Abstract
Background
Recurrence rates of FIGO stage IB-IIA and IIB-IVA cervical cancer 28–64 respectively. There is a scarcity of data on the recurrence recurrence pattern for unusual sites and theirrecurrence pattern for unusual sites and its association with survival and prognosis.
Objective
To study overall survival in patients with distant metastasis compared to local and regional nodal metastasis.
Methods
A retrospective study was done from 1/1/2017 to 30/12/22. Cervical cancer patients post primary treatments were included. Survival was analyzed with respect to 3 groups local, regional nodalconducted from 1/1/2017 to 30/12/22. Cervical cancer patients who had received primary post-primary treatments were included. Survival was analyzed with respect to three groups: local, regional nodal, and distant metastasis.
Results
225 patients had recurrences post-completion of primary treatment, of which 105 (46.6%)(46.6 %) had local, 46 (20.4%)(20.4 %) had regional nodal, and 74 (33.3 %) had distant recurrences. The median time for recurrence in local, regional nodal, and atypical recurrences were 9, 9, and 13 months (p value - <0.05), respectively. Treatment included systemic chemotherapy 122 (54.2 %), metronomic therapy 19 (8.4 %), palliative radiotherapy 44 (19.5 %), palliative surgery 8 (3.5 %) and best supportive care 30 (13.3 %) patients. Median Time to treatment-death of patients after recurrence in local, nodal and distant recurrences was 17.0 months, 18.0 months and 10.0 months respectively (p value - < 0.05). Overall Survival of patients after primary treatment with local, nodal and distant recurrences was 35.0 months, 47.0 months and 50.0 months respectively (p value <0.05).
Conclusion
Local recurrence is most common, followed by regional, nodal, and distant recurrences. Overall survival post recurrence was lowest for distant recurrences and highest for local recurrences however overall survival after primary treatment completion was highest for distant recurrence due to the late presen; however, tation of distant recurrences.
1. Background
Treatment of cervical cancer depends on the stage of the disease, with surgery reserved for early stages and concurrent chemotherapy for advanced stage. The results are very good, with minimal side effects, with the advent of newer radiotherapy techniques [1], [2], [3], [4]. Systemic chemotherapy with palliative intent is administered when there is distant metastasis [5].
Follow-up of treated patients is guided by clinical assessment with diagnostic imaging performed when there is a clinical suspicion of residual or recurrent disease, preferably targeted as per the patient’s complaints [6], [7], [8], [9]. Histological confirmation is sought depending on disease-free interval.
The recurrence rates of FIGO stage IB-IIA and IIB-IVA cervical cancer are 11 % to 22 % and 28 % to 64 % respectively [6]. Some studies had reported a recurrence rate in patients with advanced cervical cancer to be as high as 70 % [7], [10]. Most of the relapses usually occur in the first two to three years after treatment completion [11], [12], [13], [14].
Local and regional nodal metastases are common sites of recurrence whereas distant metastases are rare. Cervical cancer recurrences can be divided into two types –Typical cervical recurrences (TCR) and Atypical cervical recurrences (ACR). TCR include pelvic and regional nodal recurrences whereas ACR includes distant cervical metastasis[11]. In a review of the literature, the estimated prevalence of organ site involvement by metastatic cervical cancer was pelvic (75 %) or para-aortic nodes (62 %), lung (33–38 %), liver (33 %), peritoneum (5 to 27 %), adrenal gland (14–16 %), intestines (12 %) and skin (10 %). Treatment protocols for the management of recurrences vary depending on the presenting sites. There is a scarcity of data on the pattern of recurrence in atypical sites and prognosis.
The treatment of recurrent cervical cancer remains challenging and the prognosis of patients with recurrent cervical cancer remains poor with five year overall survival (OS) rate of less than 5 % despite intensive therapy [12]. Treatment of recurrent cervical cancer depends on the previous treatment, site of recurrence, extent of disease, duration of primary treatment and performance status of patient. Concurrent chemo-radiation, radical hysterectomy, pelvic exenteration and chemotherapy are usual treatment options for TCR. Local radiation, systemic chemotherapy and sometimes cytoreductive surgeries are the possible treatment options for regional nodal recurrences. However there are limited options for ACR and are limited to systemic chemotherapy. Recently Immunotherapy has been approved in the recurrence setting of cervical cancer [13].
2. Objective
To study the overall survival in patients with atypical recurrences (distant) compared with typical recurrences (regional nodal and pelvic metastasis).
3. Methods
A retrospective observational study was carried out at a cancer centre in North East India. Data was collected from Electronic medical records (EMR), patient files, old medical records, and followed up survival was done by telephonic conservation. Data was collected from 1st January 2017 to 30th December 2022. Patients who were diagnosed as cervical cancer with histopathology diagnosis of squamous cell carcinoma and adenocarcinoma were included. Any other histology was excluded. Only patients who completed their primary treatment were enrolled for the study. Patients who were stage IVB were excluded from the analysis. All patients who had recurrence after completion of primary treatment were included in the study. They were divided into 3 groups based on the pattern of recurrences into local, nodal and distant metastasis. Nodal and local recurrences were included in typical cervical recurrences (TCR) and distant recurrences were included in atypical cervical recurrences (ACR). Details which included Demographic and clinical characteristics, stage, histology, primary treatment administered, operative details, chemo radiotherapy given, recurrence and treatment taken, and duration of survival were recorded. Bar diagram and pie chart were used to describe the descriptive statistics. The chi-square test was used to evaluate the association between categorical variables.
Survival analysis was done using statistical software SPSS 29.0 for Windows (SPSS Inc., Chicago, IL, USA). A p value less than 0.05 was considered statistically significant at a 5 % level of significance. The primary efficacy analysis of overall survival was performed with a two-sided stratified log-rank test with a significance level of 0.05. The median time for disease recurrence (MDR) was calculated from completion of primary treatment to first recurrence. The median time for overall survival (OS) was calculated from the completion of primary treatment to the last follow-up/death. Time to next treatment-death (TNT-D) was calculated from the first recurrence to the next recurrence/death.
4. Results
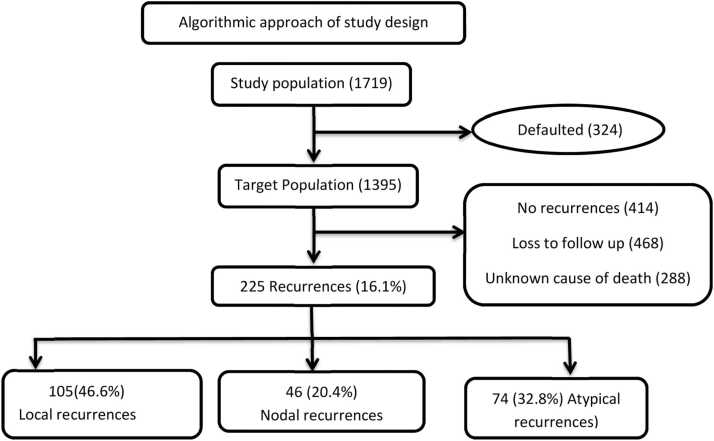
Ethical clearance from the institutional ethics committee has been obtained. As depicted in Fig. 1, A total of 1719 patients with cervical cancer were registered over 53 months out of which 324 (18.8 %) patients did not complete their primary treatment. Among the patients who had completed the treatment,414 (29.6 %) were disease free at a median follow-up of 31 months, 468 (33.5 %) were lost to follow-up. 288 (20.6 %) died without any known recurrences as they were not on regular follow and data was obtained on telephonic follow-up. A total of 225 (16.2 %) patients who had documented cervical cancer recurrences were included in the analysis. Of the 225 recurrences 105 patients (46.6 %) had local recurrences, 46 patients (20.4 %) had TCR and 74 (33.3 %) had ACR.
Fig. 1.
Algorithmic approach of study design.
It was that the median age of study population was 49.5 years (range – 45–54 years), majority of patients were stage IIB, and squamous cell carcinoma (SCC) was the most common histopathological group.
Pelvic recurrences were divided into central (cervix, vagina, bladder and rectum) and lateral local recurrences (parametrium and lateral pelvic walls). Out of the 105 local recurrences 46 patients had central recurrences and 59 had lateral recurrences. It was seen that in the central recurrences group patients only 17.3 % accepted exenteration and the rest received other modalities of treatment.
In regional nodal recurrences, most common site of nodal recurrence was in iliac groups of lymph nodes (37 %) followed by paraaortic lymph node recurrences (35 %).
We observed the sites of atypical recurrences were seen in lung, liver, bone, omentum, pericardium, adrenal gland, stomach, oesophagus, spleen, abdominal wall and breast. The most common site of atypical recurrences was Lung (30 cases – 40.5 %), followed by liver (21 cases - 28.3 %), bone (15 cases - 20 %), omentum (7 cases - 9.4 %), adrenal gland (4 cases - 5.4 %). These patients received systemic chemotherapy, oral metronomic therapy or best supportive care depending on their performance status.
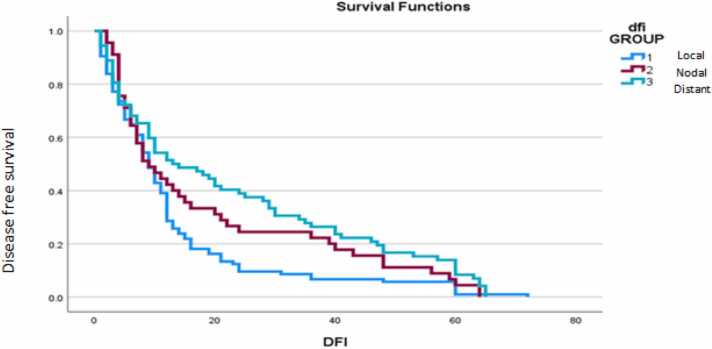
The mean DFS in local, nodal and atypical recurrences was 9 months, 9 months and 13 months respectively as shown in Fig. 2.
Fig. 2.
Median disease free recurrence in local, nodal and atypical recurrences.
It was observed that 195 patients received treatment in the form of systemic chemotherapy 122 cases (54.2 %), oral metronomic therapy 19 cases (8.4 %), palliative radiotherapy 44 cases (19.5 %) and palliative surgery 8 cases (3.5 %). The remaining 30 cases (13.3 %) patients were managed with best supportive care because of their poor performance status.
Median TNT-D of patients after recurrence in local, nodal and distant recurrences was 17 months, 18 months and 10 months respectively as shown in Fig. 3.
Fig. 3.
Time to next treatment-death (TNT-D) in local, nodal and distant recurrences.
Overall Survival of patients after primary treatment with local, nodal and distant recurrences was 35 months, 47 months and 50 months respectively as shown in Fig. 4.
Fig. 4.
Total OS.
5. Discussion
Recurrence of cancer is equal distressing for patients, caregivers and physicians. It is reported that five year survival rate ranges from 15 % and 20 % [14]. Although various options are available for primary treatment such as surgery, radiotherapy and chemotherapy, it is well known that as recurrences occur this treatment options decreases.
We observed upto 324 cases (18.8 %) of our patient defaulted. This could be attributed to the lack of knowledge and awareness and logistic reasons, financial problems, long distance of travel to cancer dedicated hospital, advanced stage of disease. Lack of awareness was also found in other studies conducted from India, Pakistan and Zambia where 40–50 % of patients were unaware about the disease and its management [15], [16], [17]. This signifies the importance of proper counselling and knowledge distribution to patients and their relatives.
In was seen 288 patients (20.6 %) died without any known factor. This could be associated with natural cause, old age and other comorbidities or to the pandemic of COVID 19, which may also be responsible for mortality especially with low immunity in cancer patients.
According to Benedet JL et al., the recurrence rate of cervical cancer is between 10 % and 20 % for International Federation of Gynaecology and Obstetrics (FIGO) stages IB to IIA and 50 % to 70 % in locally advanced disease (stages IIB-IVA) [14]. Although in our study recurrence rate is 16.1 % but this excludes practical confounders in the loss to follow up group (33.5 %) and unknown cause of death (20.6 %) patients. This highlights the importance of enquiring proper follow up of cancer patients.
Local or pelvis recurrences are most common site of involvement in cervical cancer. Next most frequent sites of involvement by recurrent cervical carcinoma are solid organ of abdomen [3], [9], [10]. Similar findings were also seen in our study with local recurrence (46.6 %), nodal recurrence (21 %), liver (21 %) and bone (15 %) of cases.
In contrast the patients who had recurrences in the central pelvis showed significantly longer survival than those with pelvic sidewall or multiple recurrences. This is because the disease in more than half of the patients who had recurrence in the central pelvis was salvageable by hysterectomy [18]. According to study conducted by seizi et al., patients who had lymph node recurrence showed significantly longer survival than those with pelvic sidewall or multiple recurrences, which may have been because it was salvageable by radiotherapy [18].
Pelvic exenteration usually represents the only therapeutic approach with curative intent for patients with central pelvic failure who have previously received irradiation [19].
In our study patients were deferred for exenteration surgery in 37 cases (80.1 %) of cases. The main reasons were social stigma, low socioeconomic status, lower education status, lack of motivation and stigma associated with diversion procedures like colostomy.
We found that lymph node metastasis are less common as compared to pelvic or local recurrences, however they had more overall survival after recurrences as compared to local and distant recurrences.
According to Cibula et-al, the prognosis of patients with recurrent disease was very poor, with an estimated overall survival of 13–17 months [20]. Similar results were observed in our study. We observed atypical recurrences occurred late which may be due to the good response of local disease to treatment but in distant recurrence survival was poor due to the involvement of multiple organs. Palliative chemotherapy is the only option for atypical cervical recurrences. Cisplatin is the most widely used drug with a response rate of 17–38 % and a median overall survival of 6.1–7.1 months [21], [22], [23], [24], [25]. Overall survival was highest for atypical recurrences due to the late presentation of atypical recurrence when compared to local and nodal recurrence.
6. Conclusion
Local pelvic recurrence was the commonest site of recurrence. Nodal recurrence have better prognosis than atypical recurrences.
CRediT authorship contribution statement
Jyotiman Nath: Supervision, Methodology, Formal analysis, Conceptualization. Duncan Khanikar: Supervision, Methodology, Formal analysis, Conceptualization. Karthik Chandra Bassetty: Writing – review & editing, Supervision, Methodology, Conceptualization. Mahendra Kumar: Writing – review & editing, Writing – original draft, Supervision, Software, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Upasana Baruah: Writing – review & editing, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Dimpy Begum: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization. Debabrata Barmon: Writing – review & editing, Supervision, Methodology, Formal analysis, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
KC helped in finding out various publication platforms and finalized to publish in this journal.
Contributor Information
Mahendra Kumar, Email: mahendrakirankargmc85@gmail.com.
Upasana Baruah, Email: drupasanabaruah@gmail.com.
Dimpy Begum, Email: drdimpyb@gmail.com.
Debabrata Barmon, Email: drdebabratabarmon@gmail.com.
Jyotiman Nath, Email: jyotimannath@gmail.com.
Duncan Khanikar, Email: duncan.gmc@gmail.com.
Karthik Chandra Bassetty, Email: kcbassetty@gmail.com.
References
- 1.Peters III WA, Liu P.Y., Barrett R.J., Stock R.J., Monk B.J., Berek J.S., Souhami L., Grigsby P., Gordon W., Jr, Alberts D.S. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Obstet Gynecol Surv. 2000;55(8):491–492. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 2.Keys H.M., Bundy B.N., Stehman F.B., Muderspach L.I., Chafe W.E., Suggs C.L., Walker J.L., Gersell D. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340(15):1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 3.Rose P.G., Bundy B.N., Watkins E.B., Thigpen J.T., Deppe G., Maiman M.A., Clarke-Pearson D.L., Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 4.Scott A.A., Weersink M., Liu Z.A., Milosevic M., Croke J., Fyles A., Lukovic J., Rink A., Beiki-Ardakani A., Borg J., Xie J. Comparing dosimetry of locally advanced cervical cancer patients treated with 3 versus 4 fractions of MRI-guided brachytherapy. Brachytherapy. 2023;22(2):146–156. doi: 10.1016/j.brachy.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Scott A.A., Weersink M., Liu Z.A., Milosevic M., Croke J., Fyles A., Lukovic J., Rink A., Beiki-Ardakani A., Borg J., Xie J. Comparing dosimetry of locally advanced cervical cancer patients treated with 3 versus 4 fractions of MRI-guided brachytherapy. Brachytherapy. 2023;22(2):146–156. doi: 10.1016/j.brachy.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Quinn M.A., Benedet J.L., Odicino F., Maisonneuve P., Beller U., Creasman W.T., Heintz A.P., Ngan H.Y., Pecorelli S. Carcinoma of the cervix uteri. Int J Gynecol Obstet. 2006;95:S43–S103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 7.Chuang L.T., Temin S., Camacho R., Dueñas-Gonzalez A., Feldman S., Gultekin M., Gupta V., Horton S., Jacob G., Kidd E.A., Lishimpi K. Management and care of women with invasive cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline. J Glob Oncol. 2016;2(5):311–340. doi: 10.1200/JGO.2016.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sankaranarayanan R., Swaminathan R., Brenner H., Chen K., Chia K.S., Chen J.G., Law S.C., Ahn Y.O., Xiang Y.B., Yeole B.B., Shin H.R. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11(2):165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 9.Lima D.P., Teixeira C.D., Abath M.D., Raposo F.A., Fontan S.B. Percutaneous nephrostomy in cervical cancer patients: a retrospective analysis. Braz J Oncol. 2023;19 1-0. [Google Scholar]
- 10.Sankaranarayanan R., Swaminathan R., Brenner H., Chen K., Chia K.S., Chen J.G., Law S.C., Ahn Y.O., Xiang Y.B., Yeole B.B., Shin H.R. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11(2):165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 11.Fulcher A.S., O'Sullivan S.G., Segreti E.M., Kavanagh B.D. Recurrent cervical carcinoma: typical and atypical manifestations. Radiographics. 1999;19(suppl_1) doi: 10.1148/radiographics.19.suppl_1.g99oc19s103. S103-16. [DOI] [PubMed] [Google Scholar]
- 12.Petignat P., Roy M. Diagnosis and management of cervical cancer. BMJ. 2007;335(7623):765–768. doi: 10.1136/bmj.39337.615197.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monk B.J., Sill M.W., Burger R.A., Gray H.J., Buekers T.E., Roman L.D. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 2009;27(7):1069. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedet J.L., Odicino F., Maisonneuve P., Beller U., Creasman W.T., Heintz A.P., Ngan H.Y., Pecorelli S. Carcinoma of the cervix uteri. Int J Gynecol Obstet. 2003;83:41–78. doi: 10.1016/s0020-7292(03)90115-9. [DOI] [PubMed] [Google Scholar]
- 15.Kadian L., Gulshan G., Sharma S., Kumari I., Yadav C., Nanda S., Yadav R. A study on knowledge and awareness of cervical cancer among females of rural and urban areas of Haryana, North India. J Cancer Educ. 2021;36(4):844–849. doi: 10.1007/s13187-020-01712-6. [DOI] [PubMed] [Google Scholar]
- 16.Riaz L., Manazir S., Jawed F., Ali S.A., Riaz R. Knowledge, perception, and prevention practices related to human papillomavirus-based cervical cancer and its socioeconomic correlates among women in Karachi, Pakistan. Cureus. 2020;12(3) doi: 10.7759/cureus.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam N.E., Islam M.S., Rayyan F., Ifa H.N., Khabir M.I., Chowdhury K., Mohiuddin A.K. Lack of knowledge is the leading key for the growing cervical cancer incidents in Bangladesh: a population based, cross-sectional study. PLOS Glob Public Health. 2022;2(1) doi: 10.1371/journal.pgph.0000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mabuchi S., Isohashi F., Yoshioka Y., Temma K., Takeda T., Yamamoto T., Enomoto T., Morishige K., Inoue T., Kimura T. Prognostic factors for survival in patients with recurrent cervical cancer previously treated with radiotherapy. Int J Gynecol Cancer. 2010;20(5):834–840. doi: 10.1111/IGC.0b013e3181dcadd1. [DOI] [PubMed] [Google Scholar]
- 19.Gadducci A., Tana R., Cosio S., Cionini L. Treatment options in recurrent cervical cancer. Oncol Lett. 2010;1(1):3–11. doi: 10.3892/ol_00000001. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cibula D., Pötter R., Planchamp F., Avall-Lundqvist E., Fischerova D., Haie-Meder C., Köhler C., Landoni F., Lax S., Lindegaard J.C., Mahantshetty U. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Virchows Arch. 2018;472:919–936. doi: 10.1007/s00428-018-2362-9. [DOI] [PubMed] [Google Scholar]
- 21.Bonomi P., Blessing J.A., Stehman F.B., DiSaia P.J., Walton L., Major F.J. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1985;3(8):1079–1085. doi: 10.1200/JCO.1985.3.8.1079. [DOI] [PubMed] [Google Scholar]
- 22.Thigpen J.T., Blessing J.A., Fowler Jr W.C., Hatch K. Phase II trials of cisplatin and piperazinedione as single agents in the treatment of advanced or recurrent non-squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. Cancer Treat Rep. 1986;70(9):1097–1100. [PubMed] [Google Scholar]
- 23.Thigpen J.T., Blessing J.A., DiSaia P.J., Fowler W.C., Jr, Hatch K.D. A randomized comparison of a rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1989;32(2):198–202. doi: 10.1016/s0090-8258(89)80033-2. [DOI] [PubMed] [Google Scholar]
- 24.Mallick S.K., Deb A.R., Nahid G.K. Treatment outcomes of concurrent weekly cisplatin with intracavitary brachytherapy in patients with cervical carcinoma, pre-treated with concurrent chemo-radiotherapy. MGM J Med Sci. 2022;9(2):141–148. [Google Scholar]
- 25.Potter M.E., Hatch K.D., Potter M.Y., Shingleton H.M., Baker V.V. Factors affecting the response of recurrent squamous cell carcinoma of the cervix to cisplatin. Cancer. 1989;63(7):1283–1286. doi: 10.1002/1097-0142(19890401)63:7<1283::aid-cncr2820630709>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]