Abstract
Background:
Fungal infections are common in HIV-infected individuals and significantly contribute to mortality. However, a substantial number of cases are undiagnosed before death.
Objective:
To determine the frequency of fungal pathogens in autopsy studies of people who died with HIV in Africa.
Methods:
We conducted a scoping review of autopsy studies conducted in Africa.
Data sources:
PubMed, Scopus, Web of Science, Embase, Google Scholar, and African Journal Online.
Study eligibility criteria:
The review encompasses studies published from inception to September 2023, and no language restrictions were imposed during the search process. We included studies that reported histopathological or microbiological evidence for the diagnosis of fungal infections and other pathogens.
Data synthesis:
Data were summarized using descriptive statistics and no meta-analysis was performed.
Results:
We examined 30 articles reporting studies conducted between 1991 and 2019, encompassing a total of 13 066 HIV-infected decedents across ten African countries. In five studies, the autopsy type was not specified. Among those studies with specified autopsy types, 20 involved complete diagnostic au topsies, whereas 5 were categorized as partial or minimally invasive autopsies. There were 2333 pathogens identified, with 946 (40.5%) being mycobacteria, 856 (36.7%) fungal, 231 (3.8%) viral, 208 (8.9%) parasitic, and 92 (3.9%) bacterial. Of the 856 fungal pathogens identified, 654 (28.0%) were Cryptococcus species, 167 (7.2%) Pneumocystis jirovecii, 16 (0.69%) Histoplasma species, 15 (0.64%) Aspergillus species, and 4 (0.17%) Candida species. Other major non-fungal pathogens identified were cytomegalovirus 172 (7.37%) and Toxoplasma gondii 173 (7.42%).
Conclusions:
Invasive fungal infections occur in over one-third of people who succumb to HIV in Africa. In addition to cryptococcosis and Pneumocystis jirovecii pneumonia, integrating other priority fungal pathogen detection and management strategies into the broader framework of HIV care in Africa is recommended. This involves increasing awareness regarding the impact of fungal infections in advanced HIV disease and strengthening diagnostic and treatment capacity.
Keywords: Advanced HIV disease, Africa, AIDS, Autopsy, Fungal pathogens, Opportunistic infections
Introduction
The burden of HIV remains substantial, with an estimated 39 million people living with HIV worldwide at the end of 2022, and approximately 82% of all people living with HIV are in Africa and Asia and the Pacific [1]. Although the advent of antiretroviral therapy (ART) has significantly improved the life expectancy and quality of life for many individuals with HIV [2,3], the interplay between HIV infection and other infectious diseases such as tuberculosis (TB) and fungal diseases such as cryptococcosis remains a critical factor influencing clinical outcomes [2,4,5].
In Africa, HIV-related mortality remains high, particularly among ART-naïve people living with HIV and those in their 1st year of ART, with 5%e30% of deaths occurring among hospitalized patients [6–9]. Although the leading causes of HIV-related deaths, such as TB, Pneumocystis jirovecii pneumonia (PCP), and cryptococcosis, are well recognized, there is growing evidence to suggest that other fungal infections such as histoplasmosis, aspergillosis, emergomycosis, and other HIV-related mycoses may be underrecognized and significant contributors to mortality among individuals with advanced HIV disease, especially in Africa where diagnostics for fungal infections are not widely available [10–12].
Pathological autopsies are the reference standard to establish causes of death and provide a unique window into the pathophysiology of fatal outcomes in individuals with HIV [13,14]. An early literature review by Cox et al. [13] identified nine complete and 11 partial or minimally invasive autopsy series. This review, which included 593 HIV-positive adults and 177 HIV-positive children, found that infectious diseases were the main causes of death in Africa, with TB being the most frequent. TB was present in 21%−54% of HIV-positive adults and was considered the cause of death in 32%−45%. However, this review included studies conducted on both HIV-infected and HIV-uninfected decedents and was published over 10 years ago. A more recent systematic review involving autopsied patients with HIV in Africa focused on only TB and non-tuberculosis mycobacterial (NTM) infections [15].
With more autopsy studies recently published [7,16–28], we conducted this scoping review aimed at synthesizing existing evidence from autopsy studies conducted in Africa to discern the role of fungal infections in contributing to mortality in individuals living with HIV. Therefore, the overall objective of this study was to assess and quantify the prevalence of infectious pathogens in autopsy studies conducted on individuals who died with HIV in Africa, with the goal of determining the proportion of fungal pathogens among all identified pathogens.
Methods
Study design
We conducted a scoping review of literature adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines [29] that ensured transparent and comprehensive reporting.
Research question
The research question for this study was designed in accordance with the Population, Exposure, and Outcome framework.
Population (P): Children and adults who died with HIV in Africa.
Exposure (E): Autopsy studies.
Outcome (O): Proportion of fungal pathogens among all pathogens identified.
The final scoping review question was then formulated as ‘What is the proportion of fungal pathogens among all pathogens identified in autopsy studies of individuals who died with HIV in Africa?’
Search strategy
With the help of a qualified medical librarian (BM), a systematic literature search was conducted using the following electronic databases: PubMed, Scopus, Embase, Web of Science, and African Journal Online. The search strategy was tailored to emphasize autopsy studies and encompassed keywords and medical subject headings related to ‘HIV’ OR ‘AIDS’, ‘autopsy’ OR ‘post-mortem’, ‘infectious diseases’ or individual aetiology such as hepatitis B virus, hepatitis C virus, cytomegalovirius (CMV), TB, etc., ‘fungi’ OR individual pathogen or disease such as ‘Pneumocystis jirovecii’ OR ‘Pneumocystosis’ OR ‘PCP’, ‘Aspergillus’ OR ‘Aspergillosis’ OR ‘Aspergill*’, etc., AND ‘Africa’. Boolean operators were used to refine the search and ensure inclusivity. No language restriction was applied.
Inclusion and exclusion criteria
We included autopsy studies conducted in Africa, involving individuals (both children and adults) diagnosed with HIV, reporting histopathological or microbiological confirmed infectious pathogens. We included both single case reports and large autopsy series. Verbal autopsies and studies not indicating how pathogens were identified or not reporting absolute number of pathogens identified were excluded.
Selection process
Two independent reviewers (WK and LA) conducted the initial screening of titles and abstracts, focusing on autopsy-related studies. Full-text assessment was performed for selected articles. Discrepancies in inclusion/exclusion decisions were resolved through discussion or consultation with a third reviewer (FB).
Data extraction
A standardized data extraction form was developed by FB, and pilot tested by WK and FB. Extracted data encompassed study characteristics, autopsy methodologies (full or minimally invasive), associated pathogen identified, and specific details on fungal contributions to mortality. Data extraction was independently conducted by two reviewers (WK and LA) and any discrepancies were discussed with and resolved by FB.
Data analysis
We conducted a narrative synthesis using Microsoft Excel summarizing key information on autopsy studies, infectious diseases, and the specific contribution of fungi to mortality as frequencies and percentages. No meta-analysis was conducted.
Ethical considerations
Because the review involves the analysis of publicly available, previously published studies, ethical approval is not applicable.
Results
Selection of sources of evidence
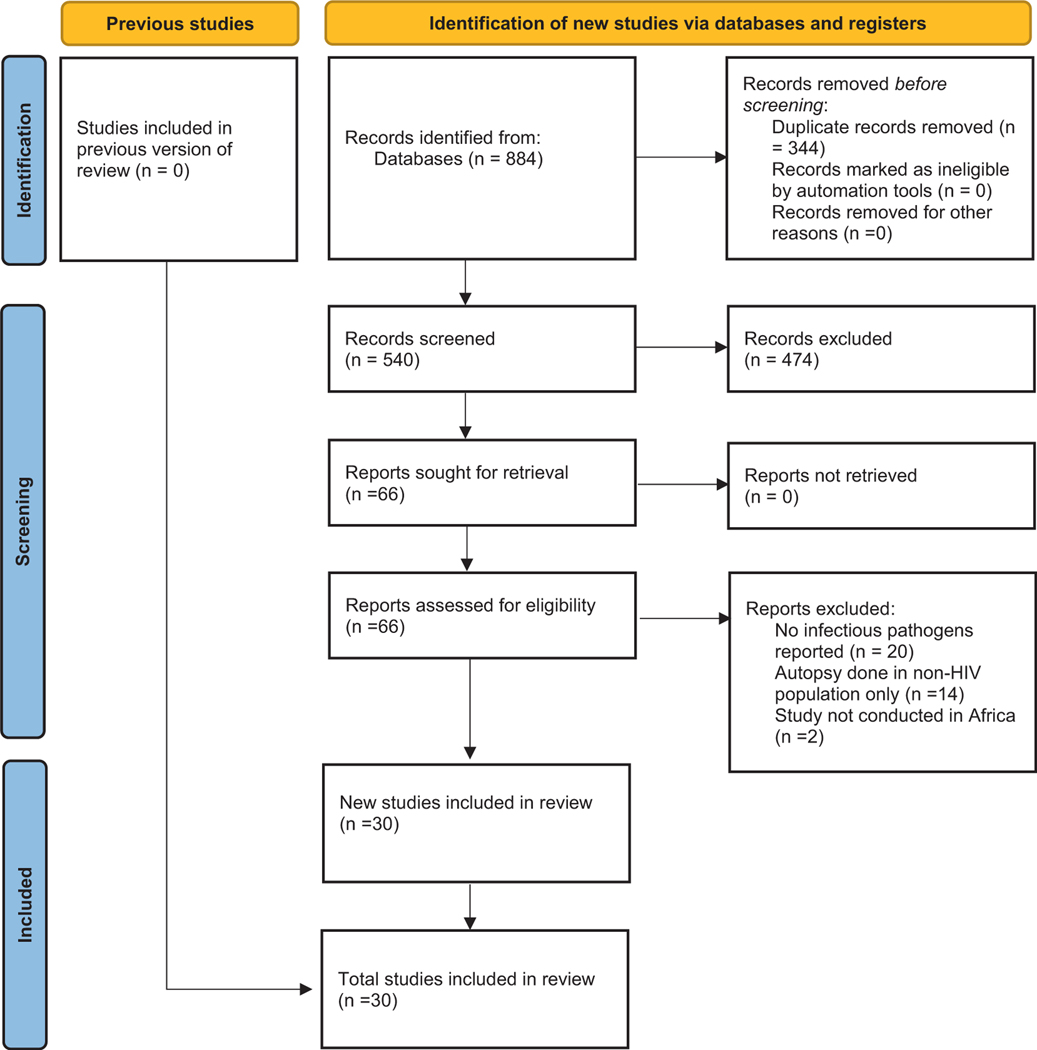
Of the 884 citations retrieved, 344 duplicate records were removed. We excluded 473 articles that were not within the scope and accessed 66 full texts for review. We excluded 26 full texts for reasons such as no infectious pathogens reported (n = 20), autopsy performed in non-HIV population only (n = 14), and study not conducted in Africa (n = 2). We included 30 unique articles in the final review (Fig. 1).
Fig. 1.
PRISMA flow diagram.
Demographics
We included 30 articles reporting studies conducted between 1991 and 2019, involving a total of 13 066 HIV-infected decedents from ten countries across Africa: South Africa (8 studies), followed by Kenya and Mozambique (4 studies each), Uganda (4 studies), Ghana (2 studies), Tanzania (3 studies), Cote d’Ivoire (2 studies), and Nigeria, Zambia, and Zimbabwe each represented by a single study (Table 1) [30–48].
Table 1.
Characteristics and summary of the studies included
| S/n | Author/year | Country | Study period | Design | Number of autopsies on people who died with HIV | Autopsy type | Fungal | Non-fungal |
|---|---|---|---|---|---|---|---|---|
| N1 | Onyango et al., 2022 [28] | Kenya | 2018–2019 | Prospective | 176 | Complete | – | K. pneumoniae (n = 16), adenovirus (n = 3), CMV (n = 3), S. pneumoniae (n = 4) |
| 2 | Letang et al., 2021 [30] | Mozambique | 2013–2015 | Prospective | 164 | Partial | Cryptococcus spp (n = 12), P. jirovecii (n = 3) | M. tuberculosis (n = 25), T. gondii (n = 15), P. falciparum (n = 3) |
| 3 | Njuguna et al., 2021 [27] | Kenya | 2014–2015 | Prospective | 64 | Complete | P. jirovecii (n = 7) | RSV (n = 7), Influenza A virus (n = 5), S. pneumoniae (n = 5) |
| 4 | Khaba et al., 2020 [31] | South Africa | NS | Case report | 1 | Complete | – | SARS-CoV-2 (n = 1) |
| 5 | Hurtado et al., 2019 [32] | Mozambique | 2013–2015 | Prospective | 284 | Complete | C. gattü (n = 5), C. neoformans var. grubii (n = 10), Cryptococcus spp (n = 2), Histoplasma spp (n = 1), Candida spp (n = 2) | T. gondii (n = 1), M tuberculosis (n = 2), S. pneumoniae (n = 1), CMV (n = 1) |
| 6 | Wake et al., 2019 [24] | South Africa | 2015–2017 | Prospective | 201 | Partial | Cryptococcus spp (n = 67), Histoplasma spp (n = 3) | – |
| 7 | Raphael et al., 2018 [33] | Nigeria | NS | Prospective | 754 | NS | Cryptococcus spp (n = 1), P. jirovecii (n = 1) | M. tuberculosis (n = 8) |
| 8 | Castillo et al., 2017 [34] | Mozambique | 2013–2015 | Prospective | 57 | Complete | Cryptococcus spp (n = 4) | M. tuberculosis (n = 5), P. falciparum (n = 4) |
| 9 | Castillo et al., 2015 [18] | Mozambique | 2013 | Prospective | 30 | Partial | (P. jirovecii (n = 2), Cryptococcus spp (n = 2) | HBV (n = 2), HHV 8 (n = 1), T. gondii (n = 2), Acinetobacter baumannii (n = 1), S. pneumoniae (n = 1), CMV (n = 1), M. tuberculosis (n = 3), M. hominis (n = 1), Enterococcus faecalis (n = 1), Streptococcus dysgalactiae (n = 1) |
| 10 | Lartey et al., 2015 [7] | Ghana | 2007 | Retrospective | 135 | Complete | Cryptococcus spp (n = 4) | M. tuberculosis (n = 69), T. gondii (n = 26) |
| 11 | Akakpo et al., 2014 [35] | Ghana | 2014 | Case Report | 1 | Complete | Cryptococcus spp (n = 1) | 0 |
| 12 | Cox et al., 2014 [36] | Uganda | 2013 | Prospective | 191 | Complete |
Cryptococcus spp (n = 13), P. jirovecii (n = 3), Aspergillus niger (n = 2), C. albicans (n = 1) |
M. tuberculosis(n = 42) |
| 13 | Kilale et al., 2013 [21] | Tanzania | NS | Prospective | 74 | NS | – | M. tuberculosis (24) |
| 14 | Wong et al., 2012 [37] | South Africa | 2009 | Prospective | 39 | Partial | Cryptococcus spp (n = 4), C. albicans (n = 1) | M. tuberculosis (n = 17), HBV (n = 1), K. pneumoniae (n = 3), A. baumannii (n = 1), Enterobacter cloacae (n = 1), E. coli (n = 2), Salmonella enterica (n = 1), Enterobacter spp (n = 2), Acinetobacter spp (n = 2), C. difficile (n = 2), Clostridium spp (n = 1), E. faecium (n = 1), Proteus mirabilis (n = 1) |
| 15 | Kabangila et al., 2011 [38] | Tanzania | NS | Case report | 1 | NS | H. capsulatum (n = 1) | – |
| 16 | Kyeyune et al., 2010 [39] | Uganda | 2007–2008 | Prospective | 407 | Complete | P. jirovecii (n = 3), Cryptococcus spp (n = 1) | M. tuberculosis (n = 63) |
| 17 | Cohen et al., 2010 [40] | South Africa | 2008–2009 | Prospective | 240 | Partial | – | M. tuberculosis (n = 189), NTM (n = 3) |
| 18 | Garcia-Jardon et al., 2010 [41] | South Africa | 2000–2008 | Prospective | 86 | Complete | Cryptococcus spp (n = 6) | M. tuberculosis (n = 32), T. gondii (n = 1) |
| 19 | Wong et al., 2007 [26] | South Africa | 1996–2000 | Prospective | 3790 | Complete | Cryptococcus spp (n = 490), P. jirovecii (n = 50), A. niger (n = 2) | M. tuberculosis (n = 41), CMV (n = 2) |
| 20 | Ng’walali et al., 2005 [42] | Tanzania | 1997–1999 | Prospective | 143 | Complete | Cryptococcus spp (n = 6) | M. tuberculosis (n = 13), T. gondii (n = 1), Bacteria unspecified (n = 10) |
| 21 | Ateenyi-Agaba et al, 2005 [43] | Uganda | 2002 | Prospective | 136 | Complete | – | HPV (n = 8) |
| 22 | Ruffini et al., 2002 [44] | South Africa | 1999 | Prospective | 121 | Partial | P. jirovecii (n = 14) | M. tuberculosis (n = 1), H. influenzae (n = 1), P. aeruginosa (n = 1), RSV (n = 1), S. pneumoniae (n = 1), CMV (n = 8), Salmonella spp (n = 1) |
| 23 | Chintu et al., 2002 [45] | Zambia | 1997–2000 | Prospective | 264 | Complete | P. jirovecii (n = 52) | M. tuberculosis (n = 32), CMV (n = 40), Measles virus (n = 5) |
| 24 | Nathoo et al., 2001 [22] | Zimbabwe | 1995 | Prospective | 24 | NS | P. jirovecii (n = 6) | K. pneumoniae (n = 2), S. aureus (n = 3), CMV (n = 2), Bacteria unspecified (n = 1) |
| 25 | Rana et al., 2000 [19] | Kenya | 1996–1997 | Prospective | 122 | Complete | – | M. tuberculosis (n = 38) |
| 26 | Orem et al., 1998 [46] | Uganda | NS | Case report | 1 | Complete | A. fumigatus (n = 1) | – |
| 27 | Bell et al., 1997 [47] | Cote d’Ivoire | NS | Prospective | 78 | Complete | P. jirovecii (n = 2) | M. tuberculosis (n = 1), CMV (n = 11), T. gondii (n = 3) |
| 28 | Rana et al., 1997 [20] | Kenya | 1995 | Prospective | 9 | Complete | P. jirovecii (n = 2), Cryptococcus spp (n = 1) | M. tuberculosis (n = 3), CMV (n = 1), K. pneumoniae (n = 1), non-typhoidal Salmonella (n = 1) |
| 29 | Jeena et al., 1996 [48] | South Africa | 1995 | Prospective | 72 | Complete | P. jirovecii (n = 2) | CMV (n = 8) |
| 30 | Lucas et al., 1993 [16] | Cote d’Ivoire | 1991 | Prospective | 5401 13 066 |
Complete | Cryptococcus spp (n = 21), Histoplasma spp (n = 11), Aspergillus spp (n = 10), P. jirovecii (n = 20) |
M. tuberculosis (n = 268), NTM (n = 14), CMV (n = 95), HSV (n = 10), VZV (n = 15), T. gondii (n = 98), Cryptosporidia spp (n = 17), S. stercoralis (n = 11), Nocardia spp (n = 23) |
CMV, cytomegalovirius; HBV, hepatitis B virus; HHV, human herpes virus; HPV, human papilloma virus; HSV-herpes simplex virus; NTM, non-tuberculosis mycobacterial; RSV, respiratory syncytial virus; VZV, varicella-zoster
Study designs and autopsy types
Of the 30 studies included, 25 were prospective case series, four were case reports, and one was a retrospective chart review. In five studies, the autopsy type was not specified. Among those specified, 20 involved complete diagnostic autopsies, whereas 5 were categorized as partial or minimally invasive autopsies.
Pathogens
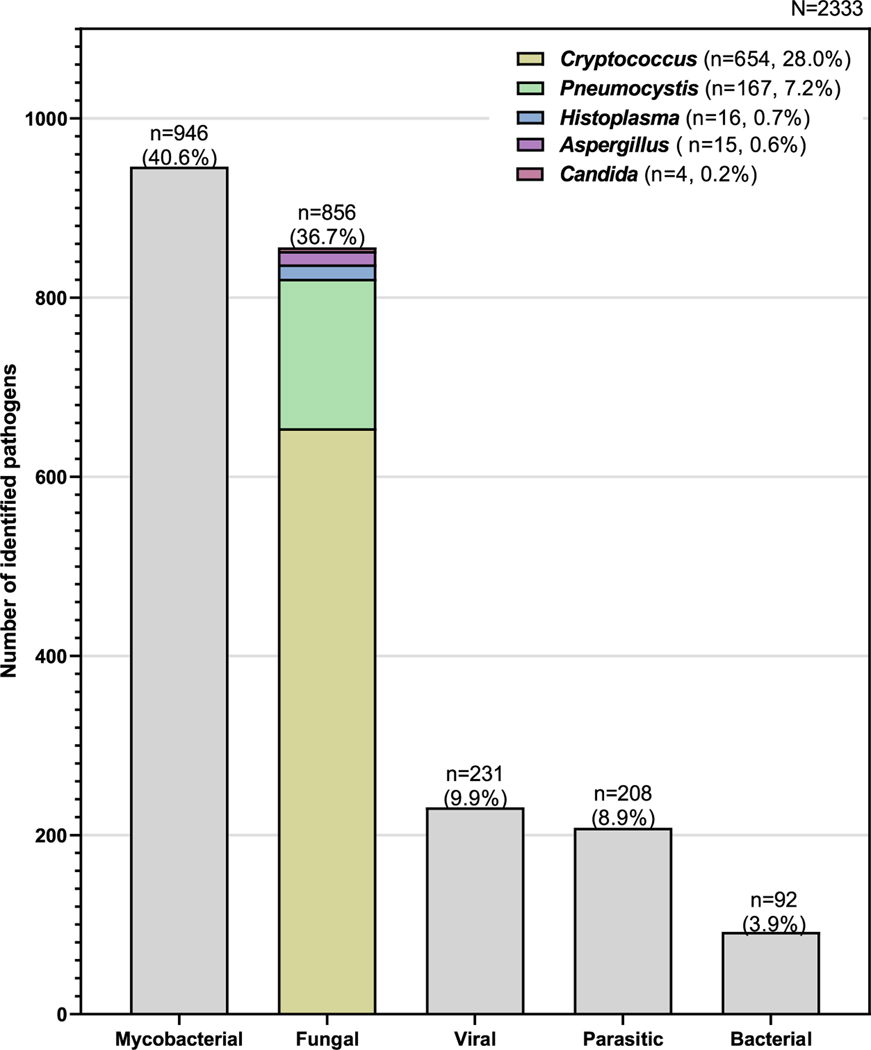
There were 2333 pathogens identified: 946 (40.5%) mycobacteria, 856 (36.7%) fungal, 231 (3.8%) viral, 208 (8.9%) parasitic, and 92 (3.9%) bacterial (Fig. 2, Table 2).
Fig. 2.
Distribution of pathogens identified following autopsy, highlighting the relative contribution of fungal pathogens.
Table 2.
Proportion of pathogens identified from autopsy samples
| Pathogen group | Frequency | %/group | %/total |
|---|---|---|---|
| Mycobacteria | |||
| Mycobacterium tuberculosis complex | 929 | 98.2 | 39.8 |
| Non-tuberculous mycobacteria | 17 | 1.8 | 0.73 |
| 946 | 40.6 | ||
| Fungi | |||
| Cryptococcus spp | 654 | 76.4 | 28.0 |
| Pneumocystis jirovecii | 167 | 19.5 | 7.16 |
| Histoplasma spp | 16 | 1.9 | 0.69 |
| Aspergillus spp | 15 | 1.8 | 0.64 |
| Candida spp | 4 | 0.5 | 0.17 |
| 856 | 36.7 | ||
| Viruses | |||
| Cytomegalovirus | 172 | 74.5 | 7.37 |
| Varicella-zoster virus | 15 | 6.5 | 0.64 |
| Herpes simplex virus | 10 | 4.3 | 0.43 |
| Human papillomavirus | 8 | 3.5 | 0.34 |
| Respiratory syncytial virus | 8 | 3.5 | 0.34 |
| Influenza A virus | 5 | 2.2 | 0.21 |
| Measles | 5 | 2.2 | 0.21 |
| Adenovirus | 3 | 1.3 | 0.13 |
| Hepatitis B virus | 3 | 1.3 | 0.13 |
| Severe acute respiratory syndrome coronavirus 2 | 1 | 0.4 | 0.04 |
| Human herpesvirus 8 | 1 | 0.4 | 0.04 |
| 231 | 9.9 | ||
| Parasites | |||
| Toxoplasma gondii | 173 | 83 | 7.42 |
| Cryptosporidium spp | 17 | 8 | 0.73 |
| Strongyloides stercoralis | 11 | 5 | 0.47 |
| Plasmodium falciparum | 7 | 3 | 0.30 |
| 208 | 8.9 | ||
| Bacteria | |||
| Nocardia spp | 23 | 25 | 0.99 |
| Klebsiella pneumoniae | 22 | 24 | 0.94 |
| Streptococcus pneumoniae | 12 | 13 | 0.51 |
| Unspecified | 11 | 12 | 0.47 |
| Enterobacter spp | 4 | 4 | 0.17 |
| Acinetobacter spp | 4 | 4 | 0.17 |
| Salmonella spp | 3 | 3 | 0.13 |
| Clostridia spp | 3 | 3 | 0.13 |
| Staphylococcus aureus | 3 | 3 | 0.13 |
| Escherichia coli | 2 | 2 | 0.09 |
| Haemophilus influenzae | 1 | 1 | 0.04 |
| Pseudomonas spp | 1 | 1 | 0.04 |
| Mycoplasma hominis | 1 | 1 | 0.04 |
| Streptococcus dysagalactiae | 1 | 1 | 0.04 |
| Proteus mirabilis | 1 | 1 | 0.04 |
| 92 | 3.9 | ||
| Grand total | 2333 | 100% |
Fungal pathogens
Of the 856 fungal pathogens identified, 654 (28.0%) were Cryptococcus spp, 167 (7.16%) Pneumocystis jirovecii, 16 (0.69%) Histoplasma species, 15 (0.64%) Aspergillus spp, and 4 (0.17%) Candida spp.
Mycobacterial
Of the 946 Mycobacteria identified, 929 (39.8%) were because of M. tuberculosis complex and 17 (0.73%) were identified as NTM.
Viral pathogens
Of the 231 viral infections identified, cytomegalovirus was the most prevalent, constituting 172 cases (7.37%). Varicella-zoster virus accounted for 15 cases (0.64%), whereas herpes simplex virus, human papillomavirus, respiratory syncytial virus, influenza A, measles virus, adenovirus, hepatitis B virus, SARS-CoV-2, and human herpesvirus eight each contributed to less than 1% of the cases.
Parasites
Of 208 parasitic infections, Toxoplasma gondii was identified in the majority, 173 cases (7.42%). Cryptosporidium species constituted 17 cases (0.73%), Strongyloides stercoralis contributed to 11 cases (0.47%), and Plasmodium falciparum was identified in seven cases (0.3%).
Bacteria
Of the 92 bacterial infections identified, Nocardia species accounted for 23 cases (0.99%), Klebsiella pneumoniae contributed to 22 cases (0.94%), Streptococcus pneumoniae accounted for 12 cases (0.51%), and unspecified bacteria were identified in 11 cases (0.47%). Other bacterial species, including Enterobacter spp., Acinetobacter spp., Salmonella spp, Clostridia spp., Staphylococcus aureus, Escherichia coli, Haemophilus influenzae, Pseudomonas spp., Mycoplasma hominis, Streptococcus dysagalactiae, and Proteus mirabilis, each contributed to less than 0.2% of the cases (Table 1).
Discussion
The AIDS epidemic, a global public health challenge for more than four decades, has resulted in approximately 40 million deaths [1,49,50]. In this review of autopsy studies involving 13 066 HIV-infected decedents, with over 2300 pathogens identified, 40.5% were mycobacterial and 36.7% were fungal. Our findings align with existing clinical epidemiological data, indicating that infectious diseases such as TB and cryptococcal meningitis continue to be leading causes of death among people with HIV globally [7,51–53]. Studies from Africa have shown that pulmonary infections account for two-thirds of pathology and central nervous system infections for approximately 20% in autopsy series [13].
Fungal infections, PCP in particular, were commonly identified in the AIDS epidemic in the early 1980s [49,54]. Before the introduction of ART, between 58% and 81% of patients with AIDS were observed to contract fungal infections at some time, and 10%−20% of them died as a direct consequence of such infections [55]. Despite widespread ART use, fungal diseases still occur in high frequency among people with advanced HIV disease, particularly in resource-limited settings where retention to care remains a challenge and almost one-third of patients enter care with advanced HIV disease [56,57]. Establishment of reflex screening for cryptococcosis and other opportunistic fungal diseases has been shown to improve diagnostic yield, early initiation of pre-emptive and targeted treatment, and improved clinical outcomes in Guatemala [58].
Cryptococcal meningitis is the leading cause of meningitis among adults living with HIV in Africa, along with toxoplasmosis and TB meningitis, they present the greatest cause of neurological morbidity and mortality [59–61]. We found cryptococcosis in 28% of all autopsied HIV individuals included in this study. An estimated 152 000 cases of cryptococcal meningitis, resulting in 112 000 cryptococcal-related deaths, accounted for 19% of AIDS-related mortality in 2020 with over 70% of these cases and substantial associated mortality occurring in Africa [5]. The introduction of cryptococcal antigen tests has simplified the screening and diagnosis of cryptococcal meningitis in resource-limited settings [62]. Therefore, most cases of cryptococcal meningitis are now diagnosed antemortem.
In this review, we found a low occurrence of histoplasmosis (0.64%), aspergillosis (0.17%), and candidiasis (0.2%). One of the earliest studies reviewed from Cote d’Ivoire found histoplasmosis in 11 cases, compared with 21 cases of cryptococcosis [16]. This speaks to under-diagnosis of histoplasmosis. Histoplasmosis in Africa has recently been highlighted by Oladele et al. [63] and Ocansey et al. [64]. However, some recent studies particularly from the West African region of Africa have shown increased report of histoplasmosis cases after advocacy, small pilot studies, and increased awareness among clinicians [65,66]. The most sensitive means of diagnosis disseminated histoplasmosis using antigen or PCR detection is not available in Africa [12]. Importantly, we have recently argued that histoplasmosis significantly overlaps with the TB-HIV syndemic in Africa owing to remarkable similarities in clinical presentation and co-infection [67]. Furthermore, a study from Calabar in Nigeria showed that approximately 13% of patients with presumptive pulmonary TB had histoplasmosis [68]. Despite a high level of prior subclinical histoplasmosis from studies utilizing histoplasmin skin tests in Africa [69,70], studies investigating active disease have yielded prevalence from as low as <1% to as high as 13% [68,71,72].
Aspergillosis has been well described to occur in advanced HIV disease [73]. A recent review of 853 cases of aspergillosis in people living with HIV from 16 countries (none from Africa) showed a very high mortality rate (707, 83%) with an average time from diagnosis to death of just over 2 months [74]. In this study, 21% of recorded cases of aspergillosis were diagnosed through post-mortem diagnosis. Bilateral pulmonary shadows, especially with cavitation, should alert clinicians to this diagnostic possibility. A multi-moded approach to the diagnosis of invasive aspergillosis involves a combination of respiratory fungal culture, serum, and bronchoalveolar lavage antigen detection and serum Aspergillus antibody is required to identify most cases. Chronic pulmonary aspergillosis did not figure in any of the studies reviewed.
TB is highly endemic in Africa and contributes to significant morbidity and mortality among both HIV-infected and HIV-uninfected people in this region [75]. We found that approximately 40% of patients with HIV who had autopsies performed had mycobacterial infection. Our findings are in line with a recent systematic review that found a prevalence of NTM infection and TB at autopsy to range from 1.3% to 27.3% and 11.8% to 48.7%, respectively [15]. PCP and acute bacterial infections, particularly TB, remain the leading respiratory causes of morbidity and mortality among people with advanced HIV disease [76,77]. In addition to early detection of drug resistance, the introduction of GeneXpert, a sensitive and rapid molecular assay, has revolutionized TB care in resource-limited settings and has significantly reduced the proportion of clinically diagnosed TB [75,78].
This review is not without limitations. First, there were limited demographic data that could be extracted from the selected papers, such as age, sex of decedents, HIV variables such as nadir CD4 count, ART status, and clinically diagnosed causes of death. Second, several studies reported bacterial infections without describing the specific pathogens and hence might have led to underreporting of bacterial and other pathogens. Third, we were unable to perform a meta-analysis given heterogeneity of the various study designs. Furthermore, small case series and case reports do not require formal assessment of quality of studies and thus this was not performed for the rest of the other studies. Also, the role of specific pathogens as contributors to HIV-associated mortality was not clearly elucidated in the autopsy studies. Finally, another major limitation of this study is that autopsies were probably performed for specific reasons such pharmacokinetic studies, evaluation of diagnostics, and others and may not necessarily represent all patients who died.
Nevertheless, we conducted extensive literature review of several databases to extract all available literature on the topic and strictly adhered to the PRISMA-ScR guidelines to ensure robustness of the review process. The findings from this scoping review highlight the contribution of fungal diseases and other co-infections to inform clinical investigations and clinical care on possible differential diagnoses and target therapy among hospitalized patients with HIV in Africa.
Conclusions
Our autopsy-based review in Africa revealed fungal pathogens, notably Cryptococcus species and Pneumocystis jirovecii, as possible contributors to mortality in advanced HIV disease. Therefore, integrating fungal pathogen detection into HIV care frameworks is crucial for clinical management. The high burden highlights the need for targeted interventions, including timely diagnosis and antifungal therapy. The study emphasizes diagnostic advancements and awareness to reduce mortality.
Acknowledgements
FB is a PhD student at the University of Manchester, United Kingdom. His work is supported by the Carigest SA Conny Naeva Charitable Foundation as part of the ‘Chronic Pulmonary Aspergillosis: Optimization of Therapy, Immunogenetic Screening, and Diagnosis in Uganda [CPA_OPTIONS_Uganda]’ project. CARIGEST SA did not play any role in the design, implementation, and analysis of the study.
Footnotes
Transparency declaration
The authors declare that they have no conflicts of interest. Research reported in this publication was supported by the Fogarty International Centre of the National Institutes of Health under Award Number D43 TW010543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
All relevant data are within the article and its supporting information files. Data are available upon reasonable request from the first author.
References
- [1].Joint United Nations Programme on HIV/AIDS. The path that ends AIDS: UNAIDS Global AIDS Update 2023. Geneva, Switzerland. 2023. [Google Scholar]
- [2].Low A, Gavriilidis G, Larke N, B-Lajoie M-R, Drouin O, Stover J, et al. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis 2016;62:1595–603. 10.1093/cid/ciw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stover J, Glaubius R, Teng Y, Kelly S, Brown T, Hallett TB, et al. Modeling the epidemiological impact of the UNAIDS 2025 targets to end AIDS as a public health threat by 2030. PLOS Med 2021;18:e1003831. 10.1371/journal.pmed.1003831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cresswell FV, Bangdiwala AS, Bahr NC, Trautner E, Nuwagira E, Ellis J, et al. Can improved diagnostics reduce mortality from tuberculous meningitis? Findings from a 6.5-year cohort in Uganda. Wellcome Open Res 2018;3:64. 10.12688/wellcomeopenres.14610.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rajasingham R, Govender NP, Jordan A, Loyse A, Shroufi A, Denning DW, et al. The global burden of HIV-associated cryptococcal infection in adults in 2020: a modelling analysis. Lancet Infect Dis 2022;22:1748–55. 10.1016/S1473-3099(22)00499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pipitò L, Zinna G, Trizzino M, Gioè C, Tolomeo M, Di Carlo P, et al. Causes of hospitalization and predictors of in-hospital mortality among people living with HIV in Sicily-Italy between 2010 and 2021. J Infect Public Health 2023;16:1703–8. 10.1016/j.jiph.2023.08.023. [DOI] [PubMed] [Google Scholar]
- [7].Lartey M, Asante-Quashie A, Essel A, Kenu E, Ganu V, Neequaye A. Causes of death in hospitalized HIV patients in the early anti-retroviral therapy era. Ghana Med J 2015;49:7–11. 10.4314/gmj.v49i1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paul S, Gilbert HM, Ziecheck W, Jacobs J, Sepkowitz KA. The impact of potent antiretroviral therapy on the characteristics of hospitalized patients with HIV infection. AIDS 1999;13:415–8. 10.1097/00002030-199902250-00015. [DOI] [PubMed] [Google Scholar]
- [9].Gaillet A, Azoulay E, de Montmollin E, Garrouste-Orgeas M, Cohen Y, Dupuis C, et al. Outcomes in critically Ill HIV-infected patients between 1997 and 2020: analysis of the OUTCOMEREA multicenter cohort. Crit Care 2023;27:108. 10.1186/s13054-023-04325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwartz IS, Kenyon C, Lehloenya R, Claasens S, Spengane Z, Prozesky H, et al. AIDS-related endemic mycoses in Western Cape, South Africa, and clinical mimics: a cross-sectional study of adults with advanced HIV and recent-onset, widespread skin lesions. Open Forum Infect Dis 2017;4:1e7. 10.1093/ofid/ofx186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bongomin F, Ekeng BE, Kibone W, Nsenga L, Olum R, Itam-Eyo A, et al. Invasive fungal diseases in Africa: a critical literature review. J Fungi (Basel) 2022;8:1236. 10.3390/jof8121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lakoh S, Kamudumuli PS, Penney ROS, Haumba SM, Jarvis JN, Hassan AJ, et al. Diagnostic capacity for invasive fungal infections in advanced HIV disease in Africa: a continent-wide survey. Lancet Infect Dis 2023;23:598–608. 10.1016/S1473-3099(22)00656-9. [DOI] [PubMed] [Google Scholar]
- [13].Cox JA, Lukande RL, Lucas S, Nelson AM, Van Marck E, Colebunders R. Autopsy causes of death in HIV-positive individuals in sub-Saharan Africa and correlation with clinical diagnoses. AIDS Rev 2010;12:183–94. [PubMed] [Google Scholar]
- [14].Antinori S, Nebuloni M, Magni C, Fasan M, Adorni F, Viola A, et al. Trends in the postmortem diagnosis of opportunistic invasive fungal infections in patients with AIDS: a retrospective study of 1,630 autopsies performed between 1984 and 2002. Am J Clin Pathol 2009;132:221–7. 10.1309/AJCPRAAE8LZ7DTNE. [DOI] [PubMed] [Google Scholar]
- [15].Chiang C-H, Tang P-U, Lee GH, Chiang T-H, Chiang C-H, Ma KS- K, et al. Prevalence of nontuberculous mycobacterium infections versus tuberculosis among autopsied HIV patients in sub-Saharan Africa: a systematic review and meta-analysis. Am J Trop Med Hyg 2021;104:628–33. 10.4269/ajtmh.20-0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lucas SB, Hounnou A, Peacock C, Beaumel A, Djomand G, N’Gbichi JM, et al. The mortality and pathology of HIV infection in a west African city. AIDS 1993;7:1569–79. [DOI] [PubMed] [Google Scholar]
- [17].Carrilho C, Monteiro E, Ussene E, Macie A, Fernandes F, Lorenzoni C, et al. Causes of death in HIV/AIDS patients in Maputo Central Hospital e a retrospective study from 2010. Histopathology 2012;61:1–2. 10.1111/j.1365-2559.2012.04359_1.x. [DOI] [Google Scholar]
- [18].Castillo P, Ussene E, Jordao D, Lovane L, Martinez MJ, Carrilho C, et al. Minimally invasive autopsy: an accurate tool for cause of death determination in developing countries. Am J Trop Med Hyg 2015;93:388. 10.1371/journal.pone.0132057. [DOI] [Google Scholar]
- [19].Rana FS, Hawken MP, Mwachari C, Bhatt SM, Abdullah F, Ng’ang’a LW, et al. Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi, Kenya. J Acquir Immune Defic Syndr 2000;24:23–9. 10.1097/00126334-200005010-00004. [DOI] [PubMed] [Google Scholar]
- [20].Rana F, Hawken MP, Meme HK, Chakaya JM, Githui WA, Odhiambo JA, et al. Autopsy findings in HIV-1-infected adults in Kenya. J Acquir Immune Defic Syndr Hum Retrovirol 1997;14:83–5. 10.1097/00042560-199701010-00017. [DOI] [PubMed] [Google Scholar]
- [21].Kilale AM, Kimaro GD, Kahwa AM, Chilagwile M, Ngowi BJ, Muller W, et al. High prevalence of tuberculosis diagnosed during autopsy examination at Muhimbili National Hospital in Dar es Salaam, Tanzania. Tanzan J Health Res 2013;15:171–7. 10.4314/thrb.v15i3.4. [DOI] [PubMed] [Google Scholar]
- [22].Nathoo KJ, Gondo M, Gwanzura L, Mhlanga BR, Mavetera T, Mason PR. Fatal Pneumocystis carinii pneumonia in HIV-seropositive infants in Harare, Zimbabwe. Trans R Soc Trop Med Hyg 2001;95:37–9. 10.1016/s0035-9203(01)90325-6. [DOI] [PubMed] [Google Scholar]
- [23].Dietrich PY, Bille J, Fontolliet C, Regamey C. Disseminated histoplasmosis due to H. capsulatum in a patient with AIDS. Schweiz Med Wochenschr 1987;117: 1289–96. [PubMed] [Google Scholar]
- [24].Wake RM, Govender NP, Omar T, Nel C, Mazanderani AH, Karat AS, et al. Cryptococcal-related mortality despite fluconazole preemptive treatment in a cryptococcal antigen screen-and-treat program. Clin Infect Dis 2020;70: 1683–90. 10.1093/cid/ciz485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Karat AS, Tlali M, Fielding KL, Charalambous S, Chihota VN, Churchyard GJ, et al. Measuring mortality due to HIV-associated tuberculosis among adults in South Africa: comparing verbal autopsy, minimally-invasive autopsy, and research data. PLOS ONE 2017;12:e0174097. 10.1371/journal.pone.0174097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wong ML, Back P, Candy G, Nelson G, Murray J. Cryptococcal pneumonia in African miners at autopsy. Int J Tuberc Lung Dis 2007;11:528–33. [PubMed] [Google Scholar]
- [27].Njuguna HN, Zaki SR, Roberts DJ, Rogena EA, Walong E, Fligner CL, et al. Postmortem study of cause of death among children hospitalized with respiratory illness in Kenya. Pediatr Infect Dis J 2021;40:715–22. 10.1097/INF.0000000000003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Onyango DO, Akelo V, van der Sande MAB, Ridzon R, Were JA, Agaya JA, et al. Causes of death in HIV-infected and HIV-uninfected children aged under-five years in western Kenya. AIDS 2022;36:59–68. 10.1097/QAD.0000000000003086. [DOI] [PubMed] [Google Scholar]
- [29].Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467. 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- [30].Letang E, Rakislova N, Martinez MJ, Carlos Hurtado J, Carrilho C, Bene R, et al. Minimally invasive tissue sampling: a tool to guide efforts to reduce AIDS-related mortality in resource-limited settings. Clin Infect Dis 2021;73: S343e50. 10.1093/cid/ciab789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Khaba MC, Ngale TC, Madala N. COVID-19 in an HIV-infected patient. Lessons learned from an autopsy case. Int J Infect Dis 2020;101:243–6. 10.1016/j.ijid.2020.09.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hurtado JC, Castillo P, Fernandes F, Navarro M, Lovane L, Casas I, et al. Mortality due to Cryptococcus neoformans and Cryptococcus gattii in low-income settings: an autopsy study. Sci Rep 2019;9:7493. 10.1038/s41598-019-43941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Raphael S, Izuchukwu AB. Autopsy findings in HIV/aids patients in lago university teaching hospital: a one year prospective study. Lab Investig 2018;98: 13. 10.1038/labinvest.2018.1. [DOI] [Google Scholar]
- [34].Castillo P, Hurtado JC, Martínez MJ, Jordao D, Lovane L, Ismail MR, et al. Validity of a minimally invasive autopsy for cause of death determination in maternal deaths in Mozambique: an observational study. PLOS Med 2017;14: e1002431. 10.1371/journal.pmed.1002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Akakpo KP, Quayson SE, Lartey M. Disseminated cryptococcosis in a patient with HIV/AIDS at a teaching hospital in Ghana. SAGE Open Med Case Rep 2015;3:2050313X14565421. 10.1177/2050313X14565421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cox JA, Lukande RL, Kalungi S, Van Marck E, Van de Vijver K, Kambugu A, et al. Needle autopsy to establish the cause of death in HIV-infected hospitalized adults in Uganda: a comparison to complete autopsy. J Acquir Immune Defic Syndr 2014;67:169–76. 10.1097/QAI.0000000000000290. [DOI] [PubMed] [Google Scholar]
- [37].Wong EB, Omar T, Setlhako GJ, Osih R, Feldman C, Murdoch DM, et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLOS ONE 2012;7:e47542. 10.1371/journal.pone.0047542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kabangila R, Semvua K, Rambau P, Jackson K, Mshana SE, Jaka H, et al. Pulmonary histoplasmosis presenting as chronic productive cough, fever, and massive unilateral consolidation in a 15-year-old immune-competent boy: a case report. J Med Case Rep 2011;5:374. 10.1186/1752-1947-5-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kyeyune R, den Boon S, Cattamanchi A, Davis JL, Worodria W, Yoo SD, et al. Causes of early mortality in HIV-infected TB suspects in an East African referral hospital. J Acquir Immune Defic Syndr 2010;55:446–50. 10.1097/qai.0b013e3181eb611a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cohen T, Murray M, Wallengren K, Alvarez GG, Samuel EY, Wilson D. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLOS Med 2010;7:e1000296. 10.1371/journal.pmed.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Garcia-Jardon M, Bhat VG, Blanco-Blanco E, Stepian A. Postmortem findings in HIV/AIDS patients in a tertiary care hospital in rural South Africa. Trop Doct 2010;40:81–4. 10.1258/td.2010.090465. [DOI] [PubMed] [Google Scholar]
- [42].Ng’walali PM, Kibayashi K, Mbonde MP, Harada S, Mwakagile D, Kitinya JN, et al. Neuropathology of human immunodeficiency virus infection: a forensic autopsy study in Dar es Salaam, Tanzania. Forensic Sci Int 2005;151:133–8. 10.1016/j.forsciint.2005.01.012. [DOI] [PubMed] [Google Scholar]
- [43].Ateenyi-Agaba C, Weiderpass E, Tommasino M, Smet A, Arslan A, Dai M, et al. Papillomavirus infection in the conjunctiva of individuals with and without AIDS: an autopsy series from Uganda. Cancer Lett 2006;239:98–102. 10.1016/j.canlet.2005.07.024. [DOI] [PubMed] [Google Scholar]
- [44].Ruffini DD, Madhi SA. The high burden of Pneumocystis carinii pneumonia in African HIV-1-infected children hospitalized for severe pneumonia. AIDS 2002;16:105–12. 10.1097/00002030-200201040-00013. [DOI] [PubMed] [Google Scholar]
- [45].Chintu C, Mudenda V, Lucas S, Nunn A, Lishimpi K, Maswahu D, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 2002;360:985–90. 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- [46].Orem J, Mpanga L, Habyara E, Nambuya A, Aisu T, Wamukota W, et al. Disseminated Aspergillus fumigatus infection: case report. East Afr Med J 1998;75:436–8. 9803639. [PubMed] [Google Scholar]
- [47].Bell JE, Lowrie S, Koffi K, Hondé M, Andoh J, De Cock KM, et al. The neuro-pathology of HIV-infected African children in Abidjan, Côte d’Ivoire. J Neuropathol Exp Neurol 1997;56:686e92. [PubMed] [Google Scholar]
- [48].Jeena PM, Coovadia HM, Chrystal V. Pneumocystis carinii and cytomegalovirus infections in severely ill, HIV-infected African infants. Ann Trop Paediatr 1996;16:361–8. 10.1080/02724936.1996.11747852. [DOI] [PubMed] [Google Scholar]
- [49].Fauci AS, Lane HC. Four decades of HIV/AIDS-dmuch accomplished, much to do. N Engl J Med 2020;383:1–4. 10.1056/NEJMp1916753. [DOI] [PubMed] [Google Scholar]
- [50].Bekker LG, Beyrer C, Mgodi N, Lewin SR, Delany-Moretlwe S, Taiwo B, et al. HIV infection. Nat Rev Dis Primers 2023;9:42. 10.1038/s41572-023-00452-3. [DOI] [PubMed] [Google Scholar]
- [51].Ford N, Meintjes G, Calmy A, Bygrave H, Migone C, Vitoria M, et al. Managing advanced HIV disease in a public health approach. Clin Infect Dis 2018;66: S106–10. 10.1093/cid/cix1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ford N, Matteelli A, Shubber Z, Hermans S, Meintjes G, Grinsztejn B, et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc 2016;19:20714. 10.7448/IAS.19.1.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Osler M, Hilderbrand K, Goemaere E, Ford N, Smith M, Meintjes G, et al. The continuing burden of advanced HIV disease over 10 years of increasing antiretroviral therapy coverage in South Africa. Clin Infect Dis 2018;66:S118–25. 10.1093/cid/cix1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Masur H, Michelis MA, Greene JB, Onorato I, Vande Stouwe RA, Holzman RS, et al. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med 1981;305: 1431–8. 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- [55].Holmberg K, Meyer RD. Fungal infections in patients with AIDS and AIDS-related complex. Scand J Infect Dis 1986;18:179–92. 10.3109/00365548609032326. [DOI] [PubMed] [Google Scholar]
- [56].Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/AIDS. Lancet Infect Dis 2017;17:e334–43. 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- [57].World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy 2017. http://www.who.int/hiv/pub/toolkits/advanced-HIV-disease-policy/en/. [Accessed 6 January 2019]. [PubMed]
- [58].Samayoa B, Aguirre L, Bonilla O, Medina N, Lau-Bonilla D, Mercado D, et al. The diagnostic laboratory hub: a new health care system reveals the incidence and mortality of tuberculosis, histoplasmosis, and cryptococcosis of PWH in Guatemala. Open Forum Infect Dis 2020;7:ofz534. 10.1093/ofid/ofz534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rutakingirwa MK, Cresswell FV, Kwizera R, Ssebambulidde K, Kagimu E, Nuwagira E, et al. Tuberculosis in HIV-associated cryptococcal meningitis is associated with an increased risk of death. J Clin Med 2020;9:781. 10.3390/jcm9030781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Britz E, Perovic O, Von Mollendorf C, Von Gottberg A, Iyaloo S, Quan V, et al. The epidemiology of meningitis among adults in a South African province with a high HIV prevalence, 2009–2012. PLOS ONE 2016;11:2009–12. 10.1371/journal.pone.0163036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Veltman JA, Bristow CC, Klausner JD. Meningitis in HIV-positive patients in sub-Saharan Africa: a review. J Int AIDS Soc 2014:1–10. 10.7448/IAS.17.1.19184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Williams DA, Kiiza T, Kwizera R, Kiggundu R, Velamakanni S, Meya DB, et al. Evaluation of fingerstick cryptococcal antigen lateral flow assay in HIV-infected persons: a diagnostic accuracy study. Clin Infect Dis 2015;61: 464–7. 10.1093/cid/civ263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Oladele RO, Ayanlowo OO, Richardson MD, Denning DW. Histoplasmosis in Africa: an emerging or a neglected disease? PLOS Negl Trop Dis 2018;12: e0006046. 10.1371/journal.pntd.0006046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ocansey BK, Kosmidis C, Agyei M, Dorkenoo AM, Ayanlowo OO, Oladele RO, et al. Histoplasmosis in Africa: current perspectives, knowledge gaps, and research priorities. PLOS Negl Trop Dis 2022;16:e0010111. 10.1371/journal.pntd.0010111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kuate MPN, Nyasa R, Mandengue C, Tendongfor N, Bongomin F, Denning DW. Screening for acute disseminated histoplasmosis in HIV disease using urinary antigen detection enzyme immunoassay: a pilot study in Cameroon. J Microbiol Methods 2021;185:106226. 10.1016/j.mimet.2021.106226. [DOI] [PubMed] [Google Scholar]
- [66].Ekeng B, Davies A, Osaigbovo I, Emanghe U, Udoh U, Alex-Wele M, et al. Current epidemiology of histoplasmosis in Nigeria: a systematic review and meta-analysis. Niger Postgrad Med J 2023;30:12. 10.4103/npmj.npmj_311_22. [DOI] [PubMed] [Google Scholar]
- [67].Kuate MPN, Ekeng BE, Kwizera R, Mandengue C, Bongomin F. Histoplasmosis overlapping with HIV and tuberculosis in sub-Saharan Africa: challenges and research priorities. Ther Adv Infect Dis 2021;8:20499361211008676. 10.1177/20499361211008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ekeng BE, Oladele RO, Emanghe UE, Ochang EA, Mirabeau TY. Prevalence of histoplasmosis and molecular characterization of histoplasma species in patients with presumptive pulmonary tuberculosis in Calabar, Nigeria. Open Forum Infect Dis 2022;9:ofac368. 10.1093/ofid/ofac368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Oladele RO, Toriello C, Ogunsola FT, Ayanlowo OO, Foden P, Fayemiwo AS, et al. Prior subclinical histoplasmosis revealed in Nigeria using histoplasmin skin testing. PLOS ONE 2018;13:e0196224. 10.1371/journal.pone.0196224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Farsey SJ, Bezjak V. Prevalence of skin sensitivity to histoplasmin and coccidioidin in various Ugandan populations. Am J Trop Med Hyg 1970;19:664–9. 10.4269/ajtmh.1970.19.664. [DOI] [PubMed] [Google Scholar]
- [71].Oladele RO, Osaigbovo II, Akanmu AS, Adekanmbi OA, Ekeng BE, Mohammed Y, et al. Prevalence of histoplasmosis among persons with advanced HIV disease, Nigeria. Emerg Infect Dis 2022;28:2261–9. 10.3201/eid2811.220542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sekar P, Nalintya E, Kwizera R, Mukashyaka C, Niyonzima G, Namakula LO, et al. Prevalence of histoplasma antigenuria among outpatient cohort with advanced HIV in Kampala, Uganda. J Fungi (Basel) 2023;9:757. 10.3390/jof9070757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Holding KJ, Dworkin MS, Wan P-CT, Hanson DL, Klevens RM, Jones JL, et al. Aspergillosis among people infected with human immunodeficiency virus: incidence and survival. Clin Infect Dis 2000;31:1253–7. 10.1086/317452. [DOI] [PubMed] [Google Scholar]
- [74].Denning DW, Morgan EF. Quantifying deaths from Aspergillosis in HIV positive people. J Fungi (Basel) 2022;8:1131. 10.3390/jof8111131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].World Health Organization. Global tuberculosis report 2022. Geneva, Switzerland. 2023. [Google Scholar]
- [76].Ford N, Doherty M. The enduring challenge of advanced HIV infection. N Engl J Med 2017;377:283–4. 10.1056/NEJMe1707598. [DOI] [PubMed] [Google Scholar]
- [77].Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 2008;22:1897–908. 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rimal R, Shrestha D, Pyakurel S, Poudel R, Shrestha P, Rai KR, et al. Diagnostic performance of GeneXpert MTB/RIF in detecting MTB in smear-negative presumptive TB patients. BMC Infect Dis 2022;22:321. 10.1186/s12879-022-07287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the article and its supporting information files. Data are available upon reasonable request from the first author.