Abstract
Proliferating nuclear cell antigen (PCNA) and replication protein A (RPA) have proven to be essential elements in many aspects of DNA metabolism including replication, repair and recombination. We have developed an in vitro assay in which the presence of an interstrand crosslink stimulates the incorporation of radiolabeled nucleotides into both damaged and undamaged plasmid DNAs. Using this assay we have investigated the roles of PCNA and RPA in crosslink-induced DNA synthesis. p21, a potent inhibitor of PCNA, was found to strongly inhibit crosslink-induced incorporation. Addition of exogenous PCNA partially restored the resynthesis activity. Likewise, neutralization of RPA by monoclonal antibodies also inhibited incorporation, but the effect was somewhat more pronounced on the undamaged plasmid than the damaged plasmid. Addition of excess RPA also partially reversed antibody inhibition. These results indicate that both PCNA and RPA are required for efficient in vitro DNA resynthesis induced by interstrand crosslinks.
INTRODUCTION
The mechanisms of repair of interstrand crosslinks in DNA remain largely unknown in eukaryotic cells. In order to investigate these mechanisms, we recently developed an in vitro cell-free assay (CRS) in which DNA synthesis was highly stimulated in plasmid DNAs by the presence of a single psoralen interstrand crosslink (1). Interestingly, this elevated DNA synthesis was also observed in undamaged plasmids co-incubated in the cellular extract, suggesting that a form of recombinational repair processing was induced by the crosslink. We also demonstrated that hamster mutants defective in ERCC1, XPF, XRCC2 or XRCC3 were deficient in this assay and that human mutants defective in XPA, XPC or XPG were proficient in the CRS assay. These in vitro results correlate well with the in vivo sensitivities of these cells to various crosslinking agents, thus validating the CRS assay as a measure of crosslink repair. In this report, we have examined the role in the CRS assay of two additional proteins known to be essential for both DNA repair and replicative synthesis, proliferating nuclear cell antigen (PCNA) and replication protein A (RPA).
PCNA has been shown to act as a processivity factor for both polymerases δ and ɛ and is an essential factor for eukaryotic DNA replication (for reviews see 2–4). From in vitro experiments, it has also proven to be an essential factor for the gap filling stage of nucleotide excision repair (NER) and may be required to recycle the excision complex to other sites of damage (5,6). The cyclin-dependent kinase inhibitor p21 (also known as Cip1, WAF1 and Sdi1) has been shown to be a potent inhibitor of PCNA mediated by a domain in its C-terminus (7,8). p21 has been shown to inhibit both SV-40 directed DNA replication and the elongation of primed DNA templates catalyzed by polymerase δ in conjunction with PCNA and replication factor C in vitro (9,10). NER has been shown to require either polymerase δ or ɛ for gap filling of the excised template (11,12), however, initial reports indicated that p21 was not an in vitro inhibitor of the resynthesis stage of NER (13,14). More recently, however, Pan et al. (15) have shown that p21 is a potent inhibitor of the gap filling step of NER in HeLa cell extracts and that this inhibition can be relieved by the addition of purified PCNA.
RPA (also known as human single-stranded DNA binding protein, HSSB) is a heterotrimeric complex required for the initiation and elongation steps of SV-40 directed DNA replication in vitro (16–18). RPA is also required for the incision steps of NER (19,20) and may play a role in damage recognition, since it has been shown to interact with xeroderma pigmentosum group A protein, forming a complex with enhanced damage recognition properties (21,22). In addition to its function in DNA replication and repair, RPA is also required for efficient presynaptic complex formation and strand exchange reactions in vitro, indicating that it likely plays a role in recombinational processes in vivo (23–25).
Because of their multifaceted roles in DNA metabolism, it is reasonable to propose that both PCNA and RPA are involved in the repair of interstrand crosslinks in DNA. Using the CRS assay, we demonstrate below that both proteins are required for crosslink-induced DNA synthesis in vitro.
MATERIALS AND METHODS
Cell-free extracts, proteins and antibodies
Whole cell extracts were prepared from HeLa cells by the method of Manley et al. (26) and stored as previously described (15). Purified PCNA and RPA were generously provided by Z.-Q. Pan. p21 was provided by J. W. Harper. The monoclonal antibody 70C, raised against the large subunit of human RPA, was provided by J. Hurwitz. The hybridoma supernatant was concentrated and partially purified by precipitation with 50% ammonium sulfate, resuspended and dialyzed against phosphate-buffered saline.
CRS assay
Preparation of the psoralen-crosslinked plasmid CLT and other substrates, which include the DT and CT plasmids, have been previously described (1). A detailed protocol for the CRS assay has also been described previously (1). Quantitation of ethidium bromide stained gels and autoradiographs was performed with the Kodak Digital Science 1D image analysis software.
RESULTS
Effect of p21 on crosslink-induced DNA synthesis
We have previously reported (1) on the development of a cell-free assay (CRS) in which a single site-specific psoralen crosslink induces DNA synthesis in both the damaged template (CLT) and an unmodified donor template (DT), as measured by the incorporation of isotopically labeled deoxyribonucleotides. Recently, using an in vitro assay for NER, Pan et al. (15) have shown that p21 is a potent inhibitor of the resynthesis stage of that pathway. These results prompted us to examine whether p21 would inhibit crosslink-induced DNA synthesis in the CRS assay.
As shown in Figure 1, increasing amounts of p21 added to HeLa whole cell extracts resulted in dramatically decreasing levels of incorporation in both the CLT and DT substrates. At 1.14 µM p21 (Fig. 1A, lane 4) the levels of incorporation into the CLT and DT substrates were essentially at background levels (lane 1). The CT substrate used for background control is identical to CLT except for the absence of the psoralen crosslink. These results were quantitated as shown in Figure 1B, which indicates incorporation independently into the CLT and DT plasmids. The levels of p21 required to inhibit the CRS assay were quite similar to the levels found to inhibit NER in vitro. Pan et al. (15) found that 0.42 and 0.84 µM p21 reduced NER synthesis to 35 and 10% of normal levels, respectively. Our results show that 0.57 and 1.14 µM reduced incorporation in the CRS assay to ~20 and 2% (average of CLT plus DT) of normal levels, respectively.
Figure 1.
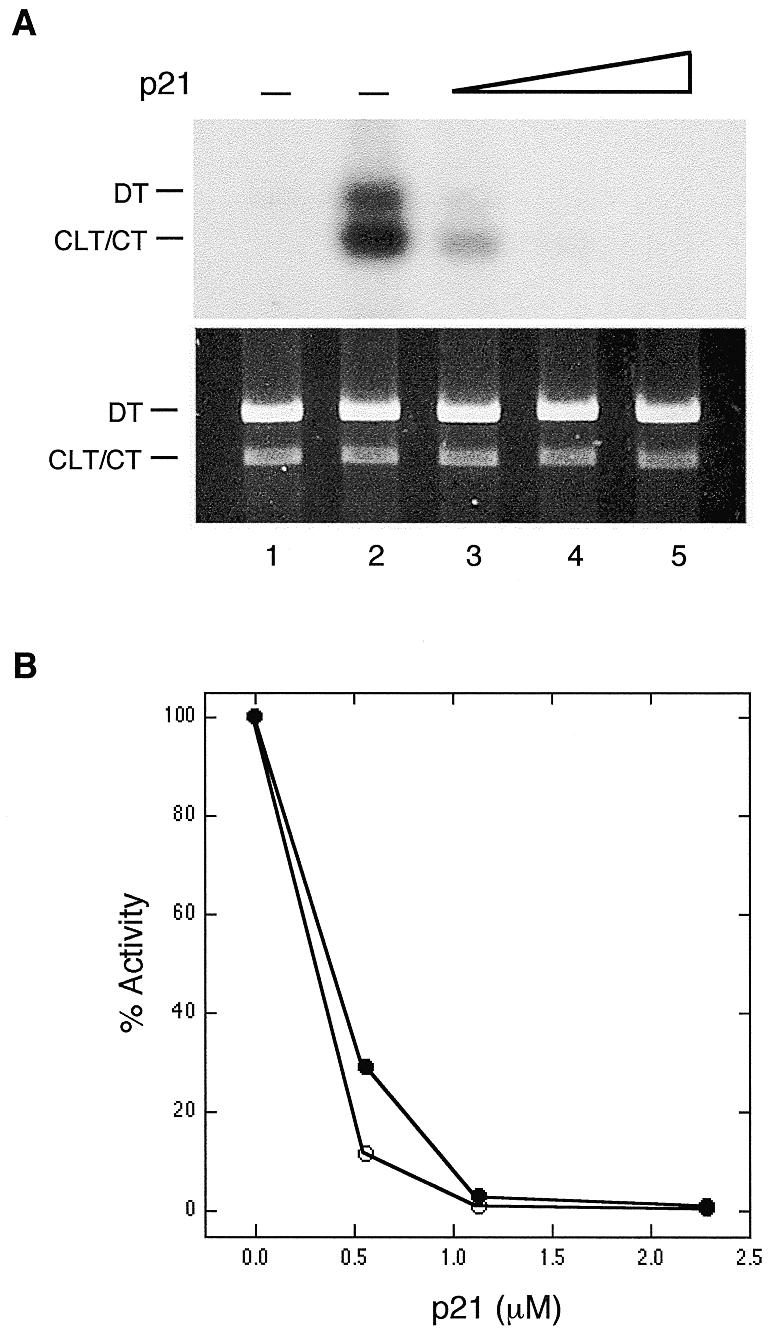
p21 inhibits interstrand crosslink-induced DNA synthesis. (A) All reactions contained HeLa cell extracts at 2.5 mg/ml and other components as described previously (1). p21 was preincubated with the HeLa cell extracts on ice for 30 min prior to addition of the other components. The reactions were subsequently incubated for 3 h at 28°C and processed as previously described (1). The addition of p21 was as follows: lanes 1 and 2, none; lane 3, 0.57 µM; lane 4, 1.14 µM; lane 5, 2.29 µM. Lane 1 contains the DT and CT plasmids and all other lanes contain the DT and CLT plasmids. Both the autoradiogram and ethidium bromide staining of the gel are shown. (B) Quantitation of the experiment shown in (A). All quantitations derived from autoradiograms were normalized for plasmid recovery as measured from the ethidium bromide stained gels. Open circle, DT plasmid; closed circle, CLT plasmid.
As previously shown by Pan et al. (15), the inhibition of NER by p21 can be reversed by addition of excess PCNA. As shown in Figure 2, addition of exogenous PCNA also reversed the inhibition of the CRS assay by p21. The reversal was not complete, but restored the CRS levels to about half of the normal levels for the CLT plasmid and about one third for the DT plasmid. Interestingly, highly excess amounts of PCNA appeared to be inhibitory. These results indicate that inhibition of crosslink-induced DNA synthesis by p21 is due to a specific interaction with PCNA, as has been found previously for SV-40 directed DNA replication and NER resynthesis (9,15).
Figure 2.
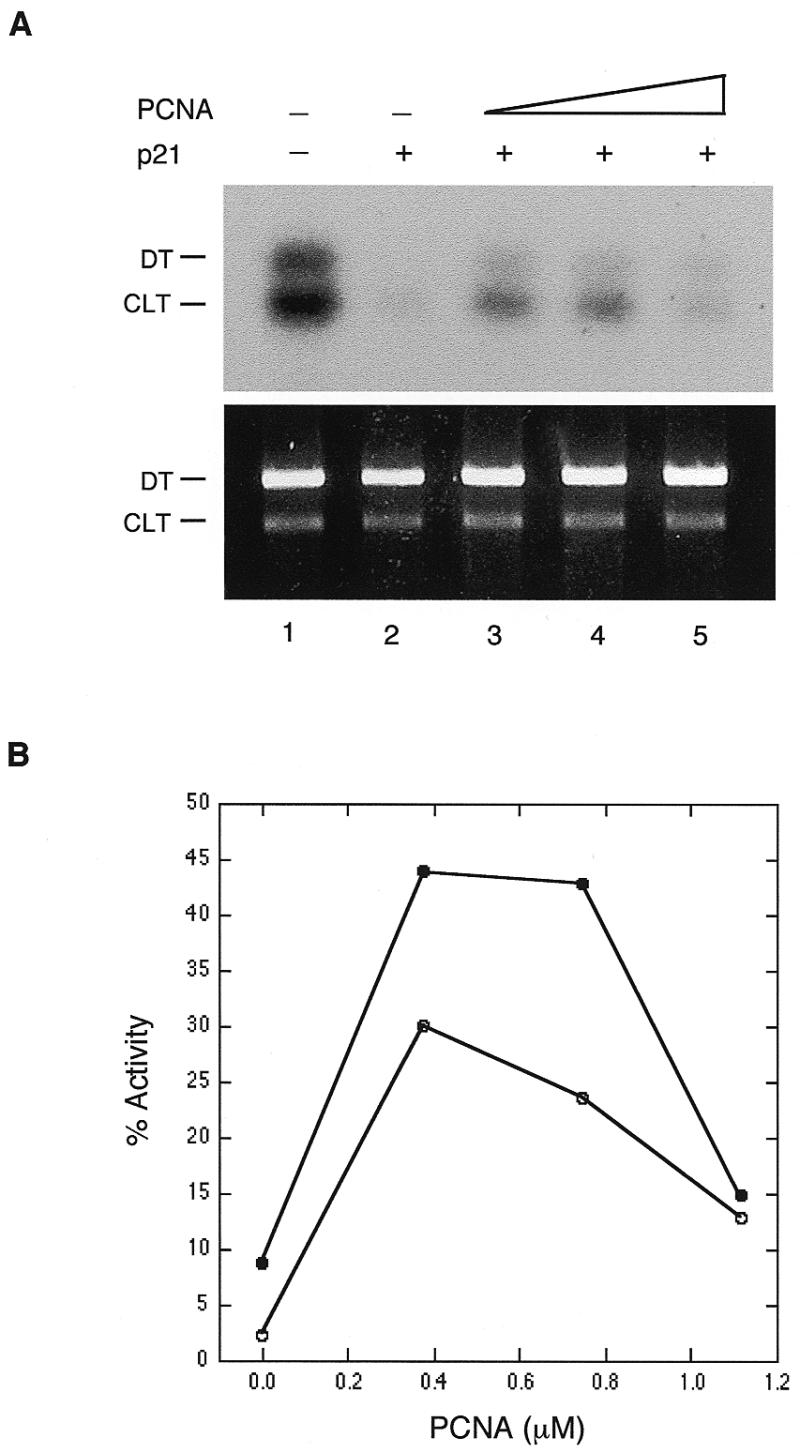
PCNA addition reverses the inhibition of crosslink-induced DNA synthesis by p21. (A) Experiments were performed as described in the legend to Figure 1. PCNA and p21 were incubated together on ice for 15 min followed by addition of HeLa cell extract and a further incubation on ice for 15 min. Other components were then added and the incubation continued for 3 h at 28°C. The addition of PCNA was as follows: lanes 1 and 2, none; lane 3, 0.37 µM; lane 4, 0.75 µM; lane 5, 1.1 µM. (B) Quantitation of the experiment shown in (A). Open circle, DT plasmid; closed circle, CLT plasmid.
Effect of neutralization of RPA on crosslink-induced DNA synthesis
Previously, Coverley et al. (19) have shown that the monoclonal antibody 70C, raised against the 70 kDa subunit of RPA, is highly inhibitory of NER in vitro. We have examined whether this same antibody is inhibitory in the CRS assay. As shown in Figure 3A, increasing amounts of 70C resulted in a progressive decrease in incorporation into both the CLT and the DT plasmids. Interestingly, the 70C antibody differentially affected incorporation into the two plasmids: the undamaged DT plasmid was more severely affected than the crosslinked CLT plasmid. Quantitation of these results is shown in Figure 3B, and indicates that at the same concentration of antibody the residual activity in the DT plasmid is approximately half of that observed in the CLT plasmid.
Figure 3.
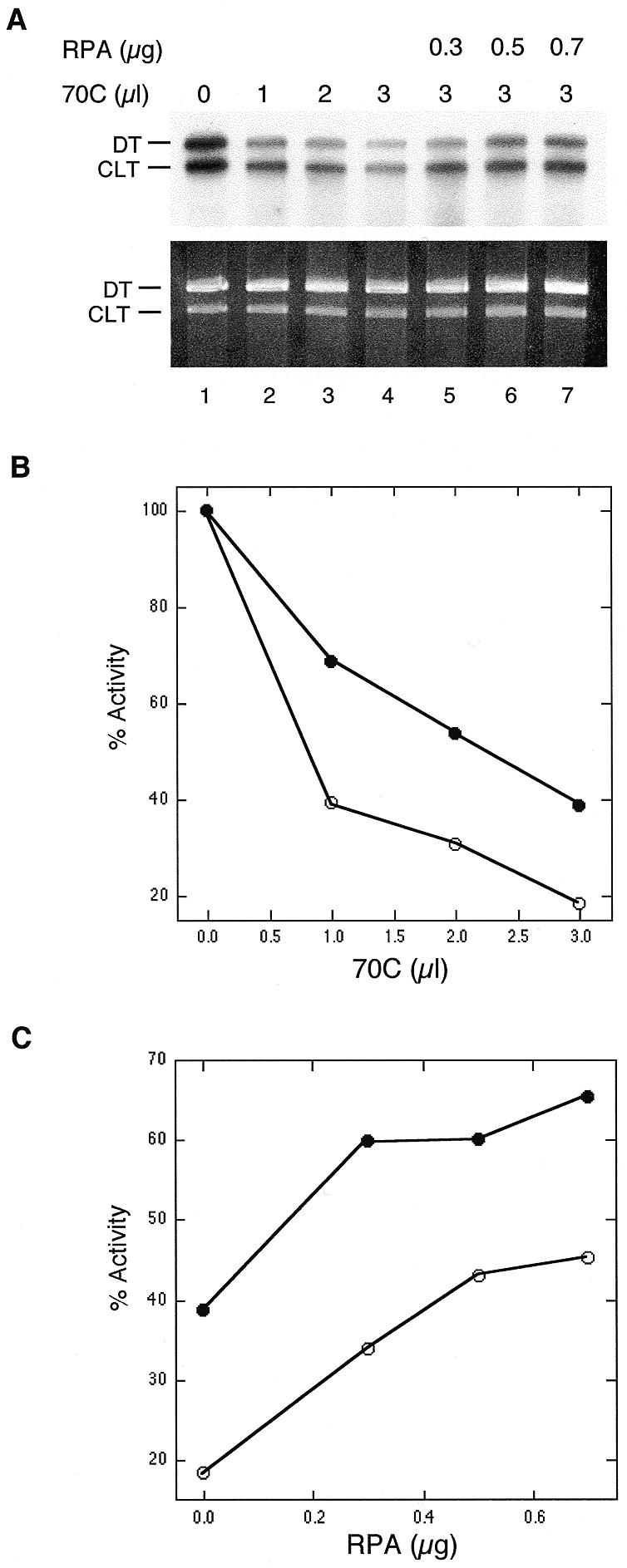
Monoclonal antibodies against RPA inhibit interstrand crosslink-induced DNA synthesis. (A) Experiments were performed as described in the legends to Figures 1 and 2. (B) Quantitation of lanes 1–4. (C) Quantitation of lanes 4–7. Open circle, DT plasmid; closed circle, CLT plasmid.
To show that inhibition of the CRS assay by the 70C antibody was due to neutralization of RPA, exogenous RPA was added to the reaction containing 3 µl of antibody. As shown in Figure 3A and C, the highest levels of excess RPA resulted in an ~2-fold recovery of incorporation in both the CLT and DT plasmids.
DISCUSSION
The results presented here demonstrate that PCNA and RPA, two proteins which are variously involved in DNA replication, repair and recombination, are required for efficient DNA synthesis in response to interstrand crosslinks. PCNA functions as a processivity factor for polymerase δ and, under physiological conditions, for polymerase ɛ as well (27–29), and both of these polymerases have been implicated in NER (11,12). The findings of Pan et al. (15) that p21 inhibits the gap filling stage of NER further supports the involvement of these polymerases in DNA repair processing. While these results contradict those reported by others (13,14), our findings confirm that p21 is a potent inhibitor of damage-induced DNA synthesis in vitro.
The mammalian polymerase(s) involved in interstrand crosslink repair remains to be identified, although our results indicate that it is likely to be a polymerase dependent upon PCNA. Recently, however, a novel polymerase has been identified from Drosophila that is defective in the mus308 mutant (30). mus308 mutants are highly sensitive to interstrand crosslinking agents such as photoactivated psoralen and diepoxybutane, but not to monofunctional alkylating agents (31). The structure of this protein is unusual in that the N-terminal domain contains motifs characteristic of RNA and DNA helicases, while the C-terminal domain contains a polymerase with sequence similarity to prokaryotic polymerase I enzymes. Interestingly, Oshige et al. (32) purified a polymerase from Drosophila cells that is apparently absent in the mus308 mutant and showed that it was resistant to aphidicolin, thus distinguishing it from polymerases α, δ and ɛ. Furthermore, PCNA and RPA did not affect activity. A mammalian homolog of the mus308 gene has yet to be identified. Our results are not consistent with the finding of a polymerase with the characteristics of the mus308 gene product, since in mammalian cell extracts we observe crosslink-induced DNA synthesis to be dependent upon both PCNA and RPA and highly sensitive to aphidicolin (unpublished results). Obviously, these differences may be accounted for by the possibility that there may be more than one polymerase involved in the complex reaction of crosslink repair or that the polymerase found in mammalian cells differs from the Drosophila enzyme.
RPA is required for both the excision and presumably the resynthesis stages of NER (19) and is also required for efficient strand exchange reactions mediated by Rad51 (23–25). In addition, both RPA and PCNA have been implicated in a recombinational repair pathway termed break-induced replication in which a migrating replication fork is established in order to effect repair synthesis (33,34). This pathway has been shown by genetic studies in yeast not to require RAD51 and we have shown in our previous study (1) that Rad51 is not required for the CRS assay. Our reported findings on the CRS assay also indicated that crosslink-stimulated incorporation occurred in both the damaged and unmodified plasmids (1). While not conclusive, it does suggest that crosslinks induce some form of recombination between the two plasmids, which may be mediated by a break-induced replication pathway. Alternatively, the function of Rad51 may be replaced by either XRCC2 and/or XRCC3, both of which have recently been shown to be required for recombination in vivo (35,36).
As discussed above, neutralization of RPA by the 70C monoclonal antibody differentially inhibited incorporation into the two plasmids: resynthesis in the unmodified DT plasmid was more severely affected than resynthesis in the crosslinked CLT plasmid. This result was also observed, but to a lesser extent, with the PCNA inhibitor p21. These observations suggest that RPA (and possibly PCNA) may play a dual role in the CRS assay in that it stimulates both DNA synthesis and recombination. The presence of the 70C antibody would affect both activities, but the inhibition of recombination might more severely affect DNA synthesis in the DT plasmid in contrast to the CLT plasmid.
REFERENCES
- 1.Li L., Peterson,C.A., Lu,X., Wei,P. and Legerski,R.J. (1999) Mol. Cell. Biol., 19, 5619–5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalberg M. and Kelly,T. (1989) Annu. Rev. Biochem., 58, 671–717. [DOI] [PubMed] [Google Scholar]
- 3.Stillman B. (1989) Annu. Rev. Cell Biol., 5, 197–245. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz J., Dean,F.B., Kwong,A.D. and Lee,S.-H. (1990) J. Biol. Chem., 265, 18043–18046. [PubMed] [Google Scholar]
- 5.Shivji M.K.K., Kenny,M.K. and Wood,R.D. (1992) Cell, 69, 367–374. [DOI] [PubMed] [Google Scholar]
- 6.Nichols A.F. and Sancar,A. (1992) Nucleic Acids Res., 20, 2441–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J., Jackson,P.K., Kirschner,M.W. and Dutta,A. (1995) Nature, 374, 386–388. [DOI] [PubMed] [Google Scholar]
- 8.Luo Y., Hurwitz,J. and Massague,J. (1995) Nature, 374, 159–161. [DOI] [PubMed] [Google Scholar]
- 9.Waga S., Hannon,G.J., Beach,D. and Stillman,B. (1994) Nature, 369, 574–578. [DOI] [PubMed] [Google Scholar]
- 10.Flores-Rozas H., Kelman,Z., Dean,F.B., Pan,Z.-Q., Harper,J.W., Elledge,S.J., O’Donnell,M. and Hurwitz,J. (1994) Proc. Natl Acad. Sci. USA, 91, 8655–8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng X.-R., Jiang,Y., Zhang,S.-J., Hao,H. and Lee,M.Y.W.T. (1994) J. Biol. Chem., 269, 13748–13751. [PubMed] [Google Scholar]
- 12.Aboussekhra A., Biggerstaff,M., Shivji,M.K.K., Vilpo,J.A., Moncollin,V., Podust,V.N., Protic,M., Hubscher,U., Egly,J.-M. and Wood,R.D. (1995) Cell, 80, 859–868. [DOI] [PubMed] [Google Scholar]
- 13.Li R., Waga,S., Hannon,G.J., Beach,D. and Stillman,B. (1994) Nature, 371, 534–537. [DOI] [PubMed] [Google Scholar]
- 14.Shivji M.K.K., Grey,S.J., Strausfeld,U.P., Wood,R.D. and Blow,J.J. (1994) Curr. Biol., 12, 1062–1068. [DOI] [PubMed] [Google Scholar]
- 15.Pan Z.-Q., Reardon,J.T., Li,L., Flores-Rozas,H., Legerski,R., Sancar,A. and Hurwitz,J. (1995) J. Biol. Chem., 270, 22008–22016. [DOI] [PubMed] [Google Scholar]
- 16.Fairman M.P. and Stillman,B. (1988) EMBO J., 7, 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waga S. and Stillman,B. (1994) Nature, 369, 207–212. [DOI] [PubMed] [Google Scholar]
- 18.Wobbe C.R., Weissbach,L., Borowiec,J.A., Dean,F.B., Murakami,Y., Bullock,P. and Hurwitz,J. (1987) Proc. Natl Acad. Sci. USA, 84, 1834–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coverley D., Kenny,M.D., Munn,M., Rupp,W.D., Lane,D.P. and Wood,R.D. (1991) Nature, 349, 538–541. [DOI] [PubMed] [Google Scholar]
- 20.Coverley D., Kenny,M.K., Lane,D.P. and Wood,R.D. (1992) Nucleic Acids Res., 15, 3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Z., Henricksen,L.A., Wold,M.S. and Ingles,C.J. (1995) Nature, 374, 566–569. [DOI] [PubMed] [Google Scholar]
- 22.Li L., Lu,X., Peterson,C.A. and Legerski,R.J. (1995) Mol. Cell. Biol., 15, 5396–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung P. (1994) Science, 265, 1241–1243. [DOI] [PubMed] [Google Scholar]
- 24.Sung P. and Robberson,D.L. (1995) Cell, 82, 453–461. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama T., Zaitseva,E.M. and Kowalczykowski,S.C. (1997) J. Biol. Chem., 272, 7940–7945. [DOI] [PubMed] [Google Scholar]
- 26.Manley J.L., Fire,A., Cano,P.A., Sharp,P.A. and Gefter,M.L. (1980) Proc. Natl Acad. Sci. USA, 77, 3855–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prelich G., Tan,C.-K., Kostura,M., Mathews,M.B., So,A.G., Downey,K.M. and Stillman,B. (1987) Nature, 326, 517–520. [DOI] [PubMed] [Google Scholar]
- 28.Bravo R., Frank,R., Blundell,P.A. and MacDonald-Bravo,H. (1987) Nature, 326, 515–517. [DOI] [PubMed] [Google Scholar]
- 29.Lee S.-H., Pan,Z.-Q., Kwong,A.D., Burgers,P.M.J. and Hurwitz,J. (1991) J. Biol. Chem., 266, 22707–22717. [PubMed] [Google Scholar]
- 30.Harris P.V., Mazina,O.M., Leonhardt,E.A., Case,R.B., Boyd,J.B. and Burtis,K.C. (1996) Mol. Cell. Biol., 16, 5764–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyd J.B., Sakaguchi,K. and Harris,P.V. (1990) Genetics, 125, 813–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oshige M., Aoyagi,N., Harris,P.V., Burtis,K.C. and Sakaguchi,K. (1999) Mutat. Res., 433, 183–192. [DOI] [PubMed] [Google Scholar]
- 33.Voelkel-Meiman K. and Roeder,G.S. (1990) Genetics, 126, 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malkova A., Ivanov,E.L. and Haber,J.E. (1996) Proc. Natl Acad. Sci. USA, 93, 7131–7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson R.D., Liu,N. and Jasin,M. (1999) Nature, 401, 397–399. [DOI] [PubMed] [Google Scholar]
- 36.Pierce A.J., Johnson,R.D., Thompson,L.H. and Jasin,M. (1999) Genes Dev., 13, 2633–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]


