Abstract
Global use of pneumococcal conjugate vaccines (PCVs) with increasingly broader serotype coverage has helped to reduce the burden of pneumococcal disease in children and adults. In clinical studies comparing PCVs, higher-valency PCVs have met noninferiority criteria (based on immunoglobulin G geometric mean concentrations and response rates) for most shared serotypes. A numeric trend of declining immunogenicity against shared serotypes with higher-valency PCVs has also been observed; however, the clinical relevance is uncertain, warranting additional research to evaluate the effectiveness of new vaccines. Novel conjugation processes, carriers, adjuvants, and vaccine platforms are approaches that could help maintain or improve immunogenicity and subsequent vaccine effectiveness while achieving broader protection with increasing valency in pneumococcal vaccines.
Keywords: immunogenicity decline, PCVs, pneumococcal vaccines, Streptococcus pneumoniae, vaccine effectiveness
Streptococcus pneumoniae is a leading cause of morbidity and mortality worldwide, especially in children <5 years of age [1, 2]. There are approximately 100 pneumococcal serotypes, a small proportion of which are responsible for most infections [3]. Pneumococcal conjugate vaccines (PCVs), routinely used in children ≥2 months of age, contain pneumococcal polysaccharides of serotypes responsible for most invasive pneumococcal disease (IPD) worldwide (Table 1, Figure 1). PCV use has been associated with a substantial reduction in the incidence of pediatric IPD caused by vaccine serotypes [4, 6, 9, 15, 16]. This reduction occurs via direct protection of vaccinated individuals and indirect protection of those unvaccinated through a decrease in nasopharyngeal carriage and subsequent transmission, particularly among vaccinated children [17]. However, nonvaccine serotypes contribute to an ongoing burden of pneumococcal disease, suggesting that PCVs with broader serotype coverage are needed.
Table 1.
Overview of PCVs
| Vaccine Name | Marketed Name | Approval Year | Age Indication | Amount of Serotype per Dose | Carrier Protein | References |
|---|---|---|---|---|---|---|
| PCV7 | Prevnar | 2000 | ≥2 mo | 2.0 µg for each except 6B (4.0 µg) | CRM197 | [4, 5] |
| PCV10 | Synflorix | 2011 | ≥6 wk–5 y | 1.0 µg for each except 4, 18C, and 19F (3 µg each) | Protein D, except for 18C (tetanus toxoid) and 19F (diphtheria toxoid) | [6–8] |
| PCV13 | Prevnar 13 | 2010 | ≥6 wk | 2.2 µg for each except 6B (4.4 µg) | CRM197 | [5, 9] |
| PCV15 (V114) | Vaxneuvance | 2021 | ≥6 wk | 2.0 µg for each except 6B (4.0 µg) | CRM197 | [10–12] |
| PCV20 | Apexxnar | 2021 | ≥18 y | 2.2 µg for each except 6B (4.4 µg) | CRM197 | [13, 14] |
| Prevnar 20 | 2023 | ≥6 wk |
Aluminum phosphate was the adjuvant for each vaccine.
Abbreviations: CRM, cross-reacting material; PCV7, 7-valent pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; V114, 15-valent pneumococcal conjugate vaccine.
Figure 1.
Serotype composition of licensed pneumococcal conjugate vaccines [4, 6, 9–11, 13, 14]. PCV7, 7-valent pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; V114, 15-valent pneumococcal conjugate vaccine.
Increasing serotype valency in new PCVs can lead to reduced immunogenicity, which may affect vaccine effectiveness (VE). However, this may not be initially apparent when licensure is based on immunobridging studies. These head-to-head studies compare the immunologic responses of a new, extended-valency PCV against a previously licensed PCV by using established correlates of efficacy that are based on antibody concentrations. The antibody concentrations identified as a correlate are used to infer similar efficacy against disease. In clinical trials, new PCVs are typically evaluated by comparing immunogenicity with previous PCVs because efficacy studies are generally not feasible [18]. A vaccine-induced serotype-specific immunoglobulin G (IgG) antibody level of 0.35 µg/mL—derived as a correlate of protection for IPD from 3 efficacy studies conducted with the 7-valent PCV (PCV7; Prevnar, Wyeth LLC, Pfizer)—is a widely accepted threshold for regulatory approval [19, 20]. Noninferiority of immune responses induced by a new PCV as compared with an established one, based on the proportion of participants with antibody levels of 0.35 µg/mL per serotype, as well as serotype-specific IgG antibody geometric mean concentration (GMC) ratios, has been used in lieu of efficacy studies in infants [18]. PCVs licensed according to immunobridging have been comparators for subsequent PCVs, creating a “bridge to a bridge.” As noninferiority analyses allow a margin of difference between PCVs, ongoing comparison of immunogenicity between a new investigational PCV and a licensed PCV that had shown lower immune responses in other comparator studies with older PCVs may lead to lower antibody levels. This reduction could lead to decreased VE if the antibody levels do not maintain a protective threshold.
This literature review examines published data on the immunogenicity of current PCVs, investigating the possible impact of increasing PCV valency on immune response and its potential clinical significance. Furthermore, we explore new vaccine technologies that may maintain or improve immunogenicity while achieving broader protection with increasing valency.
IMMUNOGENICITY OF PCVs
Immunogenicity Based on Noninferiority Criteria
Noninferiority results from key phase 3 trials comparing the immunogenicity of higher- and lower-valency PCVs are presented in Table 2. Studies used a 3 + 1 or 2 + 1 dosing schedule, and most assessed noninferiority at 1 month after the primary series whereas some assessed noninferiority at 1 month after the toddler dose. Interstudy comparisons are not made, as IgG enzyme-linked immunosorbent assays vary significantly across studies and any differences may not be clinically or statistically significant [28, 29].
Table 2.
Potential Differences in Immunogenicity Among Vaccines for Shared Serotypes in Key Phase 3 Randomized Controlled Clinical Trials
| Shared Serotypes Failing Noninferiority Criteria With Higher-Valency PCV | |||||
|---|---|---|---|---|---|
| Author | PCVs | Vaccination Schedule, mo | Timing of Assessment | Response Ratea | IgG GMC Ratiob |
| Vesikari (2009) [21] | PCV7 | 2, 3, 4 | 1 mo after primary series | 6B, 23Fc,d | NR |
| PCV10 | +12–18 | ||||
| Kieninger (2010) [22] | PCV7 | 2, 3, 4 | 1 mo after primary series | 6B | None |
| PCV13 | +11–12 | ||||
| Yeh (2010) [23] | PCV7 | 2, 4, 6 | 1 mo after primary series | 6B, 9V | None |
| PCV13 | +12–15 | ||||
| Temple (2019) [1] | PCV10 | 2, 4 | 1 mo after primary series | Noned | NR |
| PCV13 | +9.5 | ||||
| Lupinacci (2023) [24] | PCV13 | 2, 4, 6 | 1 mo after primary series | Noned | 6Ad |
| V114 | +12–15 | ||||
| Benfield (2023) [25] | PCV13 | 3, 5 | 1 mo after toddler dose | Noned | Noned |
| V114 | +12 | ||||
| Martinon-Torres (2023) [26] | PCV13 | 2, 4 | 1 mo after toddler dose | Noned | Noned |
| V114 | +11–15 | ||||
| Watson (2023) [27] | PCV13 | 2, 4, 6 | 1 mo after primary series | 1, 3, 4, 9V, 23Fd,e | None |
| PCV20 | +12–15 | 1 mo after toddler dose | NRd | Noned | |
Abbreviations: GMC, geometric mean concentration; IgG, immunoglobulin G; NR, not reported; PCV; pneumococcal conjugate vaccine; PCV7, 7-valent pneumococcal conjugate vaccine; PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; PCV20, 20-valent pneumococcal conjugate vaccine; V114, 15-valent pneumococcal conjugate vaccine.
aIgG response threshold is 0.35 μg/mL unless otherwise stated.
bNoninferiority criteria differed across studies.
cIgG response threshold is 0.20 μg/mL for noninferiority; serotypes listed did not meet noninferiority criteria.
dPrimary or coprimary immunogenicity endpoint.
eDifferent thresholds used for serotypes 5, 6B, and 19A.
Per World Health Organization (WHO) guidance, noninferiority of a higher- vs lower-valency PCV is based on the confidence intervals for the difference in IgG response rate (proportion of participants achieving a serotype-specific GMC threshold, usually ≥0.35 μg/mL) or IgG GMCs, although exact criteria can vary by study. Noninferiority is assessed per serotype: WHO guidance states that meeting either the predefined noninferiority criteria for IgG response rates or GMCs should be adequate for approval. Table 2 shows serotypes for which noninferiority criteria were not met in trials comparing higher- and lower-valency vaccines. WHO guidance also states that meeting noninferiority requirements for every serotype in a vaccine is not an absolute requirement [18]. As such, some trials have included an overall noninferiority objective requiring a predefined number of serotypes to meet noninferiority criteria [1, 21].
Noninferiority against serotype 6B was not met for 13-valent PCV (PCV13; Prevnar 13, Wyeth LLC, Pfizer) vs PCV7 after administration of the primary series across 2 studies [22, 23] and for 10-valent PCV (PCV10; Synflorix, GlaxoSmithKline Inc) [7] vs PCV7 after the administration of primary series in 1 study [21]. Responses to serotype 6B may be influenced by the infant immunization schedule used [30, 31]; however, all 3 studies assessed a 3 + 1 schedule. Although noninferiority was not assessed after the toddler dose, 95% CIs of the IgG GMCs for serotype 6B after the toddler dose did not overlap for the 2 study vaccines in 2 studies [21, 23], suggesting persistent differences in immunogenicity to this serotype between the vaccines. Serotype 6B may require a lower threshold than the standard 0.35 μg/mL for protection against IPD, with 1 study estimating a threshold of 0.16 μg/mL (95% CI, .08–2.54) for this serotype [32].
In 1 study comparing 15-valent PCV (V114, Vaxneuvance; Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc) with PCV13, noninferiority was missed with the higher-valency vaccine by a narrow margin for 1 serotype (serotype 6A). However, noninferiority based on IgG GMC ratio after dose 4 and IgG response rate was achieved [24].
Other Immunogenicity Outcomes
Several differences in absolute serotype-specific IgG GMC between higher- and lower-valency vaccines have been observed, based on 95% CIs [1, 21–27, 33]. As these data are descriptive, the statistical and clinical significance of numeric differences between vaccines cannot be determined. Higher-valency vaccines had lower GMCs for certain serotypes across several studies. Exceptions to this observation include serotype 19F, for which GMCs were frequently lower with PCV7 vs PCV10 [21, 33], and serotype 3, for which response rates and GMCs were often statistically significantly lower with PCV13 than V114 in pivotal trials [24–26]. Differences between PCVs may be related to vaccine design; for example, PCV10 utilizes different carrier proteins and conjugation chemistry and contains lower concentrations of pneumococcal polysaccharides (Table 1). Unique features of the serotype 3 capsular polysaccharide, including a high rate of capsular shedding, are a challenge for the induction of vaccine-elicited immune responses to this serotype [34, 35]. During the development of V114, these features were considered with a goal of maximizing active targets against serotype 3, which may be the reason behind more robust immune responses to serotype 3 after vaccination with V114 than PCV13 across pediatric and adult populations.
In this review, differences between higher- and lower-valency vaccines were rarely observed for opsonophagocytic activity (OPA) response rates, suggesting that functional antibody responses may be conserved. OPA responses have an important role in evaluating pediatric PCV trials and are frequently incorporated into analyses of immunogenicity. OPA assays mimic host immune responses, reflecting the ability of neutralizing antibodies to opsonize S pneumoniae and promote phagocytosis [36]. Immunogenicity against some serotypes was numerically lower with higher-valency vaccines for OPA geometric mean titers and their ratios [1, 21–25, 33, 37]. However, specific correlates of protection for OPA have not been established in children [20]. OPA is not used as a primary endpoint in pediatric studies and is often performed only in subsets of participants owing to serum volume requirements. This differs from adult studies, in which OPA is considered the principal basis for comparison [38].
RELATIONSHIPS BETWEEN MEASURES OF PCV-INDUCED IMMUNOGENICITY AND CLINICAL EFFECTIVENESS
Immunologic Thresholds of Protection
Certain serotypes may require higher concentrations of serotype-specific IgG to provide adequate protection against IPD. Based on data from England and Wales, among PCV13 serotypes, serotype-specific thresholds were highest for serotypes 3 (2.83 μg/mL), 19F (1.17 μg/mL), and 19A (1.00 μg/mL) [32]. The amount of capsular polysaccharide required to inhibit antibody-dependent bacterial killing has been shown to be considerably lower for serotype 3 vs 4 [34]. Serotype 3 also releases a greater amount of capsular polysaccharide than other serotypes, including serotypes 1, 4, 6B, and 14 [34]. This may result because serotype 3 polysaccharide is noncovalently attached to the bacterial surface, increasing shedding and thus reducing the ability of anticapsular antibodies to bind to the bacterial surface to induce killing [39]. Serotype 19F may require 6-fold-higher antibody concentrations to achieve 50% opsonophagocytic killing when compared with serotype 6B, which is attributed to resistance to complement component 3 deposition [40]. Serotypes 19A and 19F are closely related biochemically [41], suggesting that serotype 19A may be similarly resistant to opsonophagocytosis.
Vaccine Effectiveness
In a systematic review of IPD vaccine failures in children vaccinated with PCV13 or PCV10, the main serotypes associated with vaccine failure or breakthrough were serotypes 3, 19A, and 19F for PCV13 and serotypes 14 and 6B for PCV10 [42]. Except for serotype 6B, all of these serotypes are presumed to require higher protective antibody levels. For some serotypes associated with failure or breakthrough, including 19F for PCV13 [22] and 14 and 6B for PCV10 [21], immunogenicity was lower in clinical trials when compared with a lower-valency vaccine. Similarly, in a study in Canada, the most common serotypes causing breakthrough infection in children vaccinated with PCV13 during the 7 years after introduction were serotypes 3, 19A, and 19F [43].
Results from VE studies are consistent with data on vaccine failure and breakthrough disease for some serotypes and PCVs. In a retrospective study assessing the VE of PCV13 and PCV10 against IPD between 2012 and 2018 across 12 European countries [44], serotype-specific VE for PCV13 was lowest against serotype 3 (64.5%; 95% CI, 43.7%–77.6%). Serotype-specific VE of PCV13 against serotypes 3 and 19A showed a more rapid decline over the 48 months after the booster vaccination as compared with overall VE. PCV10 VE was 65.9% against serotype 19F (95% CI, −36.4% to 91.5%) [44]. In the United States, serotypes 19A and 3 were the only vaccine serotypes among the 10 most common ones causing IPD in children up to 3 years after the introduction of PCV13 [45]. In England, VE against serotype 3 IPD, calculated according to a case–control design, was 0% in participants who received ≥2 doses at <12 months of age or 1 dose at >12 months of age [46]. In Denmark, no significant changes in the incidence of serotype 3 were observed during the 3 years after the introduction of PCV13 [47], and in Sweden, PCV13 had no observable effect on serotype 3 up to 6 years after introduction [48]. In the United States and England, the majority of serotype 3 IPD cases occurred in children fully vaccinated with PCV13 [35, 46]. In Lebanon, serotype 3 IPD increased after PCV13 adoption and was a predominant cause of mortality [49].
As V114 was only recently approved for pediatric use, there are no VE data available. Modeling analyses, based on serotype-specific antibody concentrations that predicted the effectiveness of PCV13, suggest the potential for increased VE of V114 against serotype 3 as compared with PCV13, countering the trend for a decline in immunogenicity with increasing valency [50]. Similarly, 20-valent PCV was only recently approved for pediatric use; thus, no VE data were available at the time of writing.
Nasopharyngeal Carriage
Antibody levels required to prevent nasopharyngeal carriage may be higher than those needed to prevent IPD [17]. In infants in Nepal, the IgG antibody concentration associated with protection against nasopharyngeal carriage of serotype 19F was 2.54 μg/mL [51], which is greater than the reported 1.17 μg/mL correlate for serotype 19F IPD [32]. On average, protective correlates were 2.15 times higher in low/low-middle–income countries than high/upper-middle–income countries (GMC, 2.15; 95% CI, 1.46–3.17; P = .0024) [51]. Accordingly, evidence of noninferiority used for PCV licensure is insufficient to predict noninferiority against nasopharyngeal carriage [17]. Decreasing immunogenicity with higher-valency vaccines could be particularly impactful on carriage rates, given the high antibody levels required for prevention [52, 53]. Failure to prevent nasopharyngeal carriage of pneumococci in healthy individuals could reduce indirect protection, as carriage is a prerequisite for disease transmission [3].
In a study in Papua New Guinea, the proportion of infants achieving serotype-specific IgG concentrations ≥1.0 µg/mL at 9 months of age—a higher threshold than that commonly used for IPD—was significantly higher in those who received PCV10 vs PCV13 for vaccine serotypes 6B, 18C, and 19F [52]. Although the number of infants with carriage of these serotypes at 9 months of age was similar between the vaccine groups, suggesting that these differences may not necessarily affect vaccine efficacy and VE against carriage, the overall number of participants with carriage was small, limiting statistical power. PCV10 and PCV13 were administered in a 3 + 0 schedule, and antibody levels waned rapidly after completion of the series [52]. Use of a booster dose may have increased the number of infants reaching the higher threshold.
In a randomized controlled trial in Vietnam, a 1 + 1 schedule of PCV10 or PCV13 administered at 2 and 12 months of age reduced carriage of vaccine serotypes at 24 months of age (by 58% and 65%, respectively) [54]. In a randomized controlled trial comparing PCV13 and PCV7, reductions in nasopharyngeal carriage for shared serotypes were generally comparable, with a higher reduction in serotype 19F with PCV13 [55].
In addition to the traditional markers of immunogenicity assessed in PCV trials, recent studies suggest that cellular immunity may have a role in preventing pneumococcal colonization and subsequent transmission within communities. T-cell responses targeting specific pneumococcal antigens have been implicated in limiting nasopharyngeal carriage and thus can reduce the overall burden of pneumococcal disease [56]. Despite challenges with measuring cellular markers of immunogenicity in large vaccine trials, improvements in immunologic assays and methodologies will contribute to exploring cellular immunity to vaccine-induced protection [36].
Overall, evidence suggests that nasopharyngeal carriage of vaccine serotypes has decreased with the widespread use of PCVs [57, 58], including in nonvaccinated populations [59], with no obvious impact of reduced immunogenicity with higher-valency vaccines. It is possible that the impact of immunogenicity decline on nasopharyngeal carriage may be less apparent than the impact on IPD. This may be explained by the greater role of the toddler dose, and immunogenicity differences between higher- and lower-valency vaccines seem to be smaller after the toddler dose vs the primary series.
VACCINE DESIGN STRATEGIES TO MAINTAIN IMMUNOGENICITY IN HIGHER-VALENCY PCVs
While reduced immunogenicity has been observed among higher- vs lower-valency PCVs, the trend is inconsistent. Different vaccine components, platforms, technologies, and manufacturing processes can affect serotype-specific immunogenicity.
Importance of Carrier Proteins and Conjugation Methods
PCVs are manufactured by conjugating pneumococcal capsular polysaccharides to an immunogenic carrier protein, such as CRM197. Carrier proteins provide a source of T-cell help, resulting in immunoglobulin class switching to convert the polysaccharide from a T-cell–independent antigen to a T-cell–dependent antigen [60]. The choice of specific carrier proteins and conjugation processes can influence immunogenicity and the functionality of antibodies elicited after vaccination with PCVs [61].
Several hypotheses have been proposed to explain declining serotype-specific immunogenicity as valency increases. First, increasing the number of serotypes in a vaccine could reduce the potency of individual polysaccharide conjugates, due to interference among multiple polysaccharide-specific B cells competing for T-cell help from the same carrier protein [62]. Adjusting the polysaccharide dose per serotype to maintain a balanced antigen-to-carrier ratio can mitigate this immune interference in multivalent vaccines [62]. Second, the development of polysaccharide-specific B-cell and antibody responses may be hindered by immune responses to carrier proteins (carrier-induced suppression) [62, 63]. Carrier-specific immune responses that are thought to be associated with carrier-induced epitope suppression include anticarrier antibodies that prevent B cells from accessing the polysaccharide antigen, carrier-specific memory B cells that compete for T-cell help, and carrier-specific regulatory cells [62]. Third, the length of the link between polysaccharide and carrier protein, determined by the conjugation method used, may affect the immune response [63]. Last, conjugation chemistry may alter the polysaccharide structure, potentially destroying immunogenic polysaccharide epitopes [41].
Immune interference with polysaccharide responses caused by the carrier protein in PCVs is dependent on the specific carrier protein used (eg, tetanus toxoid, diphtheria toxoid, protein D, or CRM197). For CRM197, interference has primarily been observed when PCVs are mixed with other conjugate vaccines utilizing the same carrier protein [62]. Therefore, immune interference via CRM197 protein-induced suppression may occur in vaccine formulations that exceed a certain number of glycoconjugates and/or carrier protein dose. Susceptibility to immune interference may vary by serotype. For example, the serotype 6B–CRM197 conjugate is thought to be more susceptible than other serotypes [62]. PCV13 coadministered with childhood diphtheria toxoid-containing conjugate vaccines has resulted in significant reductions in immune responses for serotypes 4, 6B, and 19F as compared with coadministered PCV7 [22]. The increased carrier protein dose in PCV13 (29 µg) vs PCV7 (20 µg) and immune interference could contribute to this decline in immunogenicity [22, 62].
It is currently unclear whether carrier-induced suppression is the main mechanism underlying the reduction in immunogenicity with some higher-valency PCVs. The use of different carrier proteins, such as protein D (in PCV10) [6] or tetanus toxoid (in PCV10 and an investigational 21-valent PCV) [6, 64], could be a strategy to reduce immune interference and carrier-induced suppression, especially for populations for whom coadministration of vaccines with similar carrier proteins is generally recommended.
Strategies to Enhance PCV Immunogenicity
Conjugation Processes and Novel Carriers
Maintaining the structural integrity of the capsular polysaccharide during conjugation is important for robust T-cell–dependent immune responses that effectively recognize native polysaccharide on the bacterial surface and in generating immunologic memory [65]. Conjugate vaccines use various methods for functionalizing capsular polysaccharides prior to conjugation to the carrier protein [66]. For example, polysaccharide activation of serotype 14 is achieved via sodium periodate oxidation to introduce reactive aldehydes at vicinal hydroxyl groups in the polysaccharide chain, followed by reductive amination coupling to the carrier protein. However, depending on the chemical structure of the specific serotype, this method of polysaccharide activation can modify important immunologic epitopes or cause intrachain polysaccharide cleavage, which may reduce the ability of vaccine-elicited antibodies to recognize native polysaccharide on the infecting bacterium [66].
For PCV10, CDAP (1-cyano-4-dimethylamino-pyridinium tetrafluoroborate) is used to introduce a reactive cyano group for conjugation [41]. Cyanylation may better preserve native polysaccharide epitopes as compared with reductive amination, although data were presented only for serotype 19F [41]. Nevertheless, optimal preservation of the native polysaccharide structure is important for the effectiveness of PCVs, and novel approaches, including bioenzymatic polysaccharide activation, are being explored [66]. Galactose oxidase has been investigated for its ability to reversibly introduce site-specific aldehydes to mitigate polysaccharide degradation [66]. Preclinical results in a murine sepsis infection model showed that immunization with a pneumococcal vaccine containing a conjugate synthesized with galactose oxidase elicited increased antipneumococcal polysaccharide 14 immunoglobulin M titers [66].
Using specific linker moieties between polysaccharide and protein has been explored to increase immune response. Hydrazinepolyethylene glycol–hydrazine linkers boosted the immunogenicity of PCVs in preclinical models [63]. In a recent study in mice, PCVs containing a hydrazine–polyethylene glycol–hydrazine linker induced significantly higher IgG geometric mean titers and antibody avidity when compared with PCV only [63]. The increased size of the linker polysaccharide complexes may increase the half-life of the PCV [63].
Studies in mice have suggested that replacing carrier proteins, such as tetanus toxoid or CRM197, with 1 or more conserved S pneumoniae proteins can expand the breadth of vaccine coverage without adding additional serotype-specific conjugates [67, 68]. This could help prevent reduced immune responses attributable to high carrier protein levels [68]. Proteins such as pneumococcal surface protein A, pneumococcal histidine triad D, detoxified pneumolysin, and spr96/2021 can act as “universal” antigens and carrier proteins [15, 69].
A recent innovation in carrier protein technology utilizes eCRM carrier protein produced via cell-free synthesis, allowing the incorporation of biorthogonal amino acids at specific protein locations. Subsequent conjugation to polysaccharide can then be completed with established click chemistries. Controlling conjugation sites allows targeting outside of the primary T-cell epitope region [39]. This approach allows site-specific covalent conjugation of pneumococcal capsular polysaccharides, resulting in an increased polysaccharide-to-protein ratio and enabling inclusion of more serotypes while minimizing immune interference [39]. However, the technology requires polysaccharide activation and derivatization to introduce the cognate moiety enabling click chemistry reactions. An investigational 24-valent PCV (PCV24) uses this technology. Initial results in mice showed comparable IgG responses and OPA titers after PCV24 vaccination vs PCV13 or 23-valent pneumococcal polysaccharide vaccine [39, 70]. Cell-free synthesis of conjugate vaccine candidates has also been performed for Escherichia coli with the oligosaccharyltransferase PglB from Campylobacter jejuni, resulting in strong humoral responses [71]. It remains unclear whether changes in carrier protein alone will alter serotype-specific immune responses or VE.
Novel Vaccine Platforms
The multiple antigen-presenting system (MAPS) has been designed to present polysaccharide and protein antigen components in a modular manner. MAPS can boost the activity of B-cell and T-cell immune responses while broadening vaccine coverage via inclusion of conserved pneumococcal protein components. Furthermore, the noncovalent conjugation consists of biotinylated polysaccharides bound to bacterial rhizavidin [72]. Mice immunized with serotype 14 polysaccharide complexed with MAPS elicited an approximately 80-fold-higher IgG serum titer than those vaccinated without MAPS [72]. The MAPS construct induced multipronged immune responses, including T-helper 1 and 17 responses.
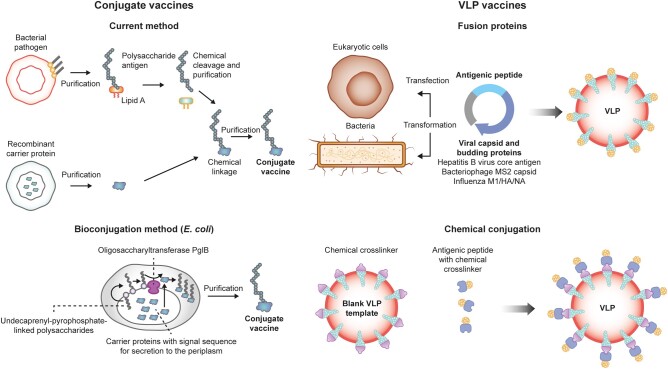
In vivo bioconjugation is an alternative to chemical conjugation with purified polysaccharide and proteins typically used in licensed pneumococcal vaccines [73]. Bioconjugation using bacterial protein glycosylation systems involves covalently linking glycans to proteins during protein glycosylation within a living cell (Figure 2). S pneumoniae is suitable for bioconjugation, as pneumococcal capsular polysaccharides use lipid-linked oligosaccharides typically assembled on the cell surface of bacterial cells prior to their polymerization and transfer to the cell surface [73]. A preclinical study evaluated the immunogenicity of a multivalent pneumococcal bioconjugate vaccine against serotypes 9V, 14, and 8 in murine models; statistically significant increases in serotype-specific IgG levels were recorded 49 days after vaccination as compared with a chemically conjugated PCV containing PCV13 serotypes [73].
Figure 2.
Schematic of platforms for the production of vaccines. VLP, virus-like particle. Reproduction based on Ihssen et al [74] and Brisse et al [75].
Virus-like particle (VLP) technology is another vaccine platform that consistently generates strong and durable antibody responses, as VLPs contain multiple protein fragments that can target immune cells and increase antigen-presenting cell uptake (Figure 2) [76]. Synthetic VLPs made from coiled coil lipopeptides provide immunologic advantages owing to their size, surface structure, and capacity to induce strong immune responses without adjuvants [76, 77]. Preclinical studies assessed the inclusion of pneumococcal surface protein A on the surface of synthetic VLPs as potential vaccine candidates. In mice, this approach resulted in significant immune responses and protection [77]. Other potential future strategies for pneumococcal vaccine development include the use of messenger RNA technology [78].
CONCLUSIONS
Based on published clinical studies, there is a trend for declining immunogenicity against shared serotypes with increasing valency of PCVs. Effectiveness data are needed to definitively elucidate the clinical relevance of this trend, which is currently unknown due to variability in protective thresholds across serotypes. However, persistence of certain serotypes in widely used PCVs, which have been predicted to have higher correlates of protection than the standard threshold of 0.35 μg/mL, suggests that immunogenicity is important.
Preservation of critical polysaccharide antigenic epitopes, choice of conjugation chemistry, and antigen concentration can affect immunogenicity, functional antibody levels, and immunologic memory. These elements must be optimized to minimize the immunogenicity decline observed in higher-valency PCVs. Novel vaccine platforms and technologies may be utilized to improve immune responses while expanding vaccine valency. Although out of the scope of this review, protein-based pneumococcal vaccines that target conserved protein antigens of S pneumoniae may represent a promising alternative strategy to PCVs [76].
Further research is needed to understand the clinical relevance of immunogenicity decline in serotype-specific responses with increasing valency. Additional data could help vaccine manufacturers improve immune responses in novel vaccines while maintaining and even enhancing coverage against persistent and emerging disease-causing serotypes.
Contributor Information
Kristen Feemster, Merck Research Laboratories, Merck & Co, Inc., Rahway, New Jersey, USA.
Ulrike K Buchwald, Merck Research Laboratories, Merck & Co, Inc., Rahway, New Jersey, USA.
Natalie Banniettis, Merck Research Laboratories, Merck & Co, Inc., Rahway, New Jersey, USA.
Joseph G Joyce, Merck Research Laboratories, Merck & Co, Inc., Rahway, New Jersey, USA.
Priscilla Velentgas, Merck Research Laboratories, Merck & Co, Inc., Rahway, New Jersey, USA.
Timothy J Chapman, Merck Research Laboratories, Merck & Co, Inc., Rahway, New Jersey, USA.
Inci Yildirim, Department of Pediatrics, School of Medicine, Yale University, New Haven, Connecticut, USA; Epidemiology of Microbial Diseases, Yale School of Public Health, New Haven, Connecticut, USA; Yale Institute for Global Health, Yale University, New Haven, Connecticut, USA; Yale Center for Infection and Immunity, Yale University School of Medicine, New Haven, Connecticut, USA.
Notes
Acknowledgments . Medical writing support, including assisting authors with the development of the initial draft and incorporation of comments, was provided by Hussain Merchant, MSc, and Rachel Wright, PhD, of Scion, and editorial support, including fact checking, referencing, figure preparation, formatting, proofreading, and submission, was provided by Ian Norton, PhD, of Scion, all according to good publication practice guidelines. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc., Rahway, New Jersey, USA.
Author contributions . All authors reviewed the literature, provided substantial input, and contributed toward the writing and development of this review article. All authors are responsible for the work described in this article. All authors were involved in drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Patient consent statement. This article does not include factors necessitating patient consent. All data used in this review were from openly available published studies.
Financial support. This work was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc., Rahway, New Jersey, USA.
References
- 1. Temple B, Toan NT, Dai VTT, et al. Immunogenicity and reactogenicity of ten-valent versus 13-valent pneumococcal conjugate vaccines among infants in Ho Chi Minh City, Vietnam: a randomised controlled trial. Lancet Infect Dis 2019; 19:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health 2018; 6:e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Pneumococcal disease. In: The Pink Book: Epidemiology and Prevention of Vaccine-Preventable Diseases. 14th ed. 2021. Available at: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html. Accessed 12 June 2023.
- 4. Food and Drug Administration . Pneumococcal 7-valent conjugate vaccine (diphtheria CRM197 protein) Prevnar. 2019. Available at: https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm137038.pdf. Accessed 20 September 2023.
- 5. Nuorti JP, Whitney CG; Centers for Disease Control and Prevention . Prevention of pneumococcal disease among infants and children: use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59:1–18. [PubMed] [Google Scholar]
- 6. GlaxoSmithKline . Summary of product information. 2019. Available at: https://www.ema.europa.eu/en/documents/product-information/synflorix-epar-product-information_en.pdf. Accessed 15 September 2023.
- 7. GlaxoSmithKline . Synflorix: pneumococcal polysaccharide and non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine, adsorbed. 2010. Available at: https://gskpro.com/content/dam/global/hcpportal/en_BD/PI/Synflorix_GDS15_IPI_15_Clean_1_03_2019_1_03_2019.pdf. Accessed 15 September 2023.
- 8. Lecrenier N, Marijam A, Olbrecht J, Soumahoro L, Nieto Guevara J, Mungall B. Ten years of experience with the pneumococcal non-typeable Haemophilus influenzae protein D–conjugate vaccine (Synflorix) in children. Expert Rev Vaccines 2020; 19:247–65. [DOI] [PubMed] [Google Scholar]
- 9. Pfizer . Prevenar13 summary of product characteristics. 2019. Available at: https://www.ema.europa.eu/en/documents/product-information/prevenar-13-epar-product-information_en.pdf. Accessed 26 May 2023.
- 10. Food and Drug Administration . Vaxneuvance (pneumococcal 15-valent conjugate vaccine) prescribing information. 2022. Available at: https://www.fda.gov/media/150819/download. Accessed 7 April 2023.
- 11. European Medicines Agency . Vaxneuvance: summary of product characteristics. 2022. Available at: https://www.ema.europa.eu/en/documents/product-information/vaxneuvance-epar-product-information_en.pdf. Accessed 7 December 2022.
- 12. Kobayashi M, Farrar JL, Gierke R, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among US adults: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfizer . Prevnar 20 package insert. 2021. Available at: https://www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20. Accessed 7 July 2023.
- 14. Pfizer . Prevnar 20 summary of product characteristics. 2021. http://www.ema.europa.eu/en/documents/product-information/apexxnar-epar-product-information_en.pdf. Accessed 7 July 2023.
- 15. Daniels CC, Rogers PD, Shelton CM. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J Pediatr Pharmacol Ther 2016; 21:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Food and Drug Administration . PREVNAR 13 (pneumococcal 13-valent conjugate vaccine [diphtheria CRM197 protein]) prescribing information. 2017. Available at: https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert------Prevnar-13.pdf. Accessed 29 March 2022.
- 17. Dagan R. Relationship between immune response to pneumococcal conjugate vaccines in infants and indirect protection after vaccine implementation. Expert Rev Vaccines 2019; 18:641–61. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization . Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines. WHO Technical Report Series No. 977, annex 3. 2013. Available at: https://cdn.who.int/media/docs/default-source/biologicals/vaccine-standardization/pneumococcus/trs_977_annex_3.pdf?sfvrsn=344f81e_3&download=true. Accessed 1 October 2023.
- 19. Siber GR, Chang I, Baker S, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine 2007; 25:3816–26. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . WHO Expert Committee on Biological Standardization. 60th rep. Geneva: World Health Organization, 2013. [PubMed] [Google Scholar]
- 21. Vesikari T, Wysocki J, Chevallier B, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28:S66–76. [DOI] [PubMed] [Google Scholar]
- 22. Kieninger DM, Kueper K, Steul K, et al. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine 2010; 28:4192–203. [DOI] [PubMed] [Google Scholar]
- 23. Yeh SH, Gurtman A, Hurley DC, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine in infants and toddlers. Pediatrics 2010; 126:e493–505. [DOI] [PubMed] [Google Scholar]
- 24. Lupinacci R, Rupp R, Wittawatmongkol O, et al. A phase 3, multicenter, randomized, double-blind, active-comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of a 4-dose regimen of V114, a 15-valent pneumococcal conjugate vaccine, in healthy infants (PNEU-PED). Vaccine 2023; 41:1142–52. [DOI] [PubMed] [Google Scholar]
- 25. Benfield T, Ramet M, Valentini P, et al. Safety, tolerability, and immunogenicity of V114 pneumococcal vaccine compared with PCV13 in a 2 + 1 regimen in healthy infants: a phase III study (PNEU-PED-EU-2). Vaccine 2023; 41:2456–65. [DOI] [PubMed] [Google Scholar]
- 26. Martinon-Torres F, Wysocki J, Szenborn L, et al. A phase III, multicenter, randomized, double-blind, active comparator-controlled study to evaluate the safety, tolerability, and immunogenicity of V114 compared with PCV13 in healthy infants (PNEU-PED-EU-1). Vaccine 2023; 41:3387–98. [DOI] [PubMed] [Google Scholar]
- 27. Watson W. 20-valent pneumococcal conjugate vaccine (PCV20) phase 3 in pediatrics. 2023. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-22/Pneumococcal-04-Watson-508.pdf. Accessed 20 June 2023.
- 28. Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother 2013; 19:412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol 2010; 17:134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huebner RE, Mbelle N, Forrest B, Madore DV, Klugman KP. Immunogenicity after one, two or three doses and impact on the antibody response to coadministered antigens of a nonavalent pneumococcal conjugate vaccine in infants of Soweto, South Africa. Pediatr Infect Dis J 2002; 21:1004–7. [DOI] [PubMed] [Google Scholar]
- 31. Kayhty H, Ahman H, Eriksson K, Sorberg M, Nilsson L. Immunogenicity and tolerability of a heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 12 months of age. Pediatr Infect Dis J 2005; 24:108–14. [DOI] [PubMed] [Google Scholar]
- 32. Andrews NJ, Waight PA, Burbidge P, et al. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014; 14:839–46. [DOI] [PubMed] [Google Scholar]
- 33. Wysocki J, Tejedor JC, Grunert D, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different Neisseria meningitidis serogroup C conjugate vaccines. Pediatr Infect Dis J 2009; 28:S77–88. [DOI] [PubMed] [Google Scholar]
- 34. Choi EH, Zhang F, Lu YJ, Malley R. Capsular polysaccharide (CPS) release by serotype 3 pneumococcal strains reduces the protective effect of anti-type 3 CPS antibodies. Clin Vaccine Immunol 2016; 23:162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lapidot R, Shea KM, Yildirim I, Cabral HJ, Pelton SI; Department of Public Health . Characteristics of serotype 3 invasive pneumococcal disease before and after universal childhood immunization with PCV13 in Massachusetts. Pathogens 2020; 9:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fletcher MA, Balmer P, Bonnet E, Dartois N. PCVs in individuals at increased risk of pneumococcal disease: a literature review. Expert Rev Vaccines 2015; 14:975–1030. [DOI] [PubMed] [Google Scholar]
- 37. Senders S, Klein NP, Lamberth E, et al. Safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in healthy infants in the United States. Pediatr Infect Dis J 2021; 40:944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LaFon DC, Nahm MH. Measuring immune responses to pneumococcal vaccines. J Immunol Methods 2018; 461:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Micoli F, Romano MR, Carboni F, Adamo R, Berti F. Strengths and weaknesses of pneumococcal conjugate vaccines. Glycoconj J 2023; 40:135–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melin M, Jarva H, Siira L, Meri S, Kayhty H, Vakevainen M. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect Immun 2009; 77:676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Poolman J, Frasch C, Nurkka A, Käyhty H, Biemans R, Schuerman L. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin Vaccine Immunol 2011; 18:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mungall BA, Hoet B, Nieto Guevara J, Soumahoro L. A systematic review of invasive pneumococcal disease vaccine failures and breakthrough with higher-valency pneumococcal conjugate vaccines in children. Expert Rev Vaccines 2022; 21:201–14. [DOI] [PubMed] [Google Scholar]
- 43. Ricketson LJ, Bettinger JA, Sadarangani M, Halperin SA, Kellner JD. Canadian immunization monitoring program AII—vaccine effectiveness of the 7-valent and 13-valent pneumococcal conjugate vaccines in Canada: an IMPACT study. Vaccine 2022; 40:2733–40. [DOI] [PubMed] [Google Scholar]
- 44. Savulescu C, Krizova P, Valentiner-Branth P, et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine 2022; 40:3963–74. [DOI] [PubMed] [Google Scholar]
- 45. Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andrews N, Kent A, Amin-Chowdhury Z, et al. Effectiveness of the seven-valent and thirteen-valent pneumococcal conjugate vaccines in England: the indirect cohort design, 2006–2018. Vaccine 2019; 37:4491–8. [DOI] [PubMed] [Google Scholar]
- 47. Harboe ZB, Dalby T, Weinberger DM, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis 2014; 59:1066–73. [DOI] [PubMed] [Google Scholar]
- 48. Naucler P, Galanis I, Morfeldt E, Darenberg J, Ortqvist A, Henriques-Normark B. Comparison of the impact of pneumococcal conjugate vaccine 10 or pneumococcal conjugate vaccine 13 on invasive pneumococcal disease in equivalent populations. Clin Infect Dis 2017; 65:1780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reslan L, Youssef N, Boutros CF, et al. The impact of vaccination on the burden of invasive pneumococcal disease from a nationwide surveillance program in Lebanon: an unexpected increase in mortality driven by non-vaccine serotypes. Expert Rev Vaccines 2022; 21:1905–21. [DOI] [PubMed] [Google Scholar]
- 50. Ryman J, Weaver J, Yee KL, Sachs JR. Predicting effectiveness of the V114 vaccine against invasive pneumococcal disease in children. Expert Rev Vaccines 2022; 21:1515–21. [DOI] [PubMed] [Google Scholar]
- 51. Voysey M, Fanshawe TR, Kelly DF, et al. Serotype-specific correlates of protection for pneumococcal carriage: an analysis of immunity in 19 countries. Clin Infect Dis 2018; 66:913–20. [DOI] [PubMed] [Google Scholar]
- 52. Pomat WS, van den Biggelaar AHJ, Wana S, et al. Safety and immunogenicity of pneumococcal conjugate vaccines in a high-risk population: a randomized controlled trial of 10-valent and 13-valent pneumococcal conjugate vaccine in Papua New Guinean infants. Clin Infect Dis 2019; 68:1472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marra LP, Sartori AL, Martinez-Silveira MS, Toscano CM, Andrade AL. Effectiveness of pneumococcal vaccines on otitis media in children: a systematic review. Value Health 2022; 25:1042–56. [DOI] [PubMed] [Google Scholar]
- 54. Temple B, Tran HP, Dai VTT, et al. Efficacy against pneumococcal carriage and the immunogenicity of reduced-dose (0 + 1 and 1 + 1) PCV10 and PCV13 schedules in Ho Chi Minh City, Viet Nam: a parallel, single-blind, randomised controlled trial. Lancet Infect Dis 2023; 23:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dagan R, Patterson S, Juergens C, et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis 2013; 57:952–62. [DOI] [PubMed] [Google Scholar]
- 56. He SWJ, van de Garde MDB, Pieren DKJ, et al. Diminished pneumococcal-specific CD4(+) T-cell response is associated with increased regulatory T cells at older age. Front Aging 2021; 2:746295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fleming-Dutra KE, Conklin L, Loo JD, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J 2014; 33(suppl 2):S152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roca A, Dione MM, Bojang A, et al. Nasopharyngeal carriage of pneumococci four years after community-wide vaccination with PCV-7 in the Gambia: long-term evaluation of a cluster randomized trial. PLoS One 2013; 8:e72198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davis SM, Deloria-Knoll M, Kassa HT, O’Brien LK. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine 2013; 32:133–45. [DOI] [PubMed] [Google Scholar]
- 60. Avci FY, Li X, Tsuji M, Kasper DL. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 2011; 17:1602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. van Westen E, Knol MJ, Wijmenga-Monsuur AJ, et al. Serotype-specific IgG antibody waning after pneumococcal conjugate primary series vaccinations with either the 10-valent or the 13-valent vaccine. Vaccines (Basel) 2018; 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: a review. Vaccine 2010; 28:5513–23. [DOI] [PubMed] [Google Scholar]
- 63. Datta A, Kapre K, Andi-Lolo I, Kapre S. Multi-valent pneumococcal conjugate vaccine for global health: from problem to platform to production. Hum Vaccin Immunother 2022; 18:2117949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sanofi . Innovation to drive sustainable growth in vaccines: part 1. 2023. Available at: https://www.sanofi.com/assets/dotcom/content-app/events/investor-presentation/2023/vaccines-investor-event/presentation-vaccines-event-2023.pdf.
- 65. Bröker M, Berti F, Costantino P. Factors contributing to the immunogenicity of meningococcal conjugate vaccines. Hum Vaccin Immunother 2016; 12:1808–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Duke JA, Paschall AV, Glushka J, Lees A, Moremen KW, Avci FY. Harnessing galactose oxidase in the development of a chemoenzymatic platform for glycoconjugate vaccine design. J Biol Chem 2022; 298:101453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Csordas FC, Perciani CT, Darrieux M, et al. Protection induced by pneumococcal surface protein A (PspA) is enhanced by conjugation to a Streptococcus pneumoniae capsular polysaccharide. Vaccine 2008; 26:2925–9. [DOI] [PubMed] [Google Scholar]
- 68. Perciani CT, Barazzone GC, Goulart C, et al. Conjugation of polysaccharide 6B from Streptococcus pneumoniae with pneumococcal surface protein A: pspA conformation and its effect on the immune response. Clin Vaccine Immunol 2013; 20:858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Broker M, Berti F, Schneider J, Vojtek I. Polysaccharide conjugate vaccine protein carriers as a “neglected valency”: potential and limitations. Vaccine 2017; 35:3286–94. [DOI] [PubMed] [Google Scholar]
- 70. Fairman J, Agarwal P, Barbanel S, et al. Non-clinical immunological comparison of a next-generation 24-valent pneumococcal conjugate vaccine (VAX-24) using site-specific carrier protein conjugation to the current standard of care (PCV13 and PPV23). Vaccine 2021; 39:3197–206. [DOI] [PubMed] [Google Scholar]
- 71. Williams AJ, Warfel KF, Desai P, et al. A low-cost recombinant glycoconjugate vaccine confers immunogenicity and protection against enterotoxigenic Escherichia coli infections in mice. Front Mol Biosci 2023; 10:1085887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang F, Lu YJ, Malley R. Multiple antigen-presenting system (MAPS) to induce comprehensive B- and T-cell immunity. Proc Natl Acad Sci USA 2013; 110:13564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harding CM, Nasr MA, Scott NE, et al. A platform for glycoengineering a polyvalent pneumococcal bioconjugate vaccine using E coli as a host. Nat Commun 2019; 10:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ihssen J, Kowarik M, Dilettoso S, Tanner C, Wacker M, Thony-Meyer L. Production of glycoprotein vaccines in Escherichia coli. Microb Cell Fact 2010; 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brisse M, Vrba SM, Kirk N, Liang Y, Ly H. Emerging concepts and technologies in vaccine development. Front Immunol 2020; 11:583077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lagousi T, Basdeki P, Routsias J, Spoulou V. Novel protein-based pneumococcal vaccines: assessing the use of distinct protein fragments instead of full-length proteins as vaccine antigens. Vaccines (Basel) 2019; 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tamborrini M, Geib N, Marrero-Nodarse A, et al. A synthetic virus-like particle streptococcal vaccine candidate using B-cell epitopes from the proline-rich region of pneumococcal surface protein A. Vaccines (Basel) 2015; 3:850–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Oliveira GS, Oliveira MLS, Miyaji EN, Rodrigues TC. Pneumococcal vaccines: past findings, present work, and future strategies. Vaccines (Basel) 2021; 9:1338. [DOI] [PMC free article] [PubMed] [Google Scholar]