., Quintanilla-Martinez L., et al. In melanoma, Hippo signaling is affected by copy number alterations and YAP1 overexpression impairs patient survival. Pigment Cell Melanoma Res. 2014;27:671–673. doi: 10.1111/pcmr.12249. [DOI] [PubMed] [Google Scholar]
- 69.Shain A.H., Yeh I., Kovalyshyn I., Sriharan A., Talevich E., Gagnon A., Dummer R., North J., Pincus L., Ruben B., et al. The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med. 2015;373:1926–1936. doi: 10.1056/NEJMoa1502583. [DOI] [PubMed] [Google Scholar]
- 70.Wang L., Tang D., Wu T., Sun F. Disruption of LTBP4 Inhibition-Induced TGFβ1 Activation Promoted Cell Proliferation and Metastasis in Skin Melanoma by Inhibiting the Activation of the Hippo-YAP1 Signaling Pathway. Front. Cell Dev. Biol. 2021;9:673904. doi: 10.3389/fcell.2021.673904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feng X., Degese M.S., Iglesias-Bartolome R., Vaque J.P., Molinolo A.A., Rodrigues M., Zaidi M.R., Ksander B.R., Merlino G., Sodhi A., et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bessler N.M., Vam Study Writing Committee Verteporfin therapy in age-related macular degeneration (VAM): an open-label multicenter photodynamic therapy study of 4,435 patients. Retina. 2004;24:512–520. doi: 10.1097/00006982-200408000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Margaron P., Sorrenti R., Levy J.G. Photodynamic therapy inhibits cell adhesion without altering integrin expression. Biochim. Biophys. Acta. 1997;1359:200–210. doi: 10.1016/s0167-4889(97)00115-8. [DOI] [PubMed] [Google Scholar]
- 74.Liu-Chittenden Y., Huang B., Shim J.S., Chen Q., Lee S.J., Anders R.A., Liu J.O., Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brodowska K., Al-Moujahed A., Marmalidou A., Meyer Zu Horste M., Cichy J., Miller J.W., Gragoudas E., Vavvas D.G. The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp. Eye Res. 2014;124:67–73. doi: 10.1016/j.exer.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gibault F., Corvaisier M., Bailly F., Huet G., Melnyk P., Cotelle P. Non-Photoinduced Biological Properties of Verteporfin. Curr. Med. Chem. 2016;23:1171–1184. doi: 10.2174/0929867323666160316125048. [DOI] [PubMed] [Google Scholar]
- 77.Felley-Bosco E., Stahel R. Hippo/YAP pathway for target ed therapy. Transl. Lung Cancer Res. 2014;3:75–83. doi: 10.3978/j.issn.2218-6751.2014.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Granville D.J., Jiang H., An M.T., Levy J.G., McManus B.M., Hunt D.W. Bcl-2 overexpression blocks caspase activation and downstream apoptotic events instigated by photodynamic therapy. Br. J. Cancer. 1999;79:95–100. doi: 10.1038/sj.bjc.6690017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nowak-Sliwinska P., Karocki A., Elas M., Pawlak A., Stochel G., Urbanska K. Verteporfin, photofrin II, and merocyanine 540 as PDT photosensitizers against melanoma cells. Biochem. Biophys. Res. Commun. 2006;349:549–555. doi: 10.1016/j.bbrc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 80.Donaldson M.J., Lim L., Harper C.A., Mackenzie J., G Campbell W. Primary treatment of choroidal amelanotic melanoma with photodynamic therapy. Clin. Exp. Ophthalmol. 2005;33:548–549. doi: 10.1111/j.1442-9071.2005.01083.x. [DOI] [PubMed] [Google Scholar]
- 81.Busetti A., Soncin M., Jori G., Rodgers M.A. High efficiency of benzoporphyrin derivative in the photodynamic therapy of pigmented malignant melanoma. Br. J. Cancer. 1999;79:821–824. doi: 10.1038/sj.bjc.6690131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Canal-Fontcuberta I., Salomão D.R., Robertson D., Cantrill H.L., Koozekanani D., Rath P.P., Pulido J.S. Clinical and histopathologic findings after photodynamic therapy of choroidal melanoma. Retina. 2012;32:942–948. doi: 10.1097/IAE.0b013e31825097c1. [DOI] [PubMed] [Google Scholar]
- 83.Lyubasyuk V., Ouyang H., Yu F.X., Guan K.L., Zhang K. YAP inhibition blocks uveal melanogenesis driven by GNAQ or GNA11 mutations. Mol. Cell. Oncol. 2015;2:e970957. doi: 10.4161/23723548.2014.970957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma Y.W., Liu Y.Z., Pan J.X. Verteporfin induces apoptosis and eliminates cancer stem-like cells in uveal melanoma in the absence of light activation. Am. J. Cancer Res. 2016;6:2816–2830. [PMC free article] [PubMed] [Google Scholar]
- 85.Fisher M.L., Grun D., Adhikary G., Xu W., Eckert R.L. Inhibition of YAP function overcomes BRAF inhibitor resistance in melanoma cancer stem cells. Oncotarget. 2017;8:110257–110272. doi: 10.18632/oncotarget.22628. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 86.Zhang H., Tu L., Ma Z., Lin Y., Tan Q. Inhibition of TAZ impairs the migration ability of melanoma cells. Open Life Sci. 2023;18:20220633. doi: 10.1515/biol-2022-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bringman-Rodenbarger L.R., Guo A.H., Lyssiotis C.A., Lombard D.B. Emerging Roles for SIRT5 in Metabolism and Cancer. Antioxid. Redox Signal. 2018;28:677–690. doi: 10.1089/ars.2017.7264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim G., Bhattarai P.Y., Lim S.C., Lee K.Y., Choi H.S. Sirtuin 5-mediated deacetylation of TAZ at K54 promotes melanoma development. Cell. Oncol. 2023;1 doi: 10.1007/s13402-023-00910. [DOI] [PubMed] [Google Scholar]
- 89.Nouri K., Azad T., Ling M., Janse van Rensburg H.J., Pipchuk A., Shen H., Hao Y., Zhang J., Yang X. Identification of Celastrol as a Novel YAP-TEAD Inhibitor for Cancer Therapy by High Throughput Screening with Ultrasensitive YAP/TAZ-TEAD Biosensors. Cancers. 2019;11:1596. doi: 10.3390/cancers11101596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu R., Li J., Guo Z., Chu D., Li C., Shi L., Zhang J., Zhu L., Li Z. Celastrol Alleviates Corneal Stromal Fibrosis by Inhibiting TGF-β1/Smad2/3-YAP/TAZ Signaling After Descemet Stripping Endothelial Keratoplasty. Invest. Ophthalmol. Vis. Sci. 2023;64:9. doi: 10.1167/iovs.64.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chitturi P., Xu S., Ahmed Abdi B., Nguyen J., Carter D.E., Sinha S., Arora R., Biernaskie J., Stratton R.J., Leask A. Tripterygium wilfordii derivative celastrol, a YAP inhibitor, has antifibrotic effects in systemic sclerosis. Ann. Rheum. Dis. 2023;82:1191–1204. doi: 10.1136/ard-2023-223859. [DOI] [PubMed] [Google Scholar]
- 92.Wang C., Dai S., Zhao X., Zhang Y., Gong L., Fu K., Ma C., Peng C., Li Y. Celastrol as an emerging anticancer agent: Current status, challenges and therapeutic strategies. Biomed. Pharmacother. 2023;163:114882. doi: 10.1016/j.biopha.2023.114882. [DOI] [PubMed] [Google Scholar]
- 93.Abbas S., Bhoumik A., Dahl R., Vasile S., Krajewski S., Cosford N.D.P., Ronai Z.A. Preclinical studies of celastrol and acetyl isogambogic acid in melanoma. Clin. Cancer Res. 2007;13:6769–6778. doi: 10.1158/1078-0432.CCR-07-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu H., Liu X.W., Cai T.Y., Cao J., Tu C.X., Lu W., He Q.J., Yang B. Celastrol acts as a potent antimetastatic agent targeting beta1 integrin and inhibiting cell-extracellular matrix adhesion, in part via the p38 mitogen-activated protein kinase pathway. J. Pharmacol. Exp. Ther. 2010;334:489–499. doi: 10.1124/jpet.110.165654. [DOI] [PubMed] [Google Scholar]
- 95.Kannaiyan R., Manu K.A., Chen L., Li F., Rajendran P., Subramaniam A., Lam P., Kumar A.P., Sethi G. Celastrol inhibits tumor cell proliferation and promotes apoptosis through the activation of c-Jun N-terminal kinase and suppression of PI3 K/Akt signaling pathways. Apoptosis. 2011;16:1028–1041. doi: 10.1007/s10495-011-0629-6. [DOI] [PubMed] [Google Scholar]
- 96.Lee J.H., Won Y.S., Park K.H., Lee M.K., Tachibana H., Yamada K., Seo K.I. Celastrol inhibits growth and induces apoptotic cell death in melanoma cells via the activation ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT signaling. Apoptosis. 2012;17:1275–1286. doi: 10.1007/s10495-012-0767-5. [DOI] [PubMed] [Google Scholar]
- 97.Cho O., Lee J.W., Jeong Y.J., Kim L.K., Jung B.K., Heo T.H. Celastrol, which targets IL-2/CD25 binding inhibition, induces T cell-mediated antitumor activity in melanoma. Eur. J. Pharmacol. 2024;962:176239. doi: 10.1016/j.ejphar.2023.176239. [DOI] [PubMed] [Google Scholar]
- 98.Wang S., Ma K., Chen L., Zhu H., Liang S., Liu M., Xu N. TAZ promotes cell growth and inhibits Celastrol-induced cell apoptosis. Biosci. Rep. 2016;36:e00386. doi: 10.1042/BSR20160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J., Jia Y., Zhang P., Yang H., Cong X., An L., Xiao C. Celastrol Self-Stabilized Nanoparticles for Effective Treatment of Melanoma. Int. J. Nanomed. 2020;15:1205–1214. doi: 10.2147/IJN.S232603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leask A. Conjunction junction, what's the function? CCN proteins as targets in fibrosis and cancers. Am. J. Physiol. Cell Physiol. 2020;318:C1046–C1054. doi: 10.1152/ajpcell.00028.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Birkeness L.B., Banerjee S., Quadir M., Banerjee S.K. The role of CCNs in controlling cellular communication in the tumor microenvironment. J. Cell Commun. Signal. 2023;17:35–45. doi: 10.1007/s12079-022-00682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chaqour B. CCN-Hippo YAP signaling in vision and its role in neuronal, glial and vascular cell function and behavior. J. Cell Commun. Signal. 2023;17:255–262. doi: 10.1007/s12079-023-00759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Braig S., Wallner S., Junglas B., Fuchshofer R., Bosserhoff A.K. CTGF is overexpressed in malignant melanoma and promotes cell invasion and migration. Br. J. Cancer. 2011;105:231–238. doi: 10.1038/bjc.2011.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peidl A., Perbal B., Leask A. Yin/Yang expression of CCN family members: Transforming growth factor beta 1, via ALK5/FAK/MEK, induces CCN1 and CCN2, yet suppresses CCN3, expression in human dermal fibroblasts. PLoS One. 2019;14:e0218178. doi: 10.1371/journal.pone.0218178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sha W., Leask A. CCN2 expression and localization in melanoma cells. J. Cell Commun. Signal. 2011;5:219–226. doi: 10.1007/s12079-011-0128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kunz M., Moeller S., Koczan D., Lorenz P., Wenger R.H., Glocker M.O., Thiesen H.J., Gross G., Ibrahim S.M. Mechanisms of hypoxic gene regulation of angiogenesis factor Cyr61 in melanoma cells. J. Biol. Chem. 2003;278:45651–45660. doi: 10.1074/jbc.M301373200. [DOI] [PubMed] [Google Scholar]
- 107.Mohammad K.S., Javelaud D., Fournier P.G.J., Niewolna M., McKenna C.R., Peng X.H., Duong V., Dunn L.K., Mauviel A., Guise T.A. TGF-beta-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res. 2011;71:175–184. doi: 10.1158/0008-5472.CAN-10-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen C.C., Mo F.E., Lau L.F. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J. Biol. Chem. 2001;276:47329–47337. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- 109.Babic A.M., Kireeva M.L., Kolesnikova T.V., Lau L.F. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc. Natl. Acad. Sci. USA. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu S., Shi-wen X., Abraham D.J., Leask A. CCN2 is required for bleomycin-induced skin fibrosis in mice. Arthritis Rheum. 2011;63:239–246. doi: 10.1002/art.30074. [DOI] [PubMed] [Google Scholar]
- 111.Quesnel K., Shi-Wen X., Hutchenreuther J., Xiao Y., Liu S., Peidl A., Naskar D., Siqueira W.L., O'Gorman D.B., Hinz B., et al. CCN1 expression by fibroblasts is required for bleomycin-induced skin fibrosis. Matrix Biol. 2019;3:100009. doi: 10.1016/j.mbplus.2019.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lau L.F. Cell surface receptors for CCN proteins. J. Cell Commun. Signal. 2016;10:121–127. doi: 10.1007/s12079-016-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li I.M.H., Horwell A.L., Chu G., de Crombrugghe B., Bou-Gharios G. Characterization of Mesenchymal-Fibroblast Cells Using the Col1a2 Promoter/Enhancer. Methods Mol. Biol. 2017;1627:139–161. doi: 10.1007/978-1-4939-7113-8_10. [DOI] [PubMed] [Google Scholar]
- 114.Hutchenreuther J., Nguyen J., Quesnel K., Vincent K.M., Petitjean L., Bourgeois S., Boyd M., Bou-Gharios G., Postovit L.M., Leask A. Cancer-associated fibroblast-specific expression of the matricellular protein CCN1 coordinates neovascularization and stroma deposition in melanoma metastasis. Cancer Res. Commun. 2024;4:556–570. doi: 10.1158/2767-9764.CRC-23-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hutchenreuther J., Vincent K.M., Carter D.E., Postovit L.M., Leask A. CCN2 Expression by Tumor Stroma Is Required for Melanoma Metastasis. J. Invest. Dermatol. 2015;135:2805–2813. doi: 10.1038/jid.2015.279. [DOI] [PubMed] [Google Scholar]
- 116.Hutchenreuther J., Vincent K., Norley C., Racanelli M., Gruber S.B., Johnson T.M., Fullen D.R., Raskin L., Perbal B., Holdsworth D.W., et al. Activation of cancer-associated fibroblasts is required for tumor neovascularization in a murine model of melanoma. Matrix Biol. 2018;74:52–61. doi: 10.1016/j.matbio.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 117.Tsang M., Quesnel K., Vincent K., Hutchenreuther J., Postovit L.M., Leask A. Insights into Fibroblast Plasticity: Cellular Communication Network 2 Is Required for Activation of Cancer-Associated Fibroblasts in a Murine Model of Melanoma. Am. J. Pathol. 2020;190:206–221. doi: 10.1016/j.ajpath.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 118.Maity G., Ghosh A., Gupta V., Haque I., Sarkar S., Das A., Dhar K., Bhavanasi S., Gunewardena S.S., Von Hoff D.D., et al. CYR61/CCN1 Regulates dCK and CTGF and Causes Gemcitabine-resistant Phenotype in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2019;18:788–800. doi: 10.1158/1535-7163.MCT-18-0899. [DOI] [PubMed] [Google Scholar]
- 119.Hesler R.A., Huang J.J., Starr M.D., Treboschi V.M., Bernanke A.G., Nixon A.B., McCall S.J., White R.R., Blobe G.C. TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3. Carcinogenesis. 2016;37:1041–1051. doi: 10.1093/carcin/bgw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leask A. A centralized communication network: Recent insights into the role of the cancer associated fibroblast in the development of drug resistance in tumors. Semin. Cell Dev. Biol. 2020;101:111–114. doi: 10.1016/j.semcdb.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 121.Neesse A., Frese K.K., Bapiro T.E., Nakagawa T., Sternlicht M.D., Seeley T.W., Pilarsky C., Jodrell D.I., Spong S.M., Tuveson D.A. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc. Natl. Acad. Sci. USA. 2013;110:12325–12330. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Finger E.C., Cheng C.F., Williams T.R., Rankin E.B., Bedogni B., Tachiki L., Spong S., Giaccia A.J., Powell M.B. CTGF is a therapeutic target for metastatic melanoma. Oncogene. 2014;33:1093–1100. doi: 10.1038/onc.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Makino K., Makino T., Stawski L., Lipson K.E., Leask A., Trojanowska M. Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis Res. Ther. 2017;19:134. doi: 10.1186/s13075-017-1356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Resovi A., Borsotti P., Ceruti T., Passoni A., Zucchetti M., Berndt A., Riser B.L., Taraboletti G., Belotti D. CCN-Based Therapeutic Peptides Modify Pancreatic Ductal Adenocarcinoma Microenvironment and Decrease Tumor Growth in Combination with Chemotherapy. Cells. 2020;9:952. doi: 10.3390/cells9040952. [DOI] [PMC free article] [PubMed] [Google Scholar]