Abstract
We present a 41-year-old female with progressive shortness of breath immediately after moving to sea level from high altitude. The patient was found to have a large PDA with systemic RV and PA pressures and pulmonary hypertension, which resolved following PDA closure.
Key Words: congenital heart defect, echocardiography, hemodynamics, hypertension, imaging, pulmonary right-sided catheterization, shortness of breath
Graphical abstract
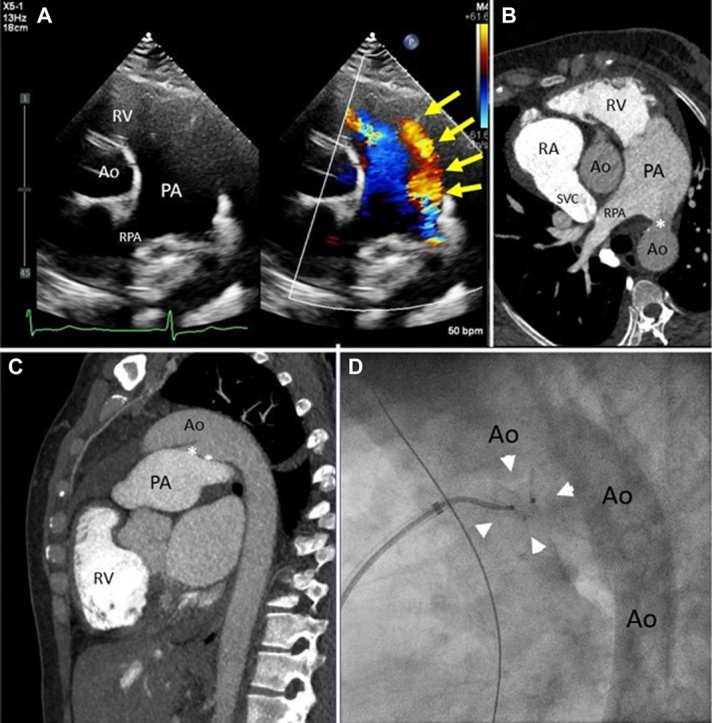
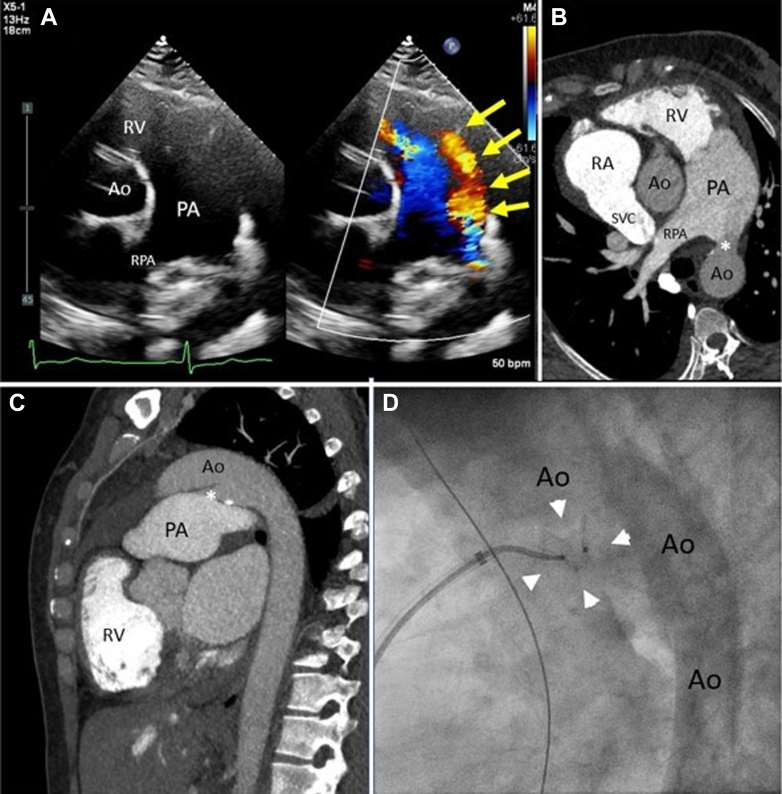
A 41-year-old woman with a history of murmur since childhood presented with progressive shortness of breath after moving to Virginia (altitude 95 m, equivalent FiO2 20.7%) from El Alto, Bolivia (altitude 4,150 m, equivalent FiO2 12.5%). The physical exam was notable for loud machinery murmur and loud S2. No cyanosis or clubbing was noted. Heart rate was 50 beats/min, blood pressure was 96/61 mm Hg, and oxygen saturation was 96%. Echocardiogram showed biventricular dilation with preserved function, 15 mm patent ductus arteriosus (PDA) with pure left-to-right shunting (Figure 1A, Video 1) and estimated pulmonary artery systolic pressure 95 mm Hg. A computed tomography of the chest (Figures 1B and 1C, Video 2) showed a large PDA 15 mm in size and a dilated pulmonary artery measuring 5 cm indicative of pulmonary hypertension and excluded coexistent congenital abnormalities. A diagnostic cardiac catheterization showed a conical PDA (type A) measuring 26 mm at the aortic end, 13 mm at the pulmonic end, and 30 mm in length. At baseline, the ascending aortic and descending thoracic aortic saturations were above 95%, indicative of a pure left-to-right shunt. The Qp:Qs ratio (pulmonary flow [Qp]:systemic flow [Qs]) was 2.2:1. Mean pulmonary artery (PA) pressure was 60 mm Hg and was unchanged with 100% oxygen and inhaled nitric oxide; however, the wedge pressure increased from 16 mm Hg to 22 mm Hg and Qp:Qs increased to 3.8:1 as pulmonary vascular resistance decreased from 6.0 to 3.7 WU (Supplemental Table 1). She was started on furosemide for pulmonary volume overload and, after a period of adequate diuresis to euvolemia, she returned for PDA closure. A 14-mm Amplatzer Muscular VSD Occluder was deployed with the proximal disc at the PA end of the PDA. Angiography through the pigtail catheter in the descending aorta and through the sheath in the main PA showed appropriate device position within the PDA (Figure 1D). The device was then released under fluoroscopy and remained in stable position. Angiography showed no aortic obstruction and only a mild residual shunt through the device. While the FiO2 was still 100%, repeat hemodynamics at the same time showed a significant decrease in right-sided pressures with mean PA pressure of 28 mm Hg and wedge pressure of 11 mm Hg. She tolerated the procedure well without complications. Cardiac catheterization hemodynamics 1-week post-procedure remained stable, showing mean PA pressure of 28 mm Hg, normal wedge pressure, pulmonary vascular resistance of 3 WU, and no hemodynamically significant shunting. Transthoracic echocardiogram 4 weeks post-procedure showed no residual flow through or around the closure device.
Figure 1.
Large Patent Ductus Arteriosus Visualization and Treatment
(A) Transthoracic echocardiogram short-axis view with and without color Doppler showing flow (yellow arrows) from the aorta (Ao) to main pulmonary artery (PA). (B) Corresponding view from computed tomography (CT) angiogram of patent ductus arteriosus (PDA) (∗). Note decreased contrast opacification of main PA from left-to-right shunting of non-opacified aortic blood compared to bright contrast opacification of the right ventricle (RV). (C) Sagittal view of CT angiogram showing PDA (∗) between Ao and main PA. (D) Invasive angiogram placement of 14-mm Amplatzer Muscular VSD Occluder (arrowheads) within the ductus arteriosus.
RA = right atrium; RPA = right main pulmonary artery; SVC = superior vena cava.
In individuals residing at high altitudes, where a hypoxic environment prevails, the pulmonary vascular constriction in response to hypoxia limits the left-to-right shunt flow even in the presence of a substantial patent ductus arteriosus. This phenomenon can contribute to an elevated presence of PDAs with larger sizes.1,2 Moreover, those with PDAs at high altitudes show an increased likelihood of developing PH.3 However, the hypoxic pulmonary vasoconstriction can mitigate permanent changes in pulmonary vascular resistance by decreasing shunt flow. Upon descending to lower altitude regions, the absence of pulmonary vasoconstriction often leads to increased left-to-right shunting, resulting in volume overload (Supplemental Figure 1). Without intervention, individuals with moderate to large PDAs may progress to heart failure, more permanent vascular disease, and potentially develop Eisenmenger’s physiology. Notably, our patient exhibited normal hemoglobin levels (12.5 g/dL) and no lab findings of iron deficiency. Additionally, saturation levels in the descending thoracic aorta matched those in the ascending aorta, confirming the absence of right-to-left shunting through the PDA. In this context, the use of typical pulmonary arterial hypertension (PAH) medications before PDA closure is contraindicated, as pulmonary vasodilation would increase left-to-right shunting and exacerbate volume overload, as evidenced by inhaled nitric oxide testing, we performed. Closure of the PDA resulted in elimination of the shunt and near normalization of the PA pressures. We demonstrate successful closure and immediate reduction in biventricular filling pressures which was sustained at follow-up. This case highlights the potential reversibility of pulmonary hypertension following PDA closure in individuals with prolonged exposure to hypoxic pulmonary vasoconstriction and significant left-to-right shunts at baseline. Closing defects characterized by pure left-to-right shunting significantly reduces the risk of developing pulmonary arterial hypertension.
Funding Support and Author Disclosures
This work was supported by the Intramural Research Program, National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (Bethesda, Maryland, USA). This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the American Association for Dental Research, the Colgate-Palmolive Company, and other private donors. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, figure, and videos, please see the online version of this paper.
Appendix
Transthoracic echocardiogram short axis view with (right panel) and without color Doppler (left panel) demonstrating continuous flow from the aorta to main pulmonary artery through the patent ductus arteriosus.
Rotating 3D surface rendering of the CT angiogram demonstrating enlarged main pulmonary artery and patent ductus arteriosus (highlighted green).
References
- 1.Tefera E., Qureshi S.A., Bermudez-Canete R., Rubio L. Percutaneous closure of patent arterial ducts in patients from high altitude: a sub-Saharan experience. Ann Pediatr Cardiol. 2015;8:196–201. doi: 10.4103/0974-2069.164690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penakiza D., Arias-Stella J. The heart and pulmonary circulation at high altitudes. Circulation. 2007;115:1132–1146. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- 3.Białkowski J., Głowacki J., Zabal C., et al. Patent ductus arteriosus at low and high altitudes: anatomical and haemodynamic features and their implications for transcatheter closure. Kardiol Pol. 2011;69(5):431–436. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiogram short axis view with (right panel) and without color Doppler (left panel) demonstrating continuous flow from the aorta to main pulmonary artery through the patent ductus arteriosus.
Rotating 3D surface rendering of the CT angiogram demonstrating enlarged main pulmonary artery and patent ductus arteriosus (highlighted green).