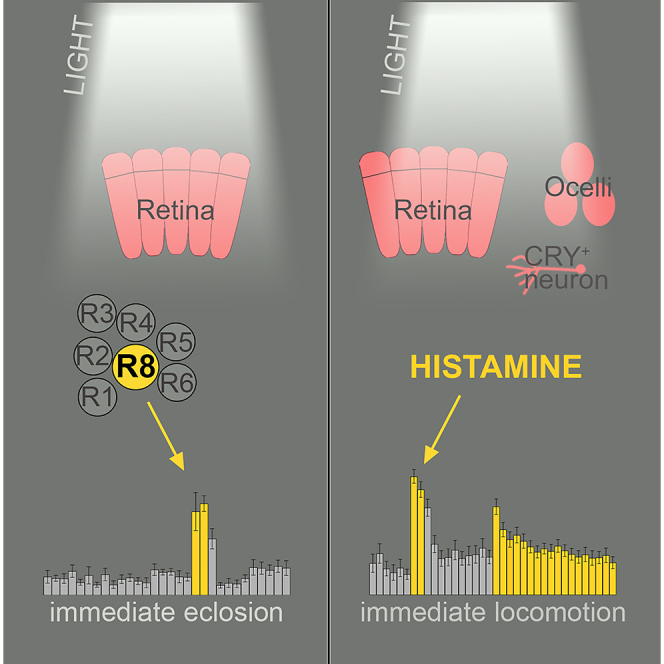
Summary
Animals need to sharpen their behavioral output in order to adapt to a variable environment. Hereby, light is one of the most pivotal environmental signals and thus behavioral plasticity in response to light can be observed in diurnal animals, including humans. Furthermore, light is the main entraining signal of the clock, yet immediate effects of light enhance or overwrite circadian output and thereby mask circadian behavior. In Drosophila, such masking effects are most evident as a lights-on response in two behavioral rhythms – the emergence of the adult insect from the pupa, called eclosion, and the diurnal rhythm of locomotor activity. Here, we show that the immediate effect of light on eclosion depends solely on R8 photoreceptors of the eyes. In contrast, the increase in activity by light at night is triggered by different cells and organs that seem to compensate for the loss of each other, potentially to ensure behavioral plasticity.
Subject areas: Biological sciences, Entomology, Neuroscience
Graphical abstract
Highlights
-
•
The compound eyes are required for the immediate effects of light on eclosion
-
•
R8 cells are necessary and sufficient for the immediate response to light
-
•
Masking of circadian locomotor activity depends on histamine-positive cells
Biological sciences; Entomology; Neuroscience
Introduction
An appropriate daily timing of behavior is of critical importance for animal fitness.1,2 Light shapes the daily timing in two ways: (1) the cyclic change in light intensity acts as an entraining signal, synchronizing the endogenous (circadian) timing system to appropriately adjust an organism to the 24 h environmental period; (2) light directly modulates behavior, i.e., increases alertness, locomotor activity, body temperature, and heart rate in diurnal animals including humans,3,4,5,6,7 while light suppresses activity and promotes sleep in nocturnal animals.8,9 These immediate light effects often obscure circadian behaviors, and are referred to as “masking”.10 The immediate light effects are essential for appropriate responses of the animal to changes in the environment and for sharpening the behavioral output. Interestingly, light responses can even provoke quasi-wild-type activity rhythms in clock-less and thus arrhythmic fruit flies and mice under natural-like conditions.11,12,13 These results were obtained in studies either carried out in the wild under natural light and temperature cycles11,12 or in the laboratory under constant temperature but with natural-like light cycles (simulating dawn and dusk).13 The latter study clearly underlines the importance of light for normal behavior.
In Drosophila melanogaster, the immediate light effects are most evident as a lights-on response in two well-described behavioral rhythms of the fly – the emergence rhythm of the adult insect from the pupa, called eclosion, and the adult activity rhythm.14,15,16,17 Eclosion is gated by the circadian clock to the early day, most probably to prevent desiccation and enhance survival rate. A light stimulus induces a rapid increase in eclosion rate (lights-on effect) that is eliminated in flies without eyes and potentially in mutants lacking ocelli.14,15 Even though the lights-on effect is gone, eclosion remains synchronized to the light-dark cycle in eyeless flies by the circadian blue-light photopigment cryptochrome (CRY,14,18,19; reviewed by Helfrich-Förster and Engelmann20). Similarly, the locomotor activity rhythm of adult eyeless flies remains synchronized to the light-dark cycle due to entrainment by CRY (reviewed by Helfrich-Förster21), but the immediate increase of activity after lights-on at ZT0, also known as “startle response”, disappears after elimination of the eyes.16,22 Subsequent studies showed that several rhodopsins contribute to this immediate light effect in adult flies.23,24
Flies receive light information via two external organs, the compound eyes and ocelli, as well as the internal extraretinal Hofbauer-Buchner (H-B) eyelets. In addition, a small subset of central brain neurons express the blue-light-sensitive photopigment CRY.18,19 The fruit fly’s compound eye consists of around 800 ommatidia, each of them equipped with six outer (R1–6) and two inner (R7, R8) photoreceptor cells. While the outer photoreceptor cells express the photoreceptor protein Rhodopsin 1 (Rh1), the inner ones express specific combinations of Rh3, Rh4, Rh5, and Rh6 (reviewed by Rister and Desplan25). Photoreceptor proteins are specifically expressed in two main ommatidia types: pale ommatidia express Rh3 in R7 and Rh5 in R8 photoreceptors and yellow ommatidia express Rh4 in R7 and Rh6 in R8 cells. A third type, the dorsal rim area (DRA) ommatidia, positioned in the DRA expresses Rh3 in R7 and R8 cells. The photoreceptors R1–6 were shown to be involved in dim light vision and the perception of motion,26 while R7/R8 are involved in color vision26,27,28 and DRA photoreceptors are involved in polarization vision.29 Besides the large compound eyes, fruit flies contain three simple dorsal eyes called ocelli. Ocelli consist of only one photoreceptor type, containing Rh2.30,31 The ocelli do not form an image or perceive objects in the environment; instead, they are sensitive to changes in light intensities and seem to respond to polarized light. As Rh2 is highly sensitive to UV light, the ocelli provide information to distinguish between sky and the ground (reviewed by Sabat et al.32). This enables the fly to maintain its orientation in space. The H-B eyelets evolve from the larval Bolwig’s organ and express Rh6.33,34,35 These extraretinal light-detecting cells are involved in entrainment at high light intensities.36 In addition to the light-detecting organs, Drosophila contains CRY-expressing neurons in the brain and compound eyes, sensitive to blue light.37,38,39,40 Beside the compound eyes, CRY is the main photoreceptor involved in light entrainment of the circadian clock.16,41
In this study, we aim to identify the photoreceptors necessary for the immediate effect of light on eclosion. In addition, we investigate if the same light-detecting cells are involved in the increase of locomotor activity to unexpected light at night. The locomotor response to light is time-of-day dependent, with increase in activity at night and absence of increase at daytimes.42,43 We provide evidence that the light effect on eclosion is mediated by Rh5-positive R8 photoreceptor neurons of the compound eyes with potential contribution of Rh6-positive R8 photoreceptor cells. In contrast, the ocelli, the H-B eyelets, as well as light-sensitive CRY-positive cells are not required to elicit this lights-on response. These results are in line with previous studies showing that the masking effect of light depends on the intact eyes.16 Interestingly, the increase in locomotor activity in response to light at night remains in flies without eyes, ocelli, or CRY-positive cells. We hypothesize redundant signaling pathways and propose that light-perceiving cells and organs compensate the loss of each other, enabling flies to react to unexpected changes in their environment. Thus, masking effects of light on circadian behaviors seem to depend on different underlying neuronal networks potentially dependent on the time of day, type of behavior, or developmental stage of the animal.
Results
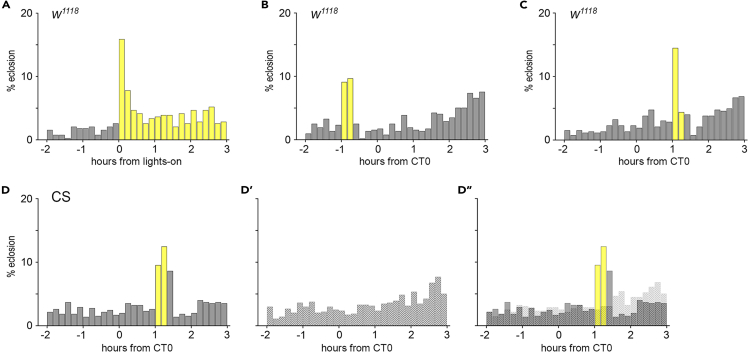
Light elicits an immediate increase in eclosion at lights-on in fly populations monitored under 14:10 light:dark cycles (Figure 1A). This immediate effect is also visible in flies kept under constant darkness when a light pulse is given at times around circadian time 0 (CT0, Figures 1B–1D). In detail, in line with previous studies,15 we could show that a 20-min white light pulse (I = 4.1 W/m2) 1 h before or after CT0 elicits an immediate eclosion response in control flies (Figures 1B–1D and S1A). The light response thus enables the flies to eclose promptly in response to their environment, reinforcing the behavior controlled by the clock. Next, we tested whether the pigmentation of the eyes plays a role in the detection of light necessary to elicit immediate eclosion. For this, we monitored eclosion in red-eyed CantonS and white-eyed (w1118) flies. Both groups showed a clear eclosion peak in response to light, even though the distribution within the 20 min is different (Figures 1C, 1D, and S1). Nevertheless, the loss of eye pigmentation does not influence the ability to react to light.
Figure 1.
The immediate light effect on eclosion behavior
(A) Eclosion pattern of Drosophila flies in 10 min intervals at the times around lights-on. Light elicits an immediate increase in eclosion.
(B and C) The lights-on response is visible in flies perceiving a 20-min light pulse (B) 1 h before (−1) or (C) 1 h after (1) expected lights-on at CT (circadian time) 0.
(D, D′) Wild-type CantonS (CS) flies show an immediate response to light (D), while flies in darkness lack the eclosion peak (D′).
(D″) The third plot combines (D) and (D′) to visualize the immediate light response in comparison to the appropriate controls monitored in darkness. Gray bars: % eclosion in dark phase, yellow bars: % eclosion in light phase; dashed bars: % eclosion in controls. n = 384 (A), 516 (B), 524 (C), 649 (D), 636 (D′). See also Figure S1.
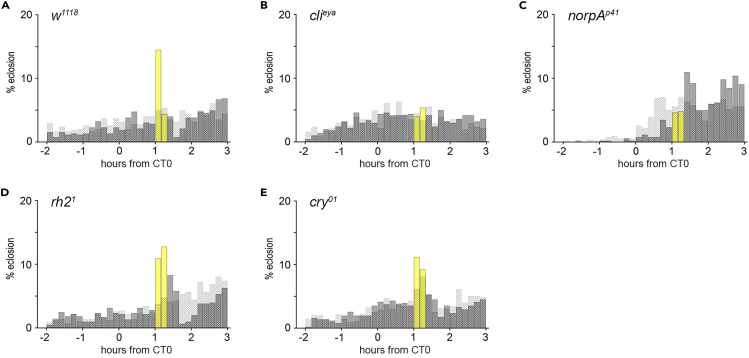
Further, we tested different mutants to discover the light-perceiving cells and organs triggering immediate eclosion. The lights-on response is gone in flies lacking the compound eyes (clieya, Figures 2B and S1C)14,15 and in norpA (no receptor potential) mutants with disturbed phospholipase C function (norpAp41, Figures 2C and S1D). In contrast, flies with disabled light perception in photoreceptors of the ocelli (rh21, Figures 2D and S1E), cry-positive cells (cry01, Figures 2E and S1F), or the Rh6-positive H-B eyelets (rh61, Figure S2), respectively, showed an immediate increase in eclosion in response to light indicating the exclusive requirement of the compound eyes in the immediate effect of light on eclosion.
Figure 2.
The immediate light effect on eclosion behavior requires the compound eyes and phospholipase C activity
(A–E) Eclosion pattern in 10 min intervals at the times around CT0. Each plot visualizes the results for the experimental (gray, yellow bars) and control groups (dashed bars) as shown in Fig.1D-D″. (B, C) Flies without eyes (B, clieya) and impaired phospholipase C activity (C, norpAp41) lack the lights-on response. (D, E) Flies lacking the rhodopsin (Rh) of the ocelli photoreceptor cells (D, rh21) or the photoprotein cryptochrome (E, cry01) respond to light. Gray bars: % eclosion in dark phase, yellow bars: % eclosion in light phase; dashed bars: % eclosion in darkness controls. nexp, nctrl = 524, 544 (A); 502, 508 (B); 520, 539 (C); 604, 584 (D); 510, 556 (E). See also Figure S1.
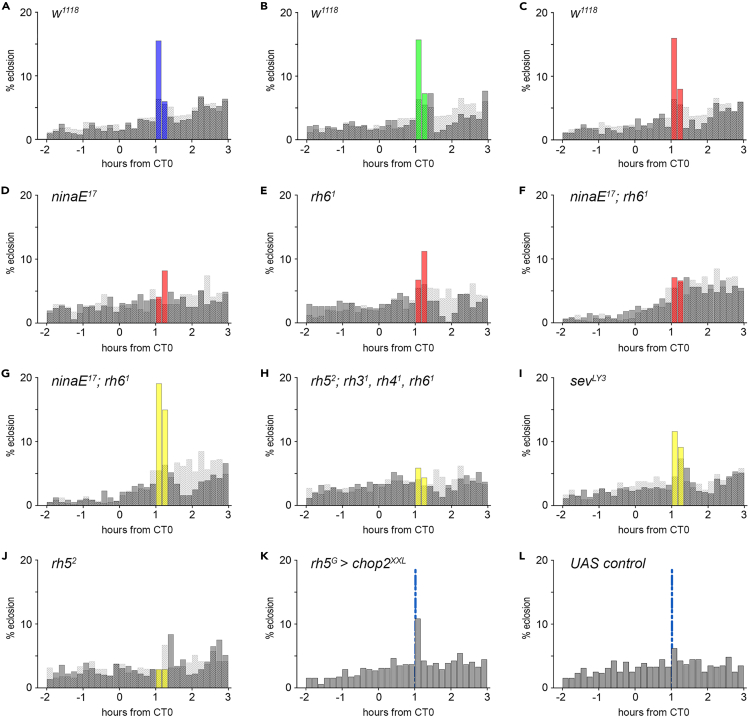
Our data suggest that the compound eyes are required for the immediate light effect on eclosion. Light information is received by photoreceptor cells of the compound eyes and converted into neuronal activity. The outer photoreceptor cells R1–R6 contain Rh1, the inner R7 cells express Rh3 (R7pale) or Rh4 (R7yellow), and proximal R8 cells express either Rh5 (R8pale) or Rh6 (R8yellow), while DRA ommatidia express Rh3 in R7 and R8. Thus, the different photoreceptor cells respond to light of a specific spectrum as the five different rhodopsins of the compound eyes have different absorption spectra.30,44,45 Therefore, illumination by monochromatic light of different wavelength will allow to address specific sets of Rh cells only and thereby give insights into the sufficiency of the different photoreceptor cells in this context. Flies showed an immediate behavioral response to 20-min blue (455–475 nm, I = 3.6 W/m2), green (510–545 nm, I = 2 W/m2), and red (625–642 nm, I = 2.3 W/m2) light pulses (Figures 3A–3C, S1G–S1I). In contrast to mammals, where immediate light effects depend exclusively on blue-light-sensitive melanopsin-expressing retinal ganglion cells,46,47,48,49,50 flies are able to additionally respond to longer wavelengths. As red light of 633 nm could only be absorbed by Rh1 or Rh6, we monitored the immediate light responses in flies without Rh1 (ninaE17), flies that lack Rh6 (rh61), and double mutants without both of these rhodopsins (ninaE17; rh61). As expected, the lights-on response to red light is missing in the rh1,rh6 double-mutant flies (Figures 3F and S1L). Lacking just one of the red-light-detecting rhodopsins leads to a strongly reduced lights-on response that was not significantly different from the controls in the rh61 mutants (Figures 3D, 3E, S1J, and S1K). This indicates at least a contribution of Rh6 to the immediate effect of red light. However, flies lacking Rh1 and Rh6 show an immediate behavioral response to a 20-min white light pulse (I = 4.1 W/m2, Figures 3G and S1M), indicating that both rhodopsins are dispensable for the immediate effect of white light on eclosion. White light includes short-wavelength light, which is mainly detected by the inner photoreceptor cells R7 and R8.44,45,46 To further disentangle the role of the R7, R8 cells, we screened rhodopsin and photoreceptor mutants for their behavioral response to white light (Figures 3G–3J). The quadruple mutant (rh52;rh31,rh41,rh61) lacks all rhodopsins of the inner photoreceptors, so that light perception is only possible via R1–R6. The lack of the lights-on response (Figures 3H and S1N) indicates that (1) R7 and/or R8 transmit light information for the lights-on response and (2) functional outer photoreceptors are not sufficient to trigger immediate eclosion. To address if R7 is involved in the immediate effect of light, we monitored eclosion in sevenless mutants (sevLY3, Figures 3I and S1O). Flies without R7 photoreceptor cells show an immediate response, suggesting that R8 cells are responsible for transmitting light information. Since the absence of Rh6 has no effect on the immediate response to white light (Figures 3G and S2), we tuned our attention to Rh5, which is also expressed in R8 cells. As expected, rh5 mutants (rh52) show no lights-on response (Figures 3J and S1P). Thus, pale R8 ommatidia expressing functional Rh5 turned out to be essential for the immediate response to white light. In line with this hypothesis, we optogenetically activated rh5-expressing R8 neurons (Figures 3K, 3L, and S2C). Photostimulation of rh5-positive neurons via Channelrhodopsin-2XXL (rh5G > chop2XXL, Figure 3K;51,52) using a short blue-light pulse of 2 min triggered immediate eclosion within the first 10 min, while this short light pulse was not sufficient to cause a significant increase in eclosion in control flies (Figures 3L, S1Q, and S1R). This demonstrates that activation of rh5-positive R8 cells can trigger immediate eclosion. Our data demonstrate that Rh5-expressing R8 cells are necessary and sufficient for the immediate effect of white light on eclosion. We cannot completely rule out a contribution from the other photoreceptors; Rh6 in particular seems to mediate the immediate effects of red light on eclosion.
Figure 3.
The immediate light effect on eclosion behavior depends on R8 cells
(A–J) Eclosion pattern in 10 min intervals at the times around CT0. Each plot visualizes the results for the experimental (gray, yellow bars) and control groups (dashed bars) as shown in Fig.1D-D″.
(A–C) Twenty-minute blue (A, 455–475 nm, I = 3.6 W/m2), green (B, 510–545 nm, I = 2 W/m2), and red (C, 625–642 nm, I = 2.3 W/m2) light pulses elicit immediate eclosion.
(D–F) The eclosion response to red light is visible in rh1 (D, ninaE17) but gone in rh6 mutants (E, rh61) and rh1, rh6 double mutants (F, ninaE1; rh61).
(G) In contrast, rh1,rh6 double mutants (ninaE1; rh61) respond with an increase of eclosion to white light (I = 4.1 W/m2).
(H) The quadruple mutant (rh52; rh31, rh41, rh61), lacking all rhodopsins of the inner photoreceptors, shows no reaction to light.
(I) The lights-on response in flies without R7 cells (sevLY3).
(J) Flies lacking Rh5 (rh52) do not respond to light with increased eclosion.
(K) Optogenetic activation of rh5-positive neurons with a 2 min blue light pulse (blue line; 455–475 nm, I = 3.41 μW/mm2) 1 h after expected lights-on elicits eclosion.
(L) Control flies without Gal4 expression (w1118; chop2XXL) do not respond to the 2 min light pulse. nexp, nctrl = 534, 1543 (A); 521, 1543 (B); 547, 1543 (C); 511, 512 (D); 579, 731 (E); 604, 567 (F); 561, 567 (G); 595, 518 (H), 525, 531 (I); 585, 506 (J); n = 516 (K), 515 (L). See also Figure S1.
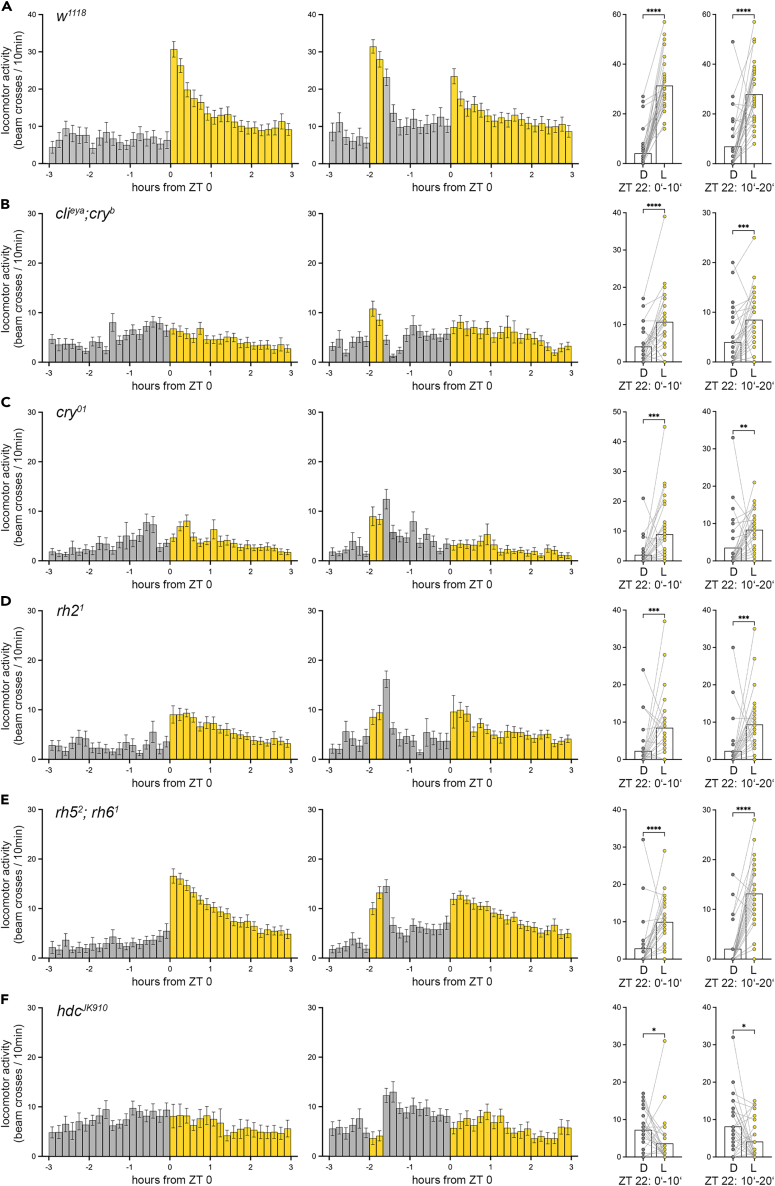
In the next step, we aimed to investigate whether the same photoreceptors that mediate immediate light effects on eclosion are generally responsible for mediating immediate light effects. For this, we focused on immediate light effects on locomotor activity. Unlike eclosion, which occurs only once in a fly’s lifetime, activity can be recorded in individual flies, and consequently, the immediate effects of light on activity can be studied multiple times per day.42,43 This allowed us to test the effects of completely unexpected light by administering the light pulse during the night to flies that are otherwise normally entrained to light-dark cycles. Previous studies have shown that flies respond strongly to unexpected light during the night.42,43,53 In contrast, responses to light during the subjective day are strongly modulated by the circadian clock and thus can be either suppressed or promoted.42,43 Importantly, the effects of light administered at CT0 are difficult to distinguish from the activity-promoting effects of the circadian clock in the morning. Therefore, we chose a different experimental paradigm from that used for the experiments on eclosion and administered a 20-min white light pulse (I = 0.923 W/m2) at ZT22, i.e., 2 h before the expected onset of light at ZT0. This paradigm even allowed us to distinguish the flies’ responses to unexpected light from the expected lights-on response observed at the transition from night to day at ZT0 (startle response, Figure 4A). It has been shown that this startle response depends on the compound eyes.16 As expected, the light pulse at ZT22 elicits an immediate increase in locomotor activity in control flies (w1118; Figure 4A). Surprisingly, the immediate light response in locomotor activity is also present in eyeless flies that in addition lack functional CRY (clieya;cryb; Figure 4B). Thus, neither photoreceptor cells of the compound eyes nor CRY are required for the increase in activity in response to light at night (Figures 4B and S3 for rh and norpA mutants). Nevertheless, these photoreceptors might contribute to the response to unexpected light, as the increase of locomotor activity in the mutants was lower than in w1118 flies (compare Figures 4A and 4B). In addition, we analyzed locomotor activity in two different cry mutants to clarify the role of photosensitive neurons outside the visual system (cryb and cry01). Flies without functional CRY responded to light at ZT22 with an immediate increased locomotor activity that was lower than that of the controls (Figures 4C and S3A). Further, immediate light effects can be seen in flies with disabled light perception in photoreceptors of the ocelli (rh21; Figure 4D) and flies without Rh5 and Rh6 and therefore without functional eyelets (rh52, rh61; Figure 4E). Thus, all the different cells and organs that perceive light appear to mediate its immediate effects and compensate for the failure of individual photoreceptors, so that flies can respond immediately to a light stimulus at night. In contrast, flies without a functional histidine decarboxylase (hdcJK910), the enzyme necessary for histamine synthesis, show no increase in locomotor activity in response to light (Figure 4F). Histamine is the main transmitter of the photoreceptor cells of the eyes, ocelli, and eyelets,54 leaving the hypothesis that cry-positive cells alone may not be sufficient to elicit the lights-on response. A closer examination of the activity pattern suggests that hdcJK910 mutants display a reduced activity during the light pulse and increase it after the light pulse has ended. CRY thus appears to be involved in the response to unexpected light, but may act in the opposite direction to the rhodopsins of the photoreceptors. Contrasting effects of CRY and the visual system on fly activity have also been observed previously55 and merit further investigation by future studies.
Figure 4.
The immediate effect of light on locomotor activity is visible in flies without functional eyes, photosensation in cry-positive cells, or ocelli
(A–F) Activity pattern in 10 min intervals at the time around zeitgeber time (ZT) 0. First and second column show bar plots of mean ± SEM activity at the day the light pulse was applied (second column) and the activity of the same flies on the previous day (first column). The third column visualizes the comparison between the mean activity at ZT22 in 10 min intervals (0′–10′ and 10′–20′) during the light pulse (L) and the previous control day in darkness (D). Data are presented as mean (bar plot) and individual values (dots). (A) Control flies (w1118) and (B) eyeless (clieya; cryb) flies, (C) flies lacking cryptochrome (cry01), (D) flies without functional ocelli photoreceptors (rh21), and (E) flies without Rh5 and Rh6 (rh52; rh61) increase activity in response to light at ZT22.
(F) The light response is absent in flies that lack the histidine decarboxylase (hdcJK910) and therefore histamine, the transmitter of photoreceptor cells. n = 27–32; asterisks denote level of significance: ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001. See also Figure S3 and Table S1.
In summary, our data provide evidence that photoreceptors of the compound eyes are involved in the masking effect of expected light, while unexpected light at night is mediated by histamine-positive cells and therefore a combination of the eyes, H-B eyelets, and potentially the ocelli and CRY.
Discussion
The immediate effect of light on locomotor activity is evident every morning in flies under light-dark rhythms at ZT0.16,17 Interestingly, former studies showed that the immediate increase in locomotion at ZT0 depends on the compound eyes and is completely absent in eyeless flies.16 Here, we observed the same: eyeless flies (clieya;cryb mutants, which lack functional CRY in addition to the eyes) as well as flies without histamine (hdcJK910 mutants) lack the startle response at ZT0 (first column, Figures 4B and 4F). Nevertheless, a light pulse during the night 2 h before lights-on at ZT22, provokes an immediate increase in activity in clieya;cryb mutants (Figure 4B). Only the disruption of neuronal communication of photoreceptor cells of the eyes, ocelli, and eyelets by the loss of histamine (hdcJK910 mutants) prevents a startle response to light at night (ZT22, Figure 4F). The immediate increase in activity in response to unexpected light at ZT22 is thus mediated by functionally redundant photoreceptor cells, whereas the startle response at ZT0 depends purely on the receptors in the eyes. The immediate effect of light on locomotion in Drosophila is modulated in a time-of-day-dependent manner: light at night increases activity, while activity is suppressed during the day.42,43 The switch in behavior seems to depend on the daily morphological changes of central clock neurons43 and on a light-mediated circuit switching in the Drosophila neuronal clock network.56 The differences in the photoreceptor requirements observed between ZT22 and ZT0 in this study might reflect the time of day and resulting morphological changes in the downstream neuronal network.
The functional redundancy of photoreceptors was also described in nocturnal mice. Here, bright light at night leads to an inhibition of locomotor activity, whereas dim light increases activity.57,58,59 Mice with degenerated retina (rd/rd mice), devoid of rods and cones, lack the increase of activity by dim light, but still show activity inhibition by bright light. In addition, mice lacking the photopigment melanopsin (Opn4−/−) in the retinal ganglion cells also show inhibition of activity by bright light, while double mutants (Opn4−/−; rd/rd) lose the ability to respond to light.48,49,60 Thus, also in mice, several photopigments contribute to the immediate light responses, and these can compensate to a certain degree the loss of each other to ensure immediate responses to unexpected external stimuli.
Interestingly, recent data suggest that CRY suppresses activity during the night in flies, while the absence of CRY enhances activity during moonlit nights.61 Flies were as active during moonlit nights as they were during the day, suggesting that CRY is important for distinguishing nocturnal low light of moonlight intensity from day light. Interestingly, this is consistent with data from marine bristle worms.61 If CRY is present, it seems to suppress activity during the night in the diurnal D. melanogaster and suppress swarming activity during the day in the nocturnal marine bristle worms. This finding for Drosophila is corroborated by the present study: cryb mutants show strong immediate light effects upon a light pulse, but flies that lack the function of all photoreceptors except of CRY (hdcJK910 mutants) have decreased responses to unexpected light. In summary, we conclude that the immediate light effects of adult flies in response to nocturnal light are mediated by all rhodopsins (those in the compound eyes, the H-B eyelets, and putatively also the ocelli), while they are inhibited by CRY. This is again different for the startle response at ZT0. Here we could not see any inhibiting effect of CRY.
The immediate effect of white light on eclosion depends on pale R8 cells in the compound eyes. White light elicits eclosion in flies without functional ocelli, H-B eyelets, and cry-positive photoreceptive cells, while flies with impaired phospholipase C activity and without eyes or Rh5 lack the lights-on response. Masking by red light, however, depends on Rh6-positive yellow R8 cells. Rh6 is also expressed in the H-B eyelet of adult flies.35,62 During metamorphosis, four larval Rh5 photoreceptors switch rhodopsin expression and become the adult Rh6-positive eyelet.34 It was demonstrated that these extraretinal photoreceptors are involved in entrainment, but they only fulfill this function a few days after eclosion because the H-B eyelets need several days to become mature.62 Therefore, it is unlikely that the H-B eyelets contribute to the light effects during eclosion.
Limitations of the study
Even though the context-dependent behavioral plasticity depends on the circadian clock, the increase in locomotor activity in response to light is still visible in flies without functional internal clocks.42,43,63 We cannot completely exclude that contextualization of the light stimulus is disturbed in hdcJK910 mutants leading to a decrease in activity in response to light at ZT22. In any case, the experiments using the hdcJK910 mutants show that the immediate effects of light on locomotion are mediated via neurotransmission through histamine, which is the main transmitter of the compound eyes, ocelli, and H-B eyelets.54 The H-B eyelets additionally use acetylcholine (ACh) as a neurotransmitter35, and a recent study shows that this is also true for the inner photoreceptors R8.64 Most interestingly, ACh appears to transmit photic input to the circadian clock, while histamine transmits visual signals for image detection and motion. Thus, consistent with our results, histamine seems to be the transmitter for the direct light responses.
For the immediate effect of white light on eclosion, only Rh5 in R8 cells of the compound eyes is needed. Possibly, not all photoreceptors are yet mature at the time of eclosion. This is certainly true for the H-B eyelets that are fully functional only several days after eclosion.62 For the other photoreceptors, such a delayed maturation is not known. R8 photoreceptors develop first, but retina formation is completed in pupal state P13/P14 including photoreceptor differentiation and rhodopsin expression.65,66,67 Nevertheless, it is possible that not all the connections to the central brain, particularly those that connect to activity-promoting centers, are fully established.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit-α-GFP | ThermoFisher Scientific | CAT#A11122; RRID: AB_221569 |
| 3C11 | Klagges et al.68 | RRID: AB_2313617 |
| goat-α-rabbit AlexaFluor 488 | ThermoFisher Scientific | CAT#A11034; RRID: AB_2576217 |
| goat-α-mouse STAR RED | Abberior | CAT#STRED-1001; RRID: AB_3068620 |
| Chemicals, peptides, and recombinant proteins | ||
| normal goat serum | Sigma-Aldrich | CAT#G9023 |
| Vectashield | Vector Laboratories | CAT#H-1000 |
| Deposited data | ||
| Eclosion Detector, Python script for Fiji | Tilman Triphan, https://zenodo.org/records/10985365 | Zenodo: https://doi.org/10.5281/zenodo.10985365 |
| Experimental models: Organisms/strains | ||
| Drosophila melanogaster: w1118 | Kittel lab | N/A |
| Drosophila melanogaster: clieya | Bonini et al.69 | N/A |
| Drosophila melanogaster: cry01 | Dolezelova et al.70 | N/A |
| Drosophila melanogaster: cryb | Stanewsky lab; Stanewsky et al.19 | N/A |
| Drosophila melanogaster: hdcJK910 | Burg et al.71 | N/A |
| Drosophila melanogaster: ninaE17 | Helfrich-Förster lab; O’Tousa et al.72 | N/A |
| Drosophila melanogaster: norpAp41 | Stanewsky lab | N/A |
| Drosophila melanogaster: rh21 | Montell lab | N/A |
| Drosophila melanogaster: rh52 | Stanewsky lab; Yamaguchi et al.73 | N/A |
| Drosophila melanogaster: rh61 | Montell lab; Cook et al.74 | N/A |
| Drosophila melanogaster: rh52; rh61 | Yamaguchi et al.73 | N/A |
| Drosophila melanogaster: ninaE17; rh61 | Hanai et al.75 | N/A |
| Drosophila melanogaster: sevLY3 | Harris et al.76 | N/A |
| Drosophila melanogaster: rh52; rh31, rh41, rh61 | Helfrich-Förster lab | N/A |
| Drosophila melanogaster: rh5G | Montell lab; Sokabe et al.,77 | BDSC #66671 |
| Drosophila melanogaster: UAS-chop2XXL | Dawydow et al.52 | N/A |
| Drosophila melanogaster: 10xUAS-IVS-myr::GFP | Pfeiffer et al.78 | BDSC #32197 |
| Software and algorithms | ||
| IC Capture 64bit | The Imaging Source Europe GmbH | RRID: SCR_016047 |
| Fiji |
http://fiji.sc Schindelin et al.79 |
RRID: SCR_002285 |
| Prism 9 | GraphPad Software, LLC | Version 9.3.1 (350); RRID: SCR_002798 |
| Drosophila Activity Monitoring System | TriKinetics Inc. | DAM2 |
| Adobe Photoshop | Adobe | Version 25.6.0 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Mareike Selcho (mareike.selcho@uni-leipzig.de).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The datasets generated and analysed during the current study are available from the lead contact on reasonable request.
-
•
All code and further information is publicly accessible and can be found at https://zenodo.org/records/10985365.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Fly stocks
Flies (Drosophila melanogaster) were raised on standard cornmeal and molasses medium at 25°C and 65% relative humidity at a 14:10 light-dark (L:D) cycle unless otherwise stated. For optogenetic experiments, vials have been covered with a light filter foil (Nr. 026; LEE Filters Worldwide, UK). The following flies were used in this study: clieya (69), cry01 (70), cryb (19, kind gift of R. Stanewsky), hdcJK910 (71), ninaE17 (72,80), norpAp41 (81,82, kind gift of R. Stanewsky), rh21 (77, kind gift of C. Montell), rh52 (73, kind gift of R. Stanewsky), rh61 (74, kind gift of C. Montell), ninaE17; rh61 (75), sevLY3 (76) and the following combinations of the mutants were used: clieya; cryb and rh52; rh61 and rh52; rh31, rh41, rh61. The Gal4- and UAS- lines used: rh5G (77, kind gift of C. Montell; BDSC #66671), UAS-chop2XXL (52), 10xUAS-IVS-myr::GFP (78; BDSC #32197).
Seven to nine days old pupae, of both sexes were used for eclosion experiments and two to four days old flies of both sexes were used for locomotor activity measurements.
Method details
Eclosion behaviour
To investigate the immediate light effect on eclosion behaviour, an eclosion monitor based on the WEclMon-System83,84 was used (Figure S4A). Experiments were performed under constant temperature (24.5°C ± 0.2°C) and humidity (around 65% RH). Temperature fluctuations upon light exposure were below 0.2°C. Seven to nine days old pupae, of both sexes, were placed onto a transparent acrylic plate. This plate was placed on an area light with an RGB or White-LED illumination (LED-color: λ(blue)= 455 – 475 nm, λ(green)= 510 – 545 nm, λ(red)= 625 – 642 nm; white LED (λ= ∼430 – 730 nm, Figure S4B); Hansen GmbH, Germany, Luminous Panel (RGBW)) and an additional IR LED strip was installed at the bottom of the lighting unit (SOLAROX® LED, λ(infrared) = 850nm, IR1-60-850, Winger Electronics GmbH & Co. KG, Germany) and covered with an aluminium box. Flies were kept in darkness and received a twenty-minute light pulse one hour after expected lights-on (CT1) on the second day, or remained in darkness (darkness control). The eclosion monitor is equipped with a camera (DMK 27AUC02 with a TPL 0420 6MP objective; DMK 37BUX287 with a TPL 0620 6MP objective, The Imaging Source Europe GmbH) that took images every two minutes (IC Capture 64bit, V2.5.1547.4007, The Imaging Source Europe GmbH, Germany). We developed a Python-script for Fiji (V2.9.0,79) that was able to independently scan a selected image sequence for eclosion events (detailed description below).
Eclosion data analysis
The detection of hatching events is based on the difference in brightness between a pupa and an empty pupal case. The latter is almost transparent while a late pupa is considerably darker (Figure S4C, empty pupal case marked with an asterisk). In the first step of the analysis all pupae are detected. The pupae are darker than the background and can be easily extracted in a single image. After setting a threshold (Figure S4D), objects are detected, and their outlines stored. Objects that are outside of the parameters defined for a pupa (area too big or too small) are excluded. Note the empty pupal case that is excluded from further analysis. As the pupae are stationary, we can follow them through time and calculate the median grey value of the area contained in their respective outlines (Figure S4E) for each frame. Alternatively, the average or mode (i.e. most frequent) grey values can be used. A large jump in brightness indicates an eclosion event (Figures S4F–S4H). The median grey values are more than doubled from around 40 to almost 100. For each eclosion event a few frames before and after the event are extracted to help with manual confirmation of real hatching events or to exclude inaccurate detections (Figure S4G). The time for each eclosion event is stored in a csv file for further analysis. Additional checks are implemented to reduce the number of incorrect detections and handle changes in lighting. See the commented source code for details. The complete workflow is implemented as a Python script for Fiji. Specific parameters that depend on camera resolution, optics and lighting can be set manually (e.g. area of the pupae in pixel, difference in brightness for full and empty pupae, etc.). All code and further information can be found at https://zenodo.org/records/10985365.
For the evaluation of eclosion events, a time window of five hours was chosen, from two hours before expected lights-on to three hours after expected lights-on. To calculate the eclosion percentage (% eclosion), the number of flies eclosed in a 10 min time interval was normalized to the number of flies eclosed during the 5 h time window. The bar plots in Figures 1D’’, 2, and 3 visualize the eclosion of flies perceiving a 20 min light pulse and of appropriate control flies kept in darkness (Figures 1D–1D″). n refers to the number of individuals tested.
To analyse changes in eclosion in response to light, eclosion percentage of experimental (L) and control (D) flies in the first 10 min (0’-10’) and second 10 min (10’-20’) interval after lights-on at CT1 was compared. Data was analysed with Excel (Microsoft) and Prism 8.2 (GraphPad). The Shapiro-Wilk test was used to analyse normal distribution and data were compared by an unpaired two-tailed t-test. Not normally distributed data were compared by a nonparametric Mann-Whitney rank sum test. Prism was used to plot the results (Figures S1 and S2). Barplots show the mean and individual values in the first and second ten minutes interval at CT1 in light and darkness. Significance levels refer to the raw p values obtained in the statistical tests. N refers to the eclosion experiments analysed.
Optogenetics
For optogenetic activation, Channelrhodopsin-2XXL (UAS-chop2XXL) has been used to depolarize rh5-positive neurons by blue light. Pupae have been collected under red light during their subjective day to monitor eclosion. One hour after expected lights-on pupae received a 2 min blue light stimulus (455 – 475 nm, Ι = 3,41 μW/mm2) and eclosion was monitored as described above. n refers to the number of individuals tested. Barplots show the mean and individual values in the ten minutes interval before and under/after the 2 min light pulse at CT1. Significance levels refer to the raw p values obtained in the statistical tests. N refers to the eclosion experiments analysed.
Locomotor activity
Flies were entrained to a 12:12 LD rhythm to be able to compare results to the data by.42,43 To investigate the lights-on effect on locomotor activity, we placed two to four days old flies of both sexes in small glass tubes with 2% agarose and 4% sugar on one side into the Drosophila Activity Monitoring System (DAM, V2, TriKinetics Inc., USA) and recorded locomotor activity for the next four night-day cycles under constant temperature and humidity (24.9 ± 0.1°C, ∼65% RH). In the first three night-day cycles the light regime did not differ from the entrained rhythm (12:12 LD). On the fourth night, a 20 min light pulse (λ= ∼435 – 780 nm, I = 0.923 W/m2, ∼100 lux) was given two hours before normal lights-on at ZT22. The data received from the DAM system was analysed by taking the number of counts of a 10 min interval of each tube and calculating the average activity. This was done for both the 6 h time window (-3 h to +3 h from ZT0) in which the 20 min light pulse was given and the same time window on the day before, which was used as control. To analyse changes in locomotor activity in response to light, the activity at ZT22 under light (L) was compared to the activity at ZT22 in darkness (D) the day before (third column Figures 4 and S3). Data was analysed with Excel (Microsoft) and Prism 8.2 (GraphPad). The Shapiro-Wilk test was used to analyse normal distribution and data was compared by a paired two-tailed t-test and for not normally distributed data by a Wilcoxon signed rank test. Prism was used to plot data. Activity levels in the first and second ten minutes interval at ZT22 are presented as bar plots showing the mean and individual values (Figures 4 and S3). Significance levels refer to the raw p values obtained in the statistical tests.
Immunohistochemistry
rh5G> 10xmyrGFP pharate flies have been used to confirm GAL4 expression in Rh5-positive R8 cells (Figure S2C). Whole heads have been fixed for 2 hours in 4% PFA. After washing in PBS (Phosphate Buffered Saline) specimens have been embedded in 7% agarose and cut into 70-100 μm sections using a vibratome (Leica VT1000S;85). Sections have been washed three times for 10 min in 0.3% PBT (PBS with 0.3% TritonX-100), blocked for 1,5 h in 5% normal goat serum in PBT. Afterwards the first antibody solution was incubated overnight at 4°C. Specimens were washed six times and the second antibody solution was added and incubated overnight at 4°C. After another washing step, specimens were mounted in Vectashield (Vector Laboratories) and stored at 4°C until scanning. Probes have been imaged using a LSM 800 (Zeiss). Afterwards the images have been edited for brightness and contrast using FIJI and Adobe Photoshop (V23.5.3).
The following antibodies were used: rabbit-α-GFP (1:1000; ThermoFisher Scientific, A11122), 3C11 α-Synapsin (1:50,68), goat-α-rabbit AlexaFluor 488 (1:250, ThermoFisher Scientific , A11034) and goat-α-mouse STAR RED (1:250, Abberior, STRED-1001).
Quantification and statistical analysis
All statistical analysis and p-values are included in Table S1. Eclosion behaviour: the Shapiro-Wilk test was used to analyse normal distribution and data were compared by an unpaired two-tailed t-test. Not normally distributed data were compared by a nonparametric Mann-Whitney rank sum test. Prism was used to calculate p-values and plot the results (Figures S1 and S2). Locomotor activity: the Shapiro-Wilk test was used to analyse normal distribution and data was compared by a paired two-tailed t-test and for not normally distributed data by a Wilcoxon signed rank test. Prism was used to calculate p-values and plot the results (Figures 4 and S3).
Acknowledgments
We thank Claude Desplan, Craig Montell, Christopher Schnaitmann, and Ralf Stanewsky for providing flies and Simon Sprecher for sharing antibodies. This work was supported by grants from the German Research Foundation (DFG) to M.S. (PA3241/2-1) and D.P. (PA1979/2-1 and PA1979/5-1). Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. This publication was supported by the Open Access Publishing Fund of Leipzig University.
Author contributions
Conceptualization, M.S.; methodology, D.P., C.H.-F., and M.S.; software, T.T.; investigation, D.B., N.-D.F., and C.M.; writing, C.H.-F., D.P., and M.S.; visualization, D.B. and M.S.; funding acquisition, C.H.-F. and M.S.
Declaration of interests
The authors declare no competing interests.
Published: April 26, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109819.
Supplemental information
References
- 1.Daan S., Tinbergen J. Young Guillemots (Uria lomvia) leaving their arctic breeding cliffs: a daily rhythm in numbers and risk. Ardea. 1979;67:96–100. [Google Scholar]
- 2.DeCoursey P.J., Walker J.K., Smith S.A. A circadian pacemaker in free-living chipmunks: essential for survival? J. Comp. Physiol. 2000;186:169–180. doi: 10.1007/s003590050017. [DOI] [PubMed] [Google Scholar]
- 3.Erkert H.G., Gröber J. Direct modulation of activity and body temperature of owl monkeys (Aotus lemurinus griseimembra) by low light intensities. Folia Primatol. 1986;47:171–188. doi: 10.1159/000156276. [DOI] [PubMed] [Google Scholar]
- 4.Aschoff J., von Goetz C. Masking of circadian activity rhythms in canaries by light and dark. J. Biol. Rhythms. 1989;4:29–38. doi: 10.1177/074873048900400102. [DOI] [PubMed] [Google Scholar]
- 5.Badia P., Myers B., Boecker M., Culpepper J., Harsh J.R. Bright light effects on body temperature, alertness, EEG and behavior. Physiol. Behav. 1991;50:583–588. doi: 10.1016/0031-9384(91)90549-4. [DOI] [PubMed] [Google Scholar]
- 6.Dijk D.J., Cajochen C., Borbély A.A. Effect of a single 3-hour exposure to bright light on core body temperature and sleep in humans. Neurosci. Lett. 1991;121:59–62. doi: 10.1016/0304-3940(91)90649-e. [DOI] [PubMed] [Google Scholar]
- 7.Cajochen C., Münch M., Kobialka S., Kräuchi K., Steiner R., Oelhafen P., Orgül S., Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J. Clin. Endocrinol. Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 8.Altimus C.M., Güler A.D., Villa K.L., McNeill D.S., Legates T.A., Hattar S. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc. Natl. Acad. Sci. USA. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mrosovsky N. In praise of masking: behavioural responses of retinally degenerate mice to dim light. Chronobiol. Int. 1994;11:343–348. doi: 10.3109/07420529409057251. [DOI] [PubMed] [Google Scholar]
- 10.Mrosovsky N. Masking: history, definitions, and measurement. Chronobiol. Int. 1999;16:415–429. doi: 10.3109/07420529908998717. [DOI] [PubMed]
- 11.Daan S., Spoelstra K., Albrecht U., Schmutz I., Daan M., Daan B., Rienks F., Poletaeva I., Dell’Omo G., Vyssotski A., Lipp H.P. Lab mice in the field: unorthodox daily activity and effects of a dysfunctional circadian clock allele. J. Biol. Rhythms. 2011;26:118–129. doi: 10.1177/0748730410397645. [DOI] [PubMed] [Google Scholar]
- 12.Vanin S., Bhutani S., Montelli S., Menegazzi P., Green E.W., Pegoraro M., Sandrelli F., Costa R., Kyriacou C.P. Unexpected features of Drosophila circadian behavioural rhythms under natural conditions. Nature. 2012;484:371–375. doi: 10.1038/nature10991. [DOI] [PubMed] [Google Scholar]
- 13.Schlichting M., Menegazzi P., Helfrich-Förster C. Normal vision can compensate for the loss of the circadian clock. Proc. Biol. Sci. 2015;282:20151846. doi: 10.1098/rspb.2015.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelmann W., Honegger H. Tagesperiodische Schlüpfrhythmik einer augenlosen Drosophila melanogaster- Mutante. Naturwissenschaften. 1966;53:588. doi: 10.1007/BF00600545. [DOI] [PubMed] [Google Scholar]
- 15.McNabb S.L., Truman J.W. Light and peptidergic eclosion hormone neurons stimulate a rapid eclosion response that masks circadian emergence in Drosophila. J. Exp. Biol. 2008;211:2263–2274. doi: 10.1242/jeb.015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rieger D., Stanewsky R., Helfrich-Förster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J. Biol. Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- 17.Wheeler D.A., Hamblen-Coyle M.J., Dushay M.S., Hall J.C. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J. Biol. Rhythms. 1993;8:67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- 18.Emery P., So W.V., Kaneko M., Hall J.C., Rosbash M. CRY, a Drosophila clock and light-regulated Cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/S0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 19.Stanewsky R., Kaneko M., Emery P., Beretta B., Wager-Smith K., Kay S.A., Rosbash M., Hall J.C. The cryb mutation identifies Cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/S0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 20.Helfrich-Förster C., Engelmann W. In: Biological Rhythms. Kumar V., editor. Springer Berlin Heidelberg; 2002. Photoreceptors for the circadian clock of the fruitfly; pp. 94–106. [DOI] [Google Scholar]
- 21.Helfrich-Förster C. The circadian system of Drosophila melanogaster and its light input pathways1. Zoology. 2002;105:297–312. doi: 10.1078/0944-2006-00074. [DOI] [PubMed] [Google Scholar]
- 22.Helfrich C., Engelmann W. Circadian rhythm of the locomotor activity in Drosophila melanogaster and its mutants ‘sine oculis’ and ‘small optic lobes’. Physiol. Entomol. 1983;8:257–272. doi: 10.1111/j.1365-3032.1983.tb00358.x. [DOI] [Google Scholar]
- 23.Schlichting M., Grebler R., Peschel N., Yoshii T., Helfrich-Förster C. Moonlight detection by Drosophila ’s endogenous clock depends on multiple photopigments in the compound eyes. J. Biol. Rhythms. 2014;29:75–86. doi: 10.1177/0748730413520428. [DOI] [PubMed] [Google Scholar]
- 24.Schlichting M., Grebler R., Menegazzi P., Helfrich-Förster C. Twilight dominates over moonlight in adjusting Drosophila ’s activity pattern. J. Biol. Rhythms. 2015;30:117–128. doi: 10.1177/0748730415575245. [DOI] [PubMed] [Google Scholar]
- 25.Rister J., Desplan C. The retinal mosaics of opsin expression in invertebrates and vertebrates. Dev. Neurobiol. 2011;71:1212–1226. doi: 10.1002/dneu.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heisenberg M., Buchner E. The rôle of retinula cell types in visual behavior of Drosophila melanogaster. J. Comp. Physiol. 1977;117:127–162. doi: 10.1007/BF00612784. [DOI] [Google Scholar]
- 27.Schnaitmann C., Garbers C., Wachtler T., Tanimoto H. Color discrimination with broadband photoreceptors. Curr. Biol. 2013;23:2375–2382. doi: 10.1016/j.cub.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Melnattur K.V., Pursley R., Lin T.-Y., Ting C.-Y., Smith P.D., Pohida T., Lee C.-H. Multiple redundant medulla projection neurons mediate color vision in Drosophila. J. Neurogenet. 2014;28:374–388. doi: 10.3109/01677063.2014.891590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wernet M.F., Velez M.M., Clark D.A., Baumann-Klausener F., Brown J.R., Klovstad M., Labhart T., Clandinin T.R. Genetic dissection reveals two separate retinal substrates for polarization vision in Drosophila. Curr. Biol. 2012;22:12–20. doi: 10.1016/j.cub.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feiler R., Harris W.A., Kirschfeld K., Wehrhahn C., Zuker C.S. Targeted misexpression of a Drosophila opsin gene leads to altered visual function. Nature. 1988;333:737–741. doi: 10.1038/333737a0. [DOI] [PubMed] [Google Scholar]
- 31.Pollock J.A., Benzer S. Transcript localization of four opsin genes in the three visual organs of Drosophila; RH2 is ocellus specific. Nature. 1988;333:779–782. doi: 10.1038/333779a0. [DOI] [PubMed] [Google Scholar]
- 32.Sabat D., Priyadarsini S., Mishra M. Understanding the structural and developmental aspect of simple eye of Drosophila: the ocelli. J. Cell Signal. 2017;1:1–10. doi: 10.4172/2576-1471.1000109. [DOI] [Google Scholar]
- 33.Hofbauer A., Buchner E. Does Drosophila have seven eyes? Naturwissenschaften. 1989;76:335–336. doi: 10.1007/BF00368438. [DOI] [Google Scholar]
- 34.Sprecher S.G., Desplan C. Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature. 2008;454:533–537. doi: 10.1038/nature07062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasuyama K., Meinertzhagen I.A. Extraretinal photoreceptors at the compound eye’s posterior margin in Drosophila melanogaster. J. Comp. Neurol. 1999;412:193–202. doi: 10.1002/(SICI)1096-9861(19990920)412:2<193::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Schlichting M., Menegazzi P., Rosbash M., Helfrich-Förster C. A distinct visual pathway mediates high light intensity adaptation of the circadian clock in Drosophila. J. Neurosci. 2019;39:1621–1630. doi: 10.1523/JNEUROSCI.1497-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshii T., Todo T., Wülbeck C., Stanewsky R., Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J. Comp. Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 38.Benito J., Houl J.H., Roman G.W., Hardin P.E. The blue-light photoreceptor CRYPTOCHROME Is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J. Biol. Rhythms. 2008;23:296–307. doi: 10.1177/0748730408318588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoang N., Schleicher E., Kacprzak S., Bouly J.-P., Picot M., Wu W., Berndt A., Wolf E., Bittl R., Ahmad M. Human and Drosophila Cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 2008;6:e160. doi: 10.1371/journal.pbio.0060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fogle K.J., Parson K.G., Dahm N.A., Holmes T.C. CRYPTOCHROME is a blue-light sensor that regulates neuronal firing rate. Science. 2011;331:1409–1413. doi: 10.1126/science.1199702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emery P., Stanewsky R., Helfrich-Förster C., Emery-Le M., Hall J.C., Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000;26:493–504. doi: 10.1016/S0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 42.Lu B., Liu W., Guo F., Guo A. Circadian modulation of light-induced locomotion responses in Drosophila melanogaster. Genes Brain Behav. 2008;7:730–739. doi: 10.1111/j.1601-183X.2008.00411.x. [DOI] [PubMed] [Google Scholar]
- 43.Song B.J., Sharp S.J., Rogulja D. Daily rewiring of a neural circuit generates a predictive model of environmental light. Sci. Adv. 2021;7:eabe4284. doi: 10.1126/sciadv.abe4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feiler R., Bjornson R., Kirschfeld K., Mismer D., Rubin G.M., Smith D.P., Socolich M., Zuker C.S. Ectopic expression of ultraviolet-rhodopsins in the blue photoreceptor cells of Drosophila: visual physiology and photochemistry of transgenic animals. J. Neurosci. 1992;12:3862–3868. doi: 10.1523/JNEUROSCI.12-10-03862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salcedo E., Huber A., Henrich S., Chadwell L.V., Chou W.H., Paulsen R., Britt S.G. Blue- and green-absorbing visual pigments of Drosophila: ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J. Neurosci. 1999;19:10716–10726. doi: 10.1523/JNEUROSCI.19-24-10716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redlin U., Vrang N., Mrosovsky N. Enhanced masking response to light in hamsters with IGL lesions. J. Comp. Physiol. 1999;184:449–456. doi: 10.1007/s003590050344. [DOI] [PubMed] [Google Scholar]
- 47.Redlin U., Mrosovsky N. Masking by light in hamsters with SCN lesions. J. Comp. Physiol. 1999;184:439–448. doi: 10.1007/s003590050343. [DOI] [PubMed] [Google Scholar]
- 48.Mrosovsky N., Hattar S. Impaired masking responses to light in melanopsin-knockout mice. Chronobiol. Int. 2003;20:989–999. doi: 10.1081/cbi-120026043. [DOI] [PubMed] [Google Scholar]
- 49.Panda S., Provencio I., Tu D.C., Pires S.S., Rollag M.D., Castrucci A.M., Pletcher M.T., Sato T.K., Wiltshire T., Andahazy M., et al. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 50.Gall A.J., Smale L., Yan L., Nunez A.A. Lesions of the intergeniculate leaflet lead to a reorganization in circadian regulation and a reversal in masking responses to photic stimuli in the nile grass rat. PLoS One. 2013;8:e67387. doi: 10.1371/journal.pone.0067387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dawydow A., Gueta R., Ljaschenko D., Ullrich S., Hermann M., Ehmann N., Gao S., Fiala A., Langenhan T., Nagel G., Kittel R.J. Channelrhodopsin-2–XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA. 2014;111:13972–13977. doi: 10.1073/pnas.1408269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim E.Y., Bae K., Ng F.S., Glossop N.R.J., Hardin P.E., Edery I. Drosophila CLOCK protein is under posttranscriptional control and influences light-induced activity. Neuron. 2002;34:69–81. doi: 10.1016/S0896-6273(02)00639-6. [DOI] [PubMed] [Google Scholar]
- 54.Pollack I., Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- 55.Kistenpfennig C., Nakayama M., Nihara R., Tomioka K., Helfrich-Förster C., Yoshii T. A tug-of-war between Cryptochrome and the visual system allows the adaptation of evening activity to long photoperiods in Drosophila melanogaster. J. Biol. Rhythms. 2018;33:24–34. doi: 10.1177/0748730417738612. [DOI] [PubMed] [Google Scholar]
- 56.Schlichting M., Weidner P., Diaz M., Menegazzi P., Dalla Benetta E., Helfrich-Förster C., Rosbash M. Light-mediated circuit switching in the Drosophila neuronal clock network. Curr. Biol. 2019;29:3266–3276.e3. doi: 10.1016/j.cub.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 57.Mrosovsky N. Contribution of classic photoreceptors to entrainment. J. Comp. Physiol. 2003;189:69–73. doi: 10.1007/s00359-002-0378-7. [DOI] [PubMed] [Google Scholar]
- 58.Redlin U., Cooper H.M., Mrosovsky N. Increased masking response to light after ablation of the visual cortex in mice. Brain Res. 2003;965:1–8. doi: 10.1016/S0006-8993(02)03844-1. [DOI] [PubMed] [Google Scholar]
- 59.Mrosovsky N., Foster R.G., Salmon P.A. Thresholds for masking responses to light in three strains of retinally degenerate mice. J. Comp. Physiol. 1999;184:423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- 60.Hattar S., Lucas R.J., Mrosovsky N., Thompson S., Douglas R.H., Hankins M.W., Lem J., Biel M., Hofmann F., Foster R.G., Yau K.W. Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zurl M., Poehn B., Rieger D., Krishnan S., Rokvic D., Veedin Rajan V.B., Gerrard E., Schlichting M., Orel L., Ćorić A., et al. Two light sensors decode moonlight versus sunlight to adjust a plastic circadian/circalunidian clock to moon phase. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2115725119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Helfrich-Förster C., Edwards T., Yasuyama K., Wisotzki B., Schneuwly S., Stanewsky R., Meinertzhagen I.A., Hofbauer A. The extraretinal eyelet of Drosophila: development, ultrastructure, and putative circadian function. J. Neurosci. 2002;22:9255–9266. doi: 10.1523/JNEUROSCI.22-21-09255.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stoleru D., Peng Y., Agosto J., Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 64.Xiao N., Xu S., Li Z.K., Tang M., Mao R., Yang T., Ma S.X., Wang P.H., Li M.T., Sunilkumar A., et al. A single photoreceptor splits perception and entrainment by cotransmission. Nature. 2023;623:562–570. doi: 10.1038/s41586-023-06681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomlinson A., Ready D.F. Neuronal differentiation in the Drosophila ommatidium. Dev. Biol. 1987;120:366–376. doi: 10.1016/0012-1606(87)90239-9. [DOI] [PubMed] [Google Scholar]
- 66.Cagan R.L., Ready D.F. The emergence of order in the Drosophila pupal retina. Dev. Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- 67.Earl J.B., Britt S.G. Expression of Drosophila rhodopsins during photoreceptor cell differentiation: insights into R7 and R8 cell subtype commitment. Gene Expr. Patterns. 2006;6:687–694. doi: 10.1016/j.modgep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 68.Klagges B.R., Heimbeck G., Godenschwege T.A., Hofbauer A., Pflugfelder G.O., Reifegerste R., Reisch D., Schaupp M., Buchner S., Buchner E. Invertebrate Synapsins: A single gene codes for several isoforms in Drosophila. J. Neurosci. 1996;16:3154–3165. doi: 10.1523/JNEUROSCI.16-10-03154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonini N.M., Leiserson W.M., Benzer S. The eyes absent gene: Genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993;72:379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- 70.Dolezelova E., Dolezel D., Hall J.C. Rhythm defects caused by newly engineered null mutations in Drosophila’s cryptochrome gene. Genetics. 2007;177:329–345. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burg M.G., Sarthy P.V., Koliantz G., Pak W.L. Genetic and molecular identification of a Drosophila histidine decarboxylase gene required in photoreceptor transmitter synthesis. EMBO J. 1993;12:911–919. doi: 10.1002/j.1460-2075.1993.tb05732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Tousa J.E., Baehr W., Martin R.L., Hirsh J., Pak W.L., Applebury M.L. The Drosophila ninaE gene encodes an opsin. Cell. 1985;40:839–850. doi: 10.1016/0092-8674(85)90343-5. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi S., Wolf R., Desplan C., Heisenberg M. Motion vision is independent of color in Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:4910–4915. doi: 10.1073/pnas.0711484105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cook T., Pichaud F., Sonneville R., Papatsenko D., Desplan C. Distinction between color photoreceptor cell fates is controlled by prospero in Drosophila. Dev. Cell. 2003;4:853–864. doi: 10.1016/S1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 75.Hanai S., Hamasaka Y., Ishida N. Circadian entrainment to red light in Drosophila: requirement of Rhodopsin 1 and Rhodopsin 6. Neuroreport. 2008;19:1441–1444. doi: 10.1097/WNR.0b013e32830e4961. [DOI] [PubMed] [Google Scholar]
- 76.Harris W.A., Stark W.S., Walker J.A. Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J. Physiol. 1976;256:415–439. doi: 10.1113/jphysiol.1976.sp011331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sokabe T., Chen H.-C., Luo J., Montell C. A switch in thermal preference in Drosophila larvae depends on multiple Rhodopsins. Cell Rep. 2016;17:336–344. doi: 10.1016/j.celrep.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pfeiffer B.D., Ngo T.-T.B., Hibbard K.L., Murphy C., Jenett A., Truman J.W., Rubin G.M. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kumar J.P., Ready D.F. Rhodopsin plays an essential structural role in Drosophila photoreceptor development. Development. 1995;121:4359–4370. doi: 10.1242/dev.121.12.4359. [DOI] [PubMed] [Google Scholar]
- 81.Pak W.L., Grossfield J., White N.V. Nonphototactic mutants in a study of vision of Drosophila. Nature. 1969;222:351–354. doi: 10.1038/222351a0. [DOI] [PubMed] [Google Scholar]
- 82.Szular J., Sehadova H., Gentile C., Szabo G., Chou W.-H., Britt S.G., Stanewsky R. Rhodopsin 5 – and Rhodopsin 6 –mediated clock synchronization in Drosophila melanogaster is independent of retinal Phospholipase C-β signaling. J. Biol. Rhythms. 2012;27:25–36. doi: 10.1177/0748730411431673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ruf F., Fraunholz M., Öchsner K., Kaderschabek J., Wegener C. WEclMon - A simple and robust camera-based system to monitor Drosophila eclosion under optogenetic manipulation and natural conditions. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selcho M., Millán C., Palacios-Muñoz A., Ruf F., Ubillo L., Chen J., Bergmann G., Ito C., Silva V., Wegener C., Ewer J. Central and peripheral clocks are coupled by a neuropeptide pathway in Drosophila. Nat. Commun. 2017;8:15563. doi: 10.1038/ncomms15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pauls D., Blechschmidt C., Frantzmann F., El Jundi B., Selcho M. A comprehensive anatomical map of the peripheral octopaminergic/tyraminergic system of Drosophila melanogaster. Sci. Rep. 2018;8:15314. doi: 10.1038/s41598-018-33686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The datasets generated and analysed during the current study are available from the lead contact on reasonable request.
-
•
All code and further information is publicly accessible and can be found at https://zenodo.org/records/10985365.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.