Abstract
Background
In the general population, up to 10% of people younger than 70 years and 15% to 20% of people older than 70 years have peripheral arterial disease (PAD). Symptomatic and asymptomatic PAD has an estimated prevalence of 13% in the over 50 years age group. However, asymptomatic PAD can account for up to 75% of PAD patients and only 10% of PAD patients have typical intermittent claudication. People with PAD are at an increased risk of death, heart and cerebrovascular disease and are recommended to receive treatment to manage their cardiac risk. They suffer from significant functional limitations in their daily activities and the most severely affected are at risk of limb loss. Many people with PAD do not have any symptoms. Only some people have discomfort or pain in the lower legs when walking, so PAD often goes undetected. Given the high incidence of asymptomatic and undiagnosed PAD, it is important to determine the effectiveness of a screening intervention in preventing cardiovascular adverse outcomes, both fatal and non‐fatal.
Objectives
To determine the effectiveness of screening for PAD in asymptomatic and undiagnosed individuals in terms of reduction of all‐cause mortality, cardiovascular events (for example myocardial infarction and stroke), morbidity from PAD (intermittent claudication, amputation, reduced walking distance) and improvement in quality of life.
Search methods
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched November 2013) and CENTRAL (2013, Issue 10).
Selection criteria
All published and unpublished randomised controlled trials (RCTs) of screening for PAD were sought without language restriction.
Data collection and analysis
Studies identified for potential inclusion in the review were independently assessed by both review authors. We planned to conduct data collection and analysis in accordance with the Cochrane Handbook for Systematic Review of Interventions.
Main results
No RCTs were identified that met the inclusion criteria.
Authors' conclusions
Unfortunately, no randomised controlled trial data are available regarding screening for PAD. Therefore, we are unable to determine the effects of screening for PAD in order to guide decision making by healthcare providers and planners. High quality randomised controlled trials evaluating the effectiveness of screening for PAD in asymptomatic and undiagnosed individuals in terms of reduction of all‐cause mortality, cardiovascular events (for example myocardial infarction and stroke), morbidity from PAD (intermittent claudication, amputation, reduced walking distance) and improvement in quality of life are needed.
Keywords: Aged, Humans, Middle Aged, Asymptomatic Diseases, Intermittent Claudication, Intermittent Claudication/etiology, Peripheral Arterial Disease, Peripheral Arterial Disease/complications, Peripheral Arterial Disease/diagnosis
Plain language summary
Screening for peripheral arterial disease
Peripheral arterial disease (PAD) is caused by fatty deposits on the walls of the arteries (or atherosclerosis) that leads to narrowing of the arteries (or stenosis) and obstructions in the major vessels supplying the lower legs. PAD can cause discomfort or pain in the lower legs when walking. People with PAD have an increased risk of death, heart and cerebrovascular disease and often receive treatment to manage their cardiac risk. They suffer from significant functional limitations in their daily activities, and the most severely affected are at risk of limb loss. Many people with PAD do not have any symptoms. Only some people have discomfort or pain in the lower legs when walking, so PAD often goes undetected. One possible way to identify this disease is to screen the population at increased risk of PAD. It is important to determine the effectiveness of screening in preventing heart and cerebrovascular diseases or further progression of PAD.
This review found no randomised controlled trial evidence on screening for PAD. High quality research is required to help healthcare providers decide whether screening for PAD in asymptomatic and undiagnosed individuals is effective in terms of reduction of all‐cause mortality, cardiovascular events (for example myocardial infarction and stroke), morbidity from PAD (intermittent claudication, amputation, reduced walking distance) and improvement in quality of life.
Background
Description of the condition
Peripheral arterial disease (PAD) is caused by atherosclerosis (fatty deposits on the walls of the arteries) that leads to stenosis (narrowing of the arteries) and occlusion (obstruction) in the major vessels supplying the lower legs. PAD can cause discomfort or pain in the lower legs when walking. This discomfort or pain is also know as intermittent claudication. While the most common cause for PAD is atherosclerosis, other causes are possible such as vasculitis (inflammation of the blood vessels), cystic adventitial disease and popliteal entrapment. In the general population, up to 10% of people younger than 70 years and 15% to 20% of people older than 70 years have PAD (Shammas 2007). Symptomatic and asymptomatic PAD has an estimated prevalence of 13% in the over 50 years age group (Hirsch 2001). However, asymptomatic PAD can account for up to 75% of PAD patients and only 10% of PAD patients have typical intermittent claudication (Beckman 2006).
People with PAD have an increased risk of mortality, myocardial infarction and cerebrovascular disease (Heald 2006) and are recommended to receive treatment to manage their cardiovascular risk (Bhasin 2007). They suffer from significant functional limitations in their daily activities, and the most severely affected are at risk of limb loss (Hooi 2004; Twine 2009). The National Institute for Health and Clinical Excellence (NICE) published guidelines on the diagnosis of lower limb PAD and assessment of patients with suspected PAD in 2012. It recommends that people with PAD should be offered a wide range of information regarding the condition including on key modifiable risk factors like smoking, control of diabetes, hyperlipidaemia, diet, body weight and exercise, and also on the secondary prevention of cardiovascular disease (NICE 2012).
One possible management strategy is to screen the population at increased risk of PAD (Mohler 2012). Given the high incidence of asymptomatic PAD, it is important to determine the effectiveness of screening in preventing cardiovascular and cerebrovascular diseases or further progression of PAD. PAD and chronic heart failure share many risks and co‐morbidities yet PAD often goes undetected (Inglis 2013).
Description of the intervention
Guidelines for PAD screening in asymptomatic adults have been developed at international (TASC 2007) and national (for example United States (ACCF/AHA 2010; USPSTF 2013); Canada (CCS 2013)) levels. These guidelines recommend various tests for PAD screening (Ferket 2012). Tests can be divided into non‐invasive and invasive vascular diagnostic tools and other methods.
Non‐invasive and invasive vascular diagnostic tools
Several vascular diagnostic tools are used to diagnose and screen for PAD. Non‐invasive diagnostic tools include ankle brachial index (ABI), toe‐brachial index (TBI), duplex ultrasound and pulse oximetry; while other diagnostic tools include contrast angiography, magnetic resonance angiography, computed tomographic angiography and photoplethysmography.
The ABI is one of the most common tools used for diagnosis of and screening for PAD, and can be used to predict the risk of cardiovascular events (Ankle Brachial Index Collaboration 2008; SIGN 2007). The ABI can be measured in different ways. The UK NICE clinical guidelines recommend that the patient is rested in a supine position and the audible systolic brachial and ankle pressures are detected with a doppler scanning probe (McDermott 2000; NICE 2012). It is recommended that the ABI of each leg should be calculated by dividing the higher of the posterior tibial artery or dorsalis pedis artery pressure by the higher of the right or left arm systolic blood pressure (Aboyans 2012). In order to classify disease, the ABI values can be divided into the four classes of normal (0.90 to 1.30), mild disease (0.7 to 0.9), moderate disease (0.41 to 0.69) and severe disease (critical limb ischaemia, less than or equal to 0.4) ranges (Macleod‐Roberts 1995).
The TBI measures the brachial systolic pressure against the large toe's systolic pressure with the brachial pressure obtained by Doppler and the toe pressure obtained by photoplethysmography. The TBI is then calculated by dividing the highest toe pressure by the highest brachial pressure. The TBI test is usually offered to patients with an abnormally high ABI (above 1.3) and who often have diabetes and calcified crural vessels which cannot be completely compressed as toe vessels are less susceptible to vessel stiffness. Guidelines and reviews recommend a cutoff value of a TBI of less than 0.70 as a diagnosis limit for PAD (Høyer 2013).
Duplex ultrasound visualises the artery with sound waves and measures the blood flow in an artery to indicate the presence of a blockage. The duplex ultrasound has a sensitivity of 80% and a specificity of up to 100% for detecting femoral and popliteal disease compared with angiography. It is less reliable for assessing the severity of stenoses in the tibial and peroneal arteries. Duplex scanning can also be used to assess carotid arteries and survey infrainguinal bypass grafts to identify sites of stenosis (Donnelly 2000).
Pulse oximetry is used to measure arterial oxygen saturation (SaO2) of the patients' index fingers and big toes in the supine position and at 12‐inch elevation. The instrument is commonly available and can be used to diagnose and screen lower extremity arterial disease in patients with diabetes. Abnormal pulse oximetry of the toes is defined as an SaO2 value of more than 2% lower than the finger value or a decrease of more than 2% on elevation of the leg (Parameswaran 2005).
Intra‐arterial contrast angiography is the reference standard for PAD imaging and diagnosis. A contrast agent is injected into the artery and X‐rays are taken to show blood flow, arteries in the legs and to pinpoint any blockages that may be present (Collins 2007).
Magnetic resonance angiography uses magnetic fields and radio waves to show blockages in the artery. It has the advantages of imaging a moving column of blood. A timed bolus of gadolinium contrast allows high quality angiographic images to be captured in a single breath hold. Depending on the vessels being studied and the field strength of the machine, this technique allows imaging sequence capture for two and three‐dimensional angiography and phase contrast (Donnelly 2000). Magnetic resonance (MR) angiography with contrast agents such as gadofosveset trisodium (MS‐325) provides significant improvements in effectiveness over unenhanced MR (and minimal and transient side effects) and it is a safe and effective form of MR evaluation of patients with aortoiliac occlusive disease (Goyen 2005).
Helical computed tomography angiography has been used for the evaluation of abdominal aortic aneurysms and it is now used in PAD. This is a multi‐detector row method that enables fine collimation to be combined with rapid (arterial phase) contrast‐enhanced scanning to determine changes in the lower limb vascular tree associated with PAD (Collins 2007).
Photoplethysmography is a quick and simple to perform optical technique used in the detection of stenotic peripheral disease (Oates 2012). Photoplethysmography uses pulse wave technology to detect changes in the blood volume in the microvascular bed of tissue (Allen 2008).
Other methods
Other methods that are used for screening for PAD and which are considered for this review are physical examination (leg symptoms, leg vascular examination); clinical history; exercise test; and questionnaires (including the World Health Organization (WHO) leg pain and Edinburgh Claudication questionnaires).
The physical examination can consist of the examination of the femoral, popliteal and foot pulses. The legs and feet of people with PAD are the same colour as the legs and feet of those with normal circulation but when elevated above the horizontal position they become pale (Mohler 2012). The legs and feet can also be examined for evidence of critical limb ischaemia, for example ulceration (NICE 2012). While specific to PAD, physical examination findings such as absence of pulses, femoral bruit and trophic skin changes have low sensitivity (Khan 2006).
History taking of claudication has a low sensitivity (54%) and positive predictive value (9%) when compared with formal non‐invasive techniques such as the ABI (Criqui 1985).
Exercise or walking tests assess the functional limitations of arterial stenoses and can differentiate occlusive arterial disease from other causes of exercise‐induced lower limb symptoms. The walking test is performed by exercising the individual on a treadmill, walking the individual, or marking time on the spot. The ABI can be measured before or after the exercise. The individual does not have occlusive arterial disease proximal to the ankle in that limb if after a five‐minute brisk walk the ankle systolic pressure does not drop (Donnelly 2000).
Standardised questionnaires such as the Edinburgh Claudication Questionnaire (ECQ) or the WHO/Rose questionnaire are other common screening measures for PAD. The ECQ is a modified format of the WHO/Rose questionnaire. The ECQ has a high sensitivity (91%) and specificity (99%) for the diagnosis of intermittent claudication compared with the diagnosis of intermittent claudication made by a physician (Leng 1992).
How the intervention might work
Mass screening programmes for PAD aim to greatly reduce the chances for further serious problems such as intermittent claudication, critical limb ischaemia, cardiovascular events, amputation, impaired ambulation or early deaths. An early diagnosis of asymptomatic PAD in the general population will increase the monitoring, surveillance and treatment of PAD in the diagnosed patients as well as address the cardiovascular disease risk factors in a group of individuals who otherwise would not be known to be at increased risk of cardio‐ and cerebrovascular disease.
Usually screening programmes must meet a set of criteria before they are introduced. In the United States, screening programmes are introduced after recommendations from the United States Preventive Services Task Force. In the United Kingdom they are introduced only after approval is received from the National Screening Committee. There are currently no systematic reviews of the evidence on screening for PAD from randomised controlled trials.
Why it is important to do this review
Given the high incidence of asymptomatic and undiagnosed PAD, it is important to determine the effectiveness of a screening intervention in preventing cardiovascular adverse outcomes, both fatal and non‐fatal.
Objectives
To determine the effectiveness of screening for PAD in asymptomatic and undiagnosed individuals in terms of reduction of all‐cause mortality, cardiovascular events (for example myocardial infarction and stroke), morbidity from PAD (intermittent claudication, amputation, reduced walking distance) and improvement in quality of life.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of population screening for PAD using any validated technique. We planned to include published studies and studies in progress if preliminary results were available. Non‐English language studies were also eligible for inclusion in the review.
Types of participants
Men and women of all ages, with or without the existing risk factors of hypertension, diabetes mellitus, dyslipidaemia or smoking history, who had not previously been diagnosed with symptomatic or asymptomatic PAD.
People who had previously been diagnosed with symptomatic or asymptomatic PAD were not eligible for inclusion.
Types of interventions
Screening for PAD using any validated method to diagnose symptomatic or asymptomatic PAD followed by appropriate treatment of affected individuals was eligible for this review. Tests used to detect PAD include:
non‐invasive and invasive vascular diagnostic tools: ABI, TBI, duplex ultrasound, pulse oximetry, magnetic resonance angiography, contrast angiography, computed tomographic angiography, contrast angiography and photoplethysmography;
other methods: physical examination (leg symptoms, leg vascular examination), clinical history, exercise test, questionnaires including the WHO/Rose and Edinburgh Claudication questionnaires.
We planned to compare screened versus unscreened participants.
Types of outcome measures
Primary outcomes
All‐cause mortality
Fatal and non‐fatal myocardial infarction and stroke (ischaemic and haemorrhagic)
Combined outcome of fatal and non‐fatal myocardial infarction, fatal and non‐fatal stroke (ischaemic and haemorrhagic) and cardiovascular death
Secondary outcomes
Progression of local disease (PAD itself)
Morbidity from PAD (intermittent claudication, amputation, walking distance, changes in ABI)
Quality of life
We planned to extract information on harms of screening (such as labelling, over‐diagnosis and over‐treatment), screening acceptance rate, and information on use of resources (such as hospital stay and use of specific facilities) from included trials where provided.
Search methods for identification of studies
There was no restriction on language. We planned to seek translations of non‐English studies. We planned to contact the authors of any relevant studies.
Electronic searches
The Cochrane Peripheral Vascular Diseases (PVD) Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched November 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2013, Issue 10), part of The Cochrane Library (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL and AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane PVD Group module in The Cochrane Library (www.thecochranelibrary.com).
The TSC searched the following trial databases for details of ongoing and unpublished studies using the terms screening and peripheral.
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
ClinicalTrials.gov (http://clinicaltrials.gov/).
Current Controlled Trials (http://www.controlled‐trials.com/).
Searching other resources
We searched the reference lists of relevant articles retrieved by the electronic searches for additional citations.
Data collection and analysis
Selection of studies
One review author (AA) used the selection criteria to identify trials for inclusion. The second review author (BF) independently confirmed this selection and any disagreements were resolved by discussion.
Data extraction and management
Both review authors (AA, BF) planned to independently extract the data. We planned to record information about the trial design, type of screening method, and baseline characteristics of participants, including information on the screening methods and outcomes (as above). We planned to contact the authors of included studies for further information if clarification was required. We planned to resolve any disagreements in data extraction and management by discussion.
Assessment of risk of bias in included studies
Both review authors (AA, BF) planned to independently use the Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011) for assessing risk of bias for each of the included studies. The tool provides a protocol for judgements on sequence generation, allocation methods, blinding, incomplete outcome data, selective outcome reporting and any other relevant biases. We planned to present the justification of the judgements of risk of bias in the 'Risk of bias' tables. We planned to resolve any disagreements by discussion.
Measures of treatment effect
We planned to base the analysis on intention‐to‐treat data from the individual clinical trials. As the primary and secondary outcomes are all binary measures, we planned to compute odds ratios (ORs) using a random‐effects model. We planned to calculate the 95% confidence intervals (CI) of the effect sizes.
Unit of analysis issues
It was planned that the unit of analysis would be the individual participant.
Dealing with missing data
We planned to seek information about dropouts, withdrawals and other missing data and, if not reported, we planned to contact the study authors.
Assessment of heterogeneity
The inclusion of studies using a wide range of screening techniques is likely to result in a high degree of heterogeneity. We planned to assess the heterogeneity between pooled studies by using the Chi2 test regarding the characteristics and quality of included studies. We planned to perform the Chi2 test to assess heterogeneity in identified subgroups, and we planned to use the I2 statistic to measure the degree of inconsistency between studies. An I2 statistic value of over 50% may represent moderate to substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
We planned to assess reporting biases such as publication bias using funnel plots if more than 10 studies were included in the review. There are many reasons for funnel plot asymmetry and we planned to consult the Cochrane Handbook for Systematic Reviews of Interventions to aid the interpretation of the results (Sterne 2011).
Data synthesis
The review authors planned to independently extract the data. One review author (AA) planned to input the data into Review Manager (RevMan 2011). The second review author (BF) planned to cross‐check data entry. We planned to resolve any discrepancies by consulting the source publication. We planned to use a random‐effects model to meta‐analyse the data. If we were unable to perform a meta‐analysis due to lack of available data we planned to present the data in a narrative manner.
Subgroup analysis and investigation of heterogeneity
Where possible, we planned to analyse clinically relevant subgroups based on type of prophylaxis and participant groupings. Possible groupings include:
hypertension;
diabetes mellitus;
dyslipidaemia;
smoking.
Sensitivity analysis
We planned to perform a sensitivity analysis to examine the stability of the results in relation to the quality of included studies. There were no sensitivity analyses required as no studies were included in this review.
Summary of findings table
We planned to present a 'Summary of findings' table including for the following primary outcomes: all‐cause mortality, fatal and non‐fatal myocardial infarction and stroke (ischaemic and haemorrhagic), combined outcome of fatal and non‐fatal myocardial infarction, fatal and non‐fatal stroke (ischaemic and haemorrhagic) and cardiovascular death.
We planned to comment on the quality of the body of evidence using the specific evidence grading system developed by the GRADE collaboration (Grade Working Group 2004). We planned to make judgements on the quality of the evidence transparent by using footnotes in the summary of findings table.
Results
Description of studies
Results of the search
See Figure 1.
1.
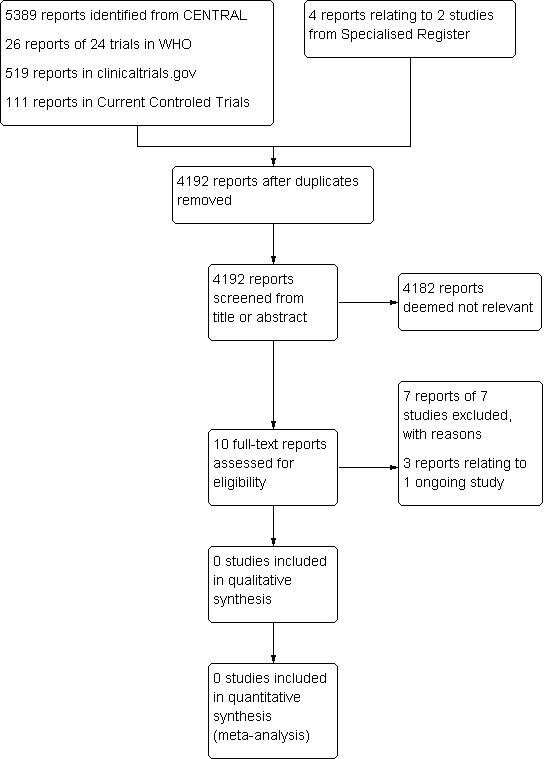
Study flow diagram.
The search results were independently reviewed by the two review authors (AA and BF). No RCTs were identified for inclusion in this review. Seven studies (Behar 2013; Bergiers 2011; Delgado‐Osorio; Farkas 2012; Kravos 2009; Mourad 2009; Taylor‐Piliae 2011) were excluded and one ongoing study was identified (Viborg 2014).
Included studies
No RCTs were identified that met the inclusion criteria.
Excluded studies
Seven studies (Behar 2013; Bergiers 2011; Delgado‐Osorio; Farkas 2012; Kravos 2009; Mourad 2009; Taylor‐Piliae 2011) were excluded. The excluded studies were not RCTs. Four studies were observational studies (Bergiers 2011; Farkas 2012; Kravos 2009; Mourad 2009) and three studies were single arm studies, including only the screened population (Behar 2013; Delgado‐Osorio; Taylor‐Piliae 2011).
Risk of bias in included studies
It was not possible to review methodological quality in the absence of studies eligible for inclusion in this review.
Effects of interventions
No published or unpublished RCTs were found on screening for PAD in asymptomatic and undiagnosed individuals.
Discussion
Summary of main results
This review documents that there are no published RCTs that assess the effectiveness of screening for PAD in asymptomatic and undiagnosed individuals in terms of reduction of all‐cause mortality, cardiovascular events (for example myocardial infarction and stroke), morbidity from PAD (intermittent claudication, amputation, reduced walking distance) and improvement in quality of life. Only one ongoing study was identified (Viborg 2014) but data are not yet available for inclusion in this review.
Guidelines for PAD screening in asymptomatic adults have been developed at international and national levels. These guidelines recommend various tests for PAD screening (Ferket 2012). Tests can be divided into non‐invasive and invasive vascular diagnostic tools and other methods. However, screening for PAD is still controversial as the published guidelines do not have similar recommendations in terms of using screening programmes for PAD in an asymptomatic population.
Overall completeness and applicability of evidence
High quality RCTs evaluating the effectiveness of screening for PAD in asymptomatic and undiagnosed individuals in terms of reduction of all‐cause mortality, cardiovascular events (for example myocardial infarction and stroke), morbidity from PAD (intermittent claudication, amputation, reduced walking distance) and improvement in quality of life are required. When designing these trials, careful consideration is required for outcomes measures. Trials should assess all relevant outcomes and should address issues related to population variability and the performance and reliability of tests.
Quality of the evidence
It was not possible to review methodological quality in the absence of studies eligible for inclusion in the review.
Potential biases in the review process
There were no included studies in this review. The PVD Group TSC performed a comprehensive search of the literature and the selection of studies was conducted according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion.
Agreements and disagreements with other studies or reviews
This review agrees with the recommendation statement of the U.S. Preventive Services Task Force (USPSTF 2013) on using ABI in screening for PAD. The USPSTF found no studies that directly address the effect of screening for PAD with ABI on future health outcomes. The recommendation concluded that it is still unclear whether measuring the ABI improves risk assessment beyond other risk factors that consider blood pressure and cholesterol levels, or that early treatment of screen‐detected PAD improves patient outcomes. Also, the recommendation considered the potential harms of screening including harms from false‐positive test results, such as anxiety and additional invasive testing to confirm the diagnosis.
One systematic review of PAD screening (Ferket 2012) identified several major guidelines (Abramson 2005; ACCF/AHA 2010; Anon 2006; Genest 2009; Grundy 2004; Hirsch 2006; NCEP 2002; TASC 2007; USPSTF 2009). The guidelines were evaluated with an Appraisal of Guidelines and Evaluation in Europe (AGREE) rigour score varying from 33% to 81%. The ABI was considered as the primary screening tool in all guidelines identified by Ferket 2012. Other tools were the use of questionnaires, considered by three guidelines (Abramson 2005; Anon 2006; Hirsch 2006), and physical examination considered by one guideline (Abramson 2005). Screening for PAD was supported only by some of the guidelines, other guidelines found insufficient evidence or were against screening. Some of the guidelines have already been updated since the publication of the review by Ferket and colleagues in 2012 as, for example, USPSTF 2009 has been replaced by USPSTF 2013 with their conclusions remaining the same.
As the recommendation of screening for PAD with ABI, or any other test, in asymptomatic individuals is still controversial, some investigators are considering pre‐screening tests such as PREVALENT or REASON. PREVALENT is a clinical prediction model that includes an asymptomatic population older than 54 years with at least one risk factor such as smoking, hypertension, diabetes or hypercholesterolaemia, but this model has not been validated (Bendermacher 2007). REASON is a risk score identifying candidates to screen for PAD using ABI in a population which is 50 to 79 years old. While the REASON model provides accurate ABI estimates with a better predictive capacity than the current Inter‐Society Consensus screening criteria, it is better at predicting low ABI in men but has only been tested on one population in Spain (Ramos 2011).
The ABI Collaboration suggests that an ABI risk model incorporating ABI and the Framingham risk score (FRS) can improve the prediction of cardiovascular events. The model is especially useful in individuals at intermediate risk. It also reassures physicians that if the FRS is not performing as expected, the ABI model can compensate for FRS deficiencies (Fowkes 2014).
Authors' conclusions
Implications for practice.
PAD is associated with an increased risk of cardiovascular events. Unfortunately, no randomised controlled trial data are available regarding screening for PAD in an asymptomatic population. Therefore, we are unable to determine the effects of screening for PAD in order to guide decision making by healthcare providers and planners.
Implications for research.
High quality randomised controlled trials evaluating the effectiveness of screening for PAD in asymptomatic and undiagnosed individuals in terms of reduction of all‐cause mortality, cardiovascular events (for example myocardial infarction and stroke), morbidity from PAD (intermittent claudication, amputation, reduced walking distance) and improvement in quality of life are needed.
Acknowledgements
We would like to thank the personnel from the Cochrane Peripheral Vascular Disease Review Group, especially Karen Welch and Marlene Stewart, for their invaluable support and advice.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Arteriosclerosis] this term only | 894 |
| #2 | MeSH descriptor: [Arteriolosclerosis] this term only | 0 |
| #3 | MeSH descriptor: [Arteriosclerosis Obliterans] this term only | 72 |
| #4 | MeSH descriptor: [Atherosclerosis] this term only | 423 |
| #5 | MeSH descriptor: [Arterial Occlusive Diseases] this term only | 775 |
| #6 | MeSH descriptor: [Intermittent Claudication] this term only | 729 |
| #7 | MeSH descriptor: [Peripheral Vascular Diseases] explode all trees | 2202 |
| #8 | MeSH descriptor: [Leg] explode all trees and with qualifiers: [Blood supply ‐ BS] | 1092 |
| #9 | MeSH descriptor: [Femoral Artery] explode all trees | 739 |
| #10 | MeSH descriptor: [Popliteal Artery] explode all trees | 263 |
| #11 | MeSH descriptor: [Iliac Artery] explode all trees | 152 |
| #12 | MeSH descriptor: [Tibial Arteries] explode all trees | 30 |
| #13 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD) | 18028 |
| #14 | (arter*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 5005 |
| #15 | (vascular) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1438 |
| #16 | (vein*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 757 |
| #17 | (veno*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1013 |
| #18 | (peripher*) near (*occlus* or steno* or obstuct* or lesio* or block* or obliter*) | 1394 |
| #19 | peripheral near/3 dis* | 3410 |
| #20 | arteriopathic | 21 |
| #21 | (claudic* or hinken*) | 1498 |
| #22 | dysvascular* | 30 |
| #23 | leg near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 192 |
| #24 | limb near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 245 |
| #25 | (lower near/3 extrem*) near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 150 |
| #26 | (iliac or femoral or popliteal or femoro* or fempop* or crural or *inguinal or infragenicular or tibio* or crural) near/3 (obstruct* or occlus* or vessel or arter*) | 1634 |
| #27 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 | 27174 |
| #28 | MeSH descriptor: [Mass Screening] explode all trees | 4600 |
| #29 | MeSH descriptor: [Early Diagnosis] this term only | 349 |
| #30 | screen*:ti,ab,kw (Word variations have been searched) | 16817 |
| #31 | identif*:ti,ab,kw (Word variations have been searched) | 34443 |
| #32 | routine* near/3 ask*:ti,ab,kw (Word variations have been searched) | 10 |
| #33 | routine* near/3 question*:ti,ab,kw (Word variations have been searched) | 45 |
| #34 | test*:ti,ab,kw (Word variations have been searched) | 135185 |
| #35 | (population or mass) near/2 assess*:ti,ab,kw (Word variations have been searched) | 259 |
| #36 | early near/3 (diagnosis or detect* or intervent*):ti,ab,kw (Word variations have been searched) | 3967 |
| #37 | periodic near/3 (exam* or evaluat* or check*):ti,ab,kw (Word variations have been searched) | 85 |
| #38 | #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 | 171207 |
| #39 | #27 and #38 in Trials | 5389 |
| #40 | sr‐pvd | 9869 |
| #41 | #39 not #40 in Trials | 3653 |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Behar 2013 | Single arm study of diabetic outpatients |
| Bergiers 2011 | Observational study |
| Delgado‐Osorio | Single arm study |
| Farkas 2012 | Observational study |
| Kravos 2009 | Observational study, one arm screened population |
| Mourad 2009 | Observational study |
| Taylor‐Piliae 2011 | Cross‐sectional study, screened population only |
Characteristics of ongoing studies [ordered by study ID]
Viborg 2014.
| Trial name or title | Randomised preventive vascular screening trial of 65 ‐ 74 year old men in the central region of Denmark |
| Methods | Allocation: Randomised Endpoint classification: Efficacy study Intervention model: Parallel assignment Masking: Single blind (investigator) Primary purpose: Screening |
| Participants | Males: aged 65 ‐ 74 years Inclusion criteria:
Exclusion criteria:
|
| Interventions | Procedure: Screening for hypertension, lower limb atherosclerosis and abdominal aortic aneurysm Invited to vascular screening
|
| Outcomes | Primary outcomes: ‐ All‐cause mortality at 3, 5 and 10 years Secondary outcomes: ‐ Cardiovascular events at 3, 5 and 10 years |
| Starting date | September 2008 |
| Contact information | Jes S Lindholt, MD PhD +45 89272447; email: Jes.S.Lindholt@Viborg.RM.DK Eskild W Henneberg, MD +45 89272445; email: Eskild.W.Henneberg@Viborg.RM.DK |
| Notes | The investigators were contacted in November 2013. No results are available yet for this study. |
Contributions of authors
AA: drafted the protocol, selected studies for inclusion and wrote the review. BF: contributed to the protocol, selected studies for inclusion and contributed to the text of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
AA is supported by a programme grant from the NIHR
-
National Institute for Health Research (NIHR), UK.
The PVD Group editorial base is supported by a programme grant from the NIHR
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office
Declarations of interest
None known
New
References
References to studies excluded from this review
Behar 2013 {published data only}
- Behar T, Bosson JL, Galanaud JP, Thoret S, Rolland C, Bura‐Rivière A, et al. Prevalence and risk factors of peripheral arterial disease in an outpatient screening campaign [Évaluation de la prévalence et des facteurs de risque de l’artériopathie oblitérante des membres inférieurs dans le cadre d’une campagne de dépistage ambulatoire]. Journal des Maladies Vasculaires 2013;38(1):22‐8. [DOI] [PubMed] [Google Scholar]
Bergiers 2011 {published data only}
- Bergiers S, Vaes B, Degryse J. To screen or not to screen for peripheral arterial disease in subjects aged 80 and over in primary health care: a cross‐sectional analysis from the BELFRAIL study. BMC Family Practice 2011;12:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delgado‐Osorio {published data only}
- Delgado‐Osorio H, Franqui‐Rivera H, García‐Palmieri MR, Díaz‐Santana MV, Alvarez M. A peripheral artery disease screening study in Puerto Rico. Boletín de la Asociación Médica de Puerto Rico 2011;103(4):17‐21. [PubMed] [Google Scholar]
Farkas 2012 {published data only}
- Farkas K, Járai Z, Kolossváry E, Ludányi A, Clement DL, Kiss I, ERV Study Group. High prevalence of peripheral arterial disease in hypertensive patients: the evaluation of ankle‐brachial index in Hungarian hypertensives screening program. Journal of Hypertension 2012;30(8):1526‐32. [DOI] [PubMed] [Google Scholar]
Kravos 2009 {published data only}
- Kravos A, Bubnič‐Sotošek K. Ankle‐brachial Index screening for peripheral artery disease in asymptomatic patients between 50 and 70 years of age. Journal of International Medical Research 2009;37(5):1611‐9. [DOI] [PubMed] [Google Scholar]
Mourad 2009 {published data only}
- Mourad JJ, Cacoub P, Collet JP, Becker F, Pinel JF, Huet D, et al. Screening of unrecognized peripheral arterial disease (PAD) using ankle‐brachial index in high cardiovascular risk patients free from symptomatic PAD. Journal of Vascular Surgery 2009;50(3):572‐80. [DOI] [PubMed] [Google Scholar]
Taylor‐Piliae 2011 {published data only}
- Taylor‐Piliae RE, Fair JM, Varady AN, Hlatky MA, Norton LC, Iribarren C, et al. Ankle brachial index screening in asymptomatic older adults. American Heart Journal 2011;161(5):979‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Viborg 2014 {published data only (unpublished sought but not used)}
- Grondal N, Henneberg EW, Lindholt JS. The Viborg vascular (VIVA) randomised screening trial: design, methods and preliminary findings. European Society for Vascular Surgery Annual Meeting; 2009; Oslo, Norway 2009.
- Grondal N, Sogaard R, Henneberg EW, Lindholt JS. The Viborg vascular (VIVA) screening trial of 65‐74 year old men in the central region of Denmark: Study protocol. Trials 2010;11:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00662480. Randomized preventive vascular screening trial of 65‐74 year old men in the central region of Denmark. NCT00662480. http://clinicaltrials.gov/ct2/show/record/NCT00662480 (accessed 9 January 2014).
Additional references
Aboyans 2012
- Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation 2012;126(24):2890‐909. [DOI] [PubMed] [Google Scholar]
Abramson 2005
- Abramson BL, Huckell V, Anand S, Forbes T, Gupta A, Harris K, et al. Canadian Cardiovascular Society Consensus Conference: peripheral arterial disease ‐ executive summary. Canadian Journal of Cardiology 2005;21:997‐1006. [PubMed] [Google Scholar]
ACCF/AHA 2010
- Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA Guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Journal of the American College of Cardiology 2010;56(25):e50‐e103. [DOI] [PubMed] [Google Scholar]
Allen 2008
- Allen J, Overbeck K, Nath AF, Murray A, Stansby G. A prospective comparison of bilateral photoplethysmography versus the ankle‐brachial pressure index for detecting and quantifying lower limb peripheral arterial disease. Journal of Vascular Surgery 2008;47(4):794‐802. [DOI] [PubMed] [Google Scholar]
Ankle Brachial Index Collaboration 2008
- The Ankle Brachial Index Collaboration, Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, et al. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: a meta‐analysis. JAMA 2008;300(2):197‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anon 2006
- Anonymous. Screening for peripheral arterial disease: recommendation statement. American Family Physician 2006;73:497‐500. [PubMed] [Google Scholar]
Beckman 2006
- Beckman JA, Jaff MR, Creager MA. The United States preventive services task force recommendation statement on screening for peripheral arterial disease: more harm than benefit?. Circulation 2006;114(8):861‐6. [DOI] [PubMed] [Google Scholar]
Bendermacher 2007
- Bendermacher BL, Teijink JA, Willigendael EM, Bartelink ML, Peters RJ, Bie RA, et al. A clinical prediction model for the presence of peripheral arterial disease‐‐the benefit of screening individuals before initiation of measurement of the ankle‐brachial index: an observational study. Vascular Medicine 2007;12:5‐11. [DOI] [PubMed] [Google Scholar]
Bhasin 2007
- Bhasin N, Scott DJA. Ankle brachial pressure index: identifying cardiovascular risk and improving diagnostic accuracy. Journal of the Royal Society of Medicine 2007;100(1):4‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
CCS 2013
- Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Canadian Journal of Cardiology 2013;29(2):151‐67. [DOI] [PubMed] [Google Scholar]
Collins 2007
- Collins R, Cranny G, Burch J, Aguiar‐Ibáñez R, Craig D, Wright K, et al. A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease. Health Technology Assessment 2007;11(20):iii‐iv, xi‐xiii, 1‐184. [DOI] [PubMed] [Google Scholar]
Criqui 1985
- Criqui MH, Fronek A, Klauber MR, Barrett‐Connor E, Gabriel S. The sensitivity, specificity, and predictive value of traditional clinical evaluation of peripheral arterial disease: results from noninvasive testing in a defined population. Circulation 1985;71(3):516‐22. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysis data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Donnelly 2000
- Donnelly R, Hinwood D, London NJM. ABC of arterial and venous disease. Non‐invasive methods of arterial and venous assessment. BMJ 2000;320(7236):698‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferket 2012
- Ferket BS, Spronk S, Colkesen EB, Hunink MG. Systematic review of guidelines on peripheral artery disease screening. American Journal of Medicine 2013;125(2):198‐208, e3. [DOI] [PubMed] [Google Scholar]
Fowkes 2014
- Fowkes F, Murray G, Butcher I, Folsom A, Hirsch A, Couper D, et al. Development and validation of an ankle brachial index risk model for the prediction of cardiovascular events. European Journal of Preventive Cardiology 2014;21(3):310‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Genest 2009
- Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult ‐ 2009 recommendations. Canadian Journal of Cardiology 2009;25:567‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goyen 2005
- Goyen M, Edelman M, Perreault P, O'Riordan E, Bertoni H, Taylor J, et al. MR angiography of aortoiliac occlusive disease: a phase III study of the safety and effectiveness of the blood‐pool contrast agent MS‐325. Radiology 2005;236(3):825‐33. [DOI] [PubMed] [Google Scholar]
Grade Working Group 2004
- GRADE Working Group 2004. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grundy 2004
- Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227‐39. [DOI] [PubMed] [Google Scholar]
Heald 2006
- Heald CL, Fowkes FG, Murray GD, Price JF, Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle‐brachial index: systematic review. Atherosclerosis 2006;189(1):61‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirsch 2001
- Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001;286(11):1317‐24. [DOI] [PubMed] [Google Scholar]
Hirsch 2006
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463‐654. [DOI] [PubMed] [Google Scholar]
Hooi 2004
- Hooi JD, Kester AD, Stoffers HE, Rinkens PE, Knottnerus JA, Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7‐year follow‐up study. Journal of Clinical Epidemiology 2004;57(3):294‐300. [DOI] [PubMed] [Google Scholar]
Høyer 2013
- Høyer C, Sandermann J, Petersen LJ. The toe‐brachial index in the diagnosis of peripheral arterial disease. Journal of Vascular Surgery 2013;58(1):231‐8. [DOI] [PubMed] [Google Scholar]
Inglis 2013
- Inglis SC, Hermis A, Shehab S, Newton PJ, Lal S, Davidson PM. Peripheral arterial disease and chronic heart failure: a dangerous mix. Heart Failure Reviews 2013;18(4):457‐64. [DOI] [PubMed] [Google Scholar]
Khan 2006
- Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease?. JAMA 2006;295(5):536‐46. [DOI] [PubMed] [Google Scholar]
Leng 1992
- Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surveys. Journal of Clinical Epidemiology 1992;45(10):1101‐9. [DOI] [PubMed] [Google Scholar]
Macleod‐Roberts 1995
- MacLeod‐Roberts J. Vascular assessment. In: Merriman LM, Tollafield DR editor(s). Assessment of the lower limb. 1st Edition. Edinburgh: Churchill Livingstone, 1995:79‐115. [Google Scholar]
McDermott 2000
- McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. Journal of Vascular Surgery 2000;32(6):1164‐71. [DOI] [PubMed] [Google Scholar]
Mohler 2012
- Mohler ER 3rd. Screening for peripheral artery disease. Circulation 2012;126:e111‐2. [DOI] [PubMed] [Google Scholar]
NCEP 2002
- National Cholesterol Education Program. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143‐421. [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Clinical Excellence. Lower limb peripheral arterial disease (CG147). NICE clinical guidelines CG147 2012.
Oates 2012
- Oates CP, Allen J, Stansby G. Beyond the ankle‐brachial pressure index for the diagnosis of peripheral arterial disease‐‐time for a new look at photoplethysmography. Angiology 2012;64(7):492‐3. [DOI] [PubMed] [Google Scholar]
Parameswaran 2005
- Parameswaran GI, Brand K, Dolan J. Pulse oximetry as a potential screening tool for lower extremity arterial disease in asymptomatic patients with diabetes mellitus. Archives of Internal Medicine 2005;165(4):442‐6. [DOI] [PubMed] [Google Scholar]
Ramos 2011
- Ramos R, Baena‐Díez JM, Quesada M, Solanas P, Subirana I, Sala J, et al. Derivation and validation of REASON: a risk score identifying candidates to screen for peripheral arterial disease using ankle brachial index. Atherosclerosis 2011;214:474‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Shammas 2007
- Shammas NW. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vascular Health Risk Management 2007;3(2):229‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2007
- Scottish Intercollegiate Guidelines Network (SIGN). Diagnosis and management of peripheral arterial disease. A national clinical guideline. www.sign.ac.uk (accessed 23 July 2013).
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Review of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
TASC 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, on behalf of the TASC II Working Group. Inter‐society consensus for the management of peripheral arterial disease (TASC II). European Journal of Vascular and Endovascular Surgery 2007;33 Suppl 1:S1‐S75. [DOI] [PubMed] [Google Scholar]
Twine 2009
- Twine CP, Coulston J, Shandall A, McLain AD. Angioplasty versus stenting for superficial femoral artery lesions. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006767.pub2] [DOI] [PubMed] [Google Scholar]
USPSTF 2009
- U.S. Preventive Services Task Force. Using nontraditional risk factors in coronary heart disease risk assessment. Annals of Internal Medicine 2009;151:474‐82. [DOI] [PubMed] [Google Scholar]
USPSTF 2013
- U.S. Preventive Services Task Force. The ankle brachial index for peripheral artery disease screening and cardiovascular disease prediction in asymptomatic adults: a systematic evidence review for the U.S. Preventive Services Task Force. Available from http://www.uspreventiveservicestaskforce.org/uspstf12/pad/pades100.pdf (accessed 16 January 2013).


