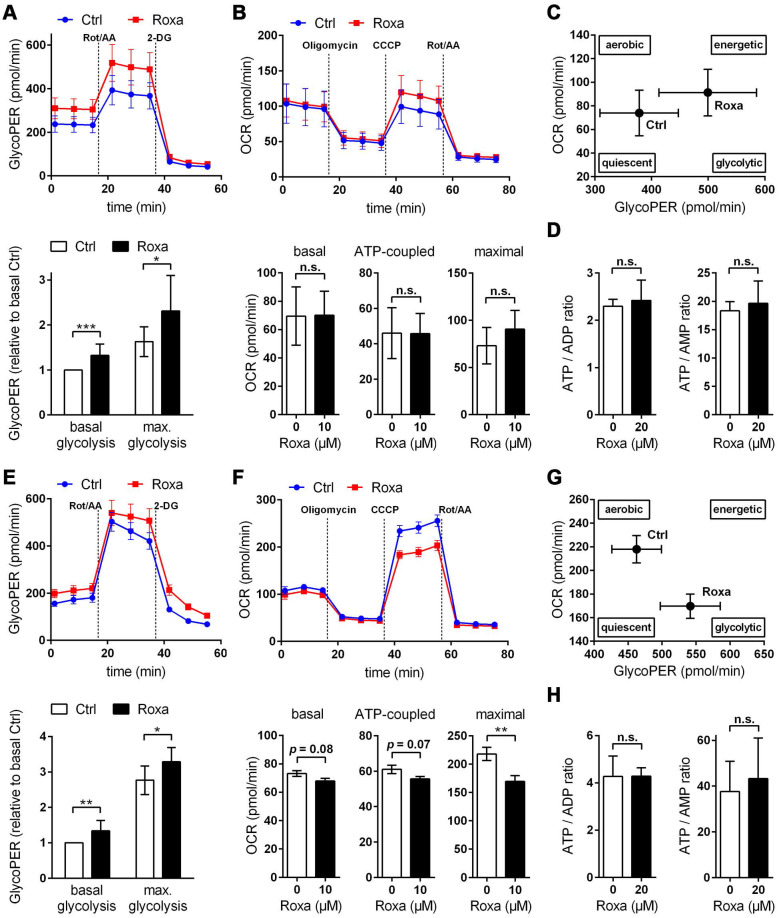
Figure 7.
Roxadustat promotes a metabolic shift to increased glycolytic energy production. The bioenergetic state of (A-C) neurons and (E-G) astrocytes treated with 10 µM roxadustat for 24 h were determined using a Seahorse XF96e Analyzer. DMSO treated cells were used as control (Ctrl). (A, E) Cell glycolytic profile was evaluated by using the Glycolytic Rate Assay. Proton efflux rate (PER) and oxygen consumption rate (OCR) were measured under basal conditions and in response to rotenone/antimycin A and 2-deoxy-D-glucose (2-DG). Glycolytic PER (GlycoPER) was calculated to determine basal glycolysis and compensatory glycolysis (in response to Rot/AA) (n = 6-12 per group; unpaired two-tailed Student's t test; * p < 0.05, ** p < 0.01, *** p < 0.001). (B, F) Cell Mito Stress Test was applied to estimate key parameters of mitochondrial respiration. OCR was measured after sequential addition of oligomycin, CCCP, and Rot/AA to calculate basal respiration (= OCRbasal - OCRRot/AA), ATP production-linked respiration (= (OCRbasal - OCROligo) - OCRRot/AA) and maximal respiration (= OCRCCCP - OCRRot/AA) (n = 8-11 per group; unpaired two-tailed Student's t test; ** p < 0.01). (C, G) Energy Map depicting the relative bioenergetic state of Ctrl and roxadustat treated cells. (D, H) Cellular AMP, ADP and ATP levels were measured by LC-MS/MS technique and the ATP/ADP and ATP/AMP ratios were calculated (n = 4-7 per group; unpaired two-tailed Student's t test).