Abstract
Background:
Alert-driven RPM or fully virtual care without routine evaluations may reduce clinic workload and promote more efficient resource allocation, principally by diminishing non-actionable patient encounters. The study objective was to conduct a cost-consequence analysis to compare three post-implant ICD follow-up strategies: (a) IPE only, (b) RPM-conventional (hybrid of IPE and RPM), and (c) RPM-alert (alert-based ICD follow up).
Methods:
We constructed a decision-analytic Markov model to estimate the costs and benefits of these three strategies over a two-year time horizon from the perspective of the US Medicare payer. Aggregate and patient-level data from the TRUST (Lumos-T Safely RedUceS RouTine Office Device Follow-up) randomized clinical trial informed clinical effectiveness model inputs. TRUST randomized 1,339 patients 2:1 to conventional RPM or IPE alone, and found that RPM was safe and reduced the number of non-actionable encounters. Cost data was obtained from the published literature. The primary outcome was incremental cost.
Results:
The mean cumulative follow-up costs per patient were $12,688 in the IPE group, $12,001 in the RPM-conventional group, and $11,011 in the RPM-alert group. Compared to the IPE group, both the RPM-conventional and RPM-alert groups were associated with lower incremental costs of −$687 (95% confidence interval [CI]-$2,138 to +$638) and −$1,677 (95% CI −$3,134 to −$304), respectively. Therefore, the RPM-alert strategy was most cost-effective with an estimated cost-savings in 99% of simulations.
Conclusions:
Alert-driven RPM was economically attractive and, if patient outcomes and safety are comparable to conventional RPM, may be the preferred strategy of ICD follow up.
Keywords: Remote monitoring, implantable cardioverter defibrillators, remote follow up, telemedicine, patient monitoring, economics, cost consequence analysis, economic evaluation
INTRODUCTION
Implantable cardioverter defibrillator (ICD) follow up is structured around 3 monthly evaluations.1, 2 This paradigm of follow up reflects technology from over 2 decades ago where ICDs required frequent threshold and battery tests as well as capacitor reformation. Advances in ICD technology have rendered this rationale obsolete yet the practice of frequent routine scheduled patient assessments persists.2
The TRUST (Lumos-T Safely RedUceS RouTine Office Device Follow-up) and other subsequent clinical trials demonstrated that remote follow up dramatically reduced the need for in person evaluation.3, 4 This laid the foundation for current guideline recommendations.1 Adoption of these recommendations requires a shift of clinician workload from visible clinic interactions to largely “invisible” patient evaluations involving management of data transmissions requiring clinic coordination and interpretation.5 Few routinely scheduled evaluations require clinical action; for example, only 6.6% of all 3-month scheduled in-person or remote transmissions in TRUST were clinically actionable. Routine evaluations represent a burden to physicians, patients and healthcare systems.4 Recent data suggests that the indirect workload may be even more substantial as an unintended consequence of alert management, continuous remote monitoring, patient-triggered transmissions, and time-intensive implantable loop recorder interpretations on top of non-actionable routinely scheduled evaluations.5
In the current environment, reimbursement appears insufficient for clinic workforce needs, forcing delegation of remote management to third parties.6 Increasing volume and complexity of patients receiving cardiac implantable electronic devices (CIEDs) will aggravate this condition.6 However, device clinic workload may be alleviated if activity is directed more to “actionable” work by reducing the number of routinely scheduled, but clinically low yield routine evaluations. Alert-based remote monitoring, without routine evaluations, has been proposed as a more efficient follow up strategy and was enacted in some clinical practices during the pandemic.7 However, the economic implications of alert-based remote monitoring are currently undefined. These requisite data are pivotal to inform policy-level health care funding decisions, and reimbursement models that directly impact the uptake of remote monitoring and device clinic structuring.
The purpose of this study is to assess the economic implications of three different strategies of ICD follow up: (1) in clinic visits only; (2) conventional remote monitoring follow-up consisting of a hybrid of in person and remote follow up; and (3) alert-driven remote monitoring, where in person visits are scheduled only if necessary. We report the findings of this cost-consequence analysis, where incremental costs and incremental benefits (i.e., quality-adjusted life years) are reported separately, rather than a cost-effectiveness analysis which provides an aggregated incremental cost effectiveness ratio.
METHODS
The study protocol and report were prepared in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement.8
Trial Design and Patient Population
The TRUST trial design and methods have been reported previously in detail.3, 4, 9 TRUST enrolled participants with single- or dual-chamber ICDs capable of home monitoring and implanted for class I/II indications. Participants from 102 centers across the US were enrolled within 45 days of successful ICD implantation and randomized in a 2:1 ratio to scheduled remote home monitoring and clinic visits (augmented by automated alert transmissions for critical events), or scheduled in-clinic visits alone. Trial enrollment occurred between November 2005 and February 2008 with follow up for 15 months. Approval of the appropriate institutional review board or ethics committee was obtained at all sites, and all patients provided written informed consent (ClinicalTrials.gov Identifier NCT00336284).
Model Design and Structure
We constructed a decision-analytic Markov model to project the costs of three follow up strategies after ICD implantation: (a) in-person evaluations (“IPE”) alone, consisting of scheduled in person visits every 3 months; (b) conventional remote patient monitoring (“RPM-conventional”), consisting of scheduled in-person visits at 3 and 12 months supplemented by remote evaluations every 3 months between clinic visits as well as unscheduled visits driven by device alerts; and (c) alert-driven only care (“RPM-alert”). In the RPM-alert strategy, there would be no scheduled in-person or remote evaluations following an initial post-operative assessment, unless a device alert prompted an unscheduled clinic visit.
A two-state Markov model was constructed in TreeAge Pro 2022 (Williamstown, MA) consisting of two health states: alive and dead. Each monthly cycle, the cohort of patients in the alive health state could be admitted to hospital (i.e., a transient health event associated with costs and decrement to patient quality of life), experience an unscheduled clinic or emergency department visit, undergoing lead or device system revision, die or remain event free (Figure 1). From the perspective of the US Medicare payer, a 2-year time horizon was chosen to reflect the follow up duration of the TRUST trial.3, 4 The primary outcome was the incremental cost per patient. The secondary outcome was incremental quality-adjusted life expectancy. We hypothesized that RPM-alert and/or RPM-conventional strategies would be associated with cost-savings, in which case a negative incremental cost-effectiveness ratio (ICER) would not be meaningful to interpret. Thus, the pre-planned economic plan was to perform a cost-consequence analysis, where costs and clinical outcomes were reported separately without the ICER summary endpoint.
Figure 1.
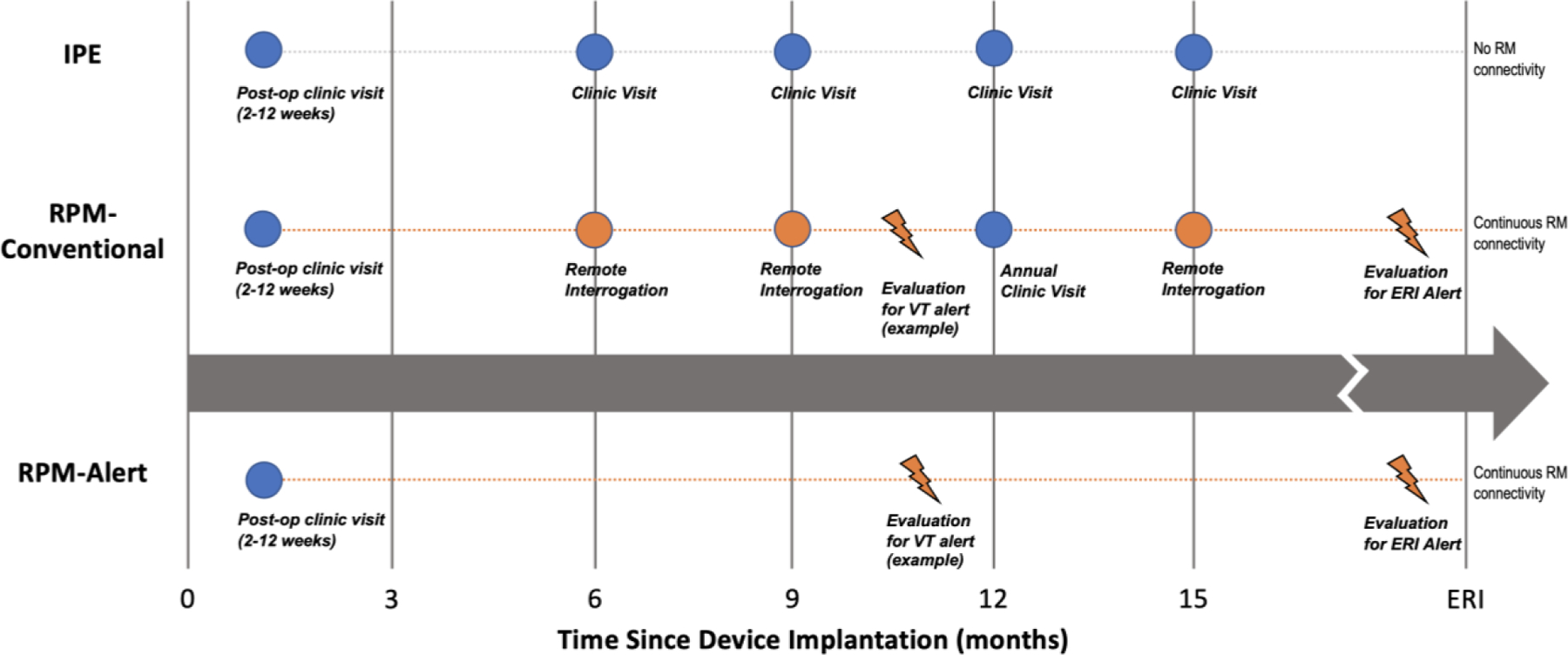
Conceptual Model of Follow Up for Patients with Implantable Cardioverter Defibrillators: IPE (In-person evaluations only), RPM- Conventional (hybrid in-person evaluations and remote evaluation), RPM-Alert (Alert-based care).
Nb. Idealized follow-up for IPE and RPM-Conventional strategies based on 2008 and 2015 Heart Rhythm Society Expert Consensus Documents. IPE versus RM-conventional directly assessed in the TRUST RCT. RM-Alert group modelled based on TRUST data. Abbreviations. ERI – end replacement indicator; RM – Remote monitoring; VT – ventricular tachycardia.
Medical Resource Use
Clinical effectiveness inputs (Table 1) were informed directly by the TRUST randomized trial, which compared a strategy of IPE to RPM-conventional (i.e., hybrid of IPE supplemented by remote evaluations). TRUST prospectively collected medical resource use data and reported the annualized rates of emergency department visits and hospitalization, total clinic visits, and device malfunction requiring lead extraction and system revision. Since TRUST reported the aggregate rates of ED and hospitalization visits, we assumed that 82% of reported visits were due to inpatient hospitalization based on medical resource use patterns from the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project Nationwide Emergency Department Sample and National Inpatient Sample that described a national cohort of patients with heart failure.10 For the RPM-alert strategy, we were required to make several model assumptions since TRUST only empirically compared the IPE and RPM-conventional strategies. Specifically, we assumed that scheduled RPM-alert strategy had similar medical resource use and clinical outcomes as the RPM-conventional strategy with regard to unscheduled emergency department visits, hospitalizations and clinic visits. Additionally, the model assumed that there was no difference in mortality between the IPE, RPM-conventional and RPM-alert strategies. This conservative assumption is consistent with the TRUST findings and prior studies reporting heterogenous impact of remote monitoring on mortality.11
Table 1.
Base case clinical and costing inputs
| Variable | Input | Range | Distribution | Reference |
|---|---|---|---|---|
| Clinical Inputs | ||||
| All-cause death at 1 year | n/a | Weibull | TRUST3 | |
| IPE Strategy | ||||
| Hospitalization / ED Visits, per patient year | 0.13 | 0.10 – 0.16 | Beta | TRUST3 |
| Clinic Visits, per patient year | 4.00 | 3.00 – 5.00 | Beta | TRUST3 |
| RPM-Conventional Strategy | ||||
| Hospitalization / ED Visits, per patient year | 0.11 | 0.08 – 0.14 | Beta | TRUST3 |
| Clinic Visits, per patient year | 2.50 | 1.88 – 3.13 | Beta | TRUST4 |
| Median Number of Alerts per patient | 1.00 | 0 – 3.00 | Triangle | TRUST4 |
| RPM-Alert Strategy | ||||
| Hospitalization / ED Visits, per patient year | 0.11 | 0.08 – 0.14 | Beta | TRUST3 |
| Clinic Visits, per patient year | 0.80 | 0.40 – 1.20 | Beta | TRUST4 |
| Median Number of Alerts per patient | 1.00 | 0 – 3.00 | Triangle | TRUST4 |
| Proportion of Hospitalization (versus ED) | 0.82 | Beta | 10 | |
| Lead / Generator Revision, per patient year | 0.02 | 0.01 – 0.06 | Beta | TRUST4 |
| Utilities | ||||
| Alive with ICD | 0.85 | SD ± 0.3 | Beta | 33 |
| Disutility HF Hospitalization | −0.0066 | −0.0135 to 0 | Beta | 34 |
| Costs (2021 USD) | ||||
| Remote Monitoring Equipment* | 2,000 | ±50% | Gamma | Assumption |
| Cost per Hospitalization | 17,389 | 13,042 – 21,736 | Gamma | 15 |
| Cost per ED Visit | 1,726 | 1,413 – 2,038 | Gamma | 14 |
| Annual Outpatient Costs of HF | 3,964 | 3,057 – 5,091 | Gamma | 35 |
| Clinic Cost, per visit | 164 | ±50% | Gamma | 13 |
| Remote monitoring cost, per assessment | 64 | ±50% | Gamma | 13 |
| Lead / Generator Revision Cost | 35,534 | 23,955 – 51,038 | Gamma | 20, 36 |
| Annual discount rate | 0.03 | n/a | n/a | 19 |
Cost of remote monitoring equipment added for sensitivity analysis.
Abbreviations: ED – emergency department; HF – heart failure; ICD – implantable cardioverter defibrillator; IPE – in-person evaluation; RPM – remote patient management.
Survival Parametrization and Model Validation
Using individual patient data from the TRUST trial, survival was modelled using a parametric model fit to a Weibull distribution, which was selected among other plausible distributions (i.e., Weibull, Log-Normal, Gompertz, Exponential) according to the smallest Akaike Information Criterion or Bayesian Information Criterion goodness-of-fit statistic.12 Parameterization of survival was performed using Stata 16 (College Station, TX).
To assess model agreement with empirical TRUST survival results, we compared the model estimates of undiscounted life expectancy to the restricted mean survival times derived directly from the TRUST trial data. The modelled and empiric survival estimates were compared over the trial follow up.
Costing Sources
Costs of outpatient encounters (such as in clinic visits or remote evaluation) were assigned using the 2021 Medicare Physician Fee Schedule based on Current Procedural Terminology codes.13 The cost of an emergency department visit was obtained from a published analysis of several national datasets including the Medical Expenditure Panel Survey, National Hospital Ambulatory Medical Care Survey, and the Healthcare Cost and Utilization Project’s Nationwide Inpatient Sample.14 For hospitalization costs, we obtained the average cost per patient of a heart failure hospitalization with a median length of stay of 5 days from an analysis of the national 5% sample of Medicare beneficiaries.15 Medication costs were not included as medication use was expected to be similar between the RM and in-clinic groups.
Device clinic nursing costs were based on the average hourly wage for a Registered Nurse reported in the 2021 Occupational Employment and Wage Statistics published by the U.S Bureau of Labor Statistics.16 The hourly wage was applied to the average visit times reported in a time and motion workflow evaluation study of in-person clinic visits and remote monitoring evaluations across 11 centers in the United States and Europe.17 In this study, the mean visit time for US centers, including direct and indirect patient care, was 12.7 minutes per remote transmission review and 51.0 minutes per in-clinic visit. Consistent with this time and motion study, our analysis assumed a two-level review process where interrogations and/or transmissions were preliminarily reviewed by device clinic nurses to determine further escalation for intervention by a physician or other clinician. Costs were valued in 2021 USD, and adjusted using the US Medical Care Consumer Price Index, where appropriate.18
Sensitivity Analyses
The base case analysis assumed that the remote monitoring equipment was bundled into the procurement cost of the implantable cardioverter defibrillation. Depending on the hospital, these equipment or infrastructure costs may be absorbed by industry or included in the marked-up procedure cost, or considered part of capital costs for existing hospital infrastructure to manage. For better understanding into the value proposition of remote monitoring, we conducted a sensitivity analysis assuming an additional cost of $2,500 for the remote monitoring equipment, which were costs assumed to be absorbed by industry or included in the device prices, or associated costs associated with hospital infrastructure.
Concordant with economic evaluation best practices,19 we conducted an exploratory analysis to project costs and outcomes over a (remaining) lifetime horizon to better reflect the potential long-term consequences of the RPM strategies. During the lifetime extrapolation, we assumed that the RPM-alert strategy would require an in-person clinic visit every 2 years. Furthermore, we included a cost of $31,503 (2021 USD) for each ICD generator replacement20 every 6 years assuming (a) an average of 6 years of battery longevity consistent with the current ICD technology during the TRUST study, and (b) that there was no substantial difference in battery longevity between the IPE and RPM strategies.21 Medical resource use observed in the TRUST trial by follow up strategy (IPE versus RPM) was assumed to be the same during the extrapolation period.
Variability and Uncertainty
All model outputs were probabilistic. Distributional assumptions of the input parameters were as follows: log-normal distributions for all hazard ratios, β-distributions to all probabilities and utilities, and γ-distributions to all costs. A Monte Carlo simulation with 10,000 iterations was used to describe the impact of uncertainty in individual model parameters on a distribution of expected costs. Deterministic one-way sensitivity analyses were also performed that varied one input parameter at a time using the lower and upper 95% interval bounds and recorded the change in the incremental cost.
Variables, for which confidence intervals were not provided, were modelled with wide distributions (±50%) to enhance external validity and to evaluate the robustness of the findings. A 3% discount rate was applied to all future costs and benefits.19
RESULTS
Model Validation
The model estimates of survival were comparable to the empirically observed survival in TRUST. Specifically, modelled life expectancies compared with restricted mean survival times were 0.496 life year (LY) versus 0.496 LY (0.493 to 0.498) at 6 months, 0.983 LY versus 0.981 LY (0.974 to 0.989) at 12 months, and 1.22 LYs versus 1.22 LYs (1.21 to 1.23) at 15 months.
Cost-Consequence Analysis
At 2 years, the mean cumulative costs per patient were $12,688 in the IPE group, $12,001 in the RPM-conventional group, and $11,011 in the RPM-alert group. Compared to the IPE group, both the RPM-conventional and RPM-alert groups were associated with lower incremental costs of −$687 (95% confidence interval (CI)-$2,138 to +$638) and −$1,677 (95% CI −$3,134 to −$304), respectively (Table 2). Of the three groups, the RPM-alert strategy was considered the preferred, optimal strategy with an estimated cost-savings in 99% of simulations.
Table 2.
Base case results (2-year time horizon).
| Strategy | Total Costs | Incremental Costs |
|---|---|---|
| IPE | 12,688 (10,490 to 15,143) | Reference |
| RPM-Conventional | 12,001 (9,955 to 14,293) | −687 (−2,138 to +638) |
| RPM-Alert | 11,011 (8,981 to 13,298) | −1,677 (−3,134 to −304) |
| Preferred Strategy | RPM-Alert: Cost Savings | |
There was no difference in discounted life expectancy between the IPE, RPM-conventional and RPM-alert arms. Quality-adjusted life expectancy between groups was also similar with an estimated 1.59 QALYs (95% CI 1.06 to 1.85) over the 2-year time horizon.
Sensitivity Analyses
For the one-way sensitivity analyses, we made the conservative assumption that emergency department visits and hospitalizations were not significantly different between the IPE and RM strategies. Under these conditions, when comparing the IPE and RPM-alert strategies, the inputs with the greatest variation effect on the model included the cost and frequency of scheduled in-person clinics (Figure 2A). However, cost-savings was estimated in all one-way sensitivity analyses across the plausible range for each model input. Similarly, across one-way sensitivity analyses, RPM-Alert was cost saving compared to the RPM-conventional strategy (Figure 2B). Notably, the cost of remote monitoring assessments did not appear to be an influential factor when varied between $32 to $96 per alert-driven assessment.
Figure 2.
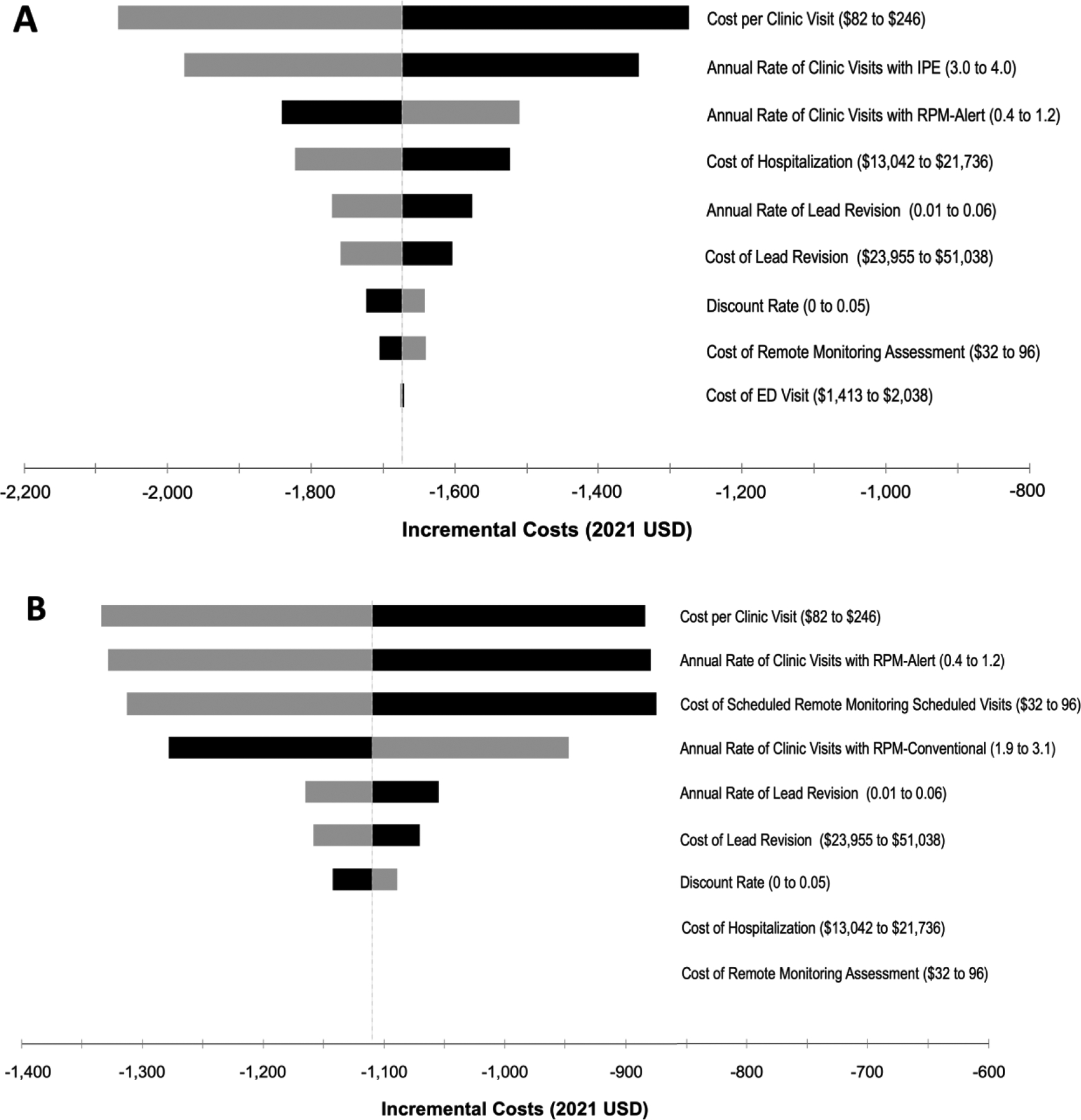
Tornado diagram summarizing one-way sensitivity analyses on incremental costs: A. RPM-Alert – IPE Strategies; B. RPM-Alert versus RPM-Conventional Strategies. Grey and black bars denote the effects of the upper and lower bounds of each variable input, respectively. That is, the upper bound may have differential effects on the incremental costs depending on the variable input. Abbreviations: ED – emergency department; IPE – in person evaluation; RPM – remote patient monitoring; USD – United States Dollar.
To assess the maximum reimbursement for a remote monitoring evaluation where the RPM strategies would still be considered cost-savings, we conducted a post-hoc threshold analysis. Reimbursement could be increased up to $162 per remote assessment, where the RPM-conventional strategy would be cost-neutral and the RPM-alert strategy would remain cost-savings compared to the IPE strategy.
While the remote monitoring equipment is often bundled into the initial cost of device, we conducted a sensitivity analysis assuming that $2,500 of additional costs for the monitoring equipment. In this scenario, the RPM-alert and RPM-conventional strategies were associated with +$827 and +$991 increased costs, respectively, compared to the IPE strategy. Thus, remote monitoring would not be considered economically attractive due to the cumulative increased costs assuming an equipment price of $2,500 over a limited 2-year time horizon.
Lifetime Horizon
The base case time horizon was limited to 2 years based on the empiric follow up of TRUST trial and to limit the uncertainty in medical resource use beyond this time period. An exploratory analysis over a lifetime horizon was conducted to assess the potential long-term consequences of the RPM strategies. Over a lifetime horizon, the cumulative costs were $114,274 for IPE, $109,449 for RM-conventional, and $105,274 for RM-alert. Compared to the IPE strategy, the total incremental cost-savings were −$4,826 (95% CI −$12,043 to +$2,056) with RPM-conventional and −$9,000 (95% CI −$16,249 to −$2,104) with RPM-alert.
There were 7.9329 discounted QALYs estimated in the IPE strategy. The RPM-alert and RPM-conventional strategies were associated with +0.0002 additional QALYs compared to IPE due to a non-significant lower rate of ED visits and hospitalizations. The RPM-alert strategy was the preferred strategy in 99% of simulations compared to both the RPM-conventional and IPE strategies due to the observed cost-savings (Figure 3).
Figure 3.
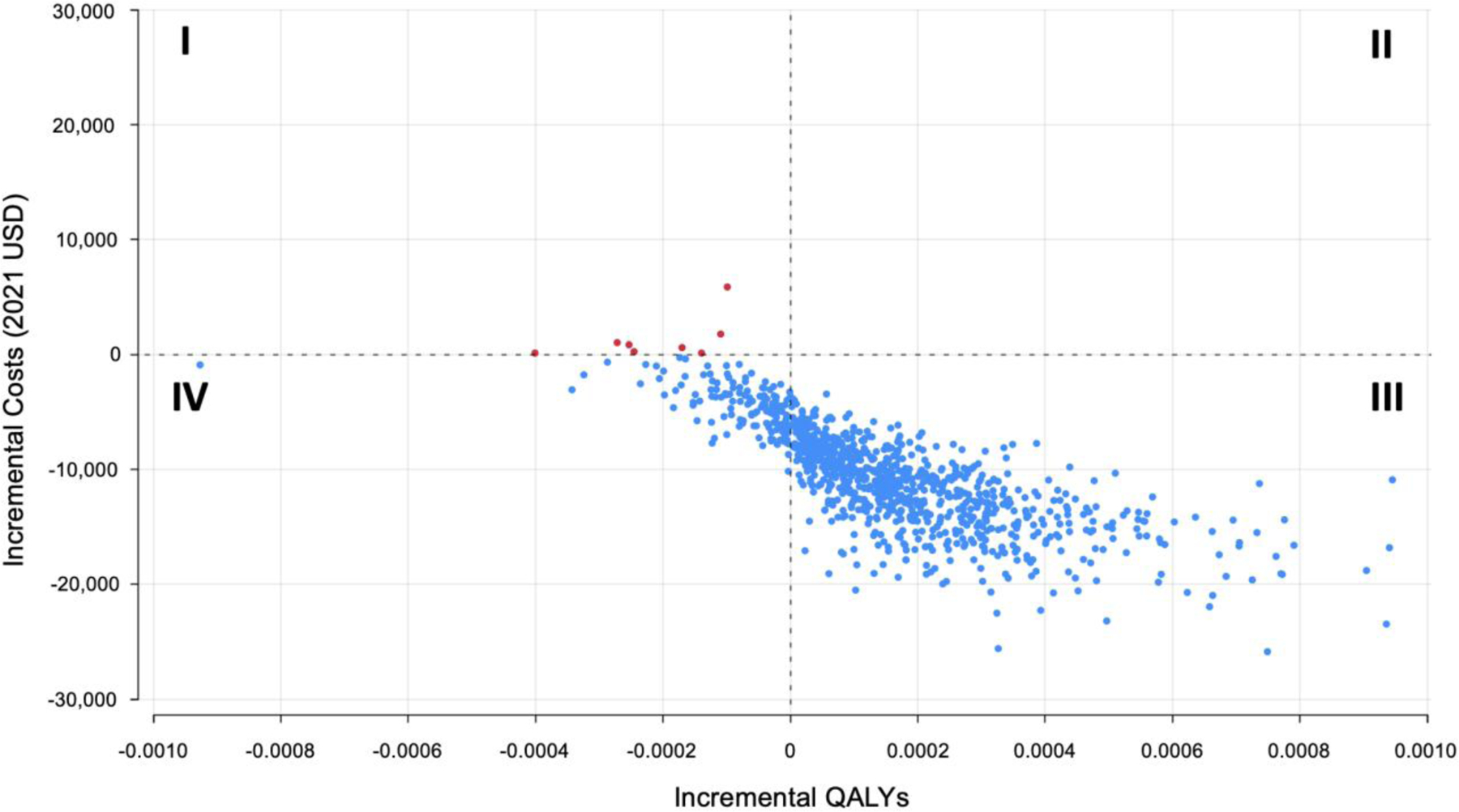
Incremental cost-effectiveness plane comparing the In-Person Evaluation (IPE) Only Strategy to Remote Patient Monitoring (RPM) Alert Strategy. Costs and benefits accrued over a lifetime horizon.
In a sensitivity analysis that included the additional fees for remote monitoring equipment, both the RPM-conventional and RPM-alert strategies were cost-savings (i.e., −$4,185 and −$6,401, respectively) compared to the IPE alone strategy.
DISCUSSION
The main finding of our analysis indicates that alert-based remote monitoring of patients with ICDs is economically attractive through provision of cost-savings to the US Medicare payor compared to either strategies of in-person evaluations alone or conventional remote monitoring consisting of scheduled remote evaluations and in-person evaluations. Due to these cost-savings, the RPM-alert strategy is considered a high value healthcare intervention as per the American College of Cardiology / American Heart Association Cost and Value Framework.22
Over 2-year time horizon of the primary analysis, the estimates of cost-savings remained consistent in the sensitivity analyses where the model inputs were varied over their reported ranges. The model inputs with the most influence on the estimated incremental costs were the cost and frequency of scheduled in-person clinics. Cost-savings would still be achieved even if reimbursement for a remote monitoring assessment was increased by over two-fold, which acknowledges the Medicare reimbursement variability by state.
This economic analysis has important implications in the United States given that over 100,000 de novo ICDs are implanted in the United States annually but implementation of remote monitoring remains limited.23 Even without considering the larger prevalent population with existing ICDs, a strategy of alert-based remote monitoring would save over 80 million dollars annually compared to a strategy to in-person evaluations alone, or 50 million dollars compared to conventional remote monitoring. These findings are particularly relevant for health policymakers when considering value-based health care initiatives.
Our results are consistent with prior cost-consequence and cost-minimization studies conducted in the United States,24 Canada,25 and Europe,26 where conventional remote monitoring (with scheduled in clinic visits) met country-specific thresholds for good value in health care. For example, remote monitoring was associated with $3,703 (2012 US dollars) in cost savings compared to in-clinic visits alone in a US nationwide cohort study of patients with pacemakers, ICDs, and cardiac resynchronization therapy using the IBM MarketScan database. These cost-savings were driven primarily through a 30% reduction in hospitalization costs associated with a remote monitoring strategy. When compared to the current economic analysis, our model provides a more conservative estimate of economic benefit when comparing remote monitoring to in-person evaluations alone. Consistent with clinical findings of the TRUST randomized clinical trial, our economic model assumed that there are no significant cost-offsets during follow up from reduced hospitalization visits, or fewer emergency department visits. However, hospital length of stay was not available in the TRUST study, which may provide additional cost benefits in favor of remote monitoring. For example, the CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) randomized study, enrolling 1,997 patients with ICDs, found that remote monitoring was associated with a significant decreased length of stay per CV hospitalization.27 Postulated mechanisms of shorter hospital length of stay include shortened response time from device alerts to clinical decision and earlier intervention for clinical deterioration enabled with remote monitoring. Additionally, TRUST did not include patients at the highest risk of HF and re-hospitalization such as those with CRT-D or pacemaker dependence. There may be a greater value proposition among those at higher HF risk due to the benefits of continuous remote monitoring and earlier intervention when acting upon alert transmissions.28
Prior economic models have not specifically assessed the cost-effectiveness of alert-based only models of care, as there is limited data available regarding clinical outcomes associated with fully virtual remote monitoring. The current study is enabled by patient-level randomized control trial data from the TRUST trial, which reported medical resource use and outcomes over a 1-year period of exclusive alert-based follow up in a post-hoc analysis.4 The finding that alert-based monitoring yields the greatest cost-savings has implications for value-based health care initiatives. That is, the current study supports the economic benefits of moving toward a solely alert-based remote monitoring model instead of conventional remote monitoring requiring a hybrid of scheduled in person clinic visits and routine transmissions; less than 7% of these routine evaluations are non-actionable.4, 29 Importantly, the economic model was limited to a 2-year time horizon to approximate the follow up of the TRUST trial. Whether an alert-based remote monitoring approach is safe and effective beyond 2 years without some form of in person assessment remains to be assessed. This form of fully virtual CIED care will be assessed in the upcoming Remote Patient Management of Cardiac Implantable Electronic Devices Tachy Study (RPM CIED Tachy) (clinicaltrials.gov NCT03405740). The advantages of alert-based care are likely to be amplified with time, since likelihood of device/lead related issues increase, battery longevity improves (and ERI can be notified by alerts promptly without intensified frequency of interrogations), unnecessary shock therapies and related hospitalizations are reduced, improved technology behind device alerts, and opportunities for disease modification (AF and HF) increase.3, 21, 28 We also did not include the reduction in patient costs (time, transportation). Therefore, our estimates are considered conservative.
Limitations
The results of the study need to be interpreted in the context of several limitations. First, the clinical effectiveness inputs were primarily based on a single randomized control trial that enrolled patients from over 10 years ago using a remote monitoring platform from a single manufacturer. However, the remote monitoring capabilities of the platform used in the TRUST study have remained largely unchanged, maintaining consistent daily connectivity, which underpins the practice of alert based follow up. The alert notification portfolio included all critical device- and arrhythmia-related parameters. Our results are considered conservative as current and future innovation could enhance remote monitoring value and efficiency, including artificial intelligence-based surveillance, longer battery longevity, and the potential for remote reprogramming. Extrapolation of the study findings to remote monitoring platforms developed by others manufacturers may be limited depending on the features included within each platform.30 Prior studies suggest that daily verification of transmissions is a key feature associated with reduction in clinical endpoints such as HF hospitalization.28, 31
Second, TRUST compared a strategy of IPE versus RPM-conventional. The RPM-alert strategy was not directly assessed in the TRUST trial, but modelled based on the outcomes of during the exclusive remote monitoring period (i.e., 3 months to 15 months of follow up) without scheduled in person clinic visits (elimination of scheduled evaluations did not compromise ability for problem discovery or patient safety). However, we were able to model an RPM-alert strategy as this analysis benefit from the use of patient-level data to simulate the outcomes associated with a solely alert-driven model of care. Effectiveness of an alert-driven model is contingent upon patient adherence and home monitoring connectivity; however, data from the TRUST trial suggests improved adherence among the RPM-conventional group compared to the IPE group.3, 4 Nonetheless, application of an actionable care model is contingent upon demonstration of the safety of alert-driven management without scheduled interrogation (in person or remote) in a longer-term study.32
Third, costing inputs for the study were obtained from external costing studies, rather than prospective bill collection alongside the clinical trial. However, the costing inputs were obtained from studies derived from Medicare or Medicaid reimbursements to ensure consistency with the study perspective of the US Medicare payer. Additionally, the value proposition of alert-based remote monitoring did not appear substantially affected by the costing inputs. Finally, we did not include indirect patient costs, such as out-of-pocket payments, clinic travel and caregiver costs, or lost productivity associated with device related care. The economic benefits of alert-based remote monitoring would likely be greater if adopting a broader societal perspective.
CONCLUSIONS
Alert-based remote patient monitoring with minimized scheduled evaluation (in person or remote assessment) is an efficient and transformative model of care. This approach is cost-savings compared to both conventional RPM and clinic only follow up strategies, with implications for value-based health care initiatives.
ACKNOWLEDGMENTS
The authors thank William Hsu, PhD, MBA for contribution to trial concept, design and execution.
Conflicts of Interest:
Dr. Piccini is supported by R01AG074185 from the National Institutes of Aging. He also receives grants for clinical research from Abbott, the American Heart Association, the Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, iRhythm, and Philips and serves as a consultant to Abbott, Abbvie, Ablacon, Altathera, ARCA biopharma, Bayer, Biotronik, Boston Scientific, Bristol Myers Squibb (Myokardia), Element Science, Itamar Medical, LivaNova, Medtronic, Milestone, ElectroPhysiology Frontiers, ReCor, Sanofi, Philips, and Up-to-Date outside of the submitted work. Mr. Michalski is an employee of Biotronik. Dr. Varma is a consultant/speaker for Abbott, Medtronic, Biotronik, Impulse Dynamics, and Boston Scientific. The remaining authors report no relevant disclosures.
Footnotes
Trial Registration: ClinicalTrials.gov NCT00336284
REFERENCES
- 1.Slotwiner D, Varma N, Akar JG, et al. HRS Expert Consensus Statement on remote interrogation and monitoring for cardiovascular implantable electronic devices. Heart Rhythm Jul 2015;12:e69–100. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman JN, Wadhera RK, Choi E, Zhao T, Secemsky EA, Fraiche AM, Shen C, Kramer DB. Trends in utilization and spending on remote monitoring of pacemakers and implantable cardioverter-defibrillators among Medicare beneficiaries. Heart Rhythm Nov 2020;17:1917–1921. [DOI] [PubMed] [Google Scholar]
- 3.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C, Investigators T. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: the Lumos-T Safely Reduces Routine Office Device Follow-up (TRUST) trial. Circulation Jul 27 2010;122:325–332. [DOI] [PubMed] [Google Scholar]
- 4.Varma N, Love CJ, Michalski J, Epstein AE, Investigators T. Alert-Based ICD Follow-Up: A Model of Digitally Driven Remote Patient Monitoring. JACC Clin Electrophysiol Aug 2021;7:976–987. [DOI] [PubMed] [Google Scholar]
- 5.O’Shea CJ, Middeldorp ME, Hendriks JM, et al. Remote Monitoring Alert Burden: An Analysis of Transmission in >26,000 Patients. JACC Clin Electrophysiol Feb 2021;7:226–234. [DOI] [PubMed] [Google Scholar]
- 6.Harvey M, Seiler A. Challenges in managing a remote monitoring device clinic. Heart Rhythm O2 Feb 2022;3:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varma N, Marrouche NF, Aguinaga L, et al. HRS/EHRA/APHRS/LAHRS/ACC/AHA Worldwide Practice Update for Telehealth and Arrhythmia Monitoring During and After a Pandemic. Circ Arrhythm Electrophysiol Jul 2020;13:e009007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E, Force CT. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ Mar 25 2013;346:f1049. [DOI] [PubMed] [Google Scholar]
- 9.Varma N Rationale and design of a prospective study of the efficacy of a remote monitoring system used in implantable cardioverter defibrillator follow-up: the Lumos-T Reduces Routine Office Device Follow-Up Study (TRUST) study. Am Heart J Dec 2007;154:1029–1034. [DOI] [PubMed] [Google Scholar]
- 10.Jackson SL, Tong X, King RJ, Loustalot F, Hong Y, Ritchey MD. National Burden of Heart Failure Events in the United States, 2006 to 2014. Circ Heart Fail Dec 2018;11:e004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alotaibi S, Hernandez-Montfort J, Ali OE, El-Chilali K, Perez BA. Remote monitoring of implantable cardiac devices in heart failure patients: a systematic review and meta-analysis of randomized controlled trials. Heart Fail Rev May 2020;25:469–479. [DOI] [PubMed] [Google Scholar]
- 12.Soikkeli F, Hashim M, Ouwens M, Postma M, Heeg B. Extrapolating Survival Data Using Historical Trial-Based a Priori Distributions. Value Health Sep 2019;22:1012–1017. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. National physician fee schedule payment amount file. 2020; Available from URL: https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed January 2020.
- 14.Galarraga JE, Pines JM. Costs of ED episodes of care in the United States. Am J Emerg Med Mar 2016;34:357–365. [DOI] [PubMed] [Google Scholar]
- 15.Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy 2017;10:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Bureau of Labor Statistics. National Occupational Employment and Wage Estimates. 2021; https://www.bls.gov/oes/current/oes_nat.htm. Accessed June 3, 2022, 2022.
- 17.Seiler A, Biundo E, Di Bacco M, Rosemas S, Nicolle E, Lanctin D, Hennion J, de Melis M, Van Heel L. Clinic Time Required for Remote and In-Person Management of Patients With Cardiac Devices: Time and Motion Workflow Evaluation. JMIR Cardio Oct 15 2021;5:e27720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.United States Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers: Medical care, not seasonally adjusted (Series ID CUUR0000SAM) 2019; https://www.bls.gov/data/ [accessed Nov 18, 2019]. Accessed November 18,, 2019.
- 19.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Jama Sep 13 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 20.Nichols CI, Vose JG, Mittal S. Incidence and Costs Related to Lead Damage Occurring Within the First Year After a Cardiac Implantable Electronic Device Replacement Procedure. J Am Heart Assoc Feb 12 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varma N, Love CJ, Schweikert R, Moll P, Michalski J, Epstein AE, Investigators T. Automatic remote monitoring utilizing daily transmissions: transmission reliability and implantable cardioverter defibrillator battery longevity in the TRUST trial. Europace Apr 1 2018;20:622–628. [DOI] [PubMed] [Google Scholar]
- 22.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation Jun 3 2014;129:2329–2345. [DOI] [PubMed] [Google Scholar]
- 23.Varma N, Piccini JP, Snell J, Fischer A, Dalal N, Mittal S. The Relationship Between Level of Adherence to Automatic Wireless Remote Monitoring and Survival in Pacemaker and Defibrillator Patients. J Am Coll Cardiol Jun 23 2015;65:2601–2610. [DOI] [PubMed] [Google Scholar]
- 24.Piccini JP, Mittal S, Snell J, Prillinger JB, Dalal N, Varma N. Impact of remote monitoring on clinical events and associated health care utilization: A nationwide assessment. Heart Rhythm Dec 2016;13:2279–2286. [DOI] [PubMed] [Google Scholar]
- 25.Chew DS, Zarrabi M, You I, Morton J, Low A, Reyes L, Yuen B, Sumner GL, Raj SR, Exner DV, Wilton SB. Clinical and Economic Outcomes Associated With Remote Monitoring for Cardiac Implantable Electronic Devices: A Population-Based Analysis. Can J Cardiol Jun 2022;38:736–744. [DOI] [PubMed] [Google Scholar]
- 26.Ricci RP, Vicentini A, D’Onofrio A, et al. Economic analysis of remote monitoring of cardiac implantable electronic devices: Results of the Health Economics Evaluation Registry for Remote Follow-up (TARIFF) study. Heart Rhythm Jan 2017;14:50–57. [DOI] [PubMed] [Google Scholar]
- 27.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH, Investigators C. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol Mar 8 2011;57:1181–1189. [DOI] [PubMed] [Google Scholar]
- 28.Hindricks G, Varma N, Kacet S, Lewalter T, Sogaard P, Guedon-Moreau L, Proff J, Gerds TA, Anker SD, Torp-Pedersen C. Daily remote monitoring of implantable cardioverter-defibrillators: insights from the pooled patient-level data from three randomized controlled trials (IN-TIME, ECOST, TRUST). Eur Heart J Jun 7 2017;38:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe E, Yamazaki F, Goto T, et al. Remote Management of Pacemaker Patients With Biennial In-Clinic Evaluation: Continuous Home Monitoring in the Japanese At-Home Study: A Randomized Clinical Trial. Circulation Arrhythmia and electrophysiology May 2020;13:e007734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ploux S, Varma N, Strik M, Lazarus A, Bordachar P. Optimizing Implantable Cardioverter-Defibrillator Remote Monitoring: A Practical Guide. JACC Clin Electrophysiol Apr 2017;3:315–328. [DOI] [PubMed] [Google Scholar]
- 31.Parthiban N, Esterman A, Mahajan R, Twomey DJ, Pathak RK, Lau DH, Roberts-Thomson KC, Young GD, Sanders P, Ganesan AN. Remote Monitoring of Implantable Cardioverter-Defibrillators: A Systematic Review and Meta-Analysis of Clinical Outcomes. J Am Coll Cardiol Jun 23 2015;65:2591–2600. [DOI] [PubMed] [Google Scholar]
- 32.Diamond J, Varma N, Kramer DB. Making the Most of Cardiac Device Remote Management: Towards an Actionable Care Model. Circ Arrhythm Electrophysiol Mar 2021;14:e009497. [DOI] [PubMed] [Google Scholar]
- 33.Mark DB, Nelson CL, Anstrom KJ, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation Jul 11 2006;114:135–142. [DOI] [PubMed] [Google Scholar]
- 34.Sandhu AT, Ollendorf DA, Chapman RH, Pearson SD, Heidenreich PA. Cost-Effectiveness of Sacubitril-Valsartan in Patients Who Have Heart Failure With Reduced Ejection Fraction. Ann Intern Med Apr 18 2017;166:607–608. [DOI] [PubMed] [Google Scholar]
- 35.Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes May 2011;4:313–320. [DOI] [PubMed] [Google Scholar]
- 36.Hosseini SM, Rozen G, Kaadan MI, Galvin J, Ruskin JN. Safety and In-Hospital Outcomes of Transvenous Lead Extraction for Cardiac Implantable Device-Related Infections: Analysis of 13 Years of Inpatient Data in the United States. JACC Clin Electrophysiol Dec 2019;5:1450–1458. [DOI] [PubMed] [Google Scholar]


