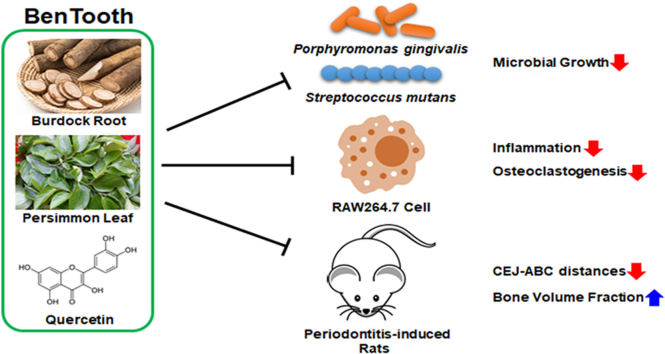
Abstract
Periodontal disease represents a condition that exhibits substantial global morbidity, and is characterized by the infection and inflammation of the periodontal tissue effectuated by bacterial pathogens. The present study aimed at evaluating the therapeutic efficacy of BenTooth, an edible natural product mixture comprising burdock root extract, persimmon leaf extract and quercetin, against periodontitis both in vitro and in vivo. BenTooth was examined for antimicrobial properties and its impact on cellular responses related to inflammation and bone resorption. Its effects were also assessed in a rat model of ligature-induced periodontitis. BenTooth demonstrated potent antimicrobial activity against P. gingivalis and S. mutans. In RAW264.7 cells, it notably diminished the expression of inducible nitric oxide synthase and cyclooxygenase-2, as well as reduced interleukin-6 and tumor necrosis factor-α levels triggered by P. gingivalis-derived lipopolysaccharide. Furthermore, BenTooth inhibited osteoclastogenesis mediated by the receptor activator of nuclear factor κB ligand. In the rat model, BenTooth consumption mitigated the ligature-induced expansion in distance between the cementoenamel junction and the alveolar bone crest and bolstered the bone volume fraction. These results present BenTooth as a potential therapeutic candidate for the prevention and remediation of periodontal diseases.
Keywords: BenTooth, Periodontal disease, Antimicrobial, Anti-inflammatory, Alveolar bone loss
Graphical abstract
1. Introduction
Periodontal disease is a globally widespread dental condition with a high prevalence in adults and has been reported to critically affect the quality of life of patients [1]. According to the Health Insurance Review and Assessment Service in Korea (https://opendata.hira.or.kr/op/opc/olapHthInsRvStatInfoTab14.do?docNo=03-021), in 2020, over 17 million Koreans were afflicted with from gingivitis and periodontal disease. Periodontitis is a multifactorial, chronic inflammatory disease affecting the periodontal tissue around the teeth. It is caused by microbial dysbiosis due to various stimuli, leading to the destruction of the supporting structures surrounding the teeth [2]. Periodontal disease is one of the primary causes of tooth loss in adults, and several lines of evidence have reported it as a significant risk factor for diseases such as cardiovascular disease and diabetes [3,4].
Although various bacteria namely Prevotella intermedia and Aggregatibacter actinomycetemcomitans serve as etiological agents of periodontitis [5], a significant body of evidence suggests that Porphyromonas gingivalis (P. gingivalis), which colonizes the oral cavity, is a predominant pathogen responsible for the onset of periodontitis [6]. P. gingivalis damages the epithelium of the gingival mucosa and endothelial cells by producing various virulence factors, impairing tissue integrity, and enhancing bacterial proliferation [7,8]. Toxic substances such as lipopolysaccharides (LPS) secreted by P. gingivalis disrupt the host immune system and increase the expression of pro-inflammatory cytokines and osteoclast differentiation factors [9,10]. Through this response, immune cells like T cells, B cells, and macrophages accumulate at the infection site, and concurrently, the secretion of inflammatory mediators also increases [11]. Another bacterium, Streptococcus mutans (S. mutans), induces dental caries by forming an oral biofilm, indirectly promoting the progression of periodontitis [12]. The formation of periodontal pockets by S. mutans provides ample nutrients for bacterial multiplication and allows for the survival of more bacteria [13]. Therefore, inhibiting the growth of these bacteria and reducing inflammation and osteoclastogenesis may help suppress the onset of periodontitis.
Although host-modulating compounds like tetracycline antibiotics and nonsteroidal anti-inflammatory drugs have been developed to treat periodontal disease, the search for safer treatments continues due to side effects such as bacterial resistance, gut dysbiosis, and hepatic or renal impairment [14,15]. Hence, we explored improving periodontal disease using safe food materials devoid of side effects. BenTooth is a blend of quercetin and extracts from the roots of Arctium lappa (burdock) and the leaves of Diospyros kaki (persimmon). Burdock roots, traditionally used as food, are known for various biological effects, including anti-inflammatory, anticancer, and antidiabetic properties [16]. These roots are rich in lignans, flavonoids, and phenolic acids [17], which exhibit anti-inflammatory, antimicrobial, and anticancer effects [18]. Persimmon leaves, utilized in herbal medicines, treat conditions such as ischemic stroke, hypertension, atherosclerosis, and infectious diseases [19]. Their extract is rich in flavonoids, tannins, and ascorbic acid, recognized for their anti-inflammatory, antioxidant, and antimicrobial properties [20]. Furthermore, prior research has provided evidence to suggest that polysaccharides isolated from persimmon leaves inhibit osteoclastogenesis [21]. Quercetin, a flavonoid, is a potent antioxidant and anti-inflammatory agent, with its derivatives exhibiting stronger antioxidant effects than vitamins C and E [22]. Additionally, quercetin has demonstrated potential for improving periodontal disease through its efficacy and antimicrobial effects [23]. Among components of BenTooth, two extracts, excluding quercetin, have not been documented to have protective effects against periodontal disease. However, their combined anti-inflammatory and antimicrobial actions are anticipated to synergize with quercetin, enhancing its efficacy against the disease. We hypothesized that BenTooth, a concoction of plant extracts and plant-derived compounds, could offer protection against periodontal disease, and we assessed its efficacy both in vitro and in animal models.
2. Materials and methods
2.1. Preparation of BenTooth
Burdock root and persimmon leaf extracts were sourced from an open market, while quercetin was acquired from Sigma-Aldrich (St. Louis, MO, USA). BenTooth was formulated by combining burdock root extract, persimmon leaf extract, and quercetin in a 3:1:1 (w/w/w) ratio.
2.2. High-Performance Liquid Chromatography (HPLC)
The chlorogenic acid, tannic acid, and quercetin in BenTooth were quantified using an 1260 Infinity II Prime LC System (Agilent 1260 series; Agilent Technologies, Santa Clara, CA, USA) and a Phenomenex Gemini C18 reverse-phase column (250 × 4.6 mm, 2.5 μm).
All components were analyzed isocratically. The mobile phase for chlorogenic acid comprised acetonitrile, acetic acid, methanol, and water in a ratio of 113:5:20:862 (v/v/v/v) at a flow rate of 1 mL/min. For tannic acid, the mobile phase was composed of 0.1 % formic acid in water (eluent A) and acetonitrile (eluent B), with elution performed at a 95:5 (v/v) ratio and a flow rate of 1 mL/min. For quercetin, the mobile phase consisted of 0.1 % phosphoric acid in water (eluent A) and 0.1 % phosphoric acid in acetonitrile (eluent B), with elution carried out at a 60:40 (v/v) ratio and a flow rate of 1 mL/min. The detection wavelengths were established at 280, 270, and 370 nm for chlorogenic acid, tannic acid, and quercetin, respectively. The components were quantified using calibration curves derived from external HPLC-grade chemical standards, and all samples were analyzed in triplicate.
2.3. Bacterial culture and antimicrobial activity
P. gingivalis (KCTC 5352) and S. mutans (KCTC 25175) were sourced from the Korean Collection for Type Cultures (Jeongeup, Korea). These strains were used to ascertain the growth inhibitory effect of BenTooth on periodontitis-inducing bacteria. P. gingivalis was cultured in tryptic soy broth (TSB) supplemented with yeast extract (5 g/L), l-cysteine (0.5 g/L), hemin (5 mg/L), and vitamin K (0.5 mg/L) and incubated for 48 h. Conversely, S. mutans was cultured in brain-heart infusion (BHI) broth for 24 h.
To assess the antimicrobial prowess of BenTooth against P. gingivalis and S. mutans, BenTooth was aliquoted in various concentrations (25, 50, and 100 μg/mL) and implemented into TSB or BHI broth. Subsequently, P. gingivalis was inoculated to achieve an initial optical density (OD) of 0.1, while S. mutans was inoculated aiming for an OD of 0.002. The OD of each bacterial sample was gauged at 600 nm: measurements for P. gingivalis were recorded every 6 h over 24 h, and for S. mutans, every 3 h over a 12 h period, using a spectrophotometer (Jenway, London, UK). The antimicrobial activity of BenTooth against each bacterial species was deduced and charted contingent upon the readings documented at 18 h and 9 h, respectively. Doxycycline served as the positive control in evaluating the antimicrobial activity.
2.4. Cell culture and cell viability
RAW264.7 cells employed in this study were procured from the Korean Cell Line Bank (40071, Seoul, Korea). The cells were cultured in Dulbecco's Modified Eagle Medium (DMEM, Sigma-Aldrich, St. Louis, MO, USA) enriched with 10 % fetal bovine serum (Capricorn Scientific, Ebsdorfergrund, Germany) and 100 units/mL of penicillin-streptomycin (Gibco, Carlsbad, CA, USA). They were maintained in an incubator (Sanyo, Tokyo, Japan) at 37 °C with 5 % CO₂.
To assess the cytotoxicity of BenTooth, RAW264.7 cells were seeded at a density of 2 × 10⁵ cells/well in a 24-well plate and incubated for 24 h. Subsequently, the cells were treated with BenTooth at concentrations of 1.25, 2.5, 5, 10, and 20 μg/mL for 30 min and incubated for an additional 24 h, either with or without 1 μg/mL of P. gingivalis-derived LPS (Pg-LPS). Following incubation, cell culture supernatants were harvested to determine nitric oxide (NO) concentration and inflammatory cytokine production. The cells were exposed to the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent for a 4-h duration. The resultant formazan crystals in the cells were solubilized in dimethyl sulfoxide, and the absorbance was gauged at 540 nm using a microplate reader (BioTek, Winooski, VT, USA).
2.5. Measurement of NO production
The cell culture supernatants were mixed with an equal volume of Griess reagent (Sigma-Aldrich, St. Louis, MO, USA) and incubated for 10 min at room temperature to quantify their NO concentration. Absorbance was measured at 540 nm using a microplate reader (BioTek, Winooski, VT, USA), and a standard curve was established using sodium nitrite.
2.6. Measurement of IL-6, TNF-α levels, and COX-2 activity
The concentrations of cytokines in cell culture supernatants were determined using the mouse IL-6 and mouse TNF-α ELISA kit (both from Thermo Fisher Scientific, Cleveland, OH, USA) as per the manufacturers' guidelines. COX-2 activity was ascertained using the COX colorimetric inhibitor screening assay kit (Cayman Chemical, Ann Arbor, MI, USA) following the manufacturer's instructions. The levels of IL-6 and TNF-α were gauged at 450 nm, while COX-2 activity was determined at 590 nm using a microplate reader (BioTek, Winooski, VT, USA).
2.7. Western blot analysis
Proteins were extracted from the RAW264.7 cells using a lysis buffer (Thermo Fisher Scientific, Cleveland, OH, USA) enriched with a protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO, USA). The total protein concentration was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). The proteins were separated using 10 % SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were then blocked for 1 h using 10 % skimmed milk in phosphate-buffered saline with Tween-20 (PBST). Subsequently, they were incubated overnight with COX-2, iNOS, or β-actin primary antibodies at 4 °C. Secondary antibodies, either goat anti-mouse IgG-HRP or goat anti-rabbit IgG-HRP, were added and incubated for 1 h. Chemiluminescence was detected using the D-Plus™ ECL Femto System (Dongin LS; Seoul, Republic of Korea) and quantified with the Image J program (National Institutes of Health).
2.8. Tartrate-Resistant acid Phosphatase (TRAP)-Positive cell staining and activity
To determine the number of TRAP-positive multinucleated cells, RAW264.7 cells were seeded on a 48-well plate at a density of 2 × 103 cells/well and incubated for 24 h. Afterward, the medium was replaced with α-Minimum Essential Medium (HyClone Laboratories, Logan, UT, USA), and the cells were exposed to varying concentrations of BenTooth along with 50 ng/ml of receptor activator of nuclear factor κB ligand (RANKL) for 5 days. On the third day, the culture medium was refreshed with new medium containing RANKL, and the samples underwent an identical treatment.
Following differentiation, osteoclasts were rinsed with PBS and fixed using a blend of citrate buffer (pH 5.4), acetone, and ethanol. TRAP staining was conducted using a TRACP and ALP double-stain kit (Takara, Tokyo, Japan) per the manufacturer's directives. Multinucleated cells, each having three or more nuclei, were enumerated by observing the stained cells under a microscope (Nikon, Tokyo, Japan).
To assess TRAP activity, RAW 264.7 cells underwent osteoclast differentiation as previously detailed. The mature osteoclasts were rinsed with PBS and fixed in 4 % paraformaldehyde. Thereafter, the fixed cells were incubated at 37 °C for 1 h with a TRAP assay buffer (10 mM tartrate in 50 mM citrate buffer) containing 5 mM 4-nitrophenyl phosphate disodium salt. The reaction was halted with 0.1 N NaOH, and the absorbance was gauged at 405 nm via a microplate reader, allowing the activity to be presented as a percentage.
2.9. Animals
Six-week-old male Sprague-Dawley rats (n = 48) were procured from Orient Bio (Seongnam, South Korea). The rats were maintained under standard conditions with a 12/12-h light/dark cycle and had access to food ad libitum. The study received approval from the Institutional Animal Care and Use Committee of DT & CRO Co. Ltd. (approval number: 210274). The animals were randomized into six groups (n = 8 per group): normal control (NC), periodontitis-induced (periodontitis), periodontitis treated with 50 mg/kg of BenTooth (BenTooth-50), periodontitis treated with 100 mg/kg of BenTooth (BenTooth-100), periodontitis treated with 200 mg/kg of BenTooth (BenTooth-200), and periodontitis treated with 20 mg/kg of doxicycline (Dox).
To induce periodontitis, the animals were anesthetized using an anesthetic solution (ketamine: Rumpun = 4:1) and a 4-0 silk ligature was tied around the cervical region of the maxillary second molar. This ligation was monitored and maintained daily throughout the experiment. Post-ligation, treatments commenced with the oral administration of either BenTooth or doxycycline for a span of 3 weeks. The NC and periodontitis groups received an orally proportional volume of vehicle for an equivalent duration. During the treatment phase, general health symptoms were monitored daily, and body weight was recorded on a weekly basis. After the 3-week treatment period, the animals were deeply anesthetized using isoflurane, euthanized by exsanguination, and their maxillae were extracted.
2.10. Alveolar bone distance measurement
The dissected maxillae were stained with 1 % methylene blue and then photographed under a microscope (Olympus, Tokyo, Japan). The distance between the cemento-enamel junction (CEJ) and the alveolar bone crest (ABC) was quantified using ImageJ 1.53n (National Institutes of Health). The CEJ-ABC distance was assessed at three sites on the 2 nd M, two sites on the 1st molar, and one site on the 3rd molar; these measurements were then summed to determine the total value.
2.11. Micro-computed tomography (CT) imaging analysis
The ABL measurement was followed by imaging of the maxillary tissue using micro-computed tomography (CT). Micro-CT images were obtained using Quantum FX (PerkinElmer, Shelton, CT, USA) at the Osong Medical Innovation Foundation (KBIO, Osong, Korea) in accordance with the manufacturer's instructions. Bone volume (BV), tissue volume (TV), and bone volume fraction (BV/TV) were evaluated using the CTAn Micro-CT Software (Bruker micro-CT, Kontich, Belgium).
2.12. Statistical analysis
Data were presented as means ± standard error of the mean (in vivo) or standard deviation (in vitro). One-way analysis of variance, followed by Tukey's post hoc test, was employed to discern the statistical significance of differences among multiple groups. A value of p < 0.05 was deemed statistically significant. All statistical analyses were conducted using the GraphPad Prism software (San Diego, CA, USA).
3. Results
3.1. HPLC analysis of marker compounds
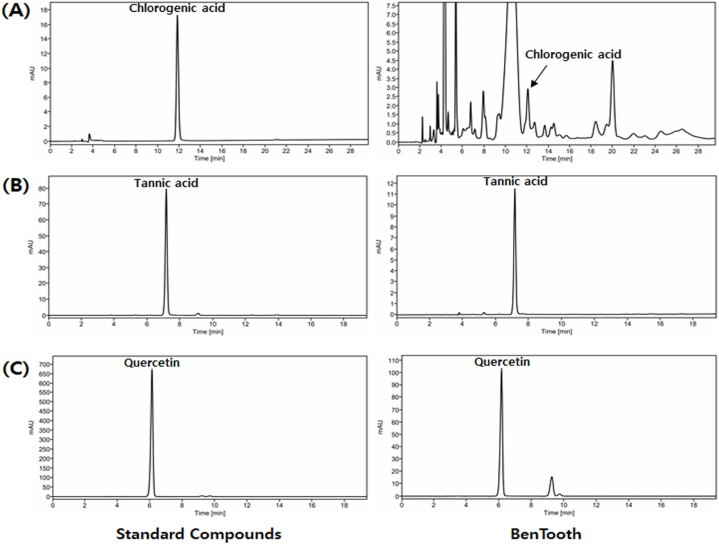
Fig. 1 and Table 1 present the results of the HPLC analysis determining the content of chlorogenic acid (Fig. 1A), tannic acid (Fig. 1B), and quercetin (Fig. 1C) in BenTooth. All three compounds were detected in BenTooth, with contents of 0.37 ± 0.03 mg/g for chlorogenic acid, 16.83 ± 0.02 mg/g for tannic acid, and 557.15 ± 0.75 mg/g for quercetin, respectively.
Fig. 1.
HPLC chromatograms of standard compounds and BenTooth. (A) Chlorogenic acid. (B) Tannin. (C) Quercetin.
Table 1.
Quantification of major components in BenTooth.
| Compound | Regression Equation | R2 | Contents (mg/g) |
|---|---|---|---|
| Chlorogenic acid | y = 30.749x - 37.649 | 0.9999 | 0.37 ± 0.03 |
| Tannin | y = 3.898x + 0.042 | 0.9999 | 16.83 ± 0.02 |
| Quercetin | y = 9.403x + 0.289 | 0.9999 | 557.15 ± 0.75 |
3.2. Antimicrobial activity of BenTooth
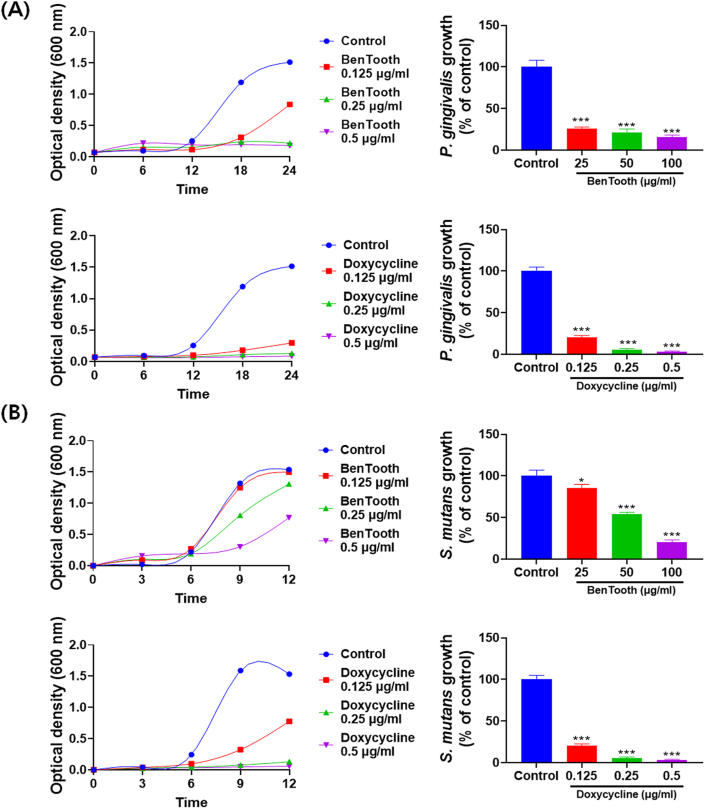
The antimicrobial activity of BenTooth against P. gingivalis and S. mutans is depicted in Fig. 2. In the control group, P. gingivalis exhibited rapid growth commencing from the 6th hour of incubation and plateaued after 18 h (Fig. 2A). BenTooth treatment markedly curtailed the microbial growth even at low concentrations, with an inhibition rate surpassing 73 % at a concentration of 25 μg/ml.
Fig. 2.
Antimicrobial activity of BenTooth. (A) Growth curve of Porphyromonas gingivalis with varying concentrations of BenTooth and Doxycycline, along with percentage growth inhibition at 18 h. (B) Growth curve of Streptococcus mutans with varying concentrations of BenTooth and Doxycycline, along with percentage growth inhibition at 9 h. Data are represented as means ± SD from three independent experiments.
Similarly, S. mutans demonstrated swift growth from the 6th hour of culture, entering a stationary phase post 9 h (Fig. 2B). Administration of BenTooth intensified the growth inhibitory activity in a dose-responsive manner, resulting in approximately 80 % growth suppression at 100 μg/ml.
3.3. Effects of BenTooth on nitric oxide and inflammatory cytokines production in RAW264.7 cells
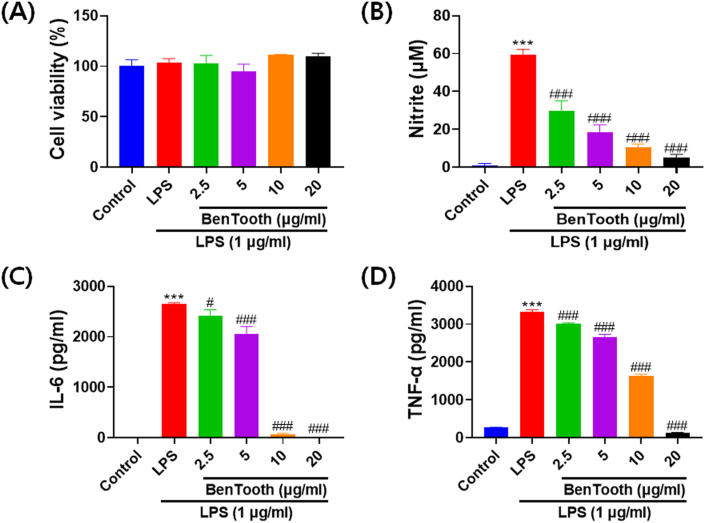
The anti-inflammatory efficacy of BenTooth was assessed by observing the production of NO and the expression of TNF-α and IL-6 (Fig. 3). Before the experiment, cell viability was ascertained using the MTT assay. Neither BenTooth nor Pg-LPS treatments exhibited toxicity at any of the tested concentrations (Fig. 3A). While Pg-LPS treatment was non-cytotoxic, it notably increased NO production. This increase was attenuated in a dose-dependent manner (Fig. 3B). Furthermore, stimulation with Pg-LPS significantly amplified the production of proinflammatory cytokines IL-6 and TNF-α (Fig. 3C and D). Treatment with BenTooth reduced the secretion of IL-6 and TNF-α dose-dependently, and at 20 μg/mL, BenTooth brought the levels akin to the control.
Fig. 3.
Effects of BenTooth on Pg-LPS-induced NO, TNF-α, and IL-6 production in RAW 264.7 macrophages. (A) Cell viability; (B) NO production; (C) TNF-α production, and (D) IL-6 production. Data are represented as means ± SD from three independent experiments.
3.4. Effects of BenTooth on COX-2 activity and expression of inflammatory mediators in Pg-LPS-induced RAW264.7 cells
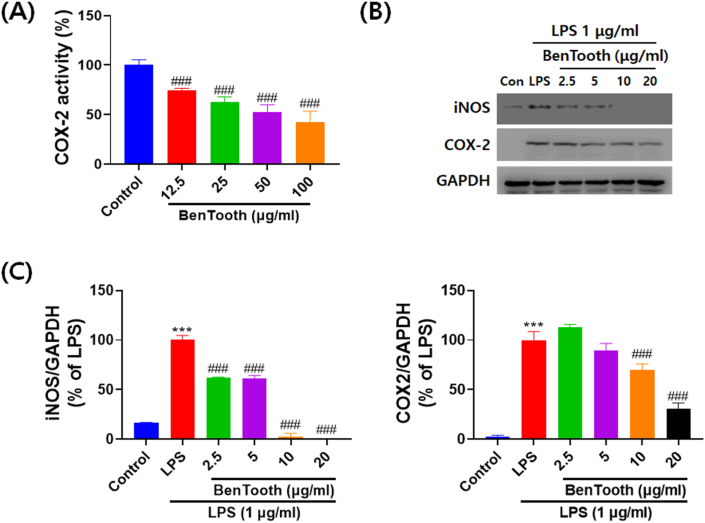
Western blotting and ELISA were utilized to determine if the inhibitory effect of BenTooth on NO production was associated with the activity and expression of iNOS and COX-2. As depicted in Fig. 4A, BenTooth reduced COX-2 activity in a dose-dependent manner, achieving more than 50 % inhibition at a concentration of 100 μg/mL. Similarly, BenTooth curtailed the Pg-LPS-induced elevation of iNOS and COX-2 protein levels in a dose-dependent fashion (Fig. 4B and C). Notably, a concentration of 20 μg/mL of BenTooth potently suppressed iNOS and COX-2 protein levels, and these findings were in line with the inhibitory effect of BenTooth on NO production.
Fig. 4.
Effects of BenTooth on COX-2 activity, as well as COX-2 and iNOS expressions. (A) COX-2 activity. (B) Representative western blots of COX-2 and iNOS. (C) Relative protein expression levels of COX-2 and iNOS. GAPDH was used for normalization, and values are expressed as a percentage of LPS ± SD from three separate experiments.
3.5. Effects of BenTooth on the formation of TRAP-positive cells and TRAP activity in RANKL-induced osteoclast differentiation
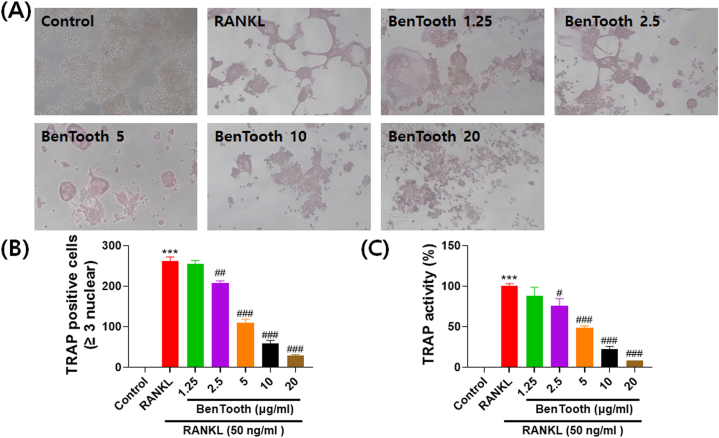
To evaluate the impact of BenTooth on osteoclastogenesis, TRAP staining was conducted, and TRAP activity was assessed. As depicted in Fig. 5A, RANKL treatment notably augmented the purplish-red area and the count of multinucleated osteoclast-like cells. Conversely, treatment with BenTooth significantly diminished both the staining area and the number of TRAP-positive multinucleated cells (Fig. 5A and B). Following RANKL treatment, there was a marked rise in TRAP activity (Fig. 5C). However, BenTooth efficaciously curtailed this activity, exhibiting this inhibitory effect in a dose-dependent manner.
Fig. 5.
Effects of BenTooth on RANKL-induced differentiation of RAW264.7 cells into osteoclasts. (A) Osteoclast morphology post-incubation with RANKL and different concentrations of BenTooth. (B) Quantitative assessment of TRAP-positive multinucleated cells (nuclei ≥3). (C) TRAP activity assay. Data are represented as percentage ± SD from three independent experiments.
3.6. Effects of BenTooth on alveolar bone loss in rats with periodontitis
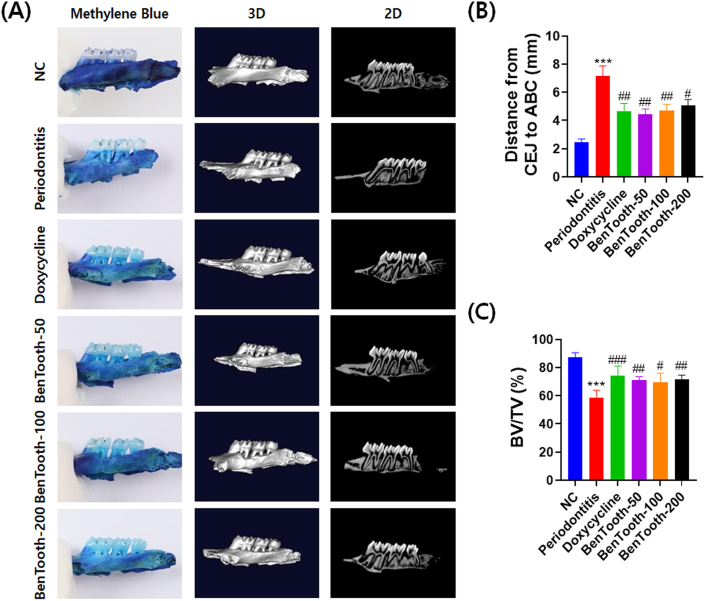
Methylene blue staining and micro-CT scanning were employed to ascertain the effects of BenTooth on periodontal tissue damage and alveolar bone loss. As depicted in Fig. 6A, the NC group exhibited intact periodontal tissue and normal alveolar bone. Contrarily, rats induced with periodontitis displayed a notable reduction in periodontal tissue and alveolar bone when compared to the NC group. Additionally, the CEJ-ABC distance in periodontitis-afflicted rats measured 7.2 mm, which is 2.9 times greater than that in NC rats (2.5 mm) (Fig. 6B). All concentrations of BenTooth markedly alleviated ligation-induced periodontal tissue damage and alveolar bone loss; the CEJ-ABC distances for the BenTooth-treated groups at 50, 100, and 200 mg/kg were 4.5, 4.7, and 5.0 mm, respectively. These values were notably lower than those of the periodontitis-induced group.
Fig. 6.
Effects of BenTooth on alveolar bone loss in periodontitis-induced rats. (A) Representative images of methylene blue staining and Micro-CT scans of maxillae. (B) CEJ-ABC distance. (C) BV/TV ratio. Data are presented as mean ± SEM for 8 rats (for CEJ-ABC distance) and 5 rats (for BV/TV ratio) per group.
Concurring with the CT images, the bone volume fraction (BV/TV) for the periodontitis-induced rats stood at 58.5 %, a value significantly reduced when juxtaposed with the NC rats' 87.3 % (Fig. 6C). The BV/TV percentages for the BenTooth-treated groups registered at 69.3 %, 71.6 %, and 74.1 %, respectively. These values underscore the effective inhibition of ligature-induced alveolar bone resorption.
4. Discussion
Periodontitis refers to a prevalent oral disease and predominant cause responsible for tooth loss in adults that affects numerous individuals globally. To ameliorate periodontitis, we formulated BenTooth by combining burdock root, persimmon leaf, and quercetin. These ingredients are recognized for their outstanding antioxidant, anti-inflammatory, and antimicrobial properties [18,20,22,23]. Initially, the antioxidant activities of BenTooth and its constituents were evaluated using DPPH and ABTS assays (Supplementary Table 1). Free radicals can impair periodontal tissue, instigate periodontal disease, or exacerbate existing periodontal conditions [24]. BenTooth markedly inhibits oxidative stress, which is believed to aid in mitigating periodontitis.
It is imperative to curtail the proliferation of detrimental bacteria to prevent or restrain the advancement of periodontal diseases. Among these bacteria, P. gingivalis and S. mutans have been identified as the etiological agents of periodontitis and periodontal caries [25]. Dental caries and periodontitis are understood to be either directly or indirectly interconnected, mainly because they share numerous contributing factors. Specifically, periodontal pockets created by caries have been reported to hasten the progression of periodontitis [26]. Hence, regulating the growth of P. gingivalis and S. mutans is vital to impede the onset of periodontitis. In our research, BenTooth demonstrated a pronounced effect in substantially inhibiting the growth of both bacteria, indicating its potential in preventing periodontitis through its antimicrobial action.
LPS in P. gingivalis is a primary pathogenic element that contributes to its virulence in causing periodontitis. It triggers an immune-inflammatory response, amplifying the production of RANKL and osteoclast differentiation [27,28]. Among various cytokines, IL-6 and TNF-α are central to bone metabolism and are known to stimulate osteoclast proliferation through the RANK-RANKL-OPG pathway [29]. Furthermore, TNF-α and IL-6 majorly induce periodontal degradation by upregulating matrix metalloproteinase (MMP) [30,31]. LPS is also known to intensify inflammation by amplifying the expression of iNOS or COX-2. Prior research has evidenced the overexpression of iNOS, an enzyme synthesizing NO, in periodontal disease patients [32]. NO is linked to bone resorption and osteoclast differentiation, while iNOS strongly correlates with alveolar bone loss [33]. COX-2 synthesizes PGE2, which surges in inflamed gingiva, playing a pivotal role in alveolar bone resorption [34]. Therefore, mitigating the inflammatory response triggered by bacterial pathogens is crucial for treating periodontitis. Our findings revealed that BenTooth significantly curtailed LPS-induced NO production without inducing cytotoxicity. These observations prompted us to surmise that BenTooth could effectively suppress inflammation. Notably, when exposed to P. gingivalis-derived LPS, there was a significant surge in IL-6 and TNF-α levels and heightened expression of iNOS and COX-2. However, BenTooth markedly improved the expression of these markers, suggesting its potential to curb periodontitis by restraining inflammatory cytokines and mediators.
Osteoclasts assume a pivotal role in alveolar bone resorption during periodontitis [35]. RANKL, crucial for their differentiation, activity, and longevity, is found in high concentrations in the biological fluids of periodontitis patients [36]. Similar to TNF-α, RANKL binds to RANK, activating osteoclastogenesis-related genes such as nuclear factor kB and mitogen-activated protein kinase [37,38], facilitating osteoclast differentiation and bone resorption. Therefore, thwarting RANKL-induced osteoclast formation is a viable strategy for periodontitis prevention and amelioration. In our experiments, RANKL treatment led to the differentiation of RAW264.7 into osteoclast-like cells, with a significant rise in TRAP activity. However, BenTooth treatment noticeably reduced both TRAP-positive cells and TRAP activity in a dose-dependent fashion, suggesting its potential to inhibit osteoclastogenesis, thereby forestalling periodontitis.
We conducted an efficacy assessment in a mouse model to discern if the in vitro effects of BenTooth also manifest in vivo and to gauge the feasibility of clinical trials for its development as a functional food ingredient. Ligature-induced periodontitis is a widely recognized method for gauging the efficacy of prospective materials for periodontal disease amelioration [39]. The CEJ-ABC distance correlates with periodontitis severity; a greater distance implies more severe periodontal tissue damage and alveolar bone loss [40]. As anticipated, the CEJ-ABC distance was significantly greater in the periodontitis group than in the NC group. Micro-CT results further revealed exacerbated alveolar bone loss due to ligature. However, BenTooth treatment not only reduced the expanded CEJ-ABC distance but also enhanced the BV/TV ratio, positioning BenTooth as a promising agent for periodontitis treatment.
In this study, BenTooth demonstrated potential efficacy in improving periodontal health. In in vivo experiments, it exhibited potent antimicrobial activity against bacterial pathogens, effectively downregulated the expression of inflammatory factors, and inhibited osteoclast formation. Moreover, BenTooth suppressed alveolar bone resorption in ligated rats, which further validated its potential for remediating periodontal disease.
The observed protective effects of BenTooth are attributable to the synergistic interactions of its constituent ingredients. Quercetin and persimmon leaf, which are pivotal components of BenTooth, portrayed remarkable antimicrobial and anti-inflammatory properties. Conversely, burdock root, evidenced a striking ability to inhibit osteoclast differentiation (Supplementary Figs. 1–4).
BenTooth may serve as a potential therapeutic candidate for ameliorating periodontitis and consequently enhancing the quality of life for individuals affected by this prevalent condition by harnessing the combined effects of its components and targeting key pathogenic mechanisms underlying periodontal disease.
5. Conclusion
The present study demonstrated that BenTooth is capable of effectively ameliorating periodontitis which can be attributed to its antimicrobial, anti-inflammatory, and anti-osteoclastic properties. Therefore, the study findings suggest that BenTooth exhibits efficacy in mitigating the development and progression of periodontitis through the combined effects of plant-derived mixtures and may serve as a potential therapeutic candidate for remediating periodontal disease.
Data availability statement
Data will be made available on request.
Ethics declarations
This study was reviewed and approved by the Institutional Animal Care and Use Committee of DT & CRO Co. Ltd. (approval number: 210274).
CRediT authorship contribution statement
Moon Ho Do: Writing – review & editing, Writing – original draft, Methodology. Hua Li: Visualization, Resources, Investigation. Soo Yong Shin: Resources, Investigation, Formal analysis. Su Yeon Cho: Resources, Investigation. Subin Oh: Investigation. Jong-Moon Jeong: Writing – review & editing, Resources, Methodology, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:The authors declare the following competing interests: M.H.D., H.L., S.Y.S., S.Y.C., S.O., and J-M.J. are employees of Ben's Lab Co., Ltd. BenTooth is related to products of Ben's Lab Co., Ltd. that are in development. However, this does not alter our adherence to Heliyon policies on sharing data and materials. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e30835.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Relvas M., López-Jarana P., Monteiro L., Pacheco J.J., Braga A.C., Salazar F. Study of prevalence, severity and risk factors of periodontal disease in a Portuguese population. J. Clin. Med. 2022;11(13):3728. doi: 10.3390/jcm11133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silva L.M., Brenchley L., Moutsopoulos N.M. Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunol. Rev. 2019;287(1):226–235. doi: 10.1111/imr.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravidà A., Qazi M., Troiano G., Saleh M.H., Greenwell H., Kornman K., Wang H.L. Using periodontal staging and grading system as a prognostic factor for future tooth loss: a long‐term retrospective study. J. Periodontol. 2020;91(4):454–461. doi: 10.1002/JPER.19-0390. [DOI] [PubMed] [Google Scholar]
- 4.Liccardo D., Cannavo A., Spagnuolo G., Ferrara N., Cittadini A., Rengo C., Rengo G. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int. J. Mol. Sci. 2019;20(6):1414. doi: 10.3390/ijms20061414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López N.J. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in progressive adult periodontitis. J. Periodontol. 2000;71(6):948–954. doi: 10.1902/jop.2000.71.6.948. [DOI] [PubMed] [Google Scholar]
- 6.Mysak J., Podzimek S., Sommerova P., Lyuya-Mi Y., Bartova J., Janatova T., Prochazkova J., Duskova J. Porphyromonas gingivalis: major periodontopathic pathogen overview. J. Immunol. Res. 2014;2014 doi: 10.1155/2014/476068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorillo L., Cervino G., Laino L., D'Amico C., Mauceri R., Tozum T.F., Gaeta M., Cicciù M. Porphyromonas gingivalis, periodontal and systemic implications: a systematic review. Dent. J. 2019;7(4):114. doi: 10.3390/dj7040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben Lagha A., Andrian E., Grenier D. Resveratrol attenuates the pathogenic and inflammatory properties of Porphyromonas gingivalis. Mol. Oral Microbiol. 2019;34(3):118–130. doi: 10.1111/omi.12260. [DOI] [PubMed] [Google Scholar]
- 9.Ren B., Lu J., Li M., Zou X., Liu Y., Wang C., Wang L. Anti-inflammatory effect of IL-1ra-loaded dextran/PLGA microspheres on Porphyromonas gingivalis lipopolysaccharide-stimulated macrophages in vitro and in vivo in a rat model of periodontitis. Biomed. Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111171. [DOI] [PubMed] [Google Scholar]
- 10.Kanzaki H., Movila A., Kayal R., Napimoga M.H., Egashira K., Dewhirst F., Sasaki H., Howait M., Al-Dharrab A., Mira A. Phosphoglycerol dihydroceramide, a distinctive ceramide produced by Porphyromonas gingivalis, promotes RANKL-induced osteoclastogenesis by acting on non-muscle myosin II-A (Myh9), an osteoclast cell fusion regulatory factor. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 2017;1862(5):452–462. doi: 10.1016/j.bbalip.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong W., Peng Y., Yue E., Huang B., Zhang W., Zhao Z., Jiang J., Wang Q., Zhao H. Gingival crevicular fluid levels of SLIT3 are increased in periodontal disease. Oral Dis. 2020;26(1):182–192. doi: 10.1111/odi.13227. [DOI] [PubMed] [Google Scholar]
- 12.Widyarman A.S., Lay S.H., Wendhita I.P., Tjakra E.E., Murdono F.I., Binartha C.T.O. Indonesian mangosteen fruit (Garcinia mangostana L.) peel extract inhibits Streptococcus mutans and Porphyromonas gingivalis in biofilms in vitro. Contemp. Clin. Dent. 2019;10(1):123. doi: 10.4103/ccd.ccd_758_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dani S., Prabhu A., Chaitra K., Desai N., Patil S.R., Rajeev R. Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: a clinico-microbiological study. Contemp. Clin. Dent. 2016;7(4):529. doi: 10.4103/0976-237X.194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golub L.M., Lee H.M. Periodontal therapeutics: current host‐modulation agents and future directions. Periodontol. 2020;82(1):186–204. doi: 10.1111/prd.12315. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graber E.M. Treating acne with the tetracycline class of antibiotics: a review. Dermatological Reviews. 2021;2(6):321–330. doi: 10.1002/der2.49. [DOI] [Google Scholar]
- 16.Mir S.A., Dar L.A., Ali T., Kareem O., Rashid R., Khan N.A., Chashoo I., Arctium lappa G. Bader. A review on its phytochemistry and pharmacology. Edible Plants in Health and Diseases. 2022:327–348. [Google Scholar]
- 17.Don R.A.S.G., Yap M.K.K. Arctium lappa L. root extract induces cell death via mitochondrial-mediated caspase-dependent apoptosis in Jurkat human leukemic T cells. Biomed. Pharmacother. 2019;110:918–929. doi: 10.1016/j.biopha.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Maheshwari M., Media J., Jia Q., Valeriote F.A. Arctin and arctigenin as a potential treatment for solid tumorsCancer Res. 2019;79(13_Supplement) doi: 10.1158/1538-7445.AM2019-366. 366-366. [DOI] [Google Scholar]
- 19.Kwon J., Park J.-E., Lee J.-S., Lee J.-H., Hwang H., Jung S.-H., Kwon H.-C., Jang D.-S. Chemical constituents of the leaves of Diospyros kaki (persimmon) Plants. 2021;10(10):2032. doi: 10.3390/plants10102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hossain A., Moon H.K., Kim J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018;27(1):177–184. doi: 10.1007/s10068-017-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang Y.-H., Ha H., Kim R., Cho C.-W., Song Y.-R., Hong H.-D., Kim T. Anti-osteoporotic effects of polysaccharides isolated from persimmon leaves via osteoclastogenesis inhibition. Nutrients. 2018;10(7):901. doi: 10.3390/nu10070901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokół-Łętowska A., Oszmiański J., Wojdyło A. Antioxidant activity of the phenolic compounds of hawthorn, pine and skullcap. Food Chem. 2007;103(3):853–859. doi: 10.1016/j.foodchem.2006.09.036. [DOI] [Google Scholar]
- 23.Xiong G., Ji W., Wang F., Zhang F., Xue P., Cheng M., Sun Y., Wang X., Zhang T. Quercetin inhibits inflammatory response induced by LPS from Porphyromonas gingivalis in human gingival fibroblasts via suppressing NF-κB signaling pathway. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/6282635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent R.R., Appukuttan D., Victor D.J., Balasundaram A. Oxidative stress in chronic periodontitis patients with type II diabetes mellitus. Eur. J. Dermatol. 2018;12(2):225–231. doi: 10.4103/ejd.ejd_244_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Y.-J., Wang B.-W., Yang C.-M., Wu C.-Y., Ou-Yang M. Autofluorescence detection method for dental plaque bacteria detection and classification: example of Porphyromonas gingivalis, aggregatibacter actinomycetemcomitans, and Streptococcus mutans. Dent. J. 2021;9(7):74. doi: 10.3390/dj9070074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Passanezi E., Sant'Ana A.C.P. Role of occlusion in periodontal disease. Periodontol. 2019;79(1):129–150. doi: 10.1111/prd.12251. 2000. [DOI] [PubMed] [Google Scholar]
- 27.Hu Y., Li H., Zhang J., Zhang X., Xia X., Qiu C., Liao Y., Chen H., Song Z., Zhou W. Periodontitis induced by P. gingivalis-LPS is associated with neuroinflammation and learning and memory impairment in Sprague-Dawley rats. Front. Neurosci. 2020;14:658. doi: 10.3389/fnins.2020.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akkaoui J., Yamada C., Duarte C., Ho A., Vardar-Sengul S., Kawai T., Movila A. Contribution of Porphyromonas gingivalis lipopolysaccharide to experimental periodontitis in relation to aging. GeroScience. 2021;43(1):367–376. doi: 10.1007/s11357-020-00258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang T., He C. TNF-α and IL-6: the link between immune and bone system. Curr. Drug Targets. 2020;21(3):213–227. doi: 10.2174/1389450120666190821161259. [DOI] [PubMed] [Google Scholar]
- 30.Molayem S., Pontes C.C. The mouth-COVID connection: H-6 levels in periodontal disease—potential role in COVID-19-related respiratory complications. Medicina stomatologică. 2020;57(4):68–80. doi: 10.1080/19424396.2020.12222617. [DOI] [Google Scholar]
- 31.Lee S.A., Park B.-R., Moon S.-M., Shin S.H., Kim J.-S., Kim D.K., Kim C.S. Cynaroside protects human periodontal ligament cells from lipopolysaccharide-induced damage and inflammation through suppression of NF-κB activation. Arch. Oral Biol. 2020;120 doi: 10.1016/j.archoralbio.2020.104944. [DOI] [PubMed] [Google Scholar]
- 32.Fernández-Rojas B., Gutiérrez-Venegas G. Flavonoids exert multiple periodontic benefits including anti-inflammatory, periodontal ligament-supporting, and alveolar bone-preserving effects. Life Sci. 2018;209:435–454. doi: 10.1016/j.lfs.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 33.Shadisvaaran S., Chin K.-Y., Shahida M.-S., Ima-Nirwana S., Leong X.-F. Effect of vitamin E on periodontitis: evidence and proposed mechanisms of action. J. Oral Biosci. 2021;63(2):97–103. doi: 10.1016/j.job.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Freire J.M., Chaves H.V., Teixeira A.H., de Sousa L.H.T., Pinto I.R., Costa J.J.d.N., de Sousa N.A., Pereira K.M.A., Girão V.C., Ferreira V.C. Protective effect of Platymiscium floribundum Vog. in tree extract on periodontitis inflammation in rats. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monasterio G., Budini V., Fernández B., Castillo F., Rojas C., Alvarez C., Cafferata E.A., Vicencio E., Cortés B.I., Cortez C. IL‐22–expressing CD 4+ AhR+ T lymphocytes are associated with RANKL‐mediated alveolar bone resorption during experimental periodontitis. J. Periodontal. Res. 2019;54(5):513–524. doi: 10.1111/jre.12654. [DOI] [PubMed] [Google Scholar]
- 36.Asif S., Ahmad B., Hamza S.A., Taib H., Kassim N.K., Zainuddin S.L.A. Investigation of salivary RANKL and OPG levels in periodontitis patients at hospital universiti sains Malaysia. Eur. J. Dermatol. 2022;16(1):173–178. doi: 10.1055/s-0041-1731930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu L., Lind T., Sundqvist A., Jacobson A., Melhus H. Retinoic acid increases proliferation of human osteoclast progenitors and inhibits RANKL-stimulated osteoclast differentiation by suppressing RANK. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braz-Silva P.H., Bergamini M.L., Mardegan A.P., De Rosa C.S., Hasseus B., Jonasson P. Inflammatory profile of chronic apical periodontitis: a literature review. Acta Odontol. Scand. 2019;77(3):173–180. doi: 10.1080/00016357.2018.1521005. [DOI] [PubMed] [Google Scholar]
- 39.Gu L., Ke Y., Gan J., Li X. Berberine suppresses bone loss and inflammation in ligature-induced periodontitis through promotion of the G protein-coupled estrogen receptor-mediated inactivation of the p38MAPK/NF-κB pathway. Arch. Oral Biol. 2021;122 doi: 10.1016/j.archoralbio.2020.104992. [DOI] [PubMed] [Google Scholar]
- 40.Nasution A.H., Amalia M., Tarigan C.C. The difference upper incisor and upper molar alveolar bone loss between smoker and non-smoker patient with chronic periodontitis. Dentika Dental Journal. 2019;22(1):6–11. doi: 10.32734/dentika.v22i1.269. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.