Abstract
English Learners (ELs), students from non-English-speaking backgrounds, are a fast-growing, understudied, group of students in the U.S. with unique learning challenges. Cognitive flexibility—the ability to switch between task demands with ease—may be an important factor in learning for ELs as they have to manage learning in their non-dominant language and access knowledge in multiple languages. We used functional MRI to measure cognitive flexibility brain activity in a group of Hispanic middle school ELs (N = 63) and related it to their academic skills. We found that brain engagement during the cognitive flexibility task was related to both out-of-scanner reading and math measures. These relationships were observed across the brain, including in cognitive control, attention, and default mode networks. This work suggests the real-world importance of cognitive flexibility for adolescent ELs, where individual differences in brain engagement were associated with educational outcomes.
English Learners (ELs) are students who speak a language other than English at home and are assessed to have English proficiency below a certain threshold when they are enrolled in school in the United States (Albus, Lazarus, & Thurlow, 2018; National Assessment of Educational Progress, 2015), although EL classification can vary by state. Overall, ELs are a fast-growing population in U.S. schools (English Learners: Demographic Trends, 2020). ELs on average have been shown to have gaps in academic achievement compared to their native-English-speaking grade-level peers, both in reading (Albus et al., 2018; Cho, Capin, Roberts, Roberts, & Vaughn, 2019; Hall et al., 2017) and math (de Araujo, Roberts, Willey, & Zahner, 2018; Freeman & Crawford, 2008), with these gaps increasing as students advance in school (de Araujo et al., 2018). There is a lot of variability among ELs in terms of their proficiency in their native language, age of enrollment in English-speaking schools, exposure to English print and language, and socio-economic background (Counts, Katsiyannis, & Whitford, 2018; Olsen, 2014). Because such varied experiences shape the neurocognitive factors important for learning, examining the neurobiological roots of those factors as they relate to academic skills in ELs can be useful for better understanding different learning outcomes and how to support these students.
Academic skills, such as reading and math, are complex acquired skills that require an orchestration of many brain systems, including sensory, memory, language, and higher-order associative systems (Landi et al., 2013; Peters & De Smedt, 2018; Price, 2012). This orchestration is thought to be facilitated by the brain’s cognitive control networks, such as the cingulo-opercular and frontoparietal networks (Engelhardt, Harden, Tucker-Drob, & Church, 2019; Petersen & Posner, 2012; Power & Petersen, 2013), which are presumed to respectively support sustained and moment-to-moment shifts in attention, both of which are critical for successful learning. In monolingual children with and without academic difficulties, cognitive control networks are engaged both during reading and math processes (Aboud, Barquero, & Cutting, 2018; Margolis et al., 2019; Roe et al., 2018; Sokolowski, Fias, Mousa, & Ansari, 2016; Wilkey & Price, 2019), and the degree of engagement in regions belonging to cognitive control networks during reading and math tasks relates to individual differences in respective academic skills (De Smedt, Holloway, & Ansari, 2011; Roe et al., 2018; Wang et al., 2019). The clear links between cognitive control brain activity and academic achievement make understanding cognitive control engagement in ELs a strong direction for supporting learning in this growing population.
There is evidence that maintenance of and access to multiple languages has both acute and long-term effects on the function and structure of cognitive control brain regions and performance on control-demanding tasks (for reviews, see Bialystok, Craik, & Luk, 2012; Calabria, Costa, Green, & Abutalebi, 2018; Vinerte & Sabourin, 2019). While it is clear that brain function, especially of language and cognitive control systems, may differ between bilingual individuals relative to monolinguals, reports of the specific differences (regions, direction, etc.) are mixed. One important factor that can contextualize the variability in the literature is that neither bilingualism nor EL status (as noted above) are uniform categorical labels (Luk & Bialystok, 2013). Further, individual differences in language proficiency within groups of bilinguals relate to brain activity, including in cognitive control regions (Jasinska & Petitto, 2013; Mouthon et al., 2020). Since primarily English-speaking classrooms are challenging for, and may demand cognitive control from, ELs, individual differences in the way cognitive control systems are engaged in this group may be key predictors of academic outcomes.
One aspect of cognitive control that is important to consider for learning is cognitive flexibility, or the ability to switch between tasks and adapt to environmental cues (Uddin, 2021). Cognitive flexibility uniquely predicts academic skills (beyond other measures of executive function), particularly in middle childhood (Magalhães, Carneiro, Limpo, & Filipe, 2020). Cognitive flexibility may be particularly important for learning in ELs, as they consistently have to manage two languages. Neuroimaging studies of cognitive flexibility tasks in bilingual adults have found different activity in frontal and subcortical regions compared to monolinguals (Garbin et al., 2010; Rodríguez-Pujadas et al., 2013). Further, individual differences in brain activity during cognitive flexibility were related to task performance in bilinguals (Garbin et al., 2010). This work suggests cognitive flexibility is a neurocognitive process that evokes patterns of brain activity with behaviorally-relevant variability in bilingual individuals.
Given that cognitive flexibility is important for learning and that brain function supporting cognitive flexibility varies in bilingual individuals, we hypothesized that brain activity during a cognitive flexibility task in ELs will relate to their academic abilities. We expected to see these effects in brain regions that support cognitive control and other domain-general processes, since using multiple languages on a daily basis may shape neural architecture in a broad way. This finding would indicate that domain-general brain systems during cognitive control-demanding contexts may also support reading and math skills in this group. Alternatively, if we find that flexibility and its accompanying brain activity are not related to (or only sporadically related to) reading and math, this will support a constrained role or lack of a role of flexibility in supporting academic learning. We also predicted that self-reported measures of English proficiency would be related to cognitive flexibility brain activity, which would indicate that proficiency in a second language is related to domain-general cognitive flexibility processes. Here, we test these hypotheses in a group of sixthand seventh-grade Hispanic ELs from Texas. Building on previous work that links bilingualism to cognitive flexibility and work that links academic skills to cognitive control, our research bridges these literatures and tests for a neurocognitive factor that could help us better understand and predict academic outcomes in an understudied and underserved group of students.
METHODS
Participants
Participants were recruited as part of a larger Texas Center for Learning Disabilities study (TCLD) in fall of 2018. The current analysis included a subset of sixth- and seventh-grade individuals who met study inclusion criteria. All students recruited for the study were currently labeled as ELs or had been previously designated as ELs within the last 2 years and were still being monitored for EL or low English proficiency classification by their school. Given an aim of the larger TCLD study, 100% of participants self-reported being of Hispanic ethnicity, including Hispanic multiracial. 95% of participants qualified as economically disadvantaged. This was a school-designated binary label based on having one or more qualifying factors: eligibility for free meals under the National School Lunch and Child Nutrition Program, being a family with an annual income at or below the official federal poverty line, being eligible for Temporary Assistance to Needy Families or other public assistance, having received a Pell Grant or comparable state program of financial assistance, being eligible for programs assisted under Title II of the Job Training Partnership Act, or being eligible for benefits under the Food Stamp Act of 1977.
Students with moderate to severe disabilities who attended a self-contained class or students with sensory impairments that prevented participation in assessment and intervention activities were excluded from the study. Participants who were interested in participating in the neuroimaging study were excluded if they were reported to have head trauma, epilepsy, MRI scanner contraindications, such as a non-removable metal implant, vision that could not be corrected with MR-compatible glasses, or were taking medication. A total of 74 participants completed MRI scans for the current study, and 64 were included in the final analysis sample (for data exclusion, see Section 1: Section 1.3.1). Participants were compensated for their time, and families were compensated for travel. The Institutional Review Board of The University of Texas Health Science Center at Houston approved the study for both Austin and Houston sites. Parents gave written consent for their child to participate in the research, and children gave their informed assent. English-Spanish bilingual research assistants conducted consent processes to ensure all families and participants were fully aware of all proceedings. Participation was voluntary, and families were informed they could terminate participation at any time.
Measures of Academic Skill and Language Proficiencies
As part of the larger TCLD study, participants completed a battery of neuropsychological tests via in-school assessment. This battery included reading measures, such as reading fluency and word reading (the Word Recognition Fluency and Letter Word Recognition subtests of the Kaufman Test of Education Achievement III (KTEA-3, Kaufman, 2014)), reading comprehension (Gates-MacGinitie Reading Test, MacGinitie, MacGinitie, Maria, & Dreyer, 2000), English Picture Vocabulary (Woodcock-Johnson III, Woodcock, McGrew, & Mather, 2001), and math measures (Math Fluency and Computation subtests of the KTEA-3). These measures were collected at the beginning of the school year (fall 2018). Additionally, a self-report measure of Spanish and English proficiency was collected 1 year (fall 2019) after baseline with an adapted version of the Language and Social Background Questionnaire (Anderson, Mak, Keyvani Chahi, & Bialystok, 2018). Self-report measures of reading, speaking, understanding, and writing in English and Spanish were combined to create one average measure of English and Spanish proficiency each. Of note, some participants did not complete the language proficiency questionnaire or academic skills battery and were excluded from those sub-analyses (Table 1).
Table 1.
Participant Demographics, Academic Skills, and Language Skills
| N | 64 |
| Female | 28 |
| % Female | 0.44 |
| Age (SD) | 12.64 (0.81) |
| Word reading fluency | 86.21 (13.78) N = 63 GSS |
| Letter word reading | 88.79 (15.16) N = 63 GSS |
| Reading comprehension | 86.60 (13.06) N = 63 GSS |
| Math fluency | 87.81 (10.31) N = 63 GSS |
| Math computation | 91.30 (12.93) N = 63 GSS |
| English vocabulary | 80.13 (10.94) N = 63 GSS |
| English proficiency | 6.87 (1.94) N = 60 ARS |
| Spanish proficiency | 7.49 (2.02) N = 60 ARS |
| Non-verbal IQ | 15.39 (3.53) N = 64 CRS |
| Hispanic/Latino | 64 |
| American Indian/Alaskan Native | 2 |
| Asian | 0 |
| Black | 0 |
| Native Hawaiian | 0 |
| White | 23 |
| More than one race | 0 |
| Other (unspecified) | 8 |
Note: Mean (and standard deviation in parentheses) are provided when applicable. Standardized measures of reading fluency, word reading, math computation, and math fluency were measured with the Kaufman Test of Education Achievement III (Kaufman, 2014); reading comprehension was measured with the Gates-MacGinitie Reading Test (MacGinitie et al., 2000); English vocabulary was measured with the Woodcock-Johnson III (Woodcock et al., 2001); and Spanish and English proficiency were measured with an adapted version of the Language and Social Background Questionnaire (Anderson et al., 2018) with a range of 1–10 with higher scores meaning higher proficiency in that language. Non-verbal IQ was measured with the Matrix Reasoning subscale from the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Race/ethnicity information other than Hispanic/Latino was not available for all participants. Abbreviations: ARS = average raw score; CRS = calculated raw score; GSS = grade standard score.
MRI Acquisition
All MRI data were acquired using Siemens 3T scanners in Houston (Prisma, N = 41) and Austin (Vida, N = 33). Total scan sessions lasted ~80 min, including structural images, diffusion-weighted images, and two runs each of resting-state, a reading and math task, and a cognitive flexibility task. The resting-state, reading and math task, and diffusion MRI data were not used for the current study. For scanning parameter details, see Appendix S1.
Cognitive Flexibility Task
To drive cognitive control systems in a non-lexical way, we used a cued switching task (Engelhardt et al., 2019; Nugiel et al., 2020) (Figure 1). This fMRI cognitive flexibility task reliably elicits putative cognitive control brain networks (Engelhardt et al., 2019; Nugiel et al., 2020). Participants were cued to focus on either the shape or color of a target stimulus for each trial. Participants then matched the subsequent target to one of two response choices (left or right) on that feature (shape or color). Each response choice matched the target on one feature (shape or color), so attention to the cued feature was critical for success. For 36 trials, a cue appeared for the first 1.5 s of the trial with a red box outline around the relevant feature to apply to the later target (“color” in Figure 1). The target stimulus appeared 0.5 s after the red box disappeared and remained on screen for 2 s, during which time the participant had to respond via button press. In 9 trials interspersed throughout the run, a target did not appear, and a red fixation cross was displayed for .5 s, followed by a white fixation cross for 0.5 s. These “cue only” trials allow for future analyses to separate cue processing from target processing, but were not used in this analysis. All trials were followed by a jitter of 0–8 s. We collected up to 2 runs per individual at 5′22″ per run (~11 min total). Task runs were excluded if accuracy was <50% or if >50% of timepoints were censored due to high motion (see Section 1.4.2). Of the 74 individuals who were scanned, 64 were included in the cognitive flexibility analysis (see Table 1). Individuals were excluded due to extreme artifacts in the data and poor data quality (N = 1), no cognitive flexibility task data collected (N = 2), too many high motion timepoints in their data (N = 2), and task performance criteria (N = 5).
Fig. 1.
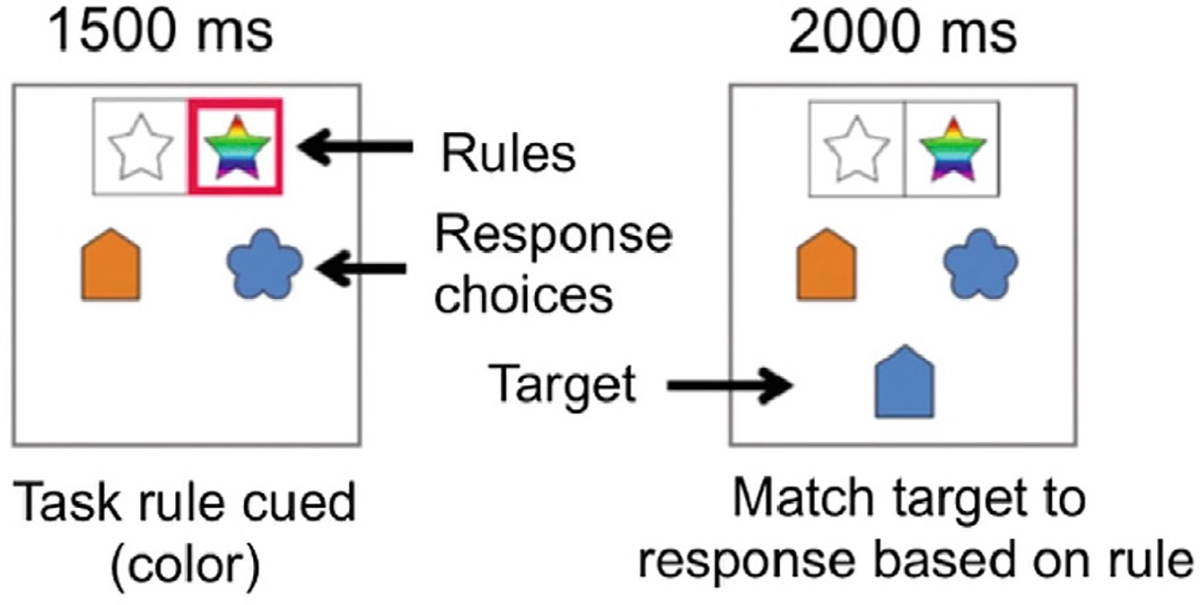
Cognitive flexibility fMRI task design. The cognitive flexibility task was event-related and consisted of 4 s cue-target paired trials (0–1,500 ms cue, 500 ms delay, 2,000 ms presentation of target) or 3 s cue only trials (0–1,500 ms cue, 500 ms delay, 500 ms of a red fixation cross, 500 ms of a white fixation cross).
fMRI Processing
Task Data Preprocessing
Data were preprocessed using the FMRIB Software Library (FSL) version 5.9 (www.fmrib.ox.ac.uk/fsl). T1 images were skull-stripped with non-brain matter removed using Freesurfer version 5.3.0 (Reuter, Rosas, & Fischl, 2010). Registration of the high resolution structural to standard space was done with FMRIB’s Linear Image Registration Tool (FLIRT, Jenkinson & Smith, 2001). Images were spatially smoothed using a Gaussian kernel of FWHM 5 mm, and the 4D dataset was grand-mean intensity normalized by a single multiplicative factor; high pass temporal filtering (Gaussian-weighted least-squares, straight line fitting, with sigma = 50 s).
Task Data First Level Individual Run Modeling
Level 1 modeling was carried out in fMRI Expert Analysis Tool (FEAT). A double-gamma HRF time-series model was carried out using FILM with local autocorrelation correction (Woolrich, Ripley, Brady, & Smith, 2001). The high-pass filter was set at 100 s. First-level models included six motion regressors and their temporal derivatives, temporal derivatives for each task regressor, and nuisance regressors that censor individual volumes identified to have excessive motion, defined as framewise displacement >0.9 mm (Siegel et al., 2014). Task runs with <50% of frames remaining after motion censoring were not included in further analyses (5 runs of the cognitive flexibility task, 2 participants dropped).
Whole Brain Correlations with Academic Skill and Language Proficiency
To test for associations between individual differences in out-of-scanner academic skills and whole-brain activity during the cognitive flexibility task, mean-centered measures of reading fluency, word reading, math computation, math fluency, reading comprehension, English vocabulary, and self-rated English and Spanish proficiency were added as third-level correlates using FLAME-1. Each academic measure was initially modeled separately to avoid model collinearity since the academic measures are correlated to each other (Figure S2). Then, secondary analyses of the significant academic measures were run, controlling for English or Spanish proficiency (two models for each significant academic measure), and English and Spanish proficiency were separately modeled while controlling for individual significant reading or math measures (see Figure S3 for English proficiency and Figure S4 for Spanish proficiency). A Z> 3.1 threshold was used to define contiguous clusters with a cluster probability of p< .05 (Eklund, Nichols, & Knutsson, 2016; Woo, Krishnan, & Wager, 2014). Gaussian random field theory was used for whole-brain multiple comparison corrections (Worsley, 2001). Separate statistical maps were generated for positive and negative correlations. FSL’s Cluster tool was used to identify the peak voxel and cluster size of clusters surviving thresholding. All clusters are reported in MNI coordinates, and brain regions were identified using the Harvard-Oxford Atlas in the FSLview software. To contextualize our findings within the brain’s functional architecture, we used a whole brain parcellation (Gordon et al., 2016) to map significant clusters onto functional brain networks. Data were projected onto inflated brains maps for visualization purposes using Caret software (Van Essen, 2012). For models yielding clusters that survived thresholding, scaled parameter estimates (PEs) were extracted from 5 mm radius spheres bloomed around the peak coordinate to depict the direction of the relation between brain activity and covariate of interest. No additional statistical tests were run on extracted PEs to avoid circularity.
RESULTS
Task Performance
Performance on the cognitive flexibility task was indexed by accuracy across all trials (M= 0.83, SD= 0.1) and response time for correct trials (M= 1.1 s, SD= 0.2 s), Figure S1. See Figure S2 for correlations between all task performance and academic measures.
Whole Brain Correlations between Academic Skills and Task-Related Brain Activity
The cognitive flexibility task versus baseline contrast elicited task-positive brain systems, including the frontoparietal and cingulo-opercular networks implicated in cognitive control and the task-negative default mode network (Figure 2). This pattern of engagement aligns closely with previous studies of this task in children (Engelhardt et al., 2019; Nugiel et al., 2020). Math fluency, reading fluency, and word reading measures were all negatively correlated with brain activity during the cognitive flexibility task. In other words, better performance on these academic measures was associated with less positive engagement, or greater suppression, of brain regions.
Fig. 2.

Main effect map of the cognitive flexibility task. Contrast task > baseline map is cluster thresholded at Z > 3.1 p < .05.
Broadly, math fluency was negatively correlated with activity in bilateral temporal cortex, medial temporal cortex, and right frontal cortex. These regions map onto the ventral attention, visual, somatomotor, retrosplenial temporal, auditory, cingulo-opercular, and default mode networks (Table 2, Figure 3a). The significance of these regions was reduced when controlling for English proficiency (Table S2), but more robust when controlling for Spanish proficiency (Table S3).
Table 2.
Peak coordinates and cluster size from whole brain correlations between academic skills measures and brain activity during the cognitive flexibility task.
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Covariate | Size | Max Z | X | Y | Z | Region | Functional network |
|
| |||||||
| Math fluency | L posterior cingulate gyrus, | Cingulo-opercular | |||||
| 291 | 4.78 | −18 | −34 | 44 | L postcentral gyrus | Somatomotor | |
| 278 | 4.43 | 34 | −52 | 12 | |||
| R angular gyrus, R middle temporal gyrus | Default mode Ventral attention Visual | ||||||
| 215 | 4.53 | −32 | −36 | −4 | L hippocampus, L parahippocampal gyrus |
Retrosplenial temporal | |
| 200 | 4.45 | 30 | 32 | 12 | R frontal white matter R inferior frontal gyrus R putamen | N/A | |
| 127 | 4.12 | 42 | 16 | −16 | R temporal pole, R insular | Auditory | |
| cortex | Cingulo-opercular | ||||||
| 126 | 4.55 | −64 | −24 | 8 | L superior temporal gyrus | Auditory | |
| 122 | 3.98 | 44 | −24 | 4 | R superior temporal gyrus | Auditory | |
| Reading fluency | 1203 | 5.12 | 26 | 12 | 6 | R putamen, R insular cortex, R | Auditory |
| superior temporal gyrus | Cingulo-opercular | ||||||
| 1198 | 5.28 | −26 | 6 | 8 | L putamen, L superior temporal | Auditory | |
| gyrus | Cingulo-opercular | ||||||
| 203 | 4.25 | −28 | 52 | 12 | L frontal pole | Fronto-parietal | |
| 195 | 4.46 | −36 | −44 | 58 | L superior parietal lobe | Dorsal attention Somatomotor | |
| 189 | 5.1 | 34 | 4 | 38 | R middle frontal gyrus | Dorsal attention Frontal-parietal | |
| 171 | 4.2 | 44 | −30 | −2 | R middle temporal gyrus | Default mode Ventral attention | |
| 155 | 4.06 | −10 | 14 | 52 | L superior frontal gyrus, | Cingulo-opercular | |
| L anterior cingulate gyrus | Default mode Dorsal attention | ||||||
| 151 | 4.31 | 26 | −46 | 62 | R superior parietal lobe | Dorsal attention Somatomotor Cingulo-opercular |
|
| 124 | 4.05 | 60 | −24 | 44 | R supramarginal gyrus | Somatomotor | |
| Word reading | Dorsal attention | ||||||
| Somatomotor | |||||||
| 184 | 4.97 | 32 | −48 | 62 | R superior parietal lobe | Visual | |
| 137 | 4.67 | −12 | −28 | 72 | L precentral gyrus | Somatomotor | |
| 124 | 4.56 | 24 | 10 | 8 | R putamen | N/A | |
Note: Size is reported in number of voxels. L=left, R=right. Activity maps were thresholded at Z >3.1 p<.05. All significant correlations between brain activity and academic skills were negative. Clusters from the correlation maps were mapped onto functional networks (Gordon et al., 2016).
Fig. 3.
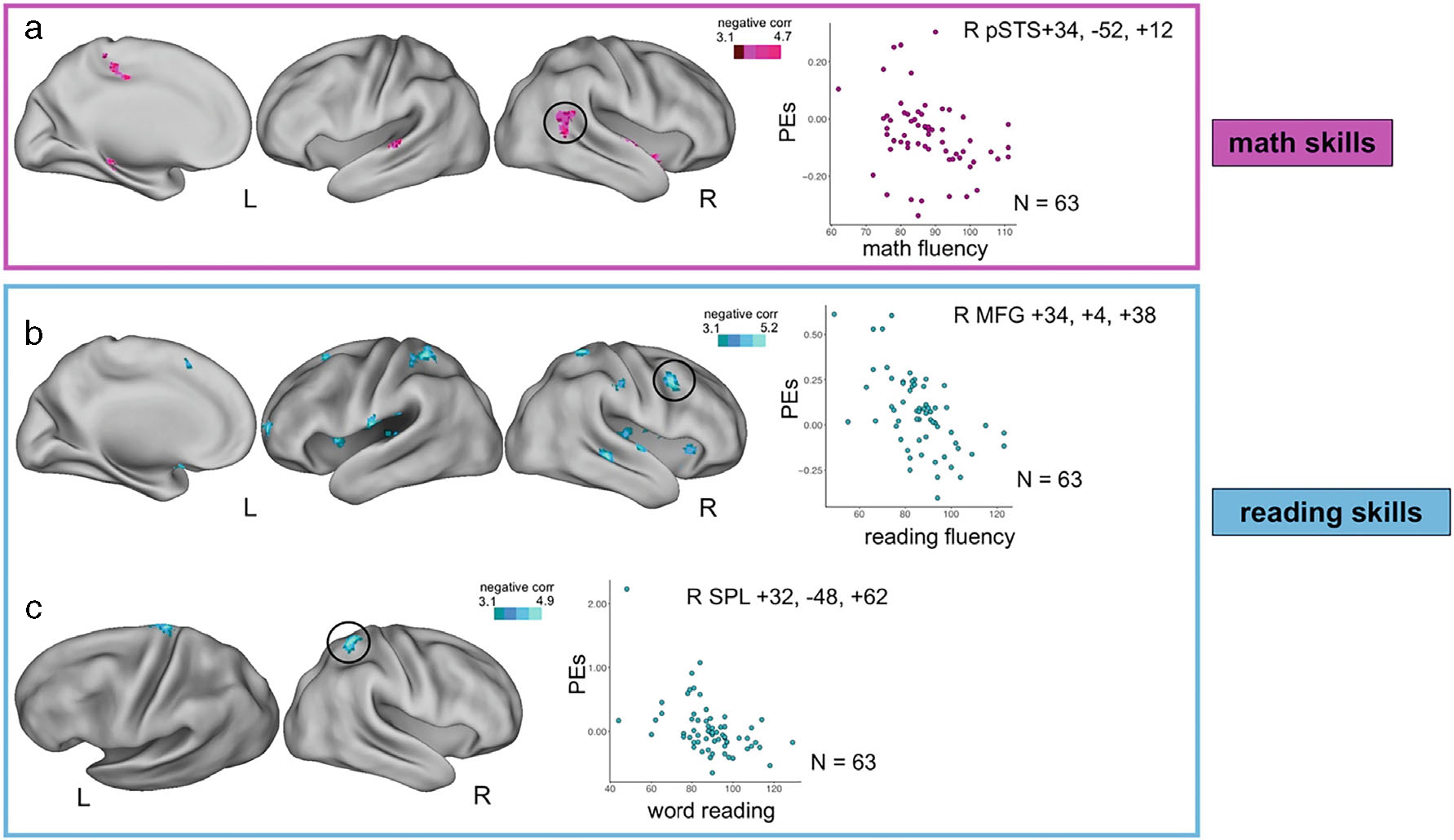
Whole brain correlations between cognitive flexibility task-related brain activity and standardized academic measures. Whole brain images and parameter estimates (PEs) of brain activity plotted with measures of academic skill from the whole brain correlational models. (a) Correlation between math fluency and activity during the cognitive flexibility task. (b) Correlation between reading fluency and activity during the cognitive flexibility task. (c) Correlation between word reading and activity during the cognitive flexibility task. Academic skills measures were all from the Kaufman Test of Education Achievement-3. Academic skills measures were mean centered when entered into the models. All brain activity maps were cluster corrected for multiple comparisons at Z > 3.1 p < .05. Scatter plots merely depict whole brain correlations; no additional statistical tests were run on these data. Scaled PEs were extracted from 5 mm radius spheres bloomed around the peak coordinate. Reported coordinates are in MNI space. pSTS = posterior superior temporal sulcus; MFG = middle frontal gyrus; SPL = superior parietal lobe.
Reading fluency was negatively correlated with activity in bilateral insulae and bilateral temporal cortex in regions of the ventral attention, cingulo-opercular, auditory, and default mode networks. Additionally, reading fluency was negatively correlated with parietal and frontal regions, including regions of the fronto-parietal, dorsal attention, and somatomotor networks (Table 2, Figure 3b). Many of the frontal and parietal regions did not remain when controlling for English or Spanish proficiency (see Tables S2 and S3).
Word reading was negatively correlated with activity in the right putamen, right superior parietal lobe, and left precentral gyrus in regions that belong to the dorsal attention, visual, and somatomotor networks (Table 2, Figure 3c). These regions were robust to controlling for English or Spanish proficiency (see Tables S2 and S3).
There was overlap in the reading fluency and word reading maps in the right superior parietal lobe and right putamen. Reading fluency and math fluency maps overlapped in the right putamen and right middle temporal gyrus. Reading fluency, word reading, and math fluency maps all overlapped in the right putamen.
Math computation and reading comprehension scores were not related to cognitive flexibility brain activity.
Whole Brain Correlations between Language Measures and Task-Related Brain Activity
There were no significant correlations between English vocabulary and brain activity during the cognitive flexibility task. There were also no significant correlations between English proficiency alone or Spanish proficiency alone and brain activity during the cognitive flexibility task (but see Appendix S1 for significance in the secondary models that tested language proficiency while controlling for standardized academic measures).
DISCUSSION
Cognitive Flexibility Relates to Academic Skills in ELs
There is mounting evidence that cognitive control plays a role in academic skills. During reading-related fMRI tasks, activity in cognitive control regions has been found to relate to out-of-scanner reading skills (Meyler et al., 2007; Roe et al., 2018). Similarly, brain activity during math tasks relates to out-of-scanner math skills (Desmet, Fias, Hartstra, & Brass, 2011). There is less work examining non-academic task brain activity related to academic outcomes, though previous work has found no relation between brain activity during a non-lexical inhibition task and reading skills (Roe et al., 2018). Critically, this question of cognitive control engagement has not been examined in Els, who make up ~10% of students in the U.S. (~17% in Texas (English Learners: Demographic Trends, 2020)). The current study addressed this gap by testing whether cognitive flexibility task-related brain activity was related to standard measures of academic skills in this group. We examined cognitive flexibility due to its specific role in learning (Kieffer, Vukovic, & Berry, 2013; Magalhães et al., 2020) and unique implications related to bilingualism (Garbin et al., 2010; Rodríguez-Pujadas et al., 2013). We found a negative relation between cognitive flexibility brain activity and reading and math skills, such that poorer performance on reading and math related to higher activation of certain brain regions during cued task switching. This negative correlation could indicate more effortful processing during the task for ELs with worse academic skills (Dunst et al., 2014; Newman & Just, 2005). This work further supports the idea that cognitive flexibility is an important skill that contributes to academic success, adding evidence from an understudied group of middle-school students.
Our neurobiological findings in ELs contrast with recent work in younger monolingual students with learning difficulties, which found no relation between a non-lexical cognitive control fMRI task (inhibition) and reading skills or response to reading intervention (Nugiel et al., 2019; Roe et al., 2018). Our study differs from these in four main ways: age, economic disadvantage, bilingualism, and task. Comparing across these studies, one could suggest that the role of cognitive control during academic learning in monolinguals is observed in domain-specific contexts (i.e., during reading tasks), while ELs exhibit these relationships across domains (i.e., during task switching). It also could be that the age tested is key: perhaps persistent reading difficulties in middle school relate to more domain-general control differences in the brain than in those in upper elementary schools. Third, it could be that probing control engagement during task switching is more specifically related to reading and math abilities than an inhibition task is related to reading, and that is why we see more results here. As noted in the introduction, cognitive flexibility may uniquely predict academic skills (Magalhães et al., 2020). These results could also be related to the difference in economic disadvantage in our sample relative to less disadvantaged samples (please see Beyond Bilingualism discussion below). We acknowledge these ideas are speculative given the multiple differences across these studies (see Limitations), but we are excited for our group and others to test these ideas in future work.
The Role of Cognitive Control, Attention, and Default Mode Networks in Reading and Math
In the current study, we found that more positive engagement of cognitive control, attention, and default mode networks during a non-academic task was related to poorer academic skills in middle school ELs. Both reading and math skills were negatively related to brain activity in the basal ganglia, cingulo-opercular cognitive control, and ventral attention networks. Previous work has found that during a sentence comprehension task, activity in the dorsal anterior cingulate, a key region of the cingulo-opercular network, was related to out-of-scanner reading comprehension ability (Roe et al., 2018). Functional connectivity of the cingulo-opercular network and ventral attention network has also been related to reading skills (Freedman, Zivan, Farah, & Horowitz-Kraus, 2020). A meta-analysis of fMRI math tasks found regions belonging to these networks were consistently engaged across studies (Arsalidou, Pawliw-Levac, Sadeghi, & Pascual-Leone, 2018). The basal ganglia and specifically the putamen have been shown to engage during number processing tasks (Arsalidou & Taylor, 2011) and show different structure and function in children with and without dyslexia (Wang et al., 2019). Our work thus adds to the growing indication of the importance of these brain networks and the basal ganglia to academic skills and finds that less engagement of these regions to accomplish a cognitive flexibility task is associated with better academic skills.
Reading skills (but not math skills) were also related to regions of the frontoparietal cognitive control network. The frontoparietal network, which is thought to support moment-to-moment cognitive control (Power & Petersen, 2013), is also related to change in reading skills over time or intervention (Aboud et al., 2018; Horowitz-Kraus, DiFrancesco, Kay, Wang, & Holland, 2015) and differs in children with reading difficulties (Margolis et al., 2019). Our results suggest that those who engaged frontoparietal regions less to perform the cognitive flexibility task were also the stronger readers.
Math fluency and reading skills were related to task-negative regions, which map onto the default mode network. Default mode brain activity has been found to relate to changes in reading skill (Nugiel et al., 2019). Default mode network functional connectivity has also been shown to predict math skills (Lynn, Wilkey, & Price, 2021). Greater suppression of default mode regions can relate to greater attention to the external rather than internal environment (Raichle, 2015). It is interesting that the greater negative activity we observe in these default mode regions yielding higher test scores would often be associated with an observation of greater engagement of task-positive control networks, but here we observe less control-related engagement being associated with better academic skills. Less brain engagement for a given task has been suggested to reflect more efficient neural processing (Dunst et al., 2014; Newman & Just, 2005) with communication across brain regions requiring less effort and resources, which is consistent with our results in task positive networks. The overall lower engagement pattern we observe during task performance associated with better academic skills may therefore reflect lower effort with the cognitive flexibility task in better-skilled ELs.
Relations between Language Proficiency and Cognitive Flexibility Brain Activity Only Emerge When Co-varying Academic Measures
There is evidence that cognitive flexibility or ‘shifting’ between languages and cognitive flexibility for non-linguistic tasks recruit similar brain architecture (Abutalebi & Green, 2016; Weissberger, Gollan, Bondi, Clark, & Wierenga, 2015), and that non-language brain systems mediate representations of two languages (Hernandez, 2009). Given this, we predicted that, along with academic skills, language proficiency would be related to cognitive flexibility task brain activity. However, we found no relation between our primary models of measures of English or Spanish language proficiency or English vocabulary and brain activity. Effects only reached significance when controlling for reading fluency or word reading (English proficiency), or math fluency (Spanish proficiency). One reason we may not have seen a simple relation in our sample is due to the developmental window we are examining. There is previous work suggesting that the interaction of bilingualism and cognitive control is non-linear across development (Hernandez, Claussenius-Kalman, Ronderos, & Vaughn, 2018). It is possible the relation between language proficiency and cognitive flexibility brain activity is stronger earlier in life as the neural architecture supporting both is developing, or later in life as the brain is changing with aging (Kupis et al., 2021). The relation between language proficiency and brain activity only after accounting for academic skill differences could also speak to a more general relation between cognitive flexibility and academic learning in this age group, irrespective of language proficiency and bilingual status. Certainly, the main results found in academic skill correlations largely held when controlling for English or Spanish proficiency in the model, though covarying with English proficiency impacted results more than covarying with Spanish. This language-specific result could be because the academic skills are taught in the English context and thus share more variance with self-rated English abilities.
Examining cognitive flexibility brain activity in a sample of students who are native English speakers would help clarify these proficiency results, though our sample was also highly economically disadvantaged, and matching socioeconomic backgrounds is challenging. It is also possible that other measures of English or Spanish proficiency that are not self-reported would show a stronger relation to cognitive flexibility. Objective measures of language processing, in particular measures of managing both languages or switching between languages on a daily basis, will be helpful in future studies of ELs.
Beyond Bilingualism – Other Factors Shaping Learning in ELs
A lot of the neuroimaging literature we used to frame the current study comes from a body of work done with bilingual individuals and a different body of work done in predominantly monolingual struggling students. While these literatures are helpful for contextualizing our findings, they do not map perfectly to our sample. ELs are a unique sample: they are bilingual but, per their label, are not fluent in both languages, which could indicate unique neurocognitive language and learning processes. Further, the ELs in our sample are all Hispanic and from Texas. 95% of our sample qualified as economically disadvantaged, and more generally, about 85% of ELs in Texas are economically disadvantaged (Du et al., 2020). Low socioeconomic status has significant effects on brain development and function (Hackman & Farah, 2009; Rakesh, Zalesky, & Whittle, 2021) and academic achievement (Romeo et al., 2017; Sirin, 2005). Thus, there are several formative interacting factors (socioeconomic status, bilingualism, and limited language proficiency) shaping neurocognitive processes and learning in our sample. The current study identifies a cognitive control task that is related to academic skills in ELs, and thus cognitive flexibility may be a factor to consider in identifying learning disabilities or designing interventions or research studies for these students. There is a lot of work to be done to both identify more factors important for learning in this group and to tease apart how these different factors shape learning in ELs. ELs are a fast-growing population in the US (English Learners: Demographic Trends, 2020), making it increasingly important to both include these students in studies of academic abilities more generally, and in specific focus on their unique needs.
Limitations
Using neuroimaging to understand individual variation and predictors of learning outcomes is a still-developing branch of cognitive neuroscience. As this field grows, it is crucial to increase diversity in the field at all levels and to include marginalized, underrepresented populations (Qu, Jorgensen, & Telzer, 2021). We belive this work makes a considerable contribution to this end by focusing on neurocognition in mostly economically disadvantged middle school ELs. One notable limitation is, the current study only has data from ELs, which prevents direct comparisons between ELs and monolingual grade-matched learners. However, we are fortunate to have a large literature of English monolingual studies, particularly in reading development, that we can use to interpret our results. Future studies that collect measures of academic skills and fMRI cognitive control tasks from multiple groups and less-represented individuals in neuroimaging (ELs, monolingual individuals, different language speakers, different socioeconomic levels, different ages, etc.) will be helpful in understanding the specificity vs. generality of our findings.
CONCLUSIONS
ELs are an understudied, underserved, and rapidly growing group of students in U.S. schools. In order to best support their learning, it is important to understand neurobiological factors that are related to their academic achievements. This analysis presents evidence that cognitive control brain engagement, as assessed during a cognitive flexibility task, is related to both reading and math skills in middle-school ELs. These effects were observed in brain regions that are also engaged in reading and math tasks in monolingual students. This work is an important step toward understanding the nuanced differences and variability within brain systems supporting academic skills in middle school and in ELs and paves the way for future studies with the ultimate goal of improving educational outcomes for these students.
Supplementary Material
Appendix S1. Supplmentary methods.
Acknowledgments—
We would like to thank the Biomedical Imaging Center at UT Austin, the CAMRI Center at the Baylor College of Medicine, and the UT Meadows Center for Prevention of Educational Risk. We thank Phillip Capin at UT Austin and Jeremy Miciak, Pat Taylor, and Henry Garcia at the University of Houston for their critical roles in the assessment and intervention arms of the TCLD project. We thank Mary Abbe Roe, Tyler Larguinho, Melissa Aristizabal, Isadora Costa, Montserrat Gonzalez Alonso, Mariana Rios, Yadira Plata, Nancy Licona, Marianne Coppola, Juan Carlos Ocampo, Arlen Rodriguez, Alice Aizza, Alejandra Gonzalez, and Miriam Ortega for assistance with data collection and management. We thank our participating families for their time and effort. The current study was supported by Eunice Kennedy Shriver National Institute for Child Health and Human Development T32HD40127 and P50HD052117 (PI: J.M. Fletcher) and NIMH 1F32MH127877-01A1 awarded to TN. The content is solely the responsibility of the authors and does not necessarily reflect the views of the funders.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
ETHICAL APPROVAL
The Institutional Review Board of the University of Texas Health Science Center at Houston approved the study for both Austin and Houston sites. Parents gave written consent for their child to participate in the research, and children gave their informed assent. English-Spanish bilingual research assistants conducted consent processes to ensure all families and participants were fully aware of all proceedings. Participation was voluntary, and families were informed they could terminate participation at any time. Participants were compensated for their time, and families were compensated for travel.
DATA AVAILABILITY STATEMENT
Upon publication acceptance, a publicly available repository on the Open Science Framework will be populated with the following: Group level thresholded and unthresholded z statistic maps for all the contrasts used in the analyses across the manuscript and supplementary materials, and behavioral variables (academic skills, language proficiency, and task performance for all participants). Since this is an ongoing longitudinal study, individual raw neuroimaging data will not be put in the repository.
REFERENCES
- Aboud KS, Barquero LA, & Cutting LE (2018). Pre-frontal mediation of the reading network predicts intervention response in dyslexia. Cortex, 101, 96–106. 10.1016/j.cortex.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutalebi J, & Green DW (2016). Neuroimaging of language control in bilinguals: Neural adaptation and reserve. Bilingualism: Language and Cognition, 19(4), 689–698. 10.1017/S1366728916000225 [DOI] [Google Scholar]
- Albus DA, Lazarus SS, & Thurlow ML (2018). 2017–18 Publicly Reported Assessment Results for Students with Disabilities and English Learners with Disabilities (NCEO Report #419). 94. [Google Scholar]
- Anderson JAE, Mak L, Keyvani Chahi A, & Bialystok E (2018). The language and social background questionnaire: Assessing degree of bilingualism in a diverse population. Behavior Research Methods, 50(1), 250–263. 10.3758/s13428-017-0867-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, Pawliw-Levac M, Sadeghi M, & Pascual-Leone J (2018). Brain areas associated with numbers and calculations in children: Meta-analyses of fMRI studies. Developmental Cognitive Neuroscience, 30, 239–250. 10.1016/j.dcn.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsalidou M, & Taylor MJ (2011). Is 2+2=4? Meta-analyses of brain areas needed for numbers and calculations. NeuroImage, 54(3), 2382–2393. 10.1016/j.neuroimage.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM, & Luk G (2012). Bilingualism: Consequences for mind and brain. Trends in Cognitive Sciences, 16(4), 240–250. 10.1016/j.tics.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria M, Costa A, Green D, & Abutalebi J (2018). Neural basis of bilingual language control. Annals of the New York Academy of Sciences, 1426, 221–235. 10.1111/nyas.13879 [DOI] [PubMed] [Google Scholar]
- Cho E, Capin P, Roberts G, Roberts GJ, & Vaughn S (2019). Examining sources and mechanisms of reading comprehension difficulties: Comparing English learners and non-English learners within the simple view of reading. Journal of Educational Psychology, 111(6), 982–1000. 10.1037/edu0000332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts J, Katsiyannis A, & Whitford DK (2018). Culturally and linguistically diverse learners in special education: English learners. NASSP Bulletin, 102(1), 5–21. 10.1177/0192636518755945 [DOI] [Google Scholar]
- de Araujo Z, Roberts SA, Willey C, & Zahner W (2018). English learners in K–12 mathematics education: A review of the literature. Review of Educational Research, 88(6), 879–919. 10.3102/0034654318798093 [DOI] [Google Scholar]
- De Smedt B, Holloway ID, & Ansari D (2011). Effects of problem size and arithmetic operation on brain activation during calculation in children with varying levels of arithmetical fluency. NeuroImage, 57(3), 771–781. 10.1016/j.neuroimage.2010.12.037 [DOI] [PubMed] [Google Scholar]
- Desmet C, Fias W, Hartstra E, & Brass M (2011). Errors and conflict at the task level and the response level. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(4), 1366–1374. 10.1523/JNEUROSCI.5371-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Murphy D, Ryon H, Wright B, Nagy S, Whalen C, & Kallus R (2020). Enrollment in Texas Public Schools, 2019–20. 78. [Google Scholar]
- Dunst B, Benedek M, Jauk E, Bergner S, Koschutnig K, Sommer M, … Neubauer AC (2014). Neural efficiency as a function of task demands. Intelligence, 42, 22–30. 10.1016/j.intell.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(33), E4929. 10.1073/pnas.1612033113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt LE, Harden KP, Tucker-Drob EM, & Church JA (2019). The neural architecture of executive functions is established by middle childhood. NeuroImage, 185(May 2018), 479–489. 10.1016/j.neuroimage.2018.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- English Learners: Demographic Trends. (2020). Nclea.Ed.Gov.
- Freedman L, Zivan M, Farah R, & Horowitz-Kraus T (2020). Greater functional connectivity within the cingulo-opercular and ventral attention networks is related to better fluent reading: A resting-state functional connectivity study. NeuroImage: Clinical, 26, 102214. 10.1016/j.nicl.2020.102214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B, & Crawford L (2008). Creating a middle school mathematics curriculum for English-language learners. Remedial and Special Education, 29(1), 9–19. 10.1177/0741932507309717 [DOI] [Google Scholar]
- Garbin G, Sanjuan A, Forn C, Bustamante JC, Rodriguez-Pujadas A, Belloch V, … Ávila C (2010). Bridging language and attention: Brain basis of the impact of bilingualism on cognitive control. NeuroImage, 53(4), 1272–1278. 10.1016/j.neuroimage.2010.05.078 [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, & Petersen SE (2016). Generation and evaluation of a cortical area Parcellation from resting-state correlations. Cerebral Cortex, 26(1), 288–303. 10.1093/cercor/bhu239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13(2), 65–73. 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Roberts GJ, Cho E, McCulley LV, Carroll M, & Vaughn S (2017). Reading instruction for English learners in the middle grades: A meta-analysis. Educational Psychology Review, 29(4), 763–794. 10.1007/s10648-016-9372-4 [DOI] [Google Scholar]
- Hernandez AE (2009). Language switching in the bilingual brain: What’s next? Brain and Language, 109(2), 133–140. 10.1016/j.bandl.2008.12.005 [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Claussenius-Kalman HL, Ronderos J, & Vaughn KA (2018). Symbiosis, parasitism and bilingual cognitive control: A Neuroemergentist perspective. Frontiers in Psychology, 9, 2171. 10.3389/fpsyg.2018.02171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, DiFrancesco M, Kay B, Wang Y, & Holland SK (2015). Increased resting-state functional connectivity of visual- and cognitive-control brain networks after training in children with reading difficulties. NeuroImage: Clinical, 8, 619–630. 10.1016/j.nicl.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska KK, & Petitto LA (2013). How age of bilingual exposure can change the neural systems for language in the developing brain: A functional near infrared spectroscopy investigation of syntactic processing in monolingual and bilingual children. Developmental Cognitive Neuroscience, 6, 87–101. 10.1016/j.dcn.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5, 143–156. [DOI] [PubMed] [Google Scholar]
- Kaufman A (2014) Kaufman Test of Educational Achievement. Bloomington, MN: NCS Pearson. [Google Scholar]
- Kieffer M, Vukovic RK, & Berry D (2013). Roles of attention shifting and inhibitory control in fourth-grade reading comprehension. Reading Research Quarterly, 48(4), 333–348. 10.1002/rrq.54 [DOI] [Google Scholar]
- Kupis L, Goodman ZT, Kornfeld S, Hoang S, Romero C, Dirks B, … Uddin LQ (2021). Brain dynamics underlying cognitive flexibility across the lifespan. Cerebral Cortex, 31, 5263–5274. 10.1093/cercor/bhab156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi N, Frost SJ, Mencl WE, Sandak R, Pugh R, Landi N, … Pugh KR (2013). Neurobiological bases of Reading comprehension: Insights from neuroimaging studies of word-level and text-level processing in skilled and impaired readers. Reading & Writing Quarterly, 3569, 145–167. 10.1080/10573569.2013.758566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G, & Bialystok E (2013). Bilingualism is not a categorical variable: Interaction between language proficiency and usage. Journal of Cognitive Psychology, 25(5), 605–621. 10.1080/20445911.2013.795574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn A, Wilkey ED, & Price GR (2021). Predicting Children’s math skills from task-based and resting-state functional brain connectivity. Cerebral Cortex, 32, 4204–4214. 10.1093/cercor/bhab476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGinitie WH, MacGinitie RK, Maria K, & Dreyer LG (2000) Gates-MacGinitie reading tests. (4th ed.). Itasca, IL: Riverside. [Google Scholar]
- Magalhães S, Carneiro L, Limpo T, & Filipe M (2020). Executive functions predict literacy and mathematics achievements: The unique contribution of cognitive flexibility in grades 2, 4, and 6. Child Neuropsychology, 26(7), 934–952. 10.1080/09297049.2020.1740188 [DOI] [PubMed] [Google Scholar]
- Margolis AE, Pagliaccio D, Davis KS, Thomas L, Banker SM, Cyr M, & Marsh R (2019). Neural correlates of cognitive control deficits in children with reading disorder. Brain Imaging and Behavior, 14, 1531–1542. 10.1007/s11682-019-00083-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, … Just MA (2007). Brain activation during sentence comprehension among good and poor readers. Cerebral Cortex, 17(12), 2780–2787. 10.1093/cercor/bhm006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouthon M, Khateb A, Lazeyras F, Pegna AJ, Lee-Jahnke H, Lehr C, & Annoni J-M (2020). Second-language proficiency modulates the brain language control network in bilingual translators: An event-related fMRI study. Bilingualism: Language and Cognition, 23(2), 251–264. 10.1017/S1366728918001141 [DOI] [Google Scholar]
- National Assessment of Educational Progress (2015) A report card for the nation and the states. Washington, DC: National Center for Educational Statistics. [Google Scholar]
- Newman S, & Just M (2005) The neural bases of intelligence: A perspective based on functional neuroimaging. In: Cognition and intelligence: Identifying the mechanisms of the mind (pp. 88–103). New York: Cambridge University Press. [Google Scholar]
- Nugiel T, Abbe M, Taylor WP, Cirino PT, Vaughn SR, Fletcher JM, … Church JA (2019). Brain activity in struggling readers before intervention relates to future reading gains. Cortex, 111, 286–302. 10.1016/j.cortex.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugiel T, Abbe Roe M, Engelhardt LE, Mitchell ME, Zheng A, & Church JA (2020). Pediatric ADHD symptom burden relates to distinct neural activity across executive function domains. NeuroImage: Clinical, 102394, 102394. 10.1016/j.nicl.2020.102394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L (2014). Meeting the unique needs of long term English language learners. Washington, DC: National Education Association. [Google Scholar]
- Peters L, & De Smedt B (2018). Arithmetic in the developing brain: A review of brain imaging studies. Developmental Cognitive Neuroscience, 30, 265–279. 10.1016/j.dcn.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35(1), 73–89. 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, & Petersen SE (2013). Control-related systems in the human brain. Current Opinion in Neurobiology, 23(2), 223–228. 10.1016/j.conb.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Jorgensen NA, & Telzer EH (2021). A call for greater attention to culture in the study of brain and development. Perspectives on Psychological Science, 16(2), 275–293. 10.1177/1745691620931461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME (2015). The brain’s default mode network. The Annual Review of Neuroscience, 38, 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Rakesh D, Zalesky A, & Whittle S (2021). Similar but distinct – Effects of different socioeconomic indicators on resting state functional connectivity: Findings from the adolescent brain cognitive development (ABCD) study®. Developmental Cognitive Neuroscience, 51, 101005. 10.1016/j.dcn.2021.101005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD, & Fischl B (2010). Highly accurate inverse consistent registration: A robust approach. NeuroImage, 53(4), 1181–1196. 10.1016/j.neuroimage.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Pujadas A, Sanjuán A, Ventura-Campos N, Román P, Martin C, Barceló F, … Ávila C (2013). Bilinguals use language-control brain areas more than monolinguals to perform non-linguistic switching tasks. PLoS One, 8(9), e73028. 10.1371/journal.pone.0073028.s004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe MA, Martinez JE, Mumford JA, Taylor WP, Cirino PT, Fletcher JM, … Church JA (2018). Control engagement during sentence and inhibition fMRI tasks in children with Reading difficulties. Cerebral Cortex, 28, 3697–3710. 10.1093/cercor/bhy170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RR, Christodoulou JA, Halverson KK, Murtagh J, Cyr AB, Schimmel C, … Gabrieli JDE (2017). Socioeconomic status and Reading disability: Neuroanatomy and plasticity in response to intervention. Cerebral Cortex, 1–16, 2297–2312. 10.1093/cercor/bhx131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JS, Power JD, Dubis JW, Vogel AC, Church JA, Schlaggar BL, & Petersen SE (2014). Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high-motion data points. Human Brain Mapping, 35(5), 1981–1996. 10.1002/hbm.22307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirin SR (2005). Socioeconomic status and academic achievement: A meta-analytic review of research. Review of Educational Research, 75(3), 417–453. 10.3102/00346543075003417 [DOI] [Google Scholar]
- Sokolowski HM, Fias W, Mousa A, & Ansari D (2016). Common and distinct brain regions in both parietal and frontal cortex support symbolic and nonsymbolic number processing in humans_ a functional neuroimaging meta-analysis. NeuroImage, 1–19, 376–394. 10.1016/j.neuroimage.2016.10.028 [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2021). Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nature Reviews Neuroscience, 1–13, 167–179. 10.1038/s41583021-00428-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC (2012). Cortical cartography and caret software. NeuroImage, 62(2), 757–764. 10.1016/j.neuroimage.2011.10.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinerte S, & Sabourin L (2019). Reviewing the bilingual cognitive control literature: Can a brain-based approach resolve the debate? Canadian Journal of Experimental Psychology, 73(2), 118–134. 10.1037/cep0000174 [DOI] [PubMed] [Google Scholar]
- Wang K, Leopold DR, Banich MT, Reineberg AE, Willcutt EG, Cutting LE, … Petrill SA (2019). Characterizing and decomposing the neural correlates of individual differences in reading ability among adolescents with task-based fMRI. Developmental Cognitive Neuroscience, 37, 100647. 10.1016/j.dcn.2019.100647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yan X, Liu Y, Spray GJ, Deng Y, & Cao F (2019). Structural and functional abnormality of the putamen in children with developmental dyslexia. Neuropsychologia, 130, 26–37. 10.1016/j.neuropsychologia.2018.07.014 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weissberger GH, Gollan TH, Bondi MW, Clark LR, & Wierenga CE (2015). Language and task switching in the bilingual brain_ bilinguals are staying, not switching, experts. Neuropsychologia, 66, 193–203. 10.1016/j.neuropsychologia.2014.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkey ED, & Price GR (2019). Attention to number: The convergence of numerical magnitude processing, attention, and mathematics in the inferior frontal gyrus. Human Brain Mapping, 40(3), 928–943. 10.1002/hbm.24422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, & Wager TD (2014). Cluster-extent based thresholding in fMRI analyses: Pitfalls and recommendations. NeuroImage, 91, 412–419. 10.1016/j.neuroimage.2013.12.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, & Mather N (2001) Woodcock-Johnson III tests of achievement. Itasca, IL: Riverside. [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, & Smith SM (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage, 14(6), 1370–1386. 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- Worsley KJ (2001). Statistical analysis of activation images. In Functional MRI: An introduction to methods. (pp. 251–270). Oxford, UK: Oxford University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplmentary methods.
Data Availability Statement
Upon publication acceptance, a publicly available repository on the Open Science Framework will be populated with the following: Group level thresholded and unthresholded z statistic maps for all the contrasts used in the analyses across the manuscript and supplementary materials, and behavioral variables (academic skills, language proficiency, and task performance for all participants). Since this is an ongoing longitudinal study, individual raw neuroimaging data will not be put in the repository.


