Abstract
Coronavirus disease 2019, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has affected 700 million people worldwide since its outbreak in 2019. The current pandemic strains, including Omicron and its large subvariant series, exhibit strong transmission and stealth. After entering the human body, the virus first infects nasal epithelial cells and invades host cells through the angiotensin‐converting enzyme 2 receptor and transmembrane serine protease 2 on the host cell surface. The nasal cavity is an important body part that protects against the virus. Immunisation of the nasal mucosa produces immunoglobulin A antibodies that effectively neutralise viruses. Saline nasal irrigation, a type of physical therapy, can reduce the viral load in the nasal cavity and prevent viral infections to some extent. As a commonly used means to fight SARS‐CoV‐2, the intramuscular (IM) vaccine can induce the human body to produce a systemic immune response and immunoglobulin G antibody; however, the antibody is difficult to distribute to the nasal mucosa in time and cannot achieve a good preventive effect. Intranasal (IN) vaccines compensate for the shortcomings of IM vaccines, induce mucosal immune responses, and have a better effect in preventing infection. In this review, we discuss the nasal defence barrier, the harm caused by SARS‐CoV‐2, the mechanism of its invasion into host cells, nasal cleaning, IM vaccines and IN vaccines, and suggest increasing the development of IN vaccines, and use of IN vaccines as a supplement to IM vaccines.
Keywords: COVID‐19, immunity, nasal cavity, SARS‐CoV‐2, vaccine
In this article, we discuss nasal mucosal immunity against SARS‐CoV‐2, the nasal cavity being an important body part that protects against the virus. Immunisation of the nasal mucosa produces immunoglobulin A antibodies that effectively neutralise viruses. Intranasal vaccine can make up for the deficiency of intramuscular vaccine in inducing nasal mucosal immunity and has a broad application prospect in the prevention and resistance of SARS‐CoV‐2.

Introduction
In 2019, the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first detected in Wuhan, China. 1 Since then, the virus has spread worldwide and become a global pandemic, infecting hundreds of millions of people. The symptoms of SARS‐CoV‐2 in humans are mainly respiratory; however, a few patients experience severe conditions, including breathing difficulties, shock, blood clotting disorders and organ failure. 2 SARS‐CoV‐2 has many variants. The Omicron variant and its subvariants have become prevalent. Mutations in the virus increase its stealth and transmission. Additionally, SARS‐CoV‐2 spreads from person to person primarily through respiratory droplets or indirect contact with virus‐infected objects and subsequently touching the mouth, nose, or eyes. 3 The nasal cavity is one of the main target organs of SARS‐CoV‐2 infection. 4 After the virus enters the nasal cavity, it first infects nasal epithelial cells. 5 Notably, the spike protein of SARS‐CoV‐2 binds to the angiotensin‐converting enzyme 2 (ACE2) receptor on the host cell surface and invades host cells via transmembrane serine protease 2 (TMPRSS2). 6 As scientists broadened their research on the mechanism of SARS‐CoV‐2 infection, we identified the importance of the nasal cavity in preventing viral infections. Besides proper mask‐wearing and hand hygiene, nasal saline irrigation can be used as a topical nasal treatment to reduce viral infections. 7
According to the World Health Organization (WHO), as of March 30, 2023, 183 vaccines have entered clinical development, and 199 vaccines have entered preclinical development. 8 Previously developed mRNA vaccines, viral vector vaccines, inactivated vaccines, protein vaccines and other vaccines are usually used in the form of intramuscular injection, which has a high effectiveness in reducing the hospitalisation rate and mortality rate of SARS‐CoV‐2 infection. 9 However, because SARS‐CoV‐2 has many variants and there may be many different circulating strains at different times, the validity and effectiveness of vaccine protection are greatly reduced. Moreover, the intramuscular (IM) vaccine has shortcomings in protecting the nasal cavity from SARS‐CoV‐2 infection in a timely and effective manner. It can induce an immunoglobulin G (IgG) immune response that protects the lower respiratory tract; however, most IM vaccines have a limited ability to induce immunoglobulin A (IgA) immune response that protects the upper respiratory tract. 10 , 11 , 12 , 13 , 14 , 15
After realising that the nasal cavity is an important link in the mechanism of viral infection, the intranasal (IN) vaccine came into being. IN vaccine can make up for the deficiency of the IM vaccine, which is difficult to induce mucosal immunity and achieve a better effect of preventing infection and preventing further transmission of virus. 16 , 17 , 18 Furthermore, IN vaccines are easy to transport and store, easy to administer and painless, allowing users to self‐administer. The numerous advantages of nasal spray vaccines make them promising for future vaccine development.
Nasal structure and defensive barrier
The human respiratory system comprises the respiratory tract and lungs. The nose, which includes the external nose, the nasal cavity and the paranasal sinuses, is the primary component of the respiratory tract. The nasal passages connect to the outer world via the nostrils and the nasopharynx through the postnasal foramen. The nasal cavity is categorised into the right and left nasal passages by the nasal septum. Each nasal cavity is split into the nasal vestibule and proper nasal cavity by the nasal threshold. The inside of the nasal vestibule is covered with nose hair to remove dust particles. Mucous membranes cover the nasal cavity and are split into the olfactory and respiratory mucous membranes. The olfactory mucosa has olfactory functions. The bulk of the nasal mucosa is respiratory mucosa, which can adhere to dust and foreign substances. The mucosal epithelium was a pseudo lamellar columnar ciliated epithelium.
The nasal mucosal epithelial tissue was composed of ciliated, goblet and basal cells (Figure 1). Cilia and microvilli, which exhibit mucociliary clearance (MCC), are found on the surface of ciliated cells. The goblet cells release mucus. The nasal mucus is separated into the following two layers: the periciliary layer of mucin molecules and the mucus layer above it. 19 When the virus enters the nasal cavity, the double layer of mucus and tightly bound ciliated goblet and basal cells are the first lines of defence. The MCC is another defence mechanism. When dust, viruses, particles and other foreign substances enter the nasal cavity and stick to the mucosa, tens of thousands of cilia of the mucosal epithelium move in unison to expel the mucus and the substances attached to it into the nasopharynx and throat, and subsequently enter the stomach through the oesophagus to be decomposed. 20 The mucosal immune response is activated when a virus penetrates the defence barrier. The main function of mucosal immunity is to remove pathogens that invade the body through the mucosal surface. Nasopharynx‐associated lymphoid tissue (NALT) is the site of the nasal mucosal immune response. NALT is the Waldeyer's ring located in the pharynx, consisting of palatine tonsils, adenoids, tubular tonsils and lingual tonsils. 20 Its interior comprises T cells, B cells and antigen‐presenting cells (APCs) such as dendritic cells and macrophages, which are covered with a layer of epithelial cells containing microfold cells (M cells). 14 M cells play a key role in triggering mucosal immunity and are key targets for viral antigens to cross the epithelial cell barrier. When the virus reaches the NALT, the soluble antigen of the virus can be recognised directly by the APCs, while particulate antigens need to be transported by the M cells, then absorbed by the APCs and presented to the CD4+ T cells in the mucosal lymphoid tissue. 21 , 22 Subsequently, CD4+ T cells interact with B cells, inducing B cells to differentiate into plasma cells capable of secreting polymeric IgA (pIgA), which passes through mucosal epithelial cells to the mucosal surface under the action of polymeric Ig receptor (pIgR), and finally forms secretory IgA (SIgA) 14 (Figure 2). It is a primary effector of mucosal immunity. 23 IgA molecules in the blood exist as monomers, whereas SIgA is a dimer formed by connecting two IgA molecules that are involved in the neutralisation and elimination of invading infections. SIgA works in three main ways: (1) preventing the attachment of foreign pathogens to mucosal epithelial cells, known as immune exclusion; (2) inhibiting the replication, transcription and assembly of intracellular viruses, or neutralising the activity of toxins, known as intracellular neutralisation; and (3) using immunoglobulin to excrete antigens from mucosal lamina propria to the mucosal surface of epithelial cells, known as antigenic excretion. 24 , 25
Figure 1.
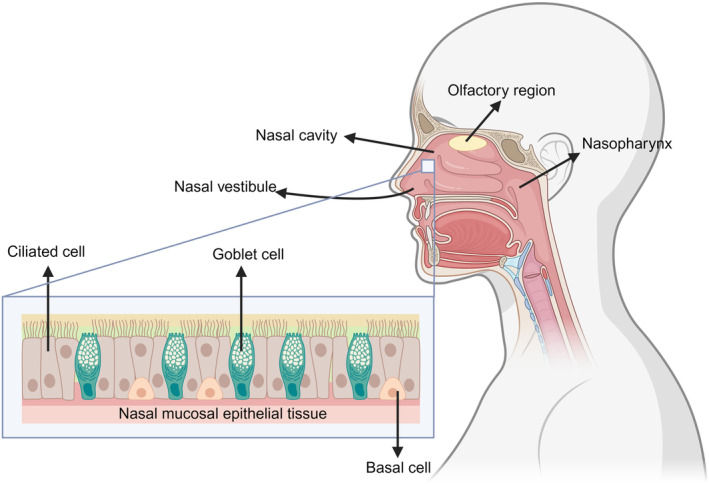
Structure of the nasal cavity. The nasal cavity is categorised into the right and left nasal passages by the nasal septum. Each nasal cavity is classified by the nasal threshold into the nasal vestibule and proper nasal cavity. The proper nasal cavity is covered by mucous membranes, which can be categorised into olfactory and respiratory mucous membranes, and respiratory mucous membranes account for most of the nasal mucous membranes. The nasal mucosal epithelium comprises ciliated goblets and basal cells.
Figure 2.

Mechanisms of IgA production by nasal mucosal immunity. When the virus antigen enters NALT, it is transported by microfold cells in the epithelial tissue, absorbed by antigen‐presenting cells such as dendritic cells and phagocytes, and presented to CD4+ T cells. CD4+ T cells promote the differentiation of B cells into plasma cells to produce pIgA and eventually form SIgA, the main effector of mucosal immunity, with the help of pIgR.
Variants of SARS‐CoV‐2
Coronavirus disease 2019 (COVID‐19) is caused by a novel type of coronavirus known as SARS‐CoV‐2. 26 According to the WHO (as of March 31, 2024), more than 700 million people have been diagnosed with COVID‐19, and more than 7 million have died of the disease. 27 This seems to be one of the toughest challenges we have ever faced. SARS‐CoV‐2, similar to all viruses, mutates over time. On May 31, 2021, the WHO announced that the name of the SARS‐CoV‐2 variant would be changed to the Greek alphabet, although this nomenclature would not replace the original scientific nomenclature. 28 In 2020, Alpha, Beta, Gamma, Delta and other mutants were discovered. The Omicron mutant, identified in November 2021, became the current pandemic strain, whereas the four preceding versions became historical epidemics. 29 Omicron is a faster and more insidious form of SARS‐CoV‐2 than earlier circulating variants. 30 Furthermore, Omicron has many sub‐variant strains, including BA.1, BA.2, BA.4 and BA.5, and each mutant has a unique set of traits. BA.5 is more infectious and transmissible than the earlier variants because it enables human nasal mucosal and airway cells to form syncytial cells to promote the spread of the virus 31 (Figure 3). However, attention has recently been focused on the new circulating strains XBB and BQ and their subvariants because of their surprising immune escape ability. BQ.1 and BQ.1.1 evolved from BA.5. XBB, XBB.1 and XBB.1.5 are recombination products of the two BA.2 pedigree variants. They have strong antibody evasion ability and can effectively evade mRNA vaccines or humoral immunity induced by natural infection. 32 BQ.1.1 is approximately six times more resistant to serum neutralisation than BA.5; however, XBB.1 is 63 and 49 times more resistant than BA.2 and BA.4/5, respectively. 33 Additionally, current drugs, such as imdevimab‐casirivimab, tixagevimab‐cilgavimab, sotrovimab and bebatelovimab, may not be effective against either BQ.1.1 or XBB. 34 Currently, the XBB series strains have the strongest antibody escape ability, far exceeding BA.5, and have reached or even exceeded the escape degree of SARS‐CoV‐1. 35 Recently, XBB.1.5 infections in the United States have increased dramatically, quickly surpassing BQ.1.1 and other XBB subtypes. On February 18, 2023, the COVID‐19 strain surveillance data from the Centers for Disease Control and Prevention revealed that XBB.1.5 mutants accounted for 80.2% of the newly detected strains in the United States. 36 Why does the XBB.1.5 have such strong propagation? This is because this strain has a higher affinity for human ACE2 receptors and a neutralising antibody escape capability similar to XBB.1. 37 ACE2 is a receptor required for SARS‐CoV‐2 to invade host cells. XBB.1.5 is not the final Omicron variation. Therefore, for public health, it is necessary to identify strategies to combat SARS‐CoV‐2 infection.
Figure 3.

BA.5 promotes viral transmission by enhancing syncytial formation. The BA.5 subvariant of Omicron can induce the fusion of nasal mucosa and airway cells to form a syncytium after invading the human body, thereby promoting the spread of the virus.
Mechanisms of SARS‐CoV‐2 infection in nasal epithelial cells
The transmission modes of SARS‐CoV‐2 are primarily respiratory and contact transmission. Virus‐carrying aerosols are the primary mode of transmission of SARS‐CoV‐2 over short and long distances. 38 , 39 People exhale aerosols when they breathe, talk, cough and sneeze, and the expelled aerosols can be transmitted through air. 40 , 41 We can become infected when we breathe in virus‐laden aerosols or touch our nose, mouth, or eyes after contact with an object infected with the virus. 3 These viral aerosols can remain infectious in the air for more than 3 h, 42 , 43 or even for several days when attached to objects. 43 , 44 , 45
In 2020, goblet and ciliated cells in the nasal airway were identified as possible initial infection sites for SARS‐CoV‐2 owing to their high expression of ACE2 and TMPRSS2. 5 The spike protein of SARS‐CoV‐2 binds to the ACE2 receptor, which acts as a door lock for the virus to enter human cells. TMPRSS2 assists in viral invasion by activating the SARS‐CoV‐2 spike protein and facilitating its entry into the cells. Currently, we have a better understanding of how SARS‐CoV‐2 infects respiratory epithelial cells. SARS‐CoV‐2 first infects respiratory ciliated cells; if the cilia are removed, infection with SARS‐CoV‐2 and other respiratory viruses can be prevented. 46 Invading viruses simultaneously activate kinases in cells that promote cytoskeleton formation and deliver newly generated viruses to the mucous layer via highly extended microvillus structures, thereby improving their ability to spread 46 (Figure 4). The nasal viral load is closely related to the severity of COVID‐19 symptoms and the transmission capacity of SARS‐CoV‐2. 47 These findings suggest that the nasal cavity plays a vital role in preventing SARS‐CoV‐2 infections.
Figure 4.

Mechanisms of virus infection in respiratory epithelial cells. SARS‐CoV‐2 first infects the respiratory ciliary cells. Viral invasion requires binding to ACE2 receptors on the surface of host cells. TMPRSS2 plays an assist role in the process of invasion. The invading virus activates p21‐activated kinases 1 and 4 (PAK1/4) to promote microvilli elongation. The newly generated virus is subsequently delivered through the microvilli to the mucus layer, allowing rapid viral spread. PCL, periciliary layer.
Nasal saline cleaning
The strong immune escape ability of the Omicron‐variant strains significantly increases the risk of reinfection and infection after vaccination; however, reducing the nasal viral load and enhancing the self‐cleaning and immunity of the nasal mucosa can reduce the risk of SARS‐CoV‐2 infection. Therefore, strengthening nasal cleaning care and enhancing nasal mucosal immunity is particularly important to preventing and treating SARS‐CoV‐2. Notably, saline cleaning of the nasal cavity can remove dust particles and air pollutants from the nasal cavity, improve the oscillating function of mucociliary cilia, reduce mucociliary edema, promote local blood circulation, and enhance the mucociliary cleaning function. 48 Simultaneously, saline increases the frequency of ciliary oscillations and reduces the production of local inflammatory mediators. 49 Currently, many studies have shown that nasal cleaning can prevent COVID‐19 to a certain extent and reduce the symptoms of SARS‐CoV‐2 infection. 7 , 50 , 51 , 52 A study found that patients who did not receive nasal irrigation had an overall risk of hospitalisation or death 8.57 times greater than those who did. 52 Moreover, saline nasal irrigation can shorten the duration of symptoms in patients 51 and can even reduce the duration of the disease by 1.9–2.5 days when combined with gargling. 50 , 53
IM vaccine
After contacting SARS‐CoV‐2, individual symptoms may be different. Most people infected have mild or moderate symptoms, such as fever, cough, tiredness, and loss of smell or taste and in a very small number, severe symptoms, including difficulty breathing, chest pain and loss of speech or movement. 2 Although most individuals have mild symptoms, damage to the human body cannot be ignored. Infection with SARS‐CoV‐2, even in mild cases, can alter an individual's immune status. Men trigger a stronger inflammatory response to other viruses, leading to more pronounced functional changes in their immune system, even long after recovery. 54 Additionally, SARS‐CoV‐2 infection can cause myocardial damage, including myocarditis, types 1 and 2 myocardial infarctions, and multisystemic inflammatory syndrome in children and adults. 55
Injectable vaccines are widely used globally to combat SARS‐CoV‐2 infection. At present, IM vaccines against SARS‐CoV‐2, such as live attenuated vaccine, inactivated vaccine, protein subunit vaccine, viral vector vaccine, DNA vaccine and mRNA vaccine, have been widely developed and used. 56 , 57 IM vaccines can prevent SARS‐CoV‐2 infection to some extent and reduce the severity of the viral infection, 58 , 59 with a good efficacy of 66.7–94.6%. 60 Moreover, studies have shown that IM vaccines can reduce the likelihood of Long COVID in patients with COVID‐19 and improve their long‐term quality of life. 61 , 62 Long COVID is widely defined as persistent symptoms after the initial infection with SARS‐CoV‐2 that last for more than 4 weeks after the initial stage of infection and progress or worsen over time, with severe and life‐threatening events likely to occur months or years after infection. 63 At the beginning of the outbreak of SARS‐CoV‐2, natural infection or vaccination can provide the body with strong short‐term immunity against later variant strains. 64 That does not mean people will not be reinfected because SARS‐CoV‐2 variants are so vast, and based on the second section above, new variants are likely to have greater immune escape. Moreover, the antibodies produced in the body after the initial infection are not permanent, and the titre of the antibodies will gradually weaken over time. 65 , 66 Multiple lines of evidence have shown that natural exposure to SARS‐CoV‐2 does not confer complete immunity. 67 , 68 , 69 , 70 , 71 , 72 Secondary infection can occur within 5–12 months of the initial infection. 73 Vaccines provide only temporary protection, and their effectiveness declines over time. 74 , 75 , 76 For the Omicron variant and the Delta variant, the vaccine efficacy of the former is generally lower than that of the latter. 77 The effectiveness of two doses of the BNT162b2 vaccine against the Omicron variant was only 8.8% after 25 weeks. 77 Moreover, IM vaccines are not good enough at stopping the spread of the virus. 14 , 78 , 79 At present, most of the IM vaccines developed in the world can only induce systemic immune response and cannot induce mucosal immune response. 10 , 11 , 12 , 13 , 14 , 15 , 80 , 81 However, as the first stop of virus invasion, mucosal immunity in the nasal cavity is crucial for neutralising the virus, preventing further transmission of the virus down the respiratory tract, and preventing disease deterioration. IM vaccines induce systemic immune responses dominated by IgG. However, IgG antibodies in the circulatory system decay quickly and cannot be distributed to the nasal cavity and respiratory mucosa in time, making it difficult to play an effective role in preventing infection. 16 , 82 Only a very high serum IgG titre can achieve complete protection of the nasal cavity. 82 Previous experiments on animals with IM vaccines have found that although the vaccine protects these animals well, the virus can still be detected in their nasal cavities. 78 , 79
IN vaccine
Intranasal vaccines may change that. IN vaccines can not only induce a systemic immune response but also induce a mucosal immune response in nasal and respiratory tissues dominated by IgA antibodies, which can quickly prevent further invasion of the virus when it enters the human body. 16 , 17 IgA can neutralise SARS‐CoV‐2. 83 , 84 , 85 SIgA antibodies, as a dimeric form of IgA, have higher viral‐neutralising activity than IgG antibodies. 86 , 87
Storage and transportation of a vast majority of currently approved IM vaccines against SARS‐CoV‐2 require cryogenic conditions. 80 Temperature control is critical to maintaining the efficacy of the vaccine. Consequently, the availability of infrastructure and technology limits vaccination coverage in remote and poor areas. However, most IN vaccines currently in development are designed to be thermostable, with less stringent temperature requirements. 80 For developing countries with underdeveloped medical conditions, the intranasal mucosal vaccine is more conducive to promotion. The nasal inhalation vaccine has low requirements for the injection personnel, is simple to operate, does not require the presence of medical personnel with professional medical training, and even the vaccinator can independently complete the vaccination at any time and anywhere. This needle‐free vaccine cannot only save human resources but also effectively reduce the recycling cost of medical waste such as needles and syringes and its secondary pollution to the environment. Furthermore, needle‐free vaccination can effectively improve the compliance of vaccinators, especially children, and can avoid local adverse reactions caused by intramuscular injection.
Some people may experience symptoms of anosmia after infection with SARS‐CoV‐2, which seriously impacts their daily lives, and its duration is highly uncertain. Although common respiratory viral infections can cause anosmia, SARS‐CoV‐2 infections are associated with a significantly higher proportion of anosmia. 88 The principle of influenza virus‐induced anosmia may be divided into two types: cell death caused by direct invasion of cells by the virus and olfactory sensory cells infected by the virus secrete a large number of proinflammatory cytokines so that they are cleared by inflammatory cells. 89 Intranasal influenza vaccination has been found to attenuate the production of influenza virus mRNA and the upregulation of inflammatory mediators in the olfactory bulb. 90 However, anosmia caused by SARS‐CoV‐2 infection does not appear to follow the same principle because ACE2 or TMPRSS2 is not expressed in olfactory sensory nerve cells, implying that SARS‐CoV‐2 cannot invade olfactory sensory nerve cells at all. 91 , 92 In fact, the olfactory epithelial barrier supporting cells are the main targets of SARS‐CoV‐2 infection, and the damage to olfactory sensory nerve cells is due to severe dysfunction of the immune system in the olfactory epithelium due to viral infection, leading to a persistent and uncontrolled inflammatory response in the olfactory epithelium. 93 The olfactory disorder caused by SARS‐CoV‐2 may be related to mucosal immune deficiency. 94 IN vaccine can enhance the function of local immunity and mucosal immunity in human body, and may be effective for the treatment of anosmia caused by SARS‐CoV‐2. 95 However, objective data on either intranasal influenza vaccines or SARS‐CoV‐2 vaccines, to further test their ability to prevent anosmia, are still lacking.
Currently, more than 10 kinds of IN vaccines have entered clinical trials worldwide. 8 Innovative and forward‐looking research on these vaccines will significantly help to improve their protection. Additionally, considering the complementary immune protection mechanism of the nasal spray and intramuscular injection vaccines, the nasal spray vaccine will become a crucial complementary point for the SARS‐CoV‐2 vaccine and a turning point in the fight against SARS‐CoV‐2.
Viral vector IN vaccine
Adenovirus (Ad) has attracted much attention since its discovery because of its strong immunogenicity and good safety. 96 Ad vaccine, as a relatively mature carrier vaccine platform, has been widely developed to combat SARS‐CoV‐2. Adenovirus serotype 5 (Ad5), one of the most commonly used Ad vectors, has also been widely used in the development of IN vaccines. The recombinant Ad5 vector vaccine Ad5.SARS‐CoV‐2‐S1, which encodes the S1 subunit antigen gene of SARS‐CoV‐2, has been shown to induce strong and long‐term specific antibody and cellular immune responses in mice. 97 Another Ad5 vector vaccine, Ad5‐S‐nb2, can induce systemic and pulmonary antibody responses in rhesus monkey experiments, which can effectively and quickly clear the virus entering the respiratory tract and lungs. 98
A vaccine developed by Bharat Biotech in India, ChAd SARS‐CoV‐2‐S (BBV154), is a recombinant chimpanzee adenovirus vector vaccine that induces long‐term, high levels of IgA with a single intranasal dose. 99 , 100 Moreover, it showed strong anti‐infection ability in protecting the upper and lower respiratory tract against SARS‐CoV‐2 variants B.1.351 (Beta) and B.1.1.28 (Gamma), compared with the lower protection of the upper respiratory tract by intramuscular injection. 99 , 100 At present, this vaccine has completed the clinical study of Phase I to Phase III. 8
Another chimpanzee adenovirus vector vaccine, ChAdOx 1 nCoV‐19 (AZD1222), was previously approved for use as an IM vaccine in the United Kingdom on December 30, 2020. 101 At present, the University of Oxford is already developing the nasal use of the vaccine and has entered Phase I clinical trials. 102 Compared with other respiratory mucosal vaccines, vaccines based on Ad5 and chimpanzee Ad have better efficacy and safety. 103 IN vaccines based on chimpanzee Ad are likely to be more effective than those based on human Ad, thus can carry on further clinical research and development. 104
There are also vaccines based on influenza viruses. CA4‐dNS1‐nCoV‐RBD, an IN vaccine based on live attenuated influenza virus vector, not only induces a rapid and durable respiratory immune response against the prototype, Beta and Omicron variants of SARS‐CoV‐2 but also has a certain influenza virus protection effect, providing a coping method for the possible coexistence of SARS‐CoV‐2 and influenza in the future. 105 Subsequent human trials established that the vaccine had a good safety profile, with an overall incidence of adverse reactions of only 19%. 106 It is the world's first IN vaccine to enter Phase III clinical trials and has already been approved for use in China in December 2022 under the name Pneucolin. 107
Live attenuated IN vaccine
Live attenuated vaccine refers to the pathogen after various treatments, will be inoculated into the human body, can imitate natural infection and induce the body's immune response, but will not cause disease. For influenza viruses, live attenuated vaccines induce an immune response that more closely resembles natural immunity than other types of vaccines. 108 , 109
MV‐014‐212, a recombinant, attenuated live IN vaccine, can produce systemic and mucosal immunity after a single intranasal dose in animal experiments, induce mucosal IgA and serum IgG, has good resistance to Alpha, Beta and Delta variants, and has now entered Phase I clinical trials. 110 Another live attenuated vaccine, COVI‐VAC, is also in clinical trials. 111
Protein subunit IN vaccine
The protein subunit vaccine platform is widely used due to its high safety and low cost. 112 , 113 The spike protein of SARS‐CoV‐2 is the most important of its four structural proteins. spike protein has two subunits: S1 subunit and S2 subunit. The S1 subunit contains the receptor binding domain (RBD), which has the function of recognising host cell surface receptors and mediating virus invasion into host cells. Therefore, the spike protein is a key target molecule for vaccine development.
Intravacc has developed an intranasal protein subunit vaccine containing a recombinant spike protein HexaPro based on a D614G mutation and based on outer membrane vesicles (OMV). 114 Intranasal injection of the vaccine can induce a large number of IgA and IgG antibodies in the respiratory tract, effectively preventing virus replication, while intramuscular injection can only cause serum IgG reaction, and the vaccine has now entered Phase I clinical trials. 114
Mambisa (CIGB‐669) is an intranasal protein subunit vaccine developed by the Cuba Center for Genetic Engineering and Biotechnology. It uses Hepatitis B virus core protein (AgnHB) as an immune vector and is effective in stimulating mucosal immune responses. 115 The vaccine is currently in Phase I/II clinical trials to investigate its immunogenicity and safety in the population. 115
ACM‐001, developed by ACM Biolabs, is an adjuvant booster vaccine against SARS‐CoV‐2 containing components from the β‐type SARS‐CoV‐2 spike protein and the immune stimulator CpG7909, which is currently in Phase I clinical trials. 116
At present, there is no intranasal mRNA vaccine against SARS‐CoV‐2 in clinical trials, but earlier studies have found the feasibility of an intranasal mRNA vaccine against tuberculosis. 117 Although mRNA vaccines have high storage temperature requirements, this problem can be improved by making certain chemical modifications to the mRNA molecules. 118 , 119 In view of the good safety and efficacy of the mRNA vaccine and its short production time, which can quickly respond to various SARS‐CoV‐2 mutants, 120 researchers can pay more attention to the development of the intranasal mRNA vaccine. Furthermore, given the complementary characteristics of the intramuscular and IN vaccines in inducing immune responses, the IN vaccine as a supplement to the IM vaccine may be a more effective measure against various variants of SARS‐CoV‐2 in the future, which may be proven by relevant clinical trials.
The development of IN vaccines faces many challenges. First of all, as mentioned above, the nasal surface has a protective barrier of cilia and mucus to prevent the intrusion of foreign substances. Therefore, increasing the residence time of the vaccine in the nasal cavity so that the antigen can be fully absorbed is one of the difficulties that researchers need to consider. Second, IN vaccines often require vaccine adjuvants, and finding vaccine adjuvants that can maintain antigen stability under the premise of fully activating the immune system is also a challenge. 10 Third, there are constraints on the size of particles ejected by IN vaccine delivery devices. Large particles tend to deposit in the front of the nasal cavity and can be easily exhaled or wiped off, while too small particles may pass directly through the nasal cavity and deposit in the lungs, leaving the upper respiratory tract unprotected. 121 Finally, the safety of vaccines cannot be ignored. At present, the vast majority of IN vaccines are still in the preclinical or early clinical trial stage, so the safety of these vaccines in humans is not clear.
Nasal spray
The spread of viral aerosols can be limited to some extent by wearing masks. 122 , 123 However, masks are no more than 70% effective in protecting the wearer. 124 Considering the importance of preventing various SARS‐CoV‐2 variants in the future, it is necessary to develop new strategies for nasal protection. Recently, an “intranasal mask” may be a good way to deal with this problem. It is a nasal spray formulation consisting of a hydrogel and tiny vesicles containing specific viral receptors that, when sprayed into the nose, can rapidly change from liquid to gel‐like attachment to the lining of the nasal cavity. 125 The hydrogel not only protects the nasal cavity from virus infection but also traps viral aerosols to prevent them from infecting the lungs, while receptors on the vesicles capture and inactivate the virus. 125 This new nasal spray can compensate for the inadequate protection of current masks and further reduce the risk of SARS‐CoV‐2 infection.
Harms of SARS‐CoV‐2 coexisting with influenza
As SARS‐CoV‐2 spreads worldwide, we should not only be vigilant about it but also take precautions against influenza viruses. Evidence exists that influenza infection promotes SARS‐CoV‐2 infection in human cells, and both may even promote infection. This mode of promotion manifested as enhanced ACE2 receptor expression. 126 , 127 The data demonstrated that the mRNA levels of ACE2 receptors in host cells increased by 2–3 times after infection with influenza and by 28 times in cases of coinfection with influenza and SARS‐CoV‐2. 127 But other studies suggest otherwise. One study found that the risk of contracting SARS‐CoV‐2 after influenza infection was reduced by 58%. 128 There is a mathematical model to support this conclusion. If one virus grows faster than the other, that virus will suppress the other virus. 129 SARS‐CoV‐2 grows much more slowly than the influenza virus; therefore, it is easy to suppress SARS‐CoV‐2 if you are infected with the influenza virus first. However, if you are infected with SARS‐CoV‐2 first, co‐infection will occur much more easily. 130 At present, it is not clear whether co‐infection will lead to more serious consequences for patients. Co‐infection will increase the severity and mortality of infected persons according to most studies. 131 , 132 , 133 , 134 , 135 Co‐infection causes twice the mortality rate of patients infected with SARS‐CoV‐2 alone. 128 However, some studies have suggested that co‐infection has no effect on the disease outcome of patients. 136 , 137 Therefore, more extensive and systematic testing of SARS‐CoV‐2 and influenza viruses is necessary, and vaccination is recommended to prevent co‐infection, given that co‐infection may be more harmful to the human body. Some intranasal influenza vaccines, such as Fluenz Tetra and FluMist Quadrivalent, have already been approved for use. 14 Moreover, the previously mentioned CA4‐dNS1‐nCoV‐RBD IN vaccine can protect against both SARS‐CoV‐2 and influenza viruses. The coexistence of SARS‐CoV‐2 and influenza viruses may become more common in the future, and the use of IN vaccines based on influenza virus vectors may become more obvious.
By March 31, 2024, more than 7 million people had died of COVID‐19 globally since the WHO declared the outbreak a global pandemic on March 11, 2020. 27 , 138 However, the global death toll from COVID‐19 reduced to its lowest level in 4 years. On April 10, 2023, United States President Joe Biden ended the nation's state of emergency in response to the coronavirus. 139 On the same day, the United States government announced that it would spend $5 billion to develop a new generation of COVID‐19 vaccines and treatments, with nasal vaccines being a major target. 140 It can be found that the IN vaccine is a significant development direction for future vaccines, which has a non‐negligible role in preventing and treating COVID‐19. IN vaccine can be used as a supplement to the IM vaccine, integrating the advantages of both vaccines to achieve better immune effects.
Conclusion
Although we can breathe a sigh of relief in the battle against SARS‐CoV‐2, COVID‐19 is still not a common cold, and its harm to the human body cannot be ignored. Therefore, more attention should be paid to the role of the nasal cavity in preventing SARS‐CoV‐2 infection. As IN vaccines can complement the shortcomings of IM vaccines regarding immune mechanisms, IN vaccines should be further studied. Future studies should consider the safety and efficacy of IN vaccines in large populations.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Ge Jin: Conceptualization; investigation; visualization; writing – original draft; writing – review and editing. Runze Wang: Conceptualization; writing – review and editing. Yi Jin: Conceptualization; investigation. Yingqiu Song: Conceptualization; project administration. Tianlu Wang: Funding acquisition; resources; supervision.
Acknowledgments
We are very grateful to the participants in this study for spending precious time participating. Figures in the paper were created with BioRender.com. This work was supported by the Fundamental Research Funds for the Central Universities (LD2023032, LD2023011) and the Ministry of Science and Technology of the People's Republic of China (G2023127016L).
References
- 1. Chen N, Zhou M, Dong X et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Coronavirus disease (COVID‐19) symptoms. Available from: https://www.who.int/health‐topics/coronavirus#tab=tab_3
- 3. World Health Organization . Coronavirus disease (COVID‐19): how is it transmitted? Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/question‐and‐answers‐hub/q‐a‐detail/coronavirus‐disease‐covid‐19‐how‐is‐it‐transmitted
- 4. Yao Y, Wang H, Liu Z. Expression of ACE2 in airways: implication for COVID‐19 risk and disease management in patients with chronic inflammatory respiratory diseases. Clin Exp Allergy 2020; 50: 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sungnak W, Huang N, Bécavin C et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020; 26: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffmann M, Kleine‐Weber H, Schroeder S et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farrell NF, Klatt‐Cromwell C, Schneider JS. Benefits and safety of nasal saline irrigations in a pandemic‐washing COVID‐19 away. JAMA Otolaryngol Head Neck Surg 2020; 146: 787–788. [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization . COVID‐19 vaccine tracker and landscape. Available from: https://www.who.int/publications/m/item/draft‐landscape‐of‐covid‐19‐candidate‐vaccines
- 9. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer‐Smadja N. Comparing COVID‐19 vaccines for their characteristics, efficacy and effectiveness against SARS‐CoV‐2 and variants of concern: a narrative review. Clin Microbiol Infect 2022; 28: 202–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chavda VP, Vora LK, Pandya AK, Patravale VB. Intranasal vaccines for SARS‐CoV‐2: from challenges to potential in COVID‐19 management. Drug Discov Today 2021; 26: 2619–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bleier BS, Ramanathan M Jr, Lane AP. COVID‐19 vaccines may not prevent nasal SARS‐CoV‐2 infection and asymptomatic transmission. Otolaryngol Head Neck Surg 2021; 164: 305–307. [DOI] [PubMed] [Google Scholar]
- 12. Tokunoh N, Tamiya S, Watanabe M et al. A nasal vaccine with inactivated whole‐virion elicits protective mucosal immunity against SARS‐CoV‐2 in mice. Front Immunol 2023; 14: 1224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krammer F. SARS‐CoV‐2 vaccines in development. Nature 2020; 586: 516–527. [DOI] [PubMed] [Google Scholar]
- 14. Tiboni M, Casettari L, Illum L. Nasal vaccination against SARS‐CoV‐2: synergistic or alternative to intramuscular vaccines? Int J Pharm 2021; 603: 120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID‐19 vaccine strategies. Nat Rev Immunol 2020; 20: 615–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waltz E. How nasal‐spray vaccines could change the pandemic. Nature 2022; 609: 240–242. [DOI] [PubMed] [Google Scholar]
- 17. Topol EJ, Iwasaki A. Operation nasal vaccine‐lightning speed to counter COVID‐19. Sci Immunol 2022; 7: eadd9947. [DOI] [PubMed] [Google Scholar]
- 18. Russell MW, Mestecky J. Mucosal immunity: the missing link in comprehending SARS‐CoV‐2 infection and transmission. Front Immunol 2022; 13: 957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr Top Otorhinolaryngol Head Neck Surg 2010; 9: Doc07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lobaina Mato Y. Nasal route for vaccine and drug delivery: features and current opportunities. Int J Pharm 2019; 572: 118813. [DOI] [PubMed] [Google Scholar]
- 21. Yusuf H, Kett V. Current prospects and future challenges for nasal vaccine delivery. Hum Vaccin Immunother 2017; 13: 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alu A, Chen L, Lei H, Wei Y, Tian X, Wei X. Intranasal COVID‐19 vaccines: from bench to bed. EBioMedicine 2022; 76: 103841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Jin L, Chen T. The effects of secretory IgA in the mucosal immune system. Biomed Res Int 2020; 2020: 2032057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol 2010; 8: 656–667. [DOI] [PubMed] [Google Scholar]
- 25. Corthésy B. Multi‐faceted functions of secretory IgA at mucosal surfaces. Front Immunol 2013; 4: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. World Health Organization . Coronavirus disease (COVID‐19) overview. Available from: https://www.who.int/health‐topics/coronavirus#tab=tab_1
- 27. World Health Organization . WHO Coronavirus (COVID‐19) dashboard. Available from: https://covid19.who.int/
- 28. World Health Organization . WHO announces simple, easy‐to‐say labels for SARS‐CoV‐2 variants of interest and concern. Available from: https://www.who.int/news/item/31‐05‐2021‐who‐announces‐simple‐easy‐to‐say‐labels‐for‐sars‐cov‐2‐variants‐of‐interest‐and‐concern
- 29. World Health Organization . Tracking SARS‐CoV‐2 variants. Available from: https://www.who.int/activities/tracking‐SARS‐CoV‐2‐variants
- 30. Rana R, Kant R, Huirem RS, Bohra D, Ganguly NK. Omicron variant: current insights and future directions. Microbiol Res 2022; 265: 127204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li C, Huang J, Yu Y et al. Human airway and nasal organoids reveal escalating replicative fitness of SARS‐CoV‐2 emerging variants. Proc Natl Acad Sci USA 2023; 120: e2300376120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Uraki R, Ito M, Furusawa Y et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB. Lancet Infect Dis 2023; 23: 30–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Q, Iketani S, Li Z et al. Alarming antibody evasion properties of rising SARS‐CoV‐2 BQ and XBB subvariants. Cell 2023; 186: 279–286.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imai M, Ito M, Kiso M et al. Efficacy of antiviral agents against omicron subvariants BQ.1.1 and XBB. N Engl J Med 2023; 388: 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao Y, Jian F, Wang J et al. Imprinted SARS‐CoV‐2 humoral immunity induces convergent omicron RBD evolution. Nature 2023; 614: 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention . COVID date tracker. Available from: https://covid.cdc.gov/covid‐data‐tracker/#variant‐proportions
- 37. Yue C, Song W, Wang L et al. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect Dis 2023; 23: 278–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS‐CoV‐2. Lancet 2021; 397: 1603–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang CC, Prather KA, Sznitman J et al. Airborne transmission of respiratory viruses. Science 2021; 373: eabd9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bu Y, Ooka R, Kikumoto H, Oh W. Recent research on expiratory particles in respiratory viral infection and control strategies: a review. Sustain Cities Soc 2021; 73: 103106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dhand R, Li J. Coughs and sneezes: their role in transmission of respiratory viral infections, including SARS‐CoV‐2. Am J Respir Crit Care Med 2020; 202: 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smither SJ, Eastaugh LS, Findlay JS, Lever MS. Experimental aerosol survival of SARS‐CoV‐2 in artificial saliva and tissue culture media at medium and high humidity. Emerg Microbes Infect 2020; 9: 1415–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Doremalen N, Bushmaker T, Morris DH et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med 2020; 382: 1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kasloff SB, Leung A, Strong JE, Funk D, Cutts T. Stability of SARS‐CoV‐2 on critical personal protective equipment. Sci Rep 2021; 11: 984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riddell S, Goldie S, Hill A, Eagles D, Drew TW. The effect of temperature on persistence of SARS‐CoV‐2 on common surfaces. Virol J 2020; 17: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu CT, Lidsky PV, Xiao Y et al. SARS‐CoV‐2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 2023; 186: 112–130.e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marks M, Millat‐Martinez P, Ouchi D et al. Transmission of COVID‐19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis 2021; 21: 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu S, Yang Y, Xu Y et al. [Expert consensus on nasal saline irrigation for the prevention of SARS‐CoV‐2 infection]. In Chinese Chin J Ophthalmol Otorhinolaryngol 2022; 22: 329–334+362. [Google Scholar]
- 49. Kanjanawasee D, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Hypertonic saline versus isotonic saline nasal irrigation: systematic review and meta‐analysis. Am J Rhinol Allergy 2018; 32: 269–279. [DOI] [PubMed] [Google Scholar]
- 50. Ramalingam S, Graham C, Dove J, Morrice L, Sheikh A. Hypertonic saline nasal irrigation and gargling should be considered as a treatment option for COVID‐19. J Glob Health 2020; 10: 010332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kimura KS, Freeman MH, Wessinger BC et al. Interim analysis of an open‐label randomized controlled trial evaluating nasal irrigations in non‐hospitalized patients with coronavirus disease 2019. Int Forum Allergy Rhinol 2020; 10: 1325–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baxter AL, Schwartz KR, Johnson RW et al. Rapid initiation of nasal saline irrigation to reduce severity in high‐risk COVID+ outpatients. Ear Nose Throat J 2022. doi: 10.1177/01455613221123737 [DOI] [PubMed] [Google Scholar]
- 53. Ramalingam S, Graham C, Dove J, Morrice L, Sheikh A. A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci Rep 2019; 9: 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sparks R, Lau WW, Liu C et al. Influenza vaccination reveals sex dimorphic imprints of prior mild COVID‐19. Nature 2023; 614: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gluckman TJ, Bhave NM, Allen LA et al. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID‐19 in adults: myocarditis and other myocardial involvement, post‐acute sequelae of SARS‐CoV‐2 infection, and return to play: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2022; 79: 1717–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soleimanpour S, Yaghoubi A. COVID‐19 vaccine: where are we now and where should we go? Expert Rev Vaccines 2021; 20: 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lurie N, Saville M, Hatchett R, Halton J. Developing Covid‐19 vaccines at pandemic speed. N Engl J Med 2020; 382: 1969–1973. [DOI] [PubMed] [Google Scholar]
- 58. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chadeau‐Hyam M, Wang H, Eales O et al. SARS‐CoV‐2 infection and vaccine effectiveness in England (REACT‐1): a series of cross‐sectional random community surveys. Lancet Respir Med 2022; 10: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. McDonald I, Murray SM, Reynolds CJ, Altmann DM, Boyton RJ. Comparative systematic review and meta‐analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS‐CoV‐2. NPJ Vaccines 2021; 6: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ayoubkhani D, Bermingham C, Pouwels KB et al. Trajectory of long covid symptoms after covid‐19 vaccination: community based cohort study. BMJ 2022; 377: e069676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tran VT, Perrodeau E, Saldanha J, Pane I, Ravaud P. Efficacy of first dose of covid‐19 vaccine versus no vaccination on symptoms of patients with long covid: target trial emulation based on ComPaRe e‐cohort. BMJ Med 2023; 2: e000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Centers for Disease Control and Prevention . Long COVID or post‐COVID conditions. Available from: https://www.cdc.gov/coronavirus/2019‐ncov/long‐term‐effects/
- 64. Tostanoski LH, Yu J, Mercado NB et al. Immunity elicited by natural infection or Ad26.COV2.S vaccination protects hamsters against SARS‐CoV‐2 variants of concern. Sci Transl Med 2021; 13: eabj3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dehgani‐Mobaraki P, Zaidi AK, Yadav N, Floridi A, Floridi E. Longitudinal observation of antibody responses for 14 months after SARS‐CoV‐2 infection. Clin Immunol 2021; 230: 108814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liew F, Talwar S, Cross A et al. SARS‐CoV‐2‐specific nasal IgA wanes 9 months after hospitalisation with COVID‐19 and is not induced by subsequent vaccination. EBioMedicine 2023; 87: 104402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tillett RL, Sevinsky JR, Hartley PD et al. Genomic evidence for reinfection with SARS‐CoV‐2: a case study. Lancet Infect Dis 2021; 21: 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. To KK, Hung IF, Ip JD et al. Coronavirus disease 2019 (COVID‐19) Re‐infection by a phylogenetically distinct severe acute respiratory syndrome coronavirus 2 strain confirmed by whole genome sequencing. Clin Infect Dis 2021; 73: e2946–e2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gupta V, Bhoyar RC, Jain A et al. Asymptomatic reinfection in 2 healthcare workers from India with genetically distinct severe acute respiratory syndrome coronavirus 2. Clin Infect Dis 2021; 73: e2823–e2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tomassini S, Kotecha D, Bird PW, Folwell A, Biju S, Tang JW. Setting the criteria for SARS‐CoV‐2 reinfection – six possible cases. J Infect 2021; 82: 282–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang J, Ding N, Ren L et al. COVID‐19 reinfection in the presence of neutralizing antibodies. Natl Sci Rev 2021; 8: nwab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harvey RA, Rassen JA, Kabelac CA et al. Association of SARS‐CoV‐2 seropositive antibody test with risk of future infection. JAMA Intern Med 2021; 181: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Milne G, Hames T, Scotton C et al. Does infection with or vaccination against SARS‐CoV‐2 lead to lasting immunity? Lancet Respir Med 2021; 9: 1450–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Singanayagam A, Hakki S, Dunning J et al. Community transmission and viral load kinetics of the SARS‐CoV‐2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 2022; 22: 183–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Eyre DW, Taylor D, Purver M et al. Effect of Covid‐19 vaccination on transmission of alpha and Delta variants. N Engl J Med 2022; 386: 744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Krause PR, Fleming TR, Longini IM et al. SARS‐CoV‐2 variants and vaccines. N Engl J Med 2021; 385: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Andrews N, Stowe J, Kirsebom F et al. Covid‐19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med 2022; 386: 1532–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mercado NB, Zahn R, Wegmann F et al. Single‐shot Ad26 vaccine protects against SARS‐CoV‐2 in rhesus macaques. Nature 2020; 586: 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Corbett KS, Flynn B, Foulds KE et al. Evaluation of the mRNA‐1273 vaccine against SARS‐CoV‐2 in nonhuman primates. N Engl J Med 2020; 383: 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kar S, Devnath P, Emran TB, Tallei TE, Mitra S, Dhama K. Oral and intranasal vaccines against SARS‐CoV‐2: current progress, prospects, advantages, and challenges. Immun Inflamm Dis 2022; 10: e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Russell MW, Moldoveanu Z, Ogra PL, Mestecky J. Mucosal immunity in COVID‐19: a neglected but critical aspect of SARS‐CoV‐2 infection. Front Immunol 2020; 11: 611337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Renegar KB, Small PA Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 2004; 173: 1978–1986. [DOI] [PubMed] [Google Scholar]
- 83. Yu HQ, Sun BQ, Fang ZF et al. Distinct features of SARS‐CoV‐2‐specific IgA response in COVID‐19 patients. Eur Respir J 2020; 56: 2001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ma H, Zeng W, He H et al. Serum IgA, IgM, and IgG responses in COVID‐19. Cell Mol Immunol 2020; 17: 773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sterlin D, Mathian A, Miyara M et al. IgA dominates the early neutralizing antibody response to SARS‐CoV‐2. Sci Transl Med 2021; 13: eabd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang Z, Lorenzi JCC, Muecksch F et al. Enhanced SARS‐CoV‐2 neutralization by dimeric IgA. Sci Transl Med 2021; 13: eabf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sun L, Kallolimath S, Palt R et al. Increased in vitro neutralizing activity of SARS‐CoV‐2 IgA1 dimers compared to monomers and IgG. Proc Natl Acad Sci USA 2021; 118: e2107148118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol 2020; 10: 806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Flanagan CE, Wise SK, DelGaudio JM, Patel ZM. Association of decreased rate of influenza vaccination with increased subjective olfactory dysfunction. JAMA Otolaryngol Head Neck Surg 2015; 141: 225–228. [DOI] [PubMed] [Google Scholar]
- 90. Zielinski MR, Souza G, Taishi P, Bohnet SG, Krueger JM. Olfactory bulb and hypothalamic acute‐phase responses to influenza virus: effects of immunization. Neuroimmunomodulation 2013; 20: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brann DH, Tsukahara T, Weinreb C et al. Non‐neuronal expression of SARS‐CoV‐2 entry genes in the olfactory system suggests mechanisms underlying COVID‐19‐associated anosmia. Sci Adv 2020; 6: eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen M, Shen W, Rowan NR et al. Elevated ACE‐2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS‐CoV‐2 entry and replication. Eur Respir J 2020; 56: 2001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Finlay JB, Brann DH, Abi Hachem R et al. Persistent post‐COVID‐19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci Transl Med 2022; 14: eadd0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. de Melo GD, Lazarini F, Levallois S et al. COVID‐19‐related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 2021; 13: eabf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sharma S, Thiriard A, Olislagers V et al. Mucosal antibody response and SARS‐CoV‐2 shedding in patients with COVID‐19 related olfactory dysfunction. J Med Virol 2024; 96: e29398. [DOI] [PubMed] [Google Scholar]
- 96. Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther 2004; 10: 616–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kim E, Weisel FJ, Balmert SC et al. A single subcutaneous or intranasal immunization with adenovirus‐based SARS‐CoV‐2 vaccine induces robust humoral and cellular immune responses in mice. Eur J Immunol 2021; 51: 1774–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Feng L, Wang Q, Shan C et al. An adenovirus‐vectored COVID‐19 vaccine confers protection from SARS‐COV‐2 challenge in rhesus macaques. Nat Commun 2020; 11: 4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hassan AO, Kafai NM, Dmitriev IP et al. A single‐dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS‐CoV‐2. Cell 2020; 183: 169–184.e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hassan AO, Shrihari S, Gorman MJ et al. An intranasal vaccine durably protects against SARS‐CoV‐2 variants in mice. Cell Rep 2021; 36: 109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Voysey M, Costa Clemens SA, Madhi SA et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet 2021; 397: 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. University of Oxford . A study of intranasal ChAdOx1 nCOV‐19. Available from: https://clinicaltrials.gov/study/NCT04816019
- 103. Afkhami S, Yao Y, Xing Z. Methods and clinical development of adenovirus‐vectored vaccines against mucosal pathogens. Mol Ther Methods Clin Dev 2016; 3: 16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Afkhami S, D'Agostino MR, Zhang A et al. Respiratory mucosal delivery of next‐generation COVID‐19 vaccine provides robust protection against both ancestral and variant strains of SARS‐CoV‐2. Cell 2022; 185: 896–915.e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen J, Wang P, Yuan L et al. A live attenuated virus‐based intranasal COVID‐19 vaccine provides rapid, prolonged, and broad protection against SARS‐CoV‐2. Sci Bull (Beijing) 2022; 67: 1372–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhu F, Zhuang C, Chu K et al. Safety and immunogenicity of a live‐attenuated influenza virus vector‐based intranasal SARS‐CoV‐2 vaccine in adults: randomised, double‐blind, placebo‐controlled, phase 1 and 2 trials. Lancet Respir Med 2022; 10: 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhang L, Jiang Y, He J et al. Intranasal influenza‐vectored COVID‐19 vaccine restrains the SARS‐CoV‐2 inflammatory response in hamsters. Nat Commun 2023; 14: 4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004; 59: 1–15. [DOI] [PubMed] [Google Scholar]
- 109. Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11: S45–S53. [DOI] [PubMed] [Google Scholar]
- 110. Tioni MF, Jordan R, Pena AS et al. Mucosal administration of a live attenuated recombinant COVID‐19 vaccine protects nonhuman primates from SARS‐CoV‐2. NPJ Vaccines 2022; 7: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wang Y, Yang C, Song Y et al. Scalable live‐attenuated SARS‐CoV‐2 vaccine candidate demonstrates preclinical safety and efficacy. Proc Natl Acad Sci USA 2021; 118: e2102775118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Soraci L, Lattanzio F, Soraci G et al. COVID‐19 vaccines: current and future perspectives. Vaccines (Basel) 2022; 10: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li M, Wang H, Tian L et al. COVID‐19 vaccine development: milestones, lessons and prospects. Signal Transduct Target Ther 2022; 7: 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. van der Ley PA, Zariri A, van Riet E, Oosterhoff D, Kruiswijk CP. An intranasal OMV‐based vaccine induces high mucosal and systemic protecting immunity against a SARS‐CoV‐2 infection. Front Immunol 2021; 12: 781280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Center for Genetic Engineering and Biotechnology (CIGB) . MAMBISA study. Available from: https://rpcec.sld.cu/en/trials/RPCEC00000345‐En
- 116. ACM Biolabs . Booster dose study to assess the safety and immunogenicity of ACM‐001 administered intramuscularly or intranasally. Available from: https://clinicaltrials.gov/study/NCT05385991
- 117. Lorenzi JC, Trombone AP, Rocha CD et al. Intranasal vaccination with messenger RNA as a new approach in gene therapy: use against tuberculosis. BMC Biotechnol 2010; 10: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kyriakidis NC, López‐Cortés A, González EV, Grimaldos AB, Prado EO. SARS‐CoV‐2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines 2021; 6: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov 2018; 17: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fathizadeh H, Afshar S, Masoudi MR et al. SARS‐CoV‐2 (Covid‐19) vaccines structure, mechanisms and effectiveness: a review. Int J Biol Macromol 2021; 188: 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xu H, Cai L, Hufnagel S, Cui Z. Intranasal vaccine: factors to consider in research and development. Int J Pharm 2021; 609: 121180. [DOI] [PubMed] [Google Scholar]
- 122. Chan JF, Yuan S, Zhang AJ et al. Surgical mask partition reduces the risk of noncontact transmission in a Golden Syrian hamster model for coronavirus disease 2019 (COVID‐19). Clin Infect Dis 2020; 71: 2139–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Cheng Y, Ma N, Witt C et al. Face masks effectively limit the probability of SARS‐CoV‐2 transmission. Science 2021; 372: 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Peeples L. Face masks: what the data say. Nature 2020; 586: 186–189. [DOI] [PubMed] [Google Scholar]
- 125. Hu X, Wang S, Fu S et al. Intranasal mask for protecting the respiratory tract against viral aerosols. Nat Commun 2023; 14: 8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kim MJ, Kim S, Kim H et al. Reciprocal enhancement of SARS‐CoV‐2 and influenza virus replication in human pluripotent stem cell‐derived lung organoids. Emerg Microbes Infect 2023; 12: 2211685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bai L, Zhao Y, Dong J et al. Coinfection with influenza A virus enhances SARS‐CoV‐2 infectivity. Cell Res 2021; 31: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stowe J, Tessier E, Zhao H et al. Interactions between SARS‐CoV‐2 and influenza, and the impact of coinfection on disease severity: a test‐negative design. Int J Epidemiol 2021; 50: 1124–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pinky L, Dobrovolny HM. Coinfections of the respiratory tract: viral competition for resources. PLoS One 2016; 11: e0155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pinky L, Dobrovolny HM. SARS‐CoV‐2 coinfections: could influenza and the common cold be beneficial? J Med Virol 2020; 92: 2623–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Swets MC, Russell CD, Harrison EM et al. SARS‐CoV‐2 co‐infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Lancet 2022; 399: 1463–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zhang AJ, Lee AC, Chan JF et al. Coinfection by severe acute respiratory syndrome coronavirus 2 and influenza A(H1N1)pdm09 virus enhances the severity of pneumonia in Golden Syrian hamsters. Clin Infect Dis 2021; 72: e978–e992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kinoshita T, Watanabe K, Sakurai Y, Nishi K, Yoshikawa R, Yasuda J. Co‐infection of SARS‐CoV‐2 and influenza virus causes more severe and prolonged pneumonia in hamsters. Sci Rep 2021; 11: 21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kim EH, Nguyen TQ, Casel MAB et al. Coinfection of SARS‐CoV‐2 and influenza A virus increases disease severity, impaired neutralizing antibody, and CD4+ T cell responses. J Virol 2022; 96: e0187321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhang G, Hu C, Luo L et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol 2020; 127: 104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Guan Z, Chen C, Li Y et al. Impact of coinfection with SARS‐CoV‐2 and influenza on disease severity: a systematic review and meta‐analysis. Front Public Health 2021; 9: 773130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cheng Y, Ma J, Wang H et al. Co‐infection of influenza A virus and SARS‐CoV‐2: a retrospective cohort study. J Med Virol 2021; 93: 2947–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed 2020; 91: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. NPR . Biden ends COVID national emergency after Congress acts. Available from: https://www.npr.org/2023/04/11/1169191865/biden‐ends‐covid‐national‐emergency
- 140. USA TODAY . White house to invest $5 billion in next‐generation COVID vaccines. Here's why we need new ones. Available from: https://www.usatoday.com/story/news/health/2023/04/10/project‐next‐generation‐coronavirus‐vaccines‐biden‐administration/11636925002/


