TO THE EDITOR:
On January 12, 2023, the U.S. government eliminated the requirement for a waiver from the Drug Enforcement Administration (DEA) to prescribe buprenorphine.1 Buprenorphine is considered to be underused for opioid use disorder and has been shown to reduce the risk of fatal overdose.2,3 The elimination of this waiver was intended to lower barriers to buprenorphine prescribing and potentially expand the pool of prescribers, which might increase buprenorphine dispensing and the initiation of treatment.
We evaluated the changes in buprenorphine prescribing patterns in relation to the change in DEA policy using the IQVIA Longitudinal Prescription Database, which captures 92% of prescriptions dispensed in U.S. retail pharmacies. For each month during 2022 and 2023, we calculated the number of prescribers accounting for buprenorphine dispensing, the number of patients with buprenorphine dispensing, and the number of new patients in whom buprenorphine treatment was initiated (filling a prescription for the first time in the previous 180 days).4 Using an interrupted-time-series design,5 we assessed whether these outcomes changed in January 2023 in comparison to the change expected from trends before policy implementation (“level change”). We also assessed whether the monthly rate of change in outcomes increased or decreased after January 2023 (“slope change”). The Supplementary Appendix, available with full text of this letter at NEJM.org, includes additional details.
Between January and December 2022, the monthly number of buprenorphine prescribers increased from 38,684 to 42,158. In January 2023, there was a level increase of 1938 prescribers (95% confidence interval [CI], 990 to 2887) and a slope increase of 595 prescribers per month (95% CI, 393 to 786) (Fig. 1A). In December 2023, there were 53,635 buprenorphine prescribers.
Figure 1. Buprenorphine Prescribers, Patients to Whom Buprenorphine Was Dispensed, and New Patients in Whom Buprenorphine Treatment Was Initiated, January 2022 through December 2023.
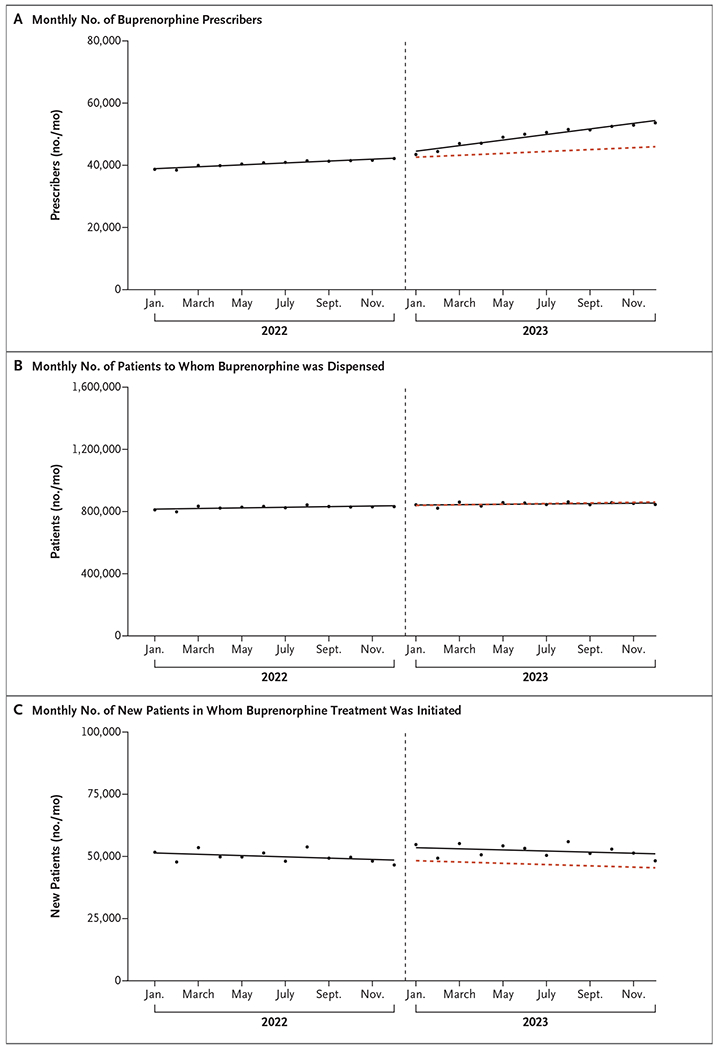
The solid lines are fitted lines from linear segmented regression models evaluating for level and slope changes in outcomes during January 2023 (vertical dashed line). The red dashed line is the counterfactual trend, or the trend that would have occurred had trends from January 2022 through December 2022 continued through December 2023. In Panel C, initiation was defined as filling a buprenorphine prescription for the first time in a 180-day period.
In contrast, between January and December 2022, the monthly number of patients to whom buprenorphine was dispensed increased only slightly, from 810,911 to 831,656. In January 2023, there was no substantial level change in the number of patients with prescriptions (2683; 95% CI, −6751 to 12,117) or slope change (−857 patients per month; 95% CI, −2943 to 1229) (Fig. 1B).
Between January and December 2022, the monthly number of patients in whom buprenorphine treatment was initiated decreased from 51,692 to 46,565. In January 2023, there was a level increase of 5245 new patients (95% CI, 3375 to 7114) but no substantial slope change (34 new patients per month; 95% CI, −336 to 404). In December 2023, buprenorphine treatment was initiated in 48,247 patients (Fig. 1C).
Therefore, after elimination of the waiver requirement, the number of buprenorphine prescribers increased. However, the number of patients in whom buprenorphine treatment was initiated increased only modestly, whereas the total number of patients using buprenorphine changed little. Limitations of our analyses include the lack of information on whether buprenorphine was prescribed for opioid use disorder or another indication, such as pain, and the possibility that the effects of waiver elimination on buprenorphine use may take longer than 1 year to manifest. These findings suggest that the policy may have reduced barriers to prescribing but was insufficient to meaningfully increase buprenorphine use through the end of 2023.
Supplementary Material
Acknowledgments
Supported by a grant (R01DA056438) from the National Institute on Drug Abuse (NIDA) to Drs. Chua, Bohnert, Conti, Lagisetty, and Nguyen. Dr. Chua is also supported by grants (R01DA057284 and K08DA048110) from the NIDA. Dr. Bohnert is also supported by grants from the National Institute of Mental Health and Blue Cross Blue Shield of Michigan. Dr. Conti is also supported by grants from the Department of Veterans Affairs, the Arnold Foundation, the National Science Foundation, and the Leukemia and Lymphoma Society. Dr. Lagisetty is also supported by a grant (K23DA047475) from the NIDA. Dr. Nguyen is also supported by a grant (R01DA057943) from the NIDA. Dr. Bicket is supported by grants from the NIDA, the Centers for Disease Control and Prevention, the Patient-Centered Outcomes Research Institute, the Food and Drug Administration, the Michigan Department of Health and Human Services, the Substance Abuse and Mental Health Services Administration, and Blue Cross Blue Shield of Michigan.
Footnotes
Contributor Information
Kao-Ping Chua, University of Michigan Medical School, Ann Arbor, MI
Amy S.B. Bohnert, University of Michigan Medical School, Ann Arbor, MI
Thuy D. Nguyen, University of Michigan School of Public Health, Ann Arbor, MI
References
- 1.117th Congress. Consolidated Appropriations Act. H.R. 2617 January 3, 2023. (https://www.congress.gov/117/bills/hr2617/BILLS-117hr2617enr.pdf).
- 2.National Academies of Science, Engineering, and Medicine. Medications for opioid use disorder save lives. Washington, DC: National Academies Press, 2019. [PubMed] [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2022 national survey on drug use and health. November 2023. (https://www.samhsa.gov/data/sites/default/files/reports/rpt42731/2022-nsduh-nnr.pdf).
- 4.Chua K-P, Nguyen TD, Zhang J, Conti RM, Lagisetty P, Bohnert AS. Trends in buprenorphine initiation and retention in the United States, 2016-2022. JAMA 2023;329:1402–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


