Abstract
Atrial fibrillation is the most common atrial arrhythmia and accounts for a significant burden of cardiovascular disease globally. With advances in implanted and wearable cardiac monitoring technology, it is now possible to readily and accurately quantify an individual’s time spent in atrial fibrillation. This review summarizes the relationship between atrial fibrillation burden and adverse cardiovascular and cerebrovascular outcomes and discusses the role of catheter ablation to mitigate the morbidity and mortality associated with greater burden of atrial fibrillation.
Keywords: Catheter ablation, mortality, stroke, heart failure
Tweet:
Catheter ablation can mitigate the adverse cardiovascular outcomes associated with greater burden of atrial fibrillation #epAblation #AFib
Introduction
Atrial fibrillation (AF) affects more than 46 million individuals globally and its prevalence and associated mortality have increased drastically from 1990 to 2010.1 With the advent of continuous cardiac monitoring, clinicians and investigators are now able to quantify the true burden of AF in patients and populations. However, the clinical application of data pertaining to AF burden remains unclear. This review will summarize current data on the association between AF burden and cardiovascular outcomes, and the effectiveness of catheter ablation in reducing AF burden and consequently improving cardiovascular outcomes.
Defining Burden
Historically, AF has been classified by the duration of individual episodes of arrhythmia. Consensus definitions classify paroxysmal AF as episodes lasting less than 7 days, persistent AF as episodes lasting greater than 7 days, and long-standing persistent AF as continuous AF lasting greater than one year in duration. Permanent AF represents AF in which the patient and clinician have decided to stop pursuing attempts to restore sinus rhythm.2 While these classifications may be useful descriptors in clinical practice, they lack discriminative ability. Their reliance on a patient’s subjective report of symptoms, which is used to infer persistence and duration of AF episodes, often leads to misclassification.3 Moreover, even in patients within the same clinical AF classification, the amount of time spent in atrial fibrillation (AF burden) can vary broadly, leading to the circumstance whereby “paroxysmal” patients with frequent short episodes may spend more time in AF than a “persistent” patient with rare longer episodes. As such, AF burden measurements can significantly overlap between clinical classifications.3, 4
With the advent of devices that continuously monitor cardiac rhythm, alternate classifications have been suggested. While atrial fibrillation burden is typically defined as the percentage of time spent in AF as detected by continuous rhythm monitoring, alternate classifications include the duration of the longest AF episode and the number of AF episodes within a period of time (figure 1). Additionally, AF can be classified into clinical AF (AF that is typically symptomatic and sustained), subclinical AF (SCAF – AF that is electrocardiographically detected in the absence of symptoms), and device-detected AF (DDAF – asymptomatic AF detected on cardiac implantable electronic devices).5
Figure 1. Clinical classifications of AF versus classification by AF burden.

Atrial fibrillation (AF) has historically been classified based on clinical presentation. Wearable and implantable cardiac monitoring devices allow for more granular classifications based on AF burden.
Determination of AF burden with intermittent cardiac monitoring is dependent on the duration of monitoring. In a post-hoc analysis of the CIRCA-DOSE (Cryoballoon vs Irrigated Radiofrequency Catheter Ablation: Double Short vs Standard Exposure Duration) trial, authors compared the effectiveness of different durations of noninvasive monitoring to determine AF burden after catheter ablation. Shorter monitoring intensities (24-/48-hour EKG monitors) were less sensitive for detecting AF recurrence and overestimated the patient’s AF burden when AF was detected. Compared to continuous implantable cardiac monitors, the optimal duration of non-invasive monitoring for accurate determination of AF burden was 28 days, typically applied as four 7-day monitors or two 14-day monitors per year.6
Cardiovascular Outcomes
Thromboembolic Risk
The association between AF burden and stroke risk has long been debated in the literature. The 2019 AHA guidelines recommend oral anticoagulation to reduce stroke risk in AF patients based on an individual’s CHA2DS2-VASc score without consideration of AF type or pattern.7 The omission of AF burden from calculation of thromboembolic risk is based on the idea that risk due to patient characteristics far outweighs risk attributable to AF pattern.8 This is supported by older studies that failed to show a significant difference in stroke risk when comparing patients with paroxysmal AF to patients with persistent or permanent AF.9–11
While the amount of AF or its pattern in patients with more than one risk factor for stroke may not alter the threshold for anticoagulation, the preponderance of evidence does suggest that longer durations of AF are associated with greater stroke risk. Post-hoc analyses of several large randomized controlled trials have observed a significantly higher risk of stroke and systemic thromboembolism in anticoagulated patients with persistent or permanent AF compared to those with paroxysmal AF.12–15 Additionally, an analysis of the ACTIVE-A (Atrial Fibrillation Clopidogrel Trial With Irbesartan for Prevention of Vascular Events) and AVERROES (Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment) trials demonstrated increasing stroke risk with more persistent forms of AF among individuals not treated with oral anticoagulation (adjusted HR 1.83, 95% CI 1.43-2.35, p < 0.001 for permanent vs. paroxysmal AF and adjusted HR 1.44, 95% CI 1.05-1.98, p = 0.02 for persistent vs. paroxysmal AF).16
Studies have yet to show a consistent minimum threshold of AF burden that is associated with a significant increase in stroke risk (table 1, figure 2). Among patients with clinical AF, greater risk of stroke has been associated with percent time in AF > 11% and continuous AF episode duration > 24 hours even after adjustment for CHA2DS2-VASc score.17, 18 Data from patients with DDAF have also demonstrated a similar association between increasing AF burden and the risk of stroke.19–21 Kaplan et al. demonstrated that the relationship between CHA2DS2-VASc score and stroke risk is mediated by DDAF burden; patients with greater AF burden were at greater risk of stroke compared to those with the same CHA2DS2-VASc score with lower AF burden.22 Tiver et al. devised a risk calculator that incorporates AF burden into the CHA2DS2-VASc score and found that AF burden may be helpful in determining the role for anticoagulation in patients with intermediate CHA2DS2-VASc scores.23
TABLE 1.
Review of studies investigating effect of AF burden on thromboembolic risk
| First Author, Year | N | Data Source | AF Burden Definition | Outcome |
|---|---|---|---|---|
| Clinical AF | ||||
| Hart,9 2000 | 2012 | SPAF I, II, III studies | Clinical classification of AF | No difference in risk of TE events with intermittent vs. sustained AF (3.2% vs. 3.3% per year) |
| Hohnholser,10 2007 | 6706 | ACTIVE-W study | Clinical classification of AF | No difference in risk of TE events with paroxysmal vs. sustained AF (RR 0.94, CI 0.63-1.40, p = 0.755) |
| Disertori,11 2013 | 1234 | GISSI study | Clinical classification of AF | No difference in risk of TE events with persistent vs. paroxysmal AF (HR 2.14, CI 0.68-6.79, p = 0.20) |
| Steinberg,12 2015 | 14062 | ROCKET-AF study | Clinical classification of AF | Increased risk of TE events with persistent vs. paroxysmal AF (HR 1.28, CI 1.01-1.64, p = 0.045) |
| Link,13 2017 | 21105 | ENGAGE AF-TIMI 48 study | Clinical classification of AF | Increased risk of TE events with persistent vs. paroxysmal AF (HR 1.27, CI 1.11-1.52, p = 0.015) and permanent vs. paroxysmal AF (HR 1.30, CI 1.15-1.43, p < 0.001) |
| Al-Khatib,14 2013 | 18201 | ARISTOTLE study | Clinical classification of AF | Increased risk of TE events with persistent or permanent AF vs. paroxysmal AF (HR 1.43, CI 1.08-1.96, p = 0.015) |
| Lip,15 2008 | 7329 | SPORTIF III and V studies | Clinical classification of AF | Increased risk of TE events with persistent vs. paroxysmal AF (HR 1.87, CI 1.04-3.36, p = 0.037) |
| Vanassche,16 2015 | 6563 | ACTIVE-A and AVERROES studies | Clinical classification of AF | Increased risk of ischemic stroke with persistent vs. paroxysmal (HR 1.44, CI 1.05-1.98, p = 0.02) and permanent vs. paroxysmal AF (HR 1.83, CI 1.43-2.35, p < 0.001) |
| Go,17 2018 | 1965 | KP-RHYTHM study | Percent time in AF | Increased risk of TE events with highest tertile of AF burden (≥11.4%) compared to lower tertiles (HR 3.16, CI 1.51-6.62) |
| Chew,18 2022 | 39710 | CIED data from Merlin.net remote monitoring database | Daily percent time in AF and maximum duration of any AF episode | Increased risk of ischemic stroke with maximum AF episode duration ≥24 hours (HR 1.366, CI 1.018-1.832, p = 0.038) |
| Device-detected AF | ||||
| Glotzer,19 2009 | 2486 | TRENDS study | Maximum daily duration of DDAF within a 30-day period | Increased risk of TE events with high AT/AF burden (≥5.5 hours) vs. no AT/AF burden (HR 2.20, CI 0.96-5.05, p = 0.06) |
| Van Gelder,20 2017 | 2580 | ASSERT study | Maximum single DDAF episode duration | Increased risk of TE events with DDAF duration > 24 hours vs. no DDAF (HR 3.24, CI 1.51-6.95, p = 0.003) |
| Boriani,21 2014 | 10016 | TRENDS, PANORAMA, and Italian ClinicalService Project studies | Maximum daily time in DDAF | Increased risk of ischemic stroke with ≥5 minutes DDAF (HR 1.76, CI 1.02-3.02, p = 0.041) |
| Kaplan,22 2019 | 21768 | Medtronic CareLink database | Maximum daily time in DDAF | Increased stroke risk (>1%/year) in patients with AF burden 6 min-23.5h and CHA2DS2VASc ≥3 and patients with AF burden >23.5h and CHA2DS2VASc ≥2 |
AF = atrial fibrillation, AT = atrial tachycardia, CIED = cardiac implantable electronic device, DDAF = device-detected atrial fibrillation, SCAF = subclinical atrial fibrillation, TE = thromboembolism
Figure 2. Effect of AF burden on stroke risk.
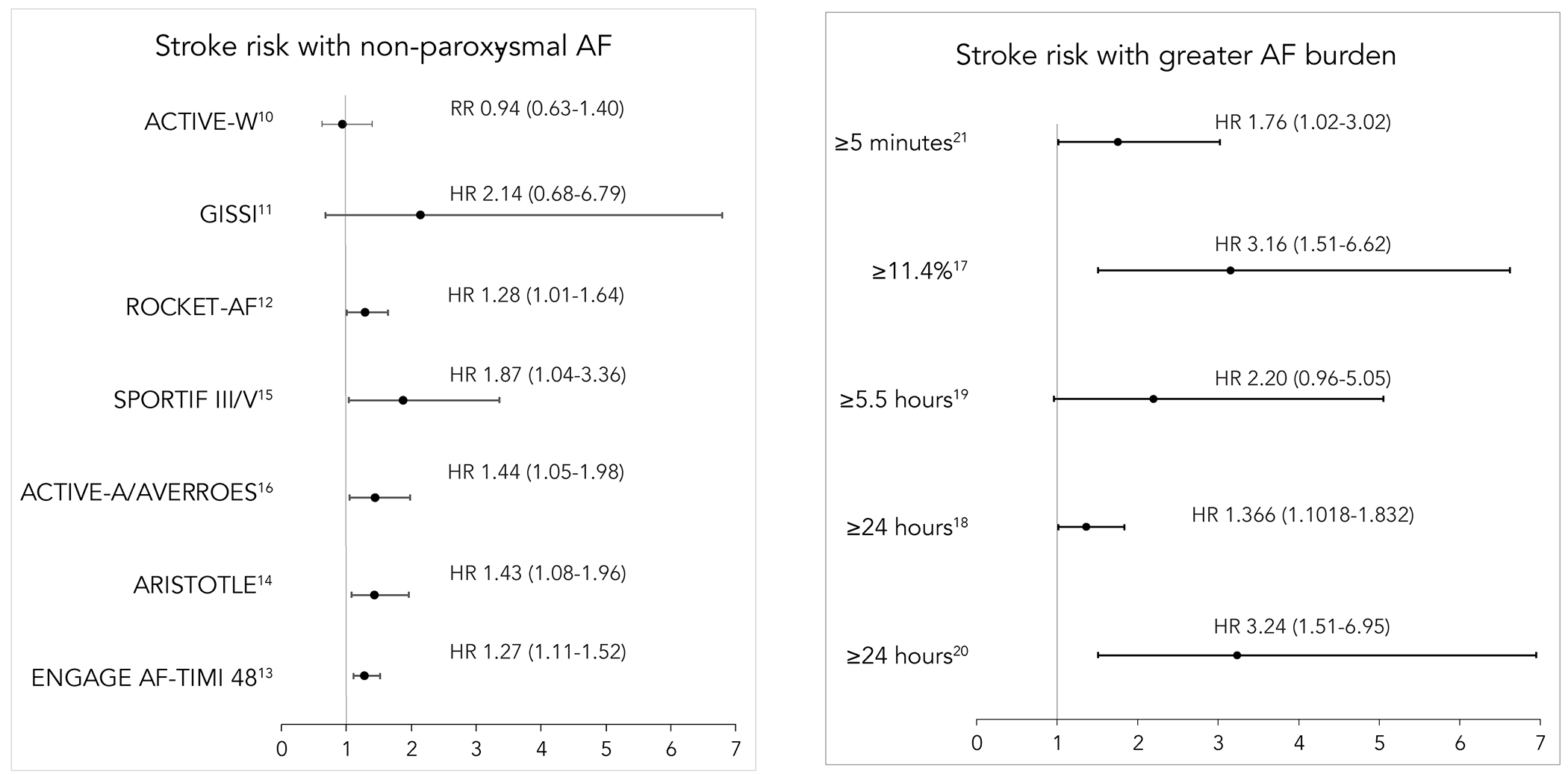
Left: stroke risk with non-paroxysmal atrial fibrillation (AF) compared to paroxysmal AF. Right: stroke risk with greater AF burden at differing cutoffs.
Mortality and Hospitalization
Accumulating evidence suggests that AF burden increases the risk of other cardiovascular complications of AF (table 2). Analysis of CIED data from 39,710 patients with a clinical diagnosis of AF demonstrated a significant association between increasing daily AF burden and all-cause mortality, all-cause hospitalization, and cardiovascular hospitalization.18 All-cause mortality at 1 year was 8.5% in patients with 0% daily AF burden, 8.9% with 0-5% daily AF burden, and 10.9% with daily AF burden >5% (p < 0.001). The association between AF burden and mortality has also been demonstrated in post-hoc analyses of several randomized controlled trials comparing the effectiveness of different oral anticoagulants. Data from the ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48) trial showed lower all-cause mortality in patients with paroxysmal AF compared to patients with persistent AF (adjusted HR 0.73, 95% CI 0.64-0.83, p < 0.001) and permanent AF (adjusted HR 0.78, 95% CI 0.69-0.87, p < 0.001).13 In the ROCKET-AF trial (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation), patients with paroxysmal AF had lower all-cause mortality than patients with persistent AF (adjusted HR 0.79, 95% CI 0.67-0.94, p = 0.006).12 AF burden therefore may represent an important risk factor for mortality.
TABLE 2.
Review of studies investigating effect of AF burden on mortality
| First Author, year | N | Data Source | AF Burden Definition | Outcome |
|---|---|---|---|---|
| Disertori,11 2013 | 1234 | GISSI study | Clinical classification of AF | No difference in mortality with persistent vs. paroxysmal AF (HR 0.52, CI 0.13-2.03, p = 0.35) |
| Steinberg,12 2015 | 14264 | ROCKET-AF study | Clinical classification of AF | Increased all-cause mortality with persistent vs. paroxysmal AF (HR 1.27, CI 1.06-1.49, p = 0.006) |
| Link,13 2017 | 21105 | ENGAGE AF-TIMI 48 study | Clinical classification of AF | Increased all-cause mortality with persistent vs. paroxysmal AF (HR 1.37, CI 1.20-1.56, p < 0.001), and permanent vs. paroxysmal AF (HR 1.28, CI 1.15-1.45, p < 0.001) |
| Lip,15 2008 | 7329 | SPORTIF III and V studies | Clinical classification of AF | No difference in mortality with paroxysmal vs. persistent AF (HR 1.24, CI 0.87-1.76, p = 0.24) |
| Chew,18 2022 | 39710 | CIED data from Merlin.net remote monitoring database | Daily percent time in AF | Increased all-cause mortality with each 10% increase in daily AF burden (HR 1.061 per 10% increase, CI 1.045-1.076, p < 0.001) |
| Park,24 2021 | 496 | Single center remote monitoring data | Total time spent in AF during follow up interval | Increased odds of composite outcome (progression to clinical AF, ischemic stroke, MI, HF-related hospitalization, or cardiac death) with high-burden SCAF (≥24 hours in 6 months) (OR 20.1, CI 7.6-52.7, p < 0.001) |
| Piccini,25 2019 | 3131 | CIED data from Merlin.net remote monitoring database | Time in AF in one week | Increased odds of death with increase in weekly AF burden > 6h (OR 2.30, CI 2.09-2.53, p < 0.001) |
AF = atrial fibrillation, CIED = cardiac implantable electronic device, HF = heart failure, MI = myocardial infarction, SCAF = subclinical atrial fibrillation, TE = thromboembolism
More recently, Park et al. reported that patients with high-burden SCAF (≥ 24 hours of SCAF in 6 months) had a significantly greater risk of the composite outcome of progression to clinical AF, ischemic stroke, myocardial infarction (MI), heart failure (HF) hospitalization, and cardiac death than those with low or no SCAF burden (p < 0.001).24 Progression of AF, measured as an increase in AF burden from week to week, has also been associated with increased mortality.25
Heart Failure Outcomes
Patients with both AF and heart failure with preserved (HFpEF) or reduced ejection fraction (HFrEF) suffer from greater morbidity and mortality than either condition alone.26, 27 Taillandier et al. demonstrated that patients with HF and permanent AF have increased all-cause mortality and readmission for HF compared to patients with HF and paroxysmal or persistent AF.28 Retrospective analysis of CIED data from over 39,000 patients with nonpermanent AF demonstrated that greater AF burden (measured as percentage of time in AF) was associated with a significantly increased risk of new-onset HF and death.29 In patients with existing HF, greater AF burden was associated with a significantly increased risk of hospitalization due to HF and death. Post-hoc analysis of ASSERT (Asymptomatic Atrial Fibrillation and Stroke Evaluation in Pacemaker Patients and the Atrial Fibrillation Reduction Atrial Pacing Trial) revealed significantly higher rates of HF hospitalization in patients with progression of DDAF to episodes >24 hours or progression to clinical AF compared to patients without progression of AF (figure 3).30 Taken together, these data suggest that AF burden is an important risk factor for the development of and hospitalization for HF.
Figure 3. Effect of AF burden on mortality and heart failure hospitalizations.
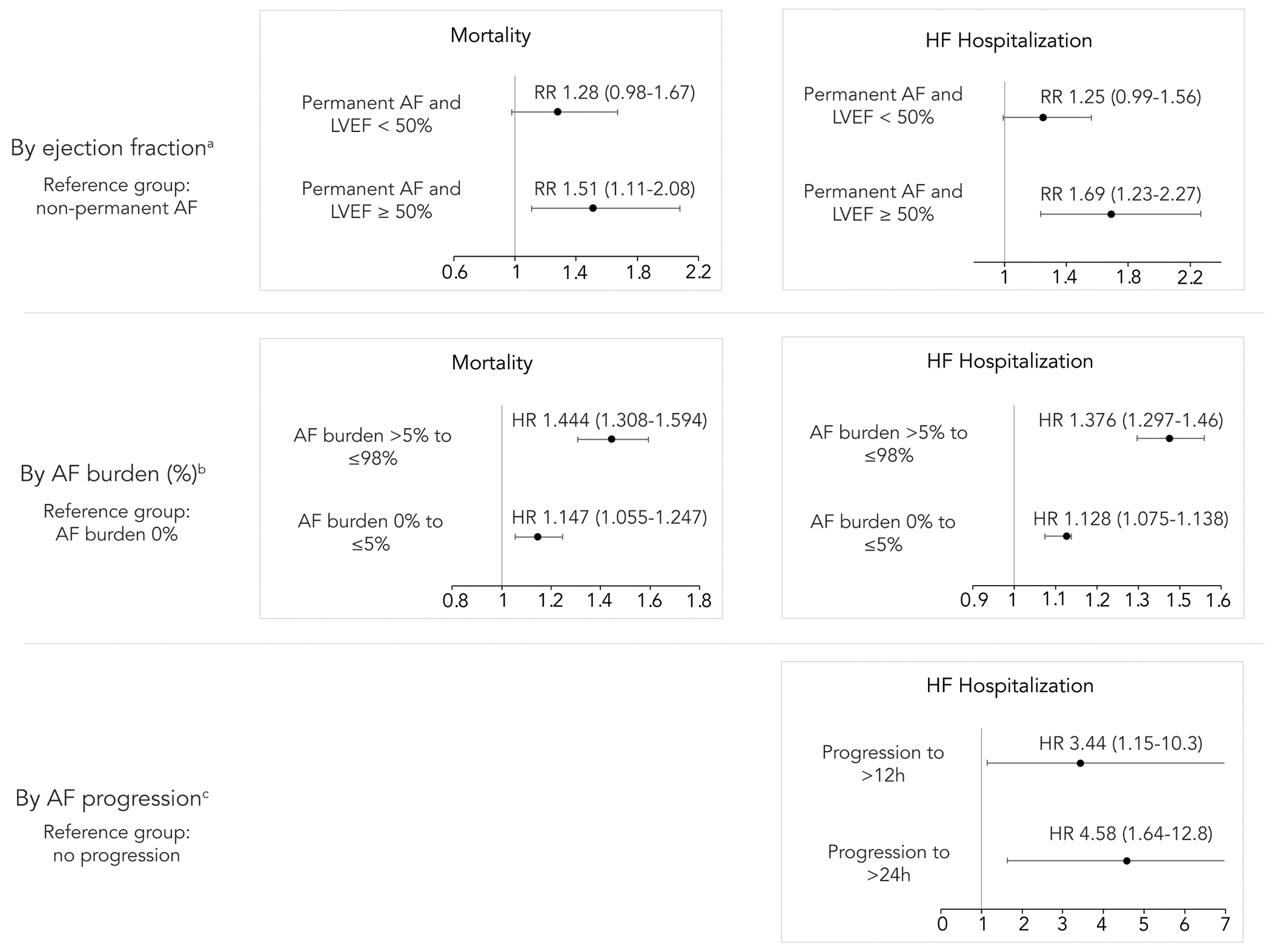
Greater atrial fibrillation (AF) burden is associated with increased risk of mortality; greater AF burden and progression are associated with increased risk of hospitalization for heart failure. The magnitude of effect is greater in patients with LVEF ≥50%. a) Data from Taillandier et al.28 b) Data from Steinberg et al.29 c) Data from Wong et al.30 h = hours, HF = heart failure, LVEF = left ventricular ejection fraction
Role of Catheter Ablation in Reducing Adverse Outcomes
Given the significant adverse effects of a greater burden of AF, patients with AF may benefit from therapies that reduce burden and prevent progression of AF. The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial showed no difference in mortality when comparing rate control and pharmacologic rhythm control strategies in patients with AF.31 However, an on-treatment analysis of the AFFIRM data showed that the presence of sinus rhythm at follow-up was associated with a decreased risk of death (adjusted HR 0.53, 99% CI 0.39-0.72, p < 0.0001).32
More recently, EAST-AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) showed benefit from an early rhythm control strategy in patients with recent diagnosis of AF within the prior 12 months.33 Patients randomized to early rhythm control in EAST had lower rates of stroke, hospitalization for worsening HF or acute coronary syndrome, and death from cardiovascular causes when compared to usual care. Importantly, the effectiveness of early rhythm control therapy was mediated by the presence of sinus rhythm in follow-up.34 While the majority of rhythm control in EAST was achieved with antiarrhythmic drug therapy, catheter ablation was also an important intervention for reducing AF burden and maintaining sinus rhythm in the trial. In the CIRCA-DOSE trial, which focused on paroxysmal AF refractory to anti-arrhythmic drug (AAD) therapy, ablation led to a >98% reduction in AF burden compared to baseline.35 Multiple other trials have shown a significant reduction in AF burden with catheter ablation compared to pharmacologic therapy (rate and/or rhythm control) (figure 4).36–38 There have also been several clinical trials of first-line ablation demonstrating significantly lower rates of arrhythmia recurrence with ablation compared to AAD therapy with similar rates of adverse safety events.39, 40 Further, ablation has been shown to delay the progression from paroxysmal AF to persistent AF more effectively than AAD therapy.41 Catheter ablation (CA) of atrial fibrillation represents a promising method for establishing and maintaining sinus rhythm in patients with AF in order to reduce AF-associated morbidity and mortality without the significant adverse effects associated with AAD therapy (central illustration).
Figure 4. Effect of catheter ablation versus medical therapy on AF burden.
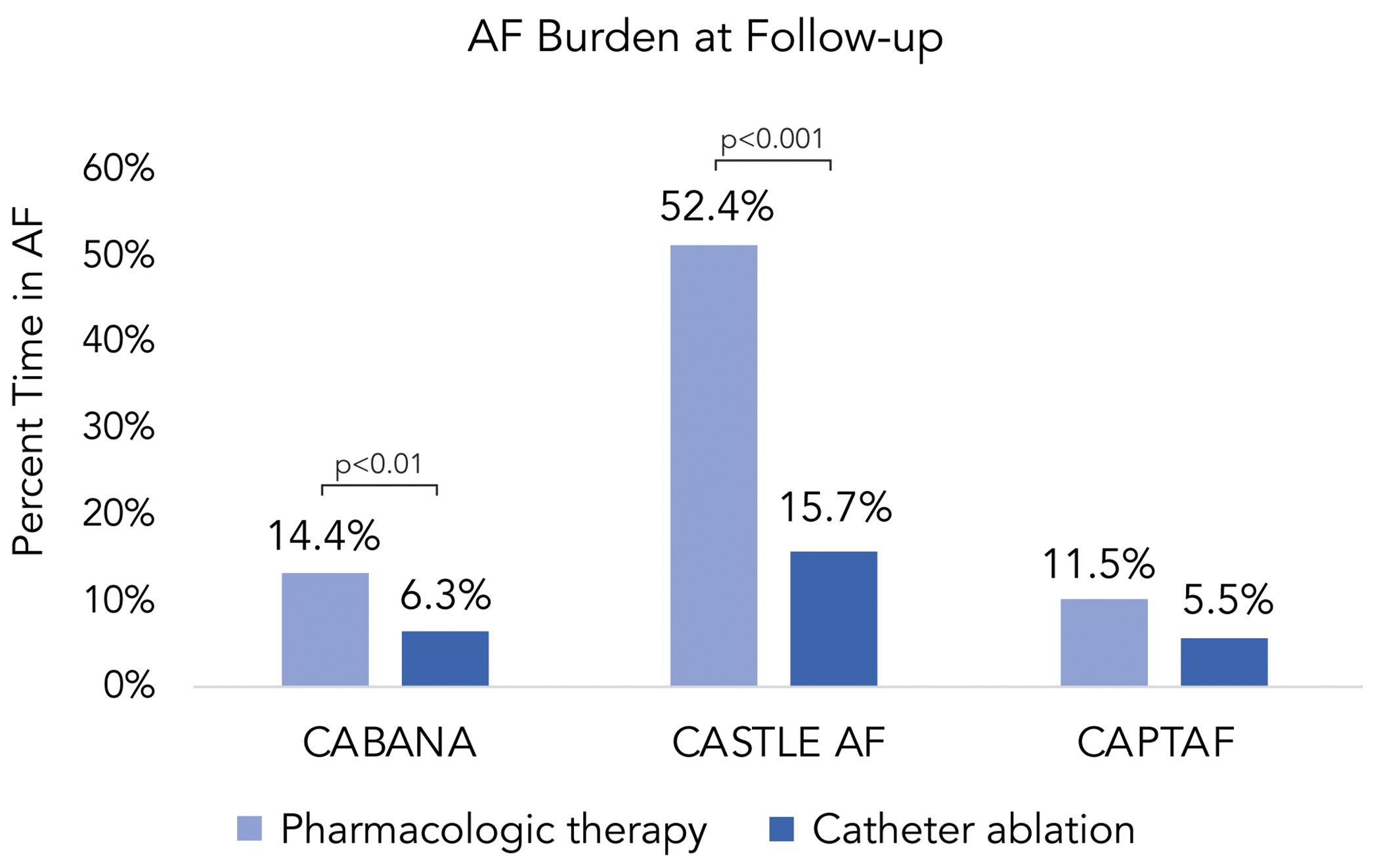
Patients treated with catheter ablation have lower atrial fibrillation (AF) burden at follow-up compared to patients treated with pharmacologic therapy.
Central illustration. Cardiovascular outcomes of AF burden and the impact of ablation.

Greater atrial fibrillation (AF) burden is associated with lower quality of life and greater risk of adverse cardiovascular outcomes. By reducing AF burden, catheter ablation of AF decreases many of the adverse effects associated with greater burden. HF = heart failure, QoL = quality of life
Improvement in Quality of Life
AHA guidelines recommend catheter ablation for patients who continue to experience symptoms of AF despite AAD therapy or in individuals who are intolerant of AAD therapy.2 Many clinical trials have demonstrated superiority of catheter ablation in those with AF that is refractory to AADs.42 This recommendation is also supported by data that demonstrate improvement in symptom burden after catheter ablation. In the CABANA (Catheter Ablation vs Antiarrhythmic Drug Therapy for Atrial Fibrillation) trial, patients with symptomatic AF treated with CA had significantly greater improvement in quality of life and symptom burden at 12 months than patients treated with medical rate or rhythm control.43 In the CIRCA-DOSE trial, a 1-point improvement in the Atrial Fibrillation Effect on Quality of Life score was observed for every absolute reduction in daily AF burden of 15.8 minutes (95% CI 7.2-24.4, p < 0.001).44 Several randomized controlled trials have also demonstrated improved quality of life, AF and HF symptom burden, and exercise tolerance (as measured by maximal oxygen consumption at peak exercise or 6-minute walk distance) in patients with AF and HF treated with CA compared to those treated with medical therapy.45–48 The DISCERN AF (Discerning Symptomatic and Asymptomatic Episodes Pre and Post Radiofrequency Ablation of Atrial Fibrillation) trial showed that daily AF burden after catheter ablation was predictive of daily physical activity levels, with a significant inverse relationship in patients with daily AF burden greater than 500 minutes.49 A prospective study of patients with symptomatic AF found that catheter ablation was associated with a significant improvement in quality of life regardless of ablative efficacy, defined as lack of recurrence of episodes of 30 seconds or more.50 These data support a shift towards primary outcomes that include AF burden and patient-reported symptoms to measure ablative success rather than the standard definition of recurrence.51
Reduction in Healthcare Utilization
Catheter ablation has also been shown to reduce healthcare utilization in patients with AF. Models of cost and healthcare utilization have demonstrated significant reductions in the number of outpatient visits, inpatient admission days, and emergency room visits with catheter ablation compared to AAD therapy in patients with AF.52, 53 Friedman et al. found that AF-related healthcare costs were significantly lower over the 18 months following ablation compared to the year prior to ablation, even when accounting for the cost of repeat ablation. Significant reductions in healthcare expenditure occurred after ablation in patients with paroxysmal and persistent AF prior to ablation, though absolute cost was higher for patients with persistent AF.54 A post-hoc analysis of the CIRCA-DOSE trial showed that healthcare utilization was significantly lower in the year following catheter ablation comparted to the year preceding ablation.55 Further analysis demonstrated that patients with AF episode duration ≤ 1 hour or an AF burden of ≤ 0.1% had rates of healthcare utilization comparable to patients without AF recurrence.56 In contrast, significantly higher rates of healthcare utilization were observed for patients with AF episode duration > 1 hour or an AF burden of > 0.1%.
Effect on Thromboembolic Risk
Data on the effect of catheter ablation on thromboembolic risk is mixed. Discerning whether catheter ablation reduces the risk of stroke may be particularly challenging given the efficacy of the direct acting oral anticoagulants for the prevention of stroke. A large retrospective analysis showed that patients with AF treated with CA had a stroke risk comparable to patients without AF (1.4% vs. 1.4%) and significantly lower than AF patients who did not undergo CA (1.4% vs. 3.5%, p < 0.001), even after adjustment for CHA2DS2-VASc score.57 However, analysis of over 3,500 patients treated with either CA or medical therapy found that there was no difference in stroke risk between the two groups after time-dependent adjustment for oral anticoagulation use.58
There is limited data exploring how AF burden might mediate the effect of catheter ablation on stroke risk. Despite greater reduction in AF burden in patients treated with CA compared to medical therapy in the CABANA trial, there was no difference in the risk of stroke between the two groups.59 Contrastingly, the EAST trial found lower stroke rates in those treated with early rhythm control (2.9% vs. 4.4%, p = 0.03).33 While EAST was not an ablation trial, it does raise the hypothesis that earlier introduction of rhythm control (with either AADs or ablation) may result in a lower risk of stroke in follow-up by reducing a patient’s AF burden.
Improvement in Mortality and Heart Failure Outcomes
Mortality benefit has not yet been demonstrated in the general population of AF patients who are treated with catheter ablation. In 2012, a Cochrane review of 32 randomized controlled trials revealed that patients treated with CA were significantly more likely to maintain normal sinus rhythm; however, there was no significant difference in mortality with CA compared to medical therapy.60 It is possible that the follow-up duration of these studies may not have been long enough to observe a difference in survival. More recently, the CABANA trial reported no significant difference in its primary end point (composite of death, disabling stroke, serious bleeding, or cardiac arrest) in symptomatic AF patients treated with CA compared to patients treated with AAD therapy.
Superiority of catheter ablation over medical therapy has been shown in patients at high risk of adverse outcomes, such as those with heart failure with reduced ejection fraction (HFrEF) and AF. The AATAC (Ablation vs. Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted Device) trial compared catheter ablation to amiodarone for treatment of persistent AF in patients with HFrEF. Treatment with catheter ablation was associated with greater maintenance of sinus rhythm, fewer unplanned hospitalizations, and lower mortality than treatment with amiodarone.47 Similarly, the CASTLE-AF (Catheter Ablation for Atrial Fibrillation with Heart Failure) trial compared CA to medical therapy for treatment of paroxysmal or persistent AF in patients with HFrEF. Treatment with CA was associated with a decrease in all-cause mortality (HR 0.53, 95% CI 0.32-0.86, p = 0.01) and risk of hospitalization for worsening HF (HR 0.56, 95% CI 0.37-0.83, p = 0.004).61
While CASTLE-AF did not observe a relationship between AF recurrence and the primary outcome (composite of all-cause mortality and hospitalization for worsening HF), there was a clear relationship between AF burden and these outcomes.36, 62 In the overall study cohort, those with AF burden > 5% had greater odds of all-cause mortality or HF hospitalization at one and two years of follow-up (OR 3.34, 95% CI, 1.5-7.46 for AF burden <5% vs. 5-80% at 1 year, and OR 2.51, 95% CI, 1.22-5.66 for AF burden <5% vs. >80% at 1 year). Of note, long-term AF burden was reduced with ablation but not with medical therapy. Among patients treated with ablation, those with AF burden <50% at 6 months had a significantly reduced risk of the primary outcome (HR 0.33, 95% CI 0.15-0.71; p = 0.01) and all-cause mortality (HR 0.23, 95% CI 0.07-0.71, p = 0.03), as well as a non-significant reduction in heart failure hospitalization (HR 0.43, 95% CI 0.17-1.06, p = 0.18). These data suggest that AF burden after catheter ablation is an important outcome with greater clinical relevance than AF recurrence.
Future directions
Many questions remain regarding the clinical application of the relationship between atrial fibrillation burden, cardiovascular outcomes, and treatment with catheter ablation. As detection of AF improves with advancements in implanted and wearable cardiac monitoring devices, providers face significant challenges determining the appropriate treatment of subclinical atrial fibrillation. Therefore, consensus is needed to establish the threshold of AF burden that warrants anticoagulation. A standard definition of and approach to AF burden is also needed to integrate AF burden into clinical decision-making, as investigators utilize different definitions in different contexts. For example, most studies on stroke prevention focus on maximal duration of AF, while studies on other outcomes (including hospitalizations or mortality) often focus on the percent of time in AF. Clinicians could use a standardized definition of AF burden to select patients that would benefit from catheter ablation, as pre-ablation AF episode duration better predicts ablative success than AF type.35
Future research should investigate therapies that slow the progression of AF and prevent the negative outcomes associated with higher AF burden. Specifically, the optimal timing of catheter ablation to improve outcomes remains unclear, particularly for younger patients with AF in whom prevention of progression will substantially decrease cumulative lifetime burden. Several studies have demonstrated higher rates of recurrence with longer time to ablation or higher AF burden,35, 63 while others have shown equivalent outcomes regardless of timing of CA or AF burden at the time of ablation.64, 65 Additionally, further work is needed to expand access to strategies that reduce AF burden in female and non-white patient populations; prior work has shown lower rates of catheter ablation in these populations66 despite similar reduction in AF burden and rates of adverse events.67–69
Finally, an alternate definition of AF recurrence after ablation is needed, as the current definition of recurrence (the presence of any AF episodes greater than 30 seconds)70 does not correlate with adverse clinical outcomes. In order to better assess the clinical success of ablation, recurrence should be defined as a burden of AF that is associated with lower quality of life, higher healthcare utilization, and higher risk of hospitalization or death.
Conclusions
With advancement in technologies that monitor atrial arrhythmias, there is now ample evidence demonstrating the association between greater AF burden and poor cardiovascular outcomes. Catheter ablation has been shown to attenuate the negative effects of AF by reducing AF burden and establishing sinus rhythm in patients with heart failure and AF. Consensus guidelines outlining the clinical implications of this data on the diagnosis and management of AF are greatly needed.
Highlight bullet points.
Data on AF burden is increasingly available with implanted and wearable monitoring devices
Greater AF burden is associated with greater risk of stroke, mortality, and HF hospitalization
Catheter ablation mitigates some of the cardiovascular outcomes associated with AF burden
Future work should integrate AF burden into clinical decision-making and measures of AF recurrence
Funding
This research did not receive financial support.
Disclosures:
HTS has no disclosures or relationships with industry. JGA receives grants and personal fees from Medtronic, grants from Baylis, personal fees from Biosense-Webster. KAW receives honoraria for speaking/consulting from Milestone Pharmaceuticals. JPP is supported by R01AG074185 from the National Institutes of Aging. He also receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, iRhythm, and Philips and serves as a consultant to Abbott, Abbvie, ARCA biopharma, Bayer, Boston Scientific, Bristol Myers Squibb (Myokardia), Element Science, Itamar Medical, LivaNova, Medtronic, Milestone, ElectroPhysiology Frontiers, ReCor, Sanofi, Philips, and Up-to-Date.
Abbreviations:
- AAD
anti-arrhythmic drug
- AF
atrial fibrillation
- CA
catheter ablation
- CIED
cardiac implantable electronic device
- DDAF
device-detected atrial fibrillation
- HF
heart failure
- SCAF
subclinical atrial fibrillation
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide Epidemiology of Atrial Fibrillation: A Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 3.Andrade JG, Yao RRJ, Deyell MW, et al. Clinical assessment of AF pattern is poorly correlated with AF burden and post ablation outcomes: A CIRCA-DOSE sub-study. J Electrocardiol. 2020;60:159–164. [DOI] [PubMed] [Google Scholar]
- 4.Charitos EI, Pürerfellner H, Glotzer TV, Ziegler PD. Clinical Classifications of Atrial Fibrillation Poorly Reflect Its Temporal Persistence. J Am Coll Cardiol. 2014;63:2840–2848. [DOI] [PubMed] [Google Scholar]
- 5.Flaker GC, Belew K, Beckman K, et al. Asymptomatic atrial fibrillation: Demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:657–663. [DOI] [PubMed] [Google Scholar]
- 6.Aguilar M, Macle L, Deyell MW, et al. Influence of Monitoring Strategy on Assessment of Ablation Success and Postablation Atrial Fibrillation Burden Assessment: Implications for Practice and Clinical Trial Design. Circulation. 2022;145:21–30. [DOI] [PubMed] [Google Scholar]
- 7.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. J Am Coll Cardiol. 2019;74:104–132. [DOI] [PubMed] [Google Scholar]
- 8.Chen LY, Chung MK, Allen LA, et al. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart RG, Pearce LA, Rothbart RM, McAnulty JH, Asinger RW, Halperin JL. Stroke with intermittent atrial fibrillation: incidence and predictors during aspirin therapy. J Am Coll Cardiol. 2000;35:183–187. [DOI] [PubMed] [Google Scholar]
- 10.Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of Stroke in Paroxysmal Versus Sustained Atrial Fibrillation in Patients Taking Oral Anticoagulation or Combined Antiplatelet Therapy. J Am Coll Cardiol. 2007;50:2156–2161. [DOI] [PubMed] [Google Scholar]
- 11.Disertori M, Franzosi MG, Barlera S, et al. Thromboembolic event rate in paroxysmal and persistent atrial fibrillation: Data from the GISSI-AF trial. BMC Cardiovasc Disord. 2013;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Link MS, Giugliano RP, Ruff CT, et al. Stroke and Mortality Risk in Patients With Various Patterns of Atrial Fibrillation: Results From the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48). Circ Arrhythm Electrophysiol. 2017;10:e004267. [DOI] [PubMed] [Google Scholar]
- 14.Al-Khatib SM, Thomas L, Wallentin L, et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34:2464–2471. [DOI] [PubMed] [Google Scholar]
- 15.Lip GYH, Frison L, Grind M, for the SPORTIF Investigators. Stroke event rates in anticoagulated patients with paroxysmal atrial fibrillation. J Intern Med. 2008;264:50–61. [DOI] [PubMed] [Google Scholar]
- 16.Vanassche T, Lauw MN, Eikelboom JW, et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J. 2015;36:281–288. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Reynolds K, Yang J, et al. Association of Burden of Atrial Fibrillation With Risk of Ischemic Stroke in Adults With Paroxysmal Atrial Fibrillation: The KP-RHYTHM Study. JAMA Cardiol. 2018;3:601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chew DS, Li Z, Steinberg BA, et al. Arrhythmic Burden and the Risk of Cardiovascular Outcomes in Patients With Paroxysmal Atrial Fibrillation and Cardiac Implanted Electronic Devices. Circ Arrhythm Electrophysiol. 2022;15:e010304. [DOI] [PubMed] [Google Scholar]
- 19.Glotzer TV, Daoud EG, Wyse DG, et al. The Relationship Between Daily Atrial Tachyarrhythmia Burden From Implantable Device Diagnostics and Stroke Risk: The TRENDS Study. Circ Arrhythm Electrophysiol. 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 20.Van Gelder IC, Healey JS, Crijns HJGM, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 21.Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10 000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke Risk as a Function of Atrial Fibrillation Duration and CHA 2 DS 2 -VASc Score. Circulation. 2019;140:1639–1646. [DOI] [PubMed] [Google Scholar]
- 23.Tiver KD, Quah J, Lahiri A, Ganesan AN, McGavigan AD. Atrial fibrillation burden: an update—the need for a CHA2DS2-VASc-AFBurden score. EP Eur. 2021;23:665–673. [DOI] [PubMed] [Google Scholar]
- 24.Park YJ, Kim JS, Park K-M, On YK, Park S-J. Subclinical Atrial Fibrillation Burden and Adverse Clinical Outcomes in Patients With Permanent Pacemakers. Stroke. 2021;52:1299–1308. [DOI] [PubMed] [Google Scholar]
- 25.Piccini JP, Passman R, Turakhia M, Connolly AT, Nabutovsky Y, Varma N. Atrial fibrillation burden, progression, and the risk of death: a case-crossover analysis in patients with cardiac implantable electronic devices. EP Eur. 2019;21:404–413. [DOI] [PubMed] [Google Scholar]
- 26.Santhanakrishnan R, Wang N, Larson MG, et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zareba W, Steinberg JS, McNitt S, Daubert JP, Piotrowicz K, Moss AJ. Implantable cardioverter-defibrillator therapy and risk of congestive heart failure or death in MADIT II patients with atrial fibrillation. Heart Rhythm. 2006;3:631–637. [DOI] [PubMed] [Google Scholar]
- 28.Taillandier S, Brunet Bernard A, Lallemand B, et al. Prognosis in Patients Hospitalized With Permanent and Nonpermanent Atrial Fibrillation in Heart Failure. Am J Cardiol. 2014;113:1189–1195. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg BA, Li Z, O’Brien EC, et al. Atrial fibrillation burden and heart failure: Data from 39,710 individuals with cardiac implanted electronic devices. Heart Rhythm. 2021;18:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong JA, Conen D, Van Gelder IC, et al. Progression of Device-Detected Subclinical Atrial Fibrillation and the Risk of Heart Failure. J Am Coll Cardiol. 2018;71:2603–2611. [DOI] [PubMed] [Google Scholar]
- 31.Wyse D, Waldo A, DiMarco J, et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 32.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. [DOI] [PubMed] [Google Scholar]
- 33.Kirchhof P, Camm AJ, Goette A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med. 2020;383:1305–1316. [DOI] [PubMed] [Google Scholar]
- 34.Eckardt L, Sehner S, Suling A, et al. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST-AFNET 4 trial. Eur Heart J. 2022;43:4127–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrade JG, Deyell MW, Verma A, et al. Association of Atrial Fibrillation Episode Duration With Arrhythmia Recurrence Following Ablation: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2020;3:e208748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brachmann J, Sohns C, Andresen D, et al. Atrial Fibrillation Burden and Clinical Outcomes in Heart Failure. JACC Clin Electrophysiol. 2021;7:594–603. [DOI] [PubMed] [Google Scholar]
- 37.Poole JE, Bahnson TD, Monahan KH, et al. Recurrence of Atrial Fibrillation After Catheter Ablation or Antiarrhythmic Drug Therapy in the CABANA Trial. J Am Coll Cardiol. 2020;75:3105–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blomström-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of Catheter Ablation vs Antiarrhythmic Medication on Quality of Life in Patients With Atrial Fibrillation: The CAPTAF Randomized Clinical Trial. JAMA. 2019;321:1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. EP Eur. 2021;23:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N Engl J Med. 2021;384:305–315. [DOI] [PubMed] [Google Scholar]
- 41.Andrade JG, Deyell MW, Macle L, et al. Progression of Atrial Fibrillation after Cryoablation or Drug Therapy. N Engl J Med. 2023;388:105–116. [DOI] [PubMed] [Google Scholar]
- 42.Calkins H, Reynolds MR, Spector P, et al. Treatment of Atrial Fibrillation With Antiarrhythmic Drugs or Radiofrequency Ablation: Two Systematic Literature Reviews and Meta-Analyses. Circ Arrhythm Electrophysiol. 2009;2:349–361. [DOI] [PubMed] [Google Scholar]
- 43.Mark DB, Anstrom KJ, Sheng S, et al. Effect of Catheter Ablation vs Medical Therapy on Quality of Life Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel M, Khairy P, Champagne J, et al. Association of Atrial Fibrillation Burden With Health-Related Quality of Life After Atrial Fibrillation Ablation: Substudy of the Cryoballoon vs Contact-Force Atrial Fibrillation Ablation (CIRCA-DOSE) Randomized Clinical Trial. JAMA Cardiol. 2021;6:1324–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hunter RJ, Berriman TJ, Diab I, et al. A Randomized Controlled Trial of Catheter Ablation Versus Medical Treatment of Atrial Fibrillation in Heart Failure (The CAMTAF Trial). Circ Arrhythm Electrophysiol. 2014;7:31–38. [DOI] [PubMed] [Google Scholar]
- 46.Parkash R, Wells GA, Rouleau J, et al. Randomized Ablation-Based Rhythm-Control Versus Rate-Control Trial in Patients With Heart Failure and Atrial Fibrillation: Results from the RAFT-AF trial. Circulation. 2022;145:1693–1704. [DOI] [PubMed] [Google Scholar]
- 47.Di Biase L, Mohanty P, Mohanty S, et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial. Circulation. 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 48.Jones DG, Haldar SK, Hussain W, et al. A Randomized Trial to Assess Catheter Ablation Versus Rate Control in the Management of Persistent Atrial Fibrillation in Heart Failure. J Am Coll Cardiol. 2013;61:1894–1903. [DOI] [PubMed] [Google Scholar]
- 49.Proietti R, Birnie D, Ziegler PD, Wells GA, Verma A. Postablation Atrial Fibrillation Burden and Patient Activity Level: Insights From the DISCERN AF Study. J Am Heart Assoc. 2018;7:e010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wokhlu A, Monahan KH, Hodge DO, et al. Long-Term Quality of Life After Ablation of Atrial Fibrillation. J Am Coll Cardiol. 2010;55:2308–2316. [DOI] [PubMed] [Google Scholar]
- 51.Blomström-Lundqvist C, Svedung Wettervik V. Reflections on the usefulness of today’s atrial fibrillation ablation procedure endpoints and patient-reported outcomes. EP Eur. 2022;24:ii29–ii43. [DOI] [PubMed] [Google Scholar]
- 52.Ladapo JA, David G, Gunnarsson CL, et al. Healthcare Utilization and Expenditures in Patients with Atrial Fibrillation Treated with Catheter Ablation. J Cardiovasc Electrophysiol. 2012;23:1–8. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds MR, Zimetbaum P, Josephson ME, Ellis E, Danilov T, Cohen DJ. Cost-Effectiveness of Radiofrequency Catheter Ablation Compared With Antiarrhythmic Drug Therapy for Paroxysmal Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2009;2:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman DJ, Field ME, Rahman M, et al. Catheter ablation and healthcare utilization and cost among patients with paroxysmal versus persistent atrial fibrillation. Heart Rhythm O2. 2021;2:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrade JG, Macle L, Verma A, et al. Quality of Life and Health Care Utilization in the CIRCA-DOSE Study. JACC Clin Electrophysiol. 2020;6:935–944. [DOI] [PubMed] [Google Scholar]
- 56.Andrade JG, Deyell MW, Macle L, et al. Healthcare utilization and quality of life for atrial fibrillation burden: the CIRCA-DOSE study. Eur Heart J. 2023;44:765–776. [DOI] [PubMed] [Google Scholar]
- 57.Bunch TJ, May HT, Bair TL, et al. Atrial fibrillation ablation patients have long-term stroke rates similar to patients without atrial fibrillation regardless of CHADS2 score. Heart Rhythm. 2013;10:1272–1277. [DOI] [PubMed] [Google Scholar]
- 58.Joza J, Samuel M, Jackevicius CA, et al. Long-term risk of stroke and bleeding post-atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2018;29:1355–1362. [DOI] [PubMed] [Google Scholar]
- 59.Packer DL, Mark DB, Robb RA, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen HS, Wen JM, Wu SN, Liu JP. Catheter ablation for paroxysmal and persistent atrial fibrillation. Cochrane Database Syst Rev. 2012:CD007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marrouche NF, Brachmann J, Andresen D, et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N Engl J Med. 2018;378:417–427. [DOI] [PubMed] [Google Scholar]
- 62.Brachmann J, et al. LBCT02-04. Presented at: Heart Rhythm Society Annual Scientific Sessions; 2018. May 9-12; Boston. [Google Scholar]
- 63.Chew DS, Black-Maier E, Loring Z, et al. Diagnosis-to-Ablation Time and Recurrence of Atrial Fibrillation Following Catheter Ablation: A Systematic Review and Meta-Analysis of Observational Studies. Circ Arrhythm Electrophysiol. 2020;13:e008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawaji T, Shizuta S, Yamagami S, et al. Early choice for catheter ablation reduced readmission in management of atrial fibrillation: Impact of diagnosis-to-ablation time. Int J Cardiol. 2019;291:69–76. [DOI] [PubMed] [Google Scholar]
- 65.Strisciuglio T, El Haddad M, Debonnaire P, et al. Paroxysmal atrial fibrillation with high vs. low arrhythmia burden: atrial remodelling and ablation outcome. EP Eur. 2020;22:1189–1196. [DOI] [PubMed] [Google Scholar]
- 66.Patel N, Deshmukh A, Thakkar B, et al. Gender, Race, and Health Insurance Status in Patients Undergoing Catheter Ablation for Atrial Fibrillation. Am J Cardiol. 2016;117:1117–1126. [DOI] [PubMed] [Google Scholar]
- 67.Russo AM, Zeitler EP, Giczewska A, et al. Association Between Sex and Treatment Outcomes of Atrial Fibrillation Ablation Versus Drug Therapy: Results From the CABANA Trial. Circulation. 2021;143:661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deng H, Shantsila A, Guo P, et al. Sex-related risks of recurrence of atrial fibrillation after ablation: Insights from the Guangzhou Atrial Fibrillation Ablation Registry. Arch Cardiovasc Dis. 2019;112:171–179. [DOI] [PubMed] [Google Scholar]
- 69.Thomas KL, Al-Khalidi HR, Silverstein AP, et al. Ablation Versus Drug Therapy for Atrial Fibrillation in Racial and Ethnic Minorities. J Am Coll Cardiol. 2021;78:126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. [DOI] [PMC free article] [PubMed] [Google Scholar]


