Abstract
The glucagon receptor family are typical class B1 G protein-coupled receptors (GPCRs) with important roles in metabolism, including the control of pancreas, brain, and liver function. As proteins with seven transmembrane domains, GPCRs are intimately in contact with lipid bilayers and therefore can be putatively regulated by interactions with their lipidic components, including cholesterol, sphingolipids, and other lipid species. Additionally, these receptors, as well as the agonists they bind to, can undergo lipid modifications, which can influence their binding capacity and/or elicit modified or biased signalling profiles. While the effect of lipids, and in particular cholesterol, has been widely studied for other GPCR classes, information about their role in regulating the glucagon receptor family is only beginning to emerge. Here we summarise our current knowledge on the effects of cholesterol modulation of glucagon receptor family signalling and trafficking profiles, as well as existing evidence for specific lipid–receptor binding and indirect effects of lipids via lipid modification of cognate agonists. Finally, we discuss the different methodologies that can be employed to study lipid–receptor interactions and summarise the importance of this area of investigation to increase our understanding of the biology of this family of metabolically relevant receptors.
Keywords: lipid, glucagon receptor, GLP-1 receptor, GIP receptor, cholesterol
Introduction
Lipids are structurally diverse organic compounds crucial for the normal functioning of all cells, as they are key constituents of membranes essential for cellular structure that enable the compartmentalisation of tightly regulated processes both within the cell and in intracellular organelles. Lipids are also major contributors to intracellular signalling, either via lipidic post-translational modifications or as allosteric modulators of membrane receptors and other factors, as well as being important sources of energy, stored within cells as lipid droplets (Shevchenko & Simons 2010, Klose et al. 2013, Muro et al. 2014, Buenaventura et al. 2019, Damian et al. 2021). The structural characteristics of the lipids present in membranes determines their mechanical properties, controlling parameters ranging from membrane fluidity and curvature to thickness and shape, as well as the propensity for the formation of rafts and other signalling nanodomains. Unique combinations of membrane lipids and proteins allow for the development of cell/organelle-specific functions, ranging from those of the plasma membrane and/or trafficking vesicles to mitochondrial membranes harbouring electron transport chains (Guo et al. 2018), or the protein folding and transport functions of endoplasmic reticulum (ER) membranes (Phillips & Miller 2021), amongst others (Shevchenko & Simons 2010, Klose et al. 2013, Muro et al. 2014, Bolla et al. 2019).
Incretins, characterised by Creutzfeldt in 1979, are peptide hormones secreted in the gastrointestinal tract in response to nutrients that potentiate the glucose-dependent secretion of insulin. The primary incretins include glucagon-like peptide 1 (GLP-1), secreted mainly from enteroendocrine L cells, with its truncated versions, GLP-1(7–37) and GLP-1(7–36)NH2, binding to and activating cognate GLP-1 receptor (GLP-1R) (Donnelly, 2012, Campbell & Drucker 2013, Paternoster & Falasca 2018), and glucose-dependent insulinotropic polypeptide (GIP), a 42-amino acid peptide (GIP(1–42)) secreted from enteroendocrine K cells that binds to and activates the GIP receptor (GIPR) (Creutzfeldt 1979, Campbell & Drucker 2013, Chia & Egan 2020). Glucagon is a closely related peptide which derives, like GLP-1, from pre-proglucagon (Müller et al. 2017) and binds to the glucagon receptor (GCGR). It is released from pancreatic alpha cells and it is best known for its ability to increase hepatic glucose production at times of hypoglycaemia (Marroqui et al. 2014). Although glucagon is not considered an incretin, it has some similar features as it is secreted in response to protein ingestion (Müller et al. 1970, Day et al. 1978, Ang et al. 2019) and is insulinotropic (Capozzi et al. 2019a, b). Although not usually associated with type 2 diabetes or obesity, the glucagon family of receptors also includes the GLP-2 receptor (GLP-2R), which is activated by GLP-2, a 33-amino acid peptide (GLP-2(1–33)) also derived from pre-proglucagon and secreted mainly from enteroendocrine L cells, like GLP-1. GLP-2 promotes intestinal growth and enhances nutrient absorption amongst other functions (Bahrami et al. 2010, Drucker & Yusta 2014, Gadgaard et al. 2023).
The GLP-1R is found mainly in pancreatic beta cells and hypothalamic neurons. Besides its endogenous activation by GLP-1, pharmacological targeting of the GLP-1R is achieved with a range of peptide agonists, including exendin-4 and semaglutide, amongst others (Campbell & Drucker 2013, Knudsen & Lau 2019). The main function of the GLP-1R is the regulation of blood glucose levels through the potentiation of insulin secretion in a glucose-dependent manner and the control of appetite through the regulation of neuronal feeding centres (Campbell & Drucker 2013, Boer & Holst 2020). The best characterised GLP-1R intracellular signalling cascade involves coupling of the active receptor to Gαs, resulting in the activation of adenylate cyclase and leading to the generation of cyclic adenosine monophosphate (cAMP). This in turn leads to activation of protein kinase A (PKA) and exchange protein activated by cAMP 2 (Epac2), followed by engagement of diverse downstream signalling networks, culminating in an increase in insulin synthesis and secretion as well as promotion of beta cell survival (Holst et al. 2009, Boer & Holst 2020, El Eid et al. 2022).
Expression of GIPR partly overlaps with that of GLP-1R, for example, in pancreatic islets and some anorectic brain regions (albeit in mainly distinct neuronal subpopulations), but GIPR is also found in non-overlapping tissues such as bone and adipose tissue although there is some uncertainty on the exact cell type that expresses the GIPR in the latter (Yip et al. 1998, Kim et al. 2013, Campbell et al. 2022). Besides activation by its cognate hormone GIP, the GIPR is activated by pharmacological peptide agonists such as the GLP-1R/GIPR dual agonist tirzepatide. However, while there is evidence for GIPR engagement by tirzepatide in ex vivo human islets (El et al. 2023), the direct engagement of GIPR by tirzepatide in vivo in humans is still debated (Drucker & Holst 2023). Like the GLP-1R, the GIPR is Gαs-coupled, with cAMP production and insulin secretion promoted downstream of its activation, which also leads to glucagon secretion from alpha cells, adipose tissue lipid deposition, and a reduction in bone resorption (Mayendraraj et al. 2022). On the other hand, GCGR is found primarily in the liver and kidney; in hepatocytes, where it is also predominantly Gαs-coupled (McGlone et al. 2021), GCGR cAMP signalling leads to PKA activation and phosphorylation of cAMP response element binding protein (CREB), which increases transcription of glucagon-activated genes such as phosphoenolpyruvate carboxykinase 1 (PCK1) and glucose-6-phosphatase (Cajulao et al. 2022), resulting in increased hepatic glucose production via simulation of glycogenolysis and gluconeogenesis, as well as hepatic amino acid uptake and ureagenesis (Marroqui et al. 2014), while reducing hepatic fat accumulation by increasing fatty acid oxidation and decreasing de novo lipogenesis (Longuet et al. 2008). GLP-2R is expressed mainly in the gastrointestinal tract and regions of the central nervous system. GLP-2R signalling, following activation by GLP-2, is mainly via Gαs coupling causing an increase in cAMP accumulation leading to increased intracellular calcium and expression of cell survival genes triggering intestinotrophic effects (Drucker & Yusta 2014, Gadgaard et al. 2022, 2023). As a result, agonists of the GLP-2R such as teduglutide have been used for the treatment of short bowel syndrome and other intestinal conditions (Bahrami et al. 2010, Drucker & Yusta 2014, Gadgaard et al. 2023).
All four receptors, GLP-1R, GIPR, GCGR, and GLP-2R, belong to the class B1/secretin-like group of G protein-coupled receptors (GPCRs), integral membrane proteins that can detect a vast array of extracellular signals and transmit them into the cells via a range of G protein signalling pathways. GPCRs are divided into four major classes, rhodopsin-like (class A), secretin-like (class B), glutamate (class C), and frizzled (class F) (Latorraca et al. 2017, Yang et al. 2021).
The glucagon family of receptors has a typical class B1 GPCR structure, with seven transmembrane domains (TMD), three extracellular and intracellular loops, a large N-terminal extracellular domain (ECD), and an intracellular C-terminal tail. Being embedded in the plasma membrane, they interact intimately with lipids, and their function is greatly affected by changes in the lipid microenvironment. There is extensive evidence to support the effect that lipids have on GPCR function, with cholesterol specifically acting as an allosteric modulator (Oates & Watts 2011, Kumar & Chattopadhyay 2021, Yeliseev et al. 2021, Baccouch et al. 2022). However, much of the work done to investigate lipid regulation of GPCR functions has focused on other GPCR classes, as highlighted in Table 1, with our understanding of lipid modulation of class B1 GPCRs only beginning to be explored. This review will focus on highlighting the effects that lipids have on the function of the glucagon receptor family (Fig. 1), including their importance in the development of treatments for type 2 diabetes and obesity, as well as a description of current techniques to study lipid–receptor interactions, as well as areas that need to be further explored.
Table 1.
Effects of lipids on the modulation of GPCR function.
| Class | GPCR | Lipid | Evidence | References |
|---|---|---|---|---|
| A | β2-Adrenergic receptor (β2AR) | Cholesterol | Cholesterol interacts with receptor; stability; allosteric modulation; dimer formation | (Hanson et al. 2008, Manna et al. 2016, Cherezov et al. 2007) |
| Phospholipids | Modulator of receptor structure and activation | (Dawaliby et al. 2016, Neale et al. 2015) | ||
| A2A adenosine receptor (A2AAR) | Cholesterol | Cholesterol interacts with receptor; cAMP modulation | (Liu et al. 2012, McGraw et al. 2022) | |
| μ-opioid receptor (OPRM1) | Cholesterol, palmitoylation | Receptor homodimerisation; receptor-Gαi2 coupling modulation | (Zheng et al. 2012) | |
| Cannabinoid receptor 1 (CB1R) | Cholesterol | Cholesterol interacts with receptor; modulates agonist binding; modulates G protein signalling via the adenylate cyclase and MAPK pathways | (Hua et al. 2017, Bari et al. 2005, Oddi et al. 2011) | |
| Cannabinoid receptor 2 (CB2R) | Cholesterol | Modulates basal activation of receptor | (Yeliseev et al. 2021) | |
| B | Glucagon-like peptide-1 receptor (GLP-1R) | Cholesterol, palmitoylation | Receptor internalisation, receptor clustering, lipid raft recruitment, modulates cAMP production | (Buenaventura et al. 2019) |
| Glucagon receptor (GCGR) | Cholesterol, PI(4,5)P2 | Cholesterol predicted to interact with receptor, modulates cAMP production; PI(4,5)P2 potentially stabilises GCGR inactivate state | (McGlone et al. 2022) | |
| Glucose-dependent insulinotropic polypeptide receptor (GIPR) | Cholesterol, palmitoylation | Lipid raft recruitment | (Buenaventura et al. 2019) | |
| Glucagon-like peptide-2 receptor | Cholesterol | Receptor internalisation | (Estall et al. 2004) | |
| C | Metabotropic glutamate receptor (mGlu1R) | Cholesterol | Cholesterol interacts with receptor; dimer formation; lipid raft recruitment | (Wu et al. 2014, Eroglu et al. 2003) |
| F | Smoothened receptor (SMO) | Cholesterol | Activation via its extracellular cysteine-rich domain | (Siebold & Rohatgi 2023, Xiao et al. 2017, Luchetti et al. 2016) |
Figure 1.
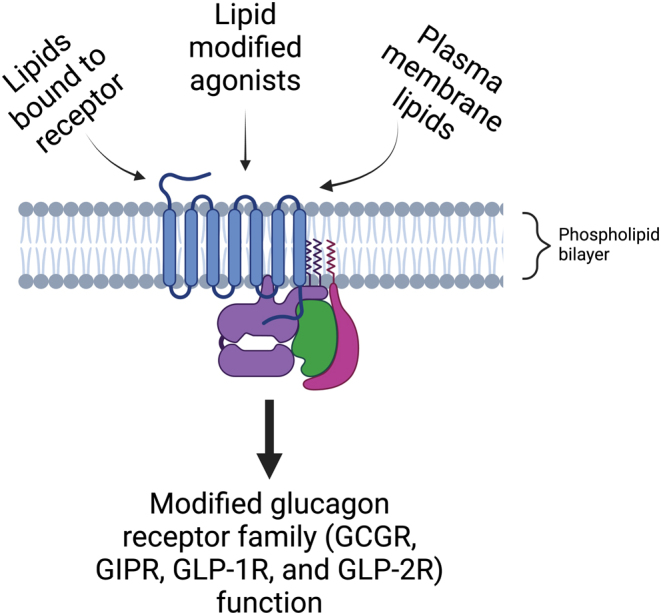
Schematic diagram summarising the potential effects of lipids on glucagon receptor family function.
Direct lipid regulation of the glucagon receptor family
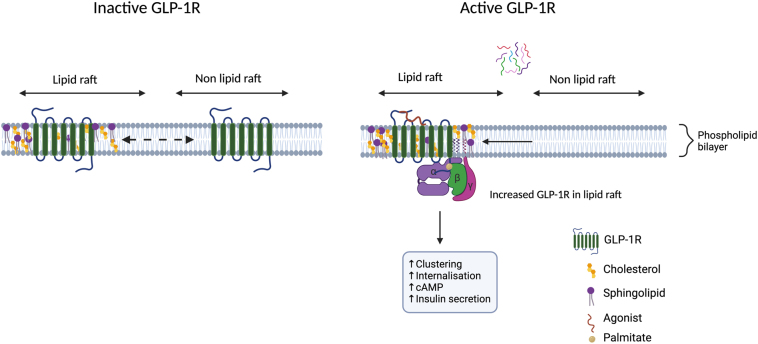
The plasma membrane is composed of a complex matrix of lipids, including glycerophospholipids (65%), sphingolipids (10%), and cholesterol (25%), and embedded proteins such as glycoproteins, ion channels, and other integral proteins like GPCRs (van Meer & de Kroon 2011, Koldsø et al. 2014). The structure and fluidity of the plasma membrane allows for the maintenance of the cellular architecture and is key in the regulation of membrane trafficking and organisation of its factors in different subdomains such as cholesterol-rich lipid rafts. Lipid rafts (also known as lipid nanodomains) are highly organised but dynamic, detergent-resistant membrane subdomains formed by the selective interaction of certain sphingolipids and cholesterol with specific membrane proteins required for signalling. These subdomains are important for the regulation of receptors like GPCRs, playing key roles in their sorting, trafficking, and signalling (Klose et al. 2013, Koldsø et al. 2014, Buenaventura et al. 2019, Kwiatkowska et al. 2020), including for the glucagon receptor family. Studies in pancreatic beta cells have shown that the GLP-1R relocates to detergent-resistant membrane fractions after stimulation with GLP-1 and the peptide agonist exendin-4 (Fig. 2). This was further confirmed via time-resolved (TR)-Förster resonance energy transfer (FRET), using Lumi4-TB labelled SNAP-tagged GLP-1R as the donor and the solvatochromic probe NR12S, which emits a blue-shifted wavelength when in a liquid-ordered membrane environment, as the acceptor in response to exendin-4 stimulation. This segregation was accompanied with increased GLP-1R clustering, measured using electron microscopy and total internal reflection fluorescence photoactivatable localisation microscopy (PALM) experiments. Disruption of the plasma membrane architecture using methyl-β-cyclodextrin (MβCD), which sequesters cholesterol, caused a dose-dependent reduction in receptor binding affinity to a fluorescent exendin-4 derivative due to faster agonist dissociation, leading to reduced cAMP signalling and inhibition of GLP-1R internalisation. Of note, given that GLP-1R signals mainly through Gαs, this G protein subunit was shown to preferentially partition into detergent-resistant membrane fractions, highlighting the importance of lipid domain organisation for the regulation of GLP-1R signalling (Buenaventura et al. 2019).
Figure 2.
Schematic of GLP-1R recruitment to lipid rafts and relevance for the control of its signalling.
The underlying mechanism of GLP-1R recruitment to lipid nanodomains has not been fully elucidated, but it was shown to involve specific receptor post-translational modifications (PTMs), which are covalent additions of a modifying group with important effects for receptor structure, localisation, and function. Amongst the different forms of GPCR PTMs, palmitoylation, also known as S-acylation, is a lipid PTM which involves the addition of a palmitate moiety by covalent linkage to cysteine residues, important for the localisation of transmembrane proteins to lipid rafts. GPCRs are usually palmitoylated in C-terminal tail cysteine residues (Levental et al. 2010, Baccouch et al. 2022). An increase in GLP-1R palmitoylation was detected after stimulation with the endogenous agonist GLP-1 as well as with exendin-4. GLP-1R contains three cysteine C-terminal residues (C438, C458, and C462), which could potentially be involved in GLP-1R palmitoylation, with the cysteine in position 438 previously shown to undergo palmitoylation (Vázquez et al. 2005). Consistently, a C438A point mutation in GLP-1R disrupted its palmitoylation without affecting receptor surface expression levels or binding affinity to fluorescently labelled exendin-4 when compared with the wild-type receptor. The palmitoylation mutant was also associated with delayed exendin-4-mediated receptor clustering, reduced recruitment to detergent-resistant membrane fractions, reduced internalisation, and reduced cAMP response and insulin secretion (Vázquez et al. 2005, Buenaventura et al. 2019). These findings highlight the importance of lipid PTMs on the control of GLP-1R association with specific lipid nanodomains to regulate its function, including glucose homeostasis.
Like GLP-1R, GIPR has also been found to localise to flotillin-positive, detergent-resistant membrane fractions in pancreatic beta cells, indicating the likely presence of the receptor in lipid rafts. However, unlike the GLP-1R, the GIPR seems to be constitutively present in rafts, and increased agonist-induced translocation to lipid nanodomains was not detected. Furthermore, GIPR was found to be constitutively palmitoylated in contrast to GLP-1R, which showed increased palmitoylation upon agonist stimulation. These findings appear to be aligned with previous results indicating that GIPR is more active at basal states compared to GLP-1R (Al-Sabah et al. 2014). There is currently limited information about the interaction of GIPR with plasma membrane and other lipids with regard to its regulation. However, given that GIPR basal activity mirrors its membrane lipid nanodomain localisation and palmitoylation status, suggesting the likely relevant functional importance of these interactions, it will be important to further investigate the regulation of GIPR by cellular lipids in the future (Al-Sabah et al. 2014, Buenaventura et al. 2019, Manchanda et al. 2023).
With regard to the effects of lipids on the regulation of GCGR function, it is worth noting that resistance to glucagon has been reported in patients with metabolic-associated steatotic liver disease (MASLD) (Suppli et al. 2020), although the mechanism underlying this effect is not completely understood. The lipid composition of the cellular membranes of hepatocytes is altered in patients with hepatic steatosis (Gorden et al. 2011); indeed, the degree of hepatic cholesterol accumulation correlates with the severity of MASLD (Puri et al. 2007). One possible explanation is that certain membrane lipids might act as allosteric modulators of the GCGR, modifying receptor outputs following their accumulation. Downstream signalling of this receptor is known to be negatively allosterically modulated by the accessory protein receptor-activity modifying protein 2 (RAMP2) (McGlone et al. 2021, Krishna Kumar et al. 2023), but evidence of a direct allosteric effect of specific lipids on the GCGR is yet to be demonstrated.
A recent combined mass spectrometry and molecular dynamics (MD) simulation study has revealed that GCGR binds with high affinity to lipids with a phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) headgroup (Kjolbye et al. 2022). The PI(4,5)P2 tail composition determines its binding affinity, which is increased for stearic and arachidonic fatty acid tails. Interestingly, the binding site of the high-affinity saturated 16:0/18:1 PI(4,5)P2, between TM6 and TM7 of GCGR, is very similar to that of the negative allosteric modulator NNC0640 (PDB 5XEZ) (Zhang et al. 2017). This could indicate that 16:0/18:1 PI(4,5)P2 can stabilise the inactive conformation of GCGR (Kjolbye et al. 2022). GCGR also binds to several other phospholipids in MD simulations, including phosphatidylglycerols (PGs; (Kjolbye et al. 2022)). As PGs are present in human and murine hepatocytes, and the relative distribution of PG species changes in disease states of steatosis and cirrhosis (Gorden et al. 2011), this interaction, if validated, could be important to determine the functional state of the GCGR under these conditions. It remains to be determined if such interactions with PI(4,5)P2 are also relevant for other members of the glucagon receptor family.
Cholesterol affects deformability and curvature of the lipid membrane, thereby modulating the agonist binding and activity of GPCRs (Harayama & Riezman 2018), and can also directly allosterically modulate certain GPCRs (van Aalst & Wylie 2021). Manipulating cholesterol levels in hepatocytes affects GCGR sensitivity – increasing cholesterol decreases cAMP production, and vice versa (McGlone et al. 2022). This relationship holds true in mouse models where liver cholesterol is manipulated using cholesterol and statin diets (McGlone et al. 2022). Whether this is due to a direct negative allosteric effect of cholesterol binding on GCGR signalling is yet to be established. Although the GCGR contains cholesterol-recognition amino-acid consensus (CRAC) and inverted CRAC (or CARC) motifs, which have been proposed to take part in cholesterol interactions, the presence of such motifs is neither sensitive nor specific to determine receptor cholesterol-binding sites (Sejdiu & Tieleman 2020). Recent MD simulations using the protein-lipid analysis toolkit, PyLipID, have however predicted several likely GCGR cholesterol-binding sites (McGlone et al. 2022). Two of these are related to the G protein binding site, which involves TM6. Experimental cholesterol loading reduced glucagon-stimulated recruitment of mini-Gs, a conformational biosensor for Gαs favouring active GPCR conformations, indicating that cholesterol might act as a negative allosteric modulator for the GCGR (McGlone et al. 2022). Further investigations using mutagenesis techniques are warranted to explore this possibility. Additionally, similar MD simulations could be applied to the rest of the glucagon receptor family to identify and screen potential cholesterol binding sites for effects in receptor function and suitability for allosteric modulation.
Finally, Estall et al. showed that, similarly to GLP-1R and GIPR, sequestering of cholesterol using MβCD or filipin significantly decreased GLP-2R internalisation after stimulation with GLP-2. However, unlike the GLP-1R, the disruption in internalisation due to cholesterol sequestering did not affect GLP-2R-dependent cAMP accumulation. GLP-2R was also shown to localise to plasma membrane lipid raft domains in vehicle conditions, a localisation which was increased after stimulation with GLP-2 (Estall et al. 2004). This further highlights the importance of lipids on the regulation of the function of all members of the glucagon receptor family. However, how these lipids, especially cholesterol, regulate GLP-2R function still needs to be thoroughly investigated.
Indirect lipid regulation of the glucagon family of receptors
Aside from the effects that specific membrane lipids have on the glucagon receptor family, lipid modifications of receptor agonists themselves, and the resulting effect that this might have on the nature of the agonist-membrane interactions, can also play an important role in modulating receptor binding kinetics and, in turn, receptor function. Incorporation of fatty acid-like moieties is a highly successful strategy that prolongs the circulatory half-lives of therapeutic peptides by allowing reversible binding to albumin, thereby preventing renal filtration via size exclusion (Knudsen & Lau 2019). Injudicious placement of the lipid modification directly affects (usually detrimentally) productive interactions between the ligand and the receptor, and there is also emerging evidence that the lipid can engage in primary interactions with the plasma membrane which, in turn, influences subsequent binding events with target receptors in the immediate vicinity. Table 2 lists the different lipid modified peptide agonist targeting the glucagon family of receptors that are currently available or being investigated.
Table 2.
Lipid modified peptide agonists targeting the glucagon family of receptors.
| Agonist | Receptor Target | Lipid Modification | References |
|---|---|---|---|
| Liraglutide | GLP-1R | C16 fatty diacid chain | (Zhao et al. 2022) |
| Semaglutide | GLP-1R | C18 diacid linked with a γGlu-2xOEG linker | (Lau et al. 2015) |
| Peptide 19 | GLP-1R and GIPR | C18 Acetyl moiety | (Zhao et al. 2022) |
| Tirzepatide | GLP-1R and GIPR | C20 fatty diacid moiety linked with γ-Glu-2xAdo | (Coskun et al. 2018, Chavda et al. 2022) |
| MEDI0382 | GLP-1R and GCGR | C16 palmitic acid with a γ-carboxylate spacer | (Li et al. 2023) |
| SAR425899 | GLP-1R and GCGR | C16 palmitic acid with a γ-carboxylate spacer | (Li et al. 2023) |
Lixisenatide, an analogue of exendin-4 with six lysine residues attached to its C-terminal tail, has a faster association rate constant compared to other receptor ligands, including exendin-4 (Zhao et al. 2022). This was proposed to be linked to the basic nature of the poly-lysine residues, which tend to bind to the plasma membrane, alluding to the role of lipids within the plasma membrane to enable this effect. Other agonists which display long residence times and varied GLP-1R outputs due to the effect of their lipid modifications include liraglutide, an analogue of GLP-1 with a C16 fatty diacid chain, and peptide-19, a dual GLP-1R and GIPR agonist with high potency at both receptors (Johnson et al. 2021), with these lipid modifications contributing to and modifying their binding properties compared to GLP-1 (Zhao et al. 2022). As long residence times are often due to slow agonist dissociation rates, we can speculate that the interaction between the lipid moiety and the cell membrane might preserve a heightened local concentration of agonist close to the membrane-embedded receptors which might contribute to explain these effects.
Other lipid-modified GLP-1R agonists with varying effects on receptor binding kinetics and function include semaglutide, a GLP-1 analogue similar to liraglutide but with two amino acid substitutions (Aib8, Arg34), which is derivatised at lysine 26 with a C18 diacid with a γGlu-2xOEG linker, as well as the dual GLP-1R/GIPR agonist LY3298176, also known as tirzepatide, which also has a fatty acid modification and varied effects on GLP-1R function (Coskun et al. 2018, Zhao et al. 2022). The lipid modifications in semaglutide have been proposed to cause increased agonist-membrane interactions, leading to increased receptor signalling (Lau et al. 2015, Zhao et al. 2022). These agonists are both clinically relevant and currently FDA-approved for type 2 diabetes, with semaglutide further approved for weight loss therapy (Lau et al. 2015, Coskun et al. 2018, Knudsen & Lau 2019, Frías et al. 2021).
Further investigations of acylated analogues of exendin-4, as well as with differentially biased GLP-1R agonists exendin-F1 and exendin-D3, have highlighted the importance of agonist lipid modifications on GLP-1R activity. Acylation of these analogues included the addition of C16 diacid at the C-terminal end through a GK linker. The exendin-4-C16 analogue caused an increase in plasma membrane interactions, accompanied with reduced GLP-1R internalisation, bias towards G protein recruitment and increased insulin secretion. The exendin-F1-C16 analogue had high affinity for the receptor compared to its non-acylated counterpart, which was accompanied by reduced internalisation and cAMP potency compared with exendin-D3-C16. Overall, lipid modifications of GLP-1R agonists appear to contribute to different agonist–receptor–plasma membrane interactions and favour specific receptor conformations, thereby contributing to modified or biased signalling (Lucey et al. 2020, 2021).
There are other lipid-modified agonists which target the GLP-1R, however these also target the GCGR, making them GLP-1R/GCGR dual agonists. Li et al. recently highlighted the importance of lipid modifications on the function of two dual peptide agonists, MEDI0382 and SAR425899, on GLP-1R and GCGR function. MEDI0382 and SAR425899 are both palmitoylated with a 16-carbon palmitic acid with a γ-carboxylate spacer at lysine 10 and 14, respectively. Lipidation of these dual agonists stabilised the binding of the peptide through increased interactions with the receptors and the plasma membrane. Non-lipidated versions of each peptide caused a significant decrease in cAMP accumulation for GLP-1R and essentially no cAMP accumulation for GCGR, highlighting the importance of these lipid modifications on altering both GLP-1R and GCGR signalling (Li et al. 2023). There is comparatively less information on the effects on lipid modifications of GCGR agonists on GCGR function alone.
A study investigating the effects of acylation of GLP-2 also highlights the importance of agonist lipid modification on GLP-2R function. Trier et al. showed that increased acyl chain length on GLP-2 caused enhanced partitioning into POPC simplified lipid membranes. This increase in membrane interaction was also established within a complex cell plasma membrane environment. The difference in acyl chain lengths also caused changes in translocation across intestinal cells when compared to non-lipid modified GLP-2 (Trier et al. 2014). Gadgaard et al. on the other hand, investigated the best position/residue for lipidation and maintained the same length of the acyl chain. GLP-2R internalisation, cAMP accumulation, and β-arrestin 1 and 2 recruitment were significantly reduced when agonists were lipidated at the N or C terminus, while changes in the middle of the agonist did not significantly affect receptor function (Gadgaard et al. 2023). These results highlight the importance of not only the type of lipid modification but also the location of this change on downstream receptor function.
It is worth noting, however, that certain lipid modifications of glucagon receptor family agonists could potentially cause biased receptor reactions or agonist-membrane interaction effects that might not always be as favourable as for some of the modifications mentioned previously.
There is presently far less information available about how lipid modification of GIPR agonists alters GIPR function or interactions with membrane lipids, probably as a result of the relatively recent interest in GIPR as a therapeutic target in type 2 diabetes and obesity compared to the GLP-1R. Currently, the focus has been on conferring an increased half-life to agonists in order to assess chronic GIPR agonism (Lafferty et al. 2023). The introduction of 2-aminoisobutyric acid and fatty acylation to GIPR agonists confers DPP4 resistance and enhanced albumin binding (Mroz et al. 2019), however whether these lipid modifications lead to altered GIPR signalling is currently unknown.
Another indirect lipid-induced regulation of GLP-1R involves the use of endocannabinoid-like lipids (Cheng et al. 2015). These lipids, which include oleoylethanolamide (OEA) and 2-oleoylglycerol (2-OG), are known to regulate food intake, potentially via increased GLP-1 release, and are structurally similar to endocannabinoids but with saturated or monounsaturated instead of polyunsaturated fatty acids, and without activating cannabinoid receptors. These are circulating lipids present in varying amounts in different tissues, with their levels being modified on demand via dietary changes in their membrane phospholipid precursors (Cheng et al. 2015, Rahman et al. 2021). Cheng et al. showed that both OEA and 2-OG can bind to GLP-1 without disrupting GLP-1 binding to GLP-1R, in a dose dependent manner, increasing the potency of GLP-1R-mediated cAMP production in the rat insulinoma RINm5F cell line. cAMP responses were not observed with the fatty acids alone or using the GLP-1R antagonist exendin-9, indicating their specificity in targeting the GLP-1R. As these lipids are differentially expressed within different tissues, they have the potential for spatial regulation of GLP-1R responses. This study also highlights their relevance as potential type 2 diabetes therapies (Cheng et al. 2015).
In normal conditions, GLP-1R activation by GLP-1 enhances glucose stimulated insulin secretion (GSIS) by potentiating the closing of ATP-sensitive potassium channels, leading to membrane depolarisation, which in turn causes an increase in intracellular calcium through the opening of voltage-gated Ca2+ channels and mobilisation of intracellular Ca2+ stores required for the exocytosis of insulin secretory vesicles (Manchanda et al. 2021). An earlier study highlighted the localisation of certain channels to plasma membrane lipid rafts as indirectly affecting GLP-1R function. Specifically, GLP-1 potentiation of GSIS was affected by the raft localisation of the L-type Ca2+ channels Cav1.2 and Cav1.3, with its disruption resulting in a significant reduction in GLP-1-mediated cAMP accumulation and GSIS potentiation (Jacobo et al. 2009). This observation highlights the importance that these lipid nanodomains have as organisers of membrane-protein microarchitecture for effective signal transmission.
Other than indirect effects of lipids via lipid modified agonists, changes in dietary lipids, specifically free fatty acids (short and/or long) and 2-monoacyl glycerol (2-MAG), can also modify GLP-1R function indirectly by regulating GLP-1 secretion. Free fatty acid-induced changes in cytosolic Ca2+ concentration have been shown to regulate GLP-1 secretion (Hirasawa et al. 2005, Abdalqadir & Adeli 2022). Activation of the GPCR GPR40 by long-chain fatty acids and GPR119 by 2-MAG have also been identified as involved in regulating GLP-1 secretion (Müller et al. 2019, Drucker & Holst 2023). This, in turn, indirectly regulates GLP-1R activation and function. GIP secretion has also been proposed to be regulated by the activation of long-chain fatty acid receptors GPR120 and GPR40, thereby also indirectly regulating GIPR function (Sankoda et al. 2019). Short chain fatty acids have also been documented to stimulate both GLP-1 and (Abdalqadir & Adeli 2022) and GLP-2 (Tappenden et al. 2003) secretion. Varying effects of dietary lipids on glucagon secretion and in turn GCGR function have also previously been outlined by Galsgaard et al. (Galsgaard et al. 2019).
Techniques to study lipid–receptor interactions
Characterising lipid–protein interactions in vitro is challenging, due to their dynamic nature and the need to use membrane mimetic environments. Structure-based approaches are one avenue for exploring specific GPCR–lipid interactions, as lipids and lipid-like species can be observed in X-ray crystallography and cryo-EM maps of GPCRs. Indeed, the progress made in recent years in our understanding of GPCR–lipid interactions can be partially attributed to the rise in GPCR structures solved by cryo-EM, allowing for co-purification with complex lipid compositions. Lipid-bound structures are now available for several class A and class C GPCRs (Duncan et al. 2020), however despite cryo-EM structures being solved for each of the 15 class B1 GPCRs since 2017 (Wootten & Miller 2020), only two of these have resolved lipid species. Six cholesterol molecules were modelled in the cryo-EM structures of PTH1R, solved by cryo-EM in detergent micelle (Zhao et al. 2019). Four cholesterol molecules were modelled into the CRF1R and CRF2R maps (Ma et al. 2020), which were also solved in detergent micelles. The cholesterol binding sites were found to overlap across these structures, with the authors of the CRF1R/CRF2R paper highlighting two conserved and well-resolved sites in TM4 helix and the ECD as possibly functionally important.
MD simulations have emerged as a powerful tool in the study of membrane proteins, as they allow the investigation of specific protein–lipid interactions at atomic or near-atomic resolution in model membranes (Corradi et al. 2018). Advances in software and hardware now allow the study of receptors in membranes of increasing complexity, towards ever-more realistic membrane models with asymmetric leaflet distributions (Marrink et al. 2019, Souza et al. 2021). Different levels of resolution have been used to study GPCR–lipid interactions, with much of the work centred around atomistic (atMD) and coarse-grained (cgMD) level resolution, which currently allows simulation around the microsecond range for nanometre membrane patches (Duncan et al. 2020). Using the cgMD Martini force field allows for longer timescales and system sizes compared to atMD, making it possible to measure statistically meaningful lipid binding affinities (Marrink et al. 2023). A key consideration in using these methods for GPCR–lipid interactions is that the Martini model has fixed secondary structure, and typically conformational changes are limited, whereas atMD simulations are limited by the shorter attainable simulation times (Borges-Araújo et al. 2023). Employing a multiscale approach combining cgMD and atMD analyses allows a more complete study of the GPCR conformational landscape (Hedger et al. 2019, Ansell et al. 2020).
High-throughput MD approaches have provided valuable insights into lipid interactions across GPCR families, with the open database ProLint containing representative lipid analyses from 20+ GPCRs (Sejdiu & Tieleman 2020). Intriguingly, the lipid sites observed in the cryo-EM structures of PTH1R (Zhao et al. 2019) and CRF1R/CRF2R (Ma et al. 2020) were not observed to bind cholesterol in these simulations. This may be due to the use of micelles during structure determination, or because the simulations are of a single state and do not include the ECD. Further work to incorporate the ECD and missing loops will be valuable for future studies, particularly as previous work has proposed that the ECD of GCGR is regulated by GM3, an area which should be further explored (Ansell et al. 2020), including for other receptors of the glucagon family. The advent of AlphaFold2 also allows more exploration of GPCR conformational spaces with models available for over 420 GPCRs in both active and inactive states on GPCRdb as of 2023 (Pándy-Szekeres et al. 2023). This opens up the possibility of extending the high-throughput approaches to find patterns in lipid interactions, including those involving multiple lipid species, hence using more complete models (Xu et al. 2021).
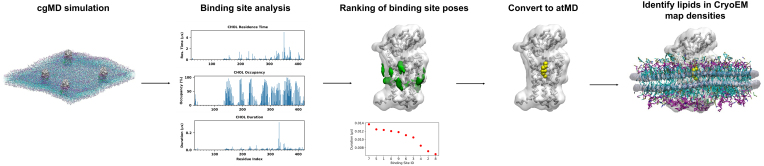
A particularly powerful approach is to combine MD simulations with cryo-EM to correctly identify lipid-like cryo-EM map densities, by following a pipeline such as the one in Fig. 3, in which cgMD simulations are used to identify state-dependent lipid binding sites. This can be done using tools such as PyLipID, which finds bound poses for the identified lipid binding sites, and calculates residence times, average duration, and number of surrounding lipids of the individual protein residues (Song et al. 2022). The final ranked binding sites can be subjected to atMD simulations to compare with densities obtained from cryo-EM. This has been exemplified by the LipIDens pipeline, which allowed an informed assignment of the correct lipid species into the map density, enabling the identification of cholesterol during refinement of the membrane protein hedgehog acyltransferase (HHAT) (Ansell et al. 2022).
Figure 3.
Example of a structure-based workflow to identify specific lipid binding sites of receptors.
Simulations of the receptor of interest can be carried out in model membranes of given complexity. Coarse-grained simulations may be used for a state-dependent analysis. Specific lipid interaction metrics can be quantified using tools such as PyLipID or Volmap (Cohen et al. 2006) to calculate average lipid occupancy around the protein. Lipid binding sites and poses may then be ranked according to these metrics. Top poses which overlap with cryo-EM map densities may be converted to atomistic resolution for further sampling of the ligand binding site. These simulations may then be used as the basis for assigning lipid densities in cryo-EM maps of the receptor of interest for further refinement, as exemplified by the LipIDens pipeline.
We anticipate that advances in cryo-EM and MD simulation approaches will continue to expand our understanding of the lipid regulation of GPCRs. This will be of particular value in the class B1 GPCR field, where a focus area should be to determine class B1 GPCR structures solved in lipid-containing environments such as nanodiscs, which provide more realistic membrane environments than detergent micelles, and allow further insights into possible lipid regulation of the ECDs (Denisov & Sligar 2016), a technique that has so far not been employed to resolve any structure for this GPCR class.
Other techniques having increasing impact on our understanding of lipid regulation of membrane proteins are NMR and mass spectrometry (MS), including native MS (nMS), HDX-MS, and lipidomics approaches. MD simulations have proven to be a highly complementary technique when used in concert with nMS to identify lipid species and binding sites. As discussed earlier, in recent work on the GCGR, cgMD simulations using different membrane compositions were used to screen for lipids with high binding affinity for the receptor. PI(4,5)P2 was identified as a strong binder, and nMS was then used to confirm this (Kjølbye et al. 2022). nMS and MD together were used to investigate the effect of tail length and saturation on binding. Recent developments in the nMS field have shown that membrane proteins can be studied directly from the membrane with no need for detergent manipulation, paving the way towards in situ structural biology (Chorev et al. 2018). We note however that cholesterol has not been readily observed in nMS of membrane proteins (Kostelic et al. 2019): in this instance, alternative NMR approaches might be useful as they have shown promise in understanding cholesterol binding, with solid-state NMR recently used to characterise binding of cholesterol to the influenza M2 channel (Elkins et al. 2017).
Click chemistry is another method of directly studying lipid–protein interactions. There are different forms of click chemistry, with one of the most common being the copper(I)-catalysed alkyne–azide cycloaddition method. For the case of lipid–protein interactions, the use of a ‘photoactivated’ click cholesterol has been established; here the structure of cholesterol was modified to include a diazirine crosslinker activated by ultraviolet light irradiation, which then binds to the surrounding molecules, including proteins. The photoactivated cholesterol also includes an alkyne group which allows for its tagging using a fluorescent azide group via copper-catalysed alkyne–azide cycloaddition (Hulce et al. 2013). This technique can be combined with pulldown of a protein of interest (including tagged receptors) using affinity beads followed by SDS PAGE and western blotting or MS to determine the level of interaction with the labelled cholesterol under different conditions and agonist stimulations. A positive of this method, when optimised, is that it is less disruptive of the ‘natural’ order of the plasma membrane as the alkyne or azide groups are small and the interactions are established in its natural environment (Liang & Astruc 2011, Hulce et al. 2013, Hofmann et al. 2014, Chauhan et al. 2020). A current limitation of this type of click chemistry is that this does not allow for the live imaging of lipid–protein interactions as cells need to be lysed prior to analysis.
Another means of investigating lipid–receptor interactions is the use of fluorescently tagged lipids or lipid-binding proteins, allowing cross-correlation or co-localisation analysis of fluorescently labelled receptors and lipids. A recent development is the use of a bioorthogonal-based cholesterol probe combined with stimulated emission depletion (STED) super-resolution microscopy in living cells. Here the cholesterol is modified to introduce an azide group in position 24. Copper-free click chemistry is then used to fluorescently label the cholesterol using dibenzocyclooctyne (DBCO) which contains a linker between the dye and the reacting group (Lorizate et al. 2021). Another fluorescence-based method is the use of purified recombinant probes based on toxins including the domain D4 of Perfringolysin O (D4H*), Ostreolysin A (OlyA), or Anthrolysin O (ALO) fused to a fluorescent protein, which bind to cholesterol-rich membrane regions and serve as cholesterol biosensors for lipid–protein interactions (Skočaj et al. 2014, Lim et al. 2019, Johnson & Radhakrishnan 2021). However, any tags used in this type of experiments should be validated to have minimal effects on the functionality of the lipids and/or the receptor, and ideally experiments should be carried out in functionally relevant cells. As previously mentioned, the use of solvatochromic and/or environmentally sensitive fluorophores like NR12S or laurdan, which can change emission depending on their location in lipid ordered vsdisordered membrane environments, can be combined with fluorescently tagged receptors to resolve the cross-correlation behaviour of the receptor with the dye to investigate lipid–protein interactions using high resolution microscopy techniques like STED (Saxena et al. 2015, Nicovich et al. 2018, Schneider et al. 2018).
Physiological and pathophysiological implications of lipids on the function of the glucagon receptor family
The incretin effect is greatly affected in patients with type 2 diabetes, with the insulinotropic influence of the GIP hormone being diminished in pancreatic beta cells and the effect of GLP-1 reduced. As previously mentioned, agonists of the GLP-1R which avoid breakdown by DPP4 have been developed as means of treatment for type 2 diabetes and obesity to restore this effect (Nauck et al. 2004, Graaf et al. 2016, Chia & Egan 2020). The glucagon family of receptors has been targeted for the treatment of various diseases involving abnormal lipid profiles, lipid content, or lipid metabolism (Mulvihill 2018, Seghieri et al. 2018, Galsgaard et al. 2019, Ammann et al. 2023, Nauck & Müller 2023). However, the physiological or pathophysiological implications of lipids themselves on the function of this family of receptors in their respective tissues still needs to be investigated. Although mostly speculative, some indirect physiological and pathophysiological implications on these receptors are outlined below.
Dyslipidaemia, usually characterised by increased levels of plasma triacylglycerols and cholesteryl esters (Goldberg 2001, Rhee et al. 2011, Klop et al. 2013, Athyros et al. 2018, Eid et al. 2019), is a common risk factor in patients with type 2 diabetes and obesity. Rhee et al. investigated the difference in LC/MS-based lipid profiles of a mixed cohort consisting of control (no diabetes) and cases (developed type 2 diabetes) and highlighted different triacylglycerol profiles between the two groups. Patients with increased risk of type 2 diabetes had more triacylglycerols of lower carbon number and double bond content compared with controls. The nature of these triacylglycerols, i.e. saturated and monosaturated fatty acids, was shown to be different up to 12 years before overtly developing the disease (Rhee et al. 2011). This indicates the potential of changes in triacylglycerol composition to impact GLP-1R and GIPR activities, thereby increasing the onset of type 2 diabetes directly or indirectly within this patient group. How the specific triacylglycerols identified in this study could potentially modify GLP-1R and GIPR function needs further investigation.
Hyperglucagonaemia is characterised by excess plasma concentration of glucagon and has been observed in individuals with MASLD (Albrechtsen et al. 2018, Grandt et al. 2023). MASLD is characterised by abnormal lipid accumulation in hepatocytes due to a combination of factors including lipotoxicity, diet, sedentary lifestyle, and genetics, amongst others (Zarghamravanbakhsh et al. 2021). It is likely that patients with MASLD have resistance to the actions of glucagon, resulting in increased circulating amino acids, which stimulate the pancreas to secrete further glucagon (Suppli et al. 2016, Wewer Albrechtsen et al. 2018). Since one of the actions of glucagon is to decrease liver fat accumulation, hepatic resistance to glucagon could contribute to the pathophysiology of MASLD. Alternatively, or additionally, lipid accumulation in hepatocytes (MASLD) could reduce their ability to respond to glucagon. Further investigation is required to properly understand the potential effects of increased liver fat content on hepatic GCGR function. A similar pathophysiology has also been identified involving increased fat accumulation in the pancreas, known as non-alcoholic fatty pancreas disease (NAFPD). NAFPD has received a lot of interest recently as it is associated with loss of beta cell function (Silva et al. 2021, Zhang et al. 2021). As the pancreas is one of the main locations for GLP-1R and GIPR function, we can speculate that this could have detrimental effects on the function of these receptors, with the potential effects that these lipid changes might have on receptor outputs warranting further examination.
Certain lipids and lipid derivatives have previously been implicated in disrupting physiological responses of incretin receptors. Specifically, increased plasma non-esterified fatty acids (NEFA) have been proposed to cause impaired beta cell function in type 2 diabetes. Kang et al., investigating the role of NEFA on disrupting incretin responses, showed that exposure of pancreatic beta cells and mouse islets to palmitate caused a reduction in the expression of Glp1r mRNA and GLP-1R protein levels without affecting Gipr levels (Kang et al. 2013). They also showed that islets isolated from diabetic mice, when compared with those from control mice, had reduced Glp1r and Gipr expression, and that this expression was partially restored after treatment with the lipid lowering agent bezafibrate. Treatment of INS-1E cells or mouse islets with palmitate also caused a reduction in both GLP-1R and GIPR potentiation of GSIS. This pattern was different for cAMP production and CREB phosphorylation in rat INS-1E vsmouse MIN6 cells, with palmitate causing a decrease in the former for both GLP-1R and GIPR but only for GLP-1R in MIN6 cells. This highlights the importance of increased NEFA on impaired beta cell function, which might be species-specific (Kang et al. 2013). A human study carried out by Astiarraga et al. also showed that acute increases in plasma NEFA levels via lipid infusion of healthy non-diabetic volunteers caused a decrease in incretin-induced potentiation of insulin secretion (Astiarraga et al. 2018, Chueire & Muscelli 2021). Tanabe et al. also noted that increased triglyceride levels impaired liraglutide efficacy in type 2 diabetes patients, potentially through a reduction in Glp1r expression (Tanabe et al. 2016), suggesting that the effects can be translated to human physiology.
Conclusion and prospective future work
Current literature highlights the importance of lipids on the function of GPCRs, with cholesterol playing a direct role as a potential allosteric modulator as well as an indirect effect related to the segregation of these receptors to cholesterol-rich nanodomains in the plasma membrane. While most of the work done regarding lipids and the glucagon receptor family has revolved around incretin receptor regulation of lipid metabolism (Yaribeygi et al. 2021), there is currently sparse information about the regulation of GLP-1R and GLP-2R by lipids, and little to no information about the regulation of GIPR, either directly or indirectly due to lipid modification of GIPR agonists. While an initial prediction of cholesterol binding sites has been performed for the GCGR, there is currently no corresponding information for the GLP-1R, GLP-2R, or GIPR. Given the importance of lipid interactions for the regulation of GPCR function (Baccouch et al. 2022), mapping cholesterol and/or other lipid binding sites is fundamental to increase our current understanding of the biology of this key family of metabolically relevant receptors. Identification of relevant and specific lipid interaction sites should be attempted using multidisciplinary structural and computational biology approaches and taking into consideration active vs inactive receptor states.
There is still a lot of research that needs to be done to fully understand the effect that lipids have on regulating the specific functions of the glucagon family of receptors besides identification of cholesterol, sphingolipid, and/or other lipid-binding sites. Important areas that need to be addressed include (i) extensive functional characterisation of lipid binding in physiologically relevant systems and determination of the effects of altering interacting lipid levels on receptor function in relevant tissues; (ii) harnessing the information generated on the effects of lipid–protein interactions as potential targets for drug development for the treatment of type 2 diabetes, obesity, and/or fatty liver disease; and (iii) human studies to investigate the effect of changes in lipid levels, either through diet or pharmacologically, on receptor function, and potential effects on patient responses to agonists targeting this family of receptors.
There might be the possibility of exploiting interactions between lipids and the glucagon family of receptors to develop small allosteric modulators that target and mimic the effect of identified lipid-binding sites, allowing for the pharmacological manipulation of receptors to either increase or reduce their lipid association to modulate receptor activity. In this context, genetic variants of these receptors (Michałowska et al. 2022, van der Velden et al. 2022, Gao et al. 2023, Lagou et al. 2023) are already known to trigger specific signalling responses; it will be interesting to determine the effects that these variants might have on receptor–lipid interactions, potentially allowing the development of personalised medicine approaches which take into consideration specific lipid associations when devising the most beneficial treatment regime. Another aspect to consider is the potential impact that lipid-modifying drugs might have on patients who are concomitantly prescribed an agonist that target the glucagon family of receptors.
This is a promising area of research not only for the development of novel treatments for metabolic diseases but also for the better fundamental understanding of lipid regulation of class B1 GPCR behaviours.
Declaration of interest
We declare no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
This work was supported by grants from the MRC (MR/X021467/1, MR/R010676/1, MR/M012646), Diabetes UK (19/0006094), an EFSD/Boehringer Ingelheim European Research Programme, a Lilly Research Award Program (LRAP), a Commonwealth PhD Scholarship to AO, and funding from the NIHR and the Academy of Medical Sciences. AT, SR, BJ, and ERM have recently received a Wellcome Trust Discovery Award (301619/Z/23/Z) to resolve all actionable cholesterol binding sites in the GLP-1R and the GCGR.
References
- Abdalqadir N & Adeli K. 2022GLP-1 and GLP-2 orchestrate intestine integrity, gut microbiota, and immune system crosstalk. Microorganisms 102061. ( 10.3390/microorganisms10102061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrechtsen NJW Junker AE Christensen M Hædersdal S Wibrand F Lund AM Galsgaard KD Holst JJ Knop FK & Vilsbøll T. 2018Hyperglucagonemia correlates with plasma levels of non-branched-chain amino acids in patients with liver disease independent of type 2 diabetes. American Journal of Physiology-Gastrointestinal and Liver Physiology 314G91–G96. ( 10.1152/ajpgi.00216.2017) [DOI] [PubMed] [Google Scholar]
- Al-Sabah S Al-Fulaij M Shaaban G Ahmed HA Mann RJ Donnelly D Bünemann M & Krasel C. 2014The GIP receptor displays higher basal activity than the GLP-1 receptor but does not recruit GRK2 or arrestin3 effectively. PLoS One 9e106890. ( 10.1371/journal.pone.0106890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammann M Santol J Pereyra D Kalchbrenner T Wuerger T Laengle J Smoot RL Hulla W Laengle F & Starlinger P. 2023Glucagon-like peptide-1 and glucagon-like peptide-2 regulation during human liver regeneration. Scientific Reports 1315980. ( 10.1038/s41598-023-43283-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang T Bruce CR & Kowalski GM. 2019Postprandial aminogenic insulin and glucagon secretion can stimulate glucose flux in humans. Diabetes 68939–946. ( 10.2337/db18-1138) [DOI] [PubMed] [Google Scholar]
- Ansell TB, Song W, Coupland CE, Carrique L, Corey RA, Duncan AL, Cassidy CK, Geurts MMG, Rasmussen T, Ward AB, et al.2022LipIDens: simulation assisted interpretation of lipid densities in cryo-EM structures of membrane proteins. Nature Communications 141–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell TB Song W & Sansom MSP. 2020The glycosphingolipid GM3 modulates conformational dynamics of the glucagon receptor. Biophysical Journal 119300–313. ( 10.1016/j.bpj.2020.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astiarraga B Chueire VB Souza AL Pereira-Moreira R Monte Alegre S Natali A Tura A Mari A Ferrannini E & Muscelli E. 2018Effects of acute NEFA manipulation on incretin-induced insulin secretion in participants with and without type 2 diabetes. Diabetologia 611829–1837. ( 10.1007/s00125-018-4633-z) [DOI] [PubMed] [Google Scholar]
- Athyros VG Doumas M Imprialos KP Stavropoulos K Georgianou E Katsimardou A & Karagiannis A. 2018Diabetes and lipid metabolism. Hormones 1761–67. ( 10.1007/s42000-018-0014-8) [DOI] [PubMed] [Google Scholar]
- Baccouch R Rascol E Stoklosa K & Alves ID. 2022The role of the lipid environment in the activity of G protein coupled receptors. Biophysical Chemistry 285106794. ( 10.1016/j.bpc.2022.106794) [DOI] [PubMed] [Google Scholar]
- Bahrami J Longuet C Baggio LL Li K & Drucker DJ. 2010Glucagon-Like peptide-2 receptor modulates islet adaptation to metabolic stress in the ob/ob Mouse. Gastroenterology 139857–868. ( 10.1053/j.gastro.2010.05.006) [DOI] [PubMed] [Google Scholar]
- Bari M Paradisi A Pasquariello N & Maccarrone M. 2005Cholesterol-dependent modulation of type 1 cannabinoid receptors in nerve cells. Journal of Neuroscience Research 81275–283. ( 10.1002/jnr.20546) [DOI] [PubMed] [Google Scholar]
- Boer GA & Holst JJ. 2020Incretin hormones and type 2 diabetes—mechanistic insights and therapeutic approaches. Biology 9473. ( 10.3390/biology9120473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla JR Agasid MT Mehmood S & Robinson CV. 2019Membrane protein–lipid interactions probed using mass spectrometry. Annual Review of Biochemistry 8885–111. ( 10.1146/annurev-biochem-013118-111508) [DOI] [PubMed] [Google Scholar]
- Borges-Araújo L, Patmanidis I, Singh AP, Santos LHS, Sieradzan AK, Vanni S, Czaplewski C, Pantano S, Shinoda W, Monticelli L, et al.2023Pragmatic coarse-graining of proteins: models and applications. Journal of Chemical Theory and Computation 197112–7135. ( 10.1021/acs.jctc.3c00733) [DOI] [PubMed] [Google Scholar]
- Buenaventura T, Bitsi S, Laughlin WE, Burgoyne T, Lyu Z, Oqua AI, Norman H, McGlone ER, Klymchenko AS, Corrêa IR, et al.2019Agonist-induced membrane nanodomain clustering drives GLP-1 receptor responses in pancreatic beta cells. PLoS Biology 17e3000097. ( 10.1371/journal.pbio.3000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajulao JMB Hernandez E von Zastrow ME & Sanchez EL. 2022Glucagon receptor-mediated regulation of gluconeogenic gene transcription is endocytosis-dependent in primary hepatocytes. Molecular Biology of the Cell 33ar90. ( 10.1091/mbc.E21-09-0430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JE, Beaudry JL, Svendsen B, Baggio LL, Gordon AN, Ussher JR, Wong CK, Gribble FM, D’Alessio DA, Reimann F, et al.2022GIPR is predominantly localized to nonadipocyte cell types within white adipose tissue. Diabetes 711115–1127. ( 10.2337/db21-1166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JE & Drucker DJ. 2013Pharmacology, physiology, and mechanisms of incretin hormone action. Electronic 17819–837. ( 10.1016/j.cmet.2013.04.008) [DOI] [PubMed] [Google Scholar]
- Capozzi ME, Svendsen B, Encisco SE, Lewandowski SL, Martin MD, Lin H, Jaffe JL, Coch RW, Haldeman JM, MacDonald PE, et al.2019aβ Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 4e126742. ( 10.1172/jci.insight.126742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi ME Wait JB Koech J Gordon AN Coch RW Svendsen B Finan B D’Alessio DA & Campbell JE. 2019bGlucagon lowers glycemia when β-cells are active. JCI Insight 5e129954. ( 10.1172/jci.insight.129954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan N Sere YY Sokol AM Graumann J & Menon AK. 2020A PhotoClick cholesterol‐based quantitative proteomics screen for cytoplasmic sterol‐binding proteins in Saccharomyces cerevisiae. Yeast 3715–25. ( 10.1002/yea.3448) [DOI] [PubMed] [Google Scholar]
- Chavda VP Ajabiya J Teli D Bojarska J & Apostolopoulos V. 2022Tirzepatide, a new era of dual-targeted treatment for diabetes and obesity: a mini-review. Molecules 274315. ( 10.3390/molecules27134315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YH Ho MS Huang WT Chou YT & King K. 2015Modulation of glucagon-like peptide-1 (GLP-1) potency by endocannabinoid-like lipids represents a novel mode of regulating GLP-1 receptor signaling. Journal of Biological Chemistry 29014302–14313. ( 10.1074/jbc.M115.655662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, et al.2007High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 3181258–1265. ( 10.1126/science.1150577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia CW & Egan JM. 2020Incretins in obesity and diabetes. Annals of the New York Academy of Sciences 1461104–126. ( 10.1111/nyas.14211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorev DS, Baker LA, Wu D, Beilsten-Edmands V, Rouse SL, Zeev-Ben-Mordehai T, Jiko C, Samsudin F, Gerle C, Khalid S, et al.2018Protein assemblies ejected directly from native membranes yield complexes for mass spectrometry. Science 362829–834. ( 10.1126/science.aau0976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chueire VB & Muscelli E. 2021Effect of free fatty acids on insulin secretion, insulin sensitivity and incretin effect – a narrative review. Archives of Endocrinology and Metabolism 6524–31. ( 10.20945/2359-3997000000313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J Arkhipov A Braun R & Schulten K. 2006Imaging the migration pathways for O2, CO, NO, and Xe inside myoglobin. Biophysical Journal 911844–1857. ( 10.1529/biophysj.106.085746) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi V, Mendez-Villuendas E, Ingólfsson HI, Gu RX, Siuda I, Melo MN, Moussatova A, DeGagné LJ, Sejdiu BI, Singh G, et al.2018Lipid–protein interactions are unique fingerprints for membrane proteins. ACS Central Science 4709–717. ( 10.1021/acscentsci.8b00143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun T, Sloop KW, Loghin C, Alsina-Fernandez J, Urva S, Bokvist KB, Cui X, Briere DA, Cabrera O, Roell WC, et al.2018LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Molecular Metabolism 183–14. ( 10.1016/j.molmet.2018.09.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt W.1979The incretin concept today. Diabetologia 1675–85. ( 10.1007/BF01225454) [DOI] [PubMed] [Google Scholar]
- Damian M, Louet M, Gomes AAS, M’Kadmi C, Denoyelle S, Cantel S, Mary S, Bisch PM, Fehrentz JA, Catoire LJ, et al.2021Allosteric modulation of ghrelin receptor signaling by lipids. Nature Communications 123938. ( 10.1038/s41467-021-23756-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawaliby R Trubbia C Delporte C Masureel M Van Antwerpen P Kobilka BK & Govaerts C. 2016Allosteric regulation of G protein–coupled receptor activity by phospholipids. Nature Chemical Biology 1235–39. ( 10.1038/nchembio.1960) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JL Johansen K Ganda OP Soeldner JS Gleason RE & Midgley W. 1978Factors governing insulin and glucagon responses during normal meals. Clinical Endocrinology 9443–454. ( 10.1111/j.1365-2265.1978.tb03584.x) [DOI] [PubMed] [Google Scholar]
- Denisov IG & Sligar SG. 2016Nanodiscs for structural and functional studies of membrane proteins. Nature Structural and Molecular Biology 23481–486. ( 10.1038/nsmb.3195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D.2012The structure and function of the glucagon-like peptide-1 receptor and its ligands. British Journal of Pharmacology 16627–41. ( 10.1111/j.1476-5381.2011.01687.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ & Holst JJ. 2023The expanding incretin universe: from basic biology to clinical translation. Diabetologia 661765–1779. ( 10.1007/s00125-023-05906-7) [DOI] [PubMed] [Google Scholar]
- Drucker DJ & Yusta B. 2014Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annual Review of Physiology 76561–583. ( 10.1146/annurev-physiol-021113-170317) [DOI] [PubMed] [Google Scholar]
- Duncan AL Song W & Sansom MSP. 2020Lipid-dependent regulation of ion channels and G protein-coupled receptors: insights from structures and simulations. Annual Review of Pharmacology and Toxicology 6031–50. ( 10.1146/annurev-pharmtox-010919-023411) [DOI] [PubMed] [Google Scholar]
- Eid S Sas KM Abcouwer SF Feldman EL Gardner TW Pennathur S & Fort PE. 2019New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia 621539–1549. ( 10.1007/s00125-019-4959-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Eid L Reynolds CA Tomas A & Ben J. 2022Biased agonism and polymorphic variation at the GLP-1 receptor: implications for the development of personalised therapeutics. Pharmacological Research 184106411. ( 10.1016/j.phrs.2022.106411) [DOI] [PubMed] [Google Scholar]
- El K, Douros JD, Willard FS, Novikoff A, Sargsyan A, Perez-Tilve D, Wainscott DB, Yang B, Chen A, Wothe D, et al.2023The incretin co-agonist tirzepatide requires GIPR for hormone secretion from human islets. Nature Metabolism 5945–954. ( 10.1038/s42255-023-00811-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins MR Williams JK Gelenter MD Dai P Kwon B Sergeyev IV Pentelute BL & Hong M. 2017Cholesterol-binding site of the influenza M2 protein in lipid bilayers from solid-state NMR. PNAS 11412946–12951. ( 10.1073/pnas.1715127114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu Ç Brügger B Wieland F & Sinning I. 2003Glutamate-binding affinity of Drosophila metabotropic glutamate receptor is modulated by association with lipid rafts. PNAS 10010219–10224. ( 10.1073/pnas.1737042100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estall JL Yusta B & Drucker DJ. 2004Lipid raft-dependent glucagon-like peptide-2 receptor trafficking occurs independently of agonist-induced desensitization. Molecular Biology of the Cell 153673–3687. ( 10.1091/mbc.e03-11-0825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, Liu B, Cui X, Brown K. & SURPASS-2 Investigators 2021Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. New England Journal of Medicine 385503–515. ( 10.1056/NEJMoa2107519) [DOI] [PubMed] [Google Scholar]
- Gadgaard S, van der Velden WJC, Schiellerup SP, Hunt JE, Gabe MBN, Windeløv JA, Boer GA, Kissow H, Ørskov C, Holst JJ, et al.2022Novel agonist and antagonist radioligands for the GLP-2 receptor. Useful tools for studies of basic GLP-2 receptor pharmacology. British Journal of Pharmacology 1791998–2015. ( 10.1111/bph.15766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgaard S Windeløv JA Schiellerup SP Holst JJ Hartmann B & Rosenkilde MM. 2023Long-acting agonists of human and rodent GLP-2 receptors for studies of the physiology and pharmacological potential of the GLP-2 system. Biomedicine and Pharmacotherapy 160114383. ( 10.1016/j.biopha.2023.114383) [DOI] [PubMed] [Google Scholar]
- Galsgaard KD Pedersen J Knop FK Holst JJ & Wewer Albrechtsen NJ. 2019Glucagon receptor signaling and lipid metabolism. Frontiers in Physiology 10413. ( 10.3389/fphys.2019.00413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Liu L, Huh E, Gbahou F, Cecon E, Oshima M, Houzé L, Katsonis P, Hegron A, Fan Z, et al.2023Human GLP1R variants affecting GLP1R cell surface expression are associated with impaired glucose control and increased adiposity. Nature Metabolism 51673–1684. ( 10.1038/s42255-023-00889-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg IJ.2001Clinical review 124: Diabetic dyslipidemia: causes and consequences. Journal of Clinical Endocrinology and Metabolism 86965–971. ( 10.1210/jcem.86.3.7304) [DOI] [PubMed] [Google Scholar]
- Gorden DL Ivanova PT Myers DS McIntyre JO VanSaun MN Wright JK Matrisian LM & Brown HA. 2011Increased diacylglycerols characterize hepatic lipid changes in progression of human nonalcoholic fatty liver disease; comparison to a murine model. PLoS One 6e22775. ( 10.1371/journal.pone.0022775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graaf Cd, Donnelly D, Wootten D, Lau J, Sexton PM, Miller LJ, Ahn JM, Liao J, Fletcher MM, Yang D, et al.2016Glucagon-like peptide-1 and its class B G protein–coupled receptors: a long march to therapeutic successes. Pharmacological Reviews 68954–1013. ( 10.1124/pr.115.011395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandt J, Jensen A-SH, Werge MP, Rashu EB, Møller A, Junker AE, Hobolth L, Mortensen C, Johansen CD, Vyberg M, et al.2023Postprandial dysfunction in fatty liver disease. Physiological Reports 11e15653. ( 10.14814/phy2.15653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R Gu J Zong S Wu M & Yang M. 2018Structure and mechanism of mitochondrial electron transport chain. Biomedical Journal 419–20. ( 10.1016/j.bj.2017.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MA Cherezov V Griffith MT Roth CB Jaakola VP Chien EYT Velasquez J Kuhn P & Stevens RC. 2008A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure 16897–905. ( 10.1016/j.str.2008.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama T & Riezman H. 2018Understanding the diversity of membrane lipid composition. Nature Reviews 19281–296. ( 10.1038/nrm.2017.138) [DOI] [PubMed] [Google Scholar]
- Hedger G Koldsø H Chavent M Siebold C Rohatgi R & Sansom MSP. 2019Cholesterol interaction sites on the transmembrane domain of the hedgehog signal transducer and class F G protein-coupled receptor smoothened. Structure 27549–559.. ( 10.1016/j.str.2018.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa A Tsumaya K Awaji T Katsuma S Adachi T Yamada M Sugimoto Y Miyazaki S & Tsujimoto G. 2005Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Medicine 1190–94. ( 10.1038/nm1168) [DOI] [PubMed] [Google Scholar]
- Hofmann K Thiele C Schött HF Gaebler A Schoene M Kiver Y Friedrichs S Lütjohann D & Kuerschner L. 2014A novel alkyne cholesterol to trace cellular cholesterol metabolism and localization. Journal of Lipid Research 55583–591. ( 10.1194/jlr.D044727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst JJ Vilsbøll T & Deacon CF. 2009The incretin system and its role in type 2 diabetes mellitus. Molecular and Cellular Endocrinology 297127–136. ( 10.1016/j.mce.2008.08.012) [DOI] [PubMed] [Google Scholar]
- Hua T, Vemuri K, Nikas SP, Laprairie RB, Wu Y, Qu L, Pu M, Korde A, Jiang S, Ho JH, et al.2017Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 547468–471. ( 10.1038/nature23272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulce JJ Cognetta AB Niphakis MJ Tully SE & Cravatt BF. 2013Proteome-wide mapping of cholesterol-interacting proteins in mammalian cells. Nature Methods 10259–264. ( 10.1038/nmeth.2368) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo SM Guerra ML Jarrard RE Przybyla JA Liu G Watts VJ & Hockerman GH. 2009The intracellular II-III loops of Cav1.2 and Cav1.3 uncouple L-type voltage-gated Ca2+ channels from glucagon-like Peptide-1 potentiation of insulin secretion in INS-1 cells via displacement from lipid rafts. Journal of Pharmacology and Experimental Therapeutics 330283–293. ( 10.1124/jpet.109.150672) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA & Radhakrishnan A. 2021The use of anthrolysin O and ostreolysin A to study cholesterol in cell membranes. Methods in Enzymology 649543–566. ( 10.1016/bs.mie.2021.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RM Zhang X Piper SJ Nettleton TJ Vandekolk TH Langmead CJ Danev R Sexton PM & Wootten D. 2021Cryo-EM structure of the dual incretin receptor agonist, peptide-19, in complex with the glucagon-like peptide-1 receptor. Biochemical and Biophysical Research Communications 57884–90. ( 10.1016/j.bbrc.2021.09.016) [DOI] [PubMed] [Google Scholar]
- Kang ZF Deng Y Zhou Y Fan RR Chan JC Laybutt DR Luzuriaga J & Xu G. 2013Pharmacological reduction of NEFA restores the efficacy of incretin-based therapies through GLP-1 receptor signalling in the beta cell in mouse models of diabetes. Diabetologia 56423–433. ( 10.1007/s00125-012-2776-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ Nian C & McIntosh CH. 2013Resistin knockout mice exhibit impaired adipocyte glucose-dependent insulinotropic polypeptide receptor (GIPR) expression. Diabetes 62471–477. ( 10.2337/db12-0257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjolbye LR Sorensen L Yan J Berglund NA Ferkinghoff-Borg J Robinson CV & Schiøtt B. 2022Lipid modulation of a class B GPCR: elucidating the modulatory role of PI(4,5)P(2) lipids. Journal of Chemical Information and Modeling 626788–6802. ( 10.1021/acs.jcim.2c00635) [DOI] [PubMed] [Google Scholar]
- Klop B Elte JW & Cabezas MC. 2013Dyslipidemia in obesity: mechanisms and potential targets. Nutrients 51218–1240. ( 10.3390/nu5041218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose C Surma MA & Simons K. 2013Organellar lipidomics—background and perspectives. Current Opinion in Cell Biology 25406–413. ( 10.1016/j.ceb.2013.03.005) [DOI] [PubMed] [Google Scholar]
- Knudsen LB & Lau J. 2019The discovery and development of liraglutide and semaglutide. Frontiers in Endocrinology 10155. ( 10.3389/fendo.2019.00155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldsø H Shorthouse D Hélie J & Sansom MSP. 2014Lipid clustering correlates with membrane curvature as revealed by molecular simulations of complex lipid bilayers. PLoS Computational Biology 10e1003911. ( 10.1371/journal.pcbi.1003911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostelic MM Ryan AM Reid DJ Noun JM & Marty MT. 2019Expanding the types of lipids amenable to native mass spectrometry of lipoprotein complexes. Journal of the American Society for Mass Spectrometry 301416–1425. ( 10.1007/s13361-019-02174-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna Kumar K, O’Brien ES, Habrian CH, Latorraca NR, Wang H, Tuneew I, Montabana E, Marqusee S, Hilger D, Isacoff EY, et al.2023Negative allosteric modulation of the glucagon receptor by RAMP2. Cell 1861465–1477.. ( 10.1016/j.cell.2023.02.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GA & Chattopadhyay A. 2021Cholesterol-dependent endocytosis of GPCRs: implications in pathophysiology and therapeutics. Biophysical Reviews 131007–1017. ( 10.1007/s12551-021-00878-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska K Matveichuk OV Fronk J & Ciesielska A. 2020Flotillins: at the intersection of protein S-palmitoylation and lipid-mediated signaling. International Journal of Molecular Sciences 21. ( 10.3390/ijms21072283) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafferty RA Flatt PR & Irwin N. 2023GLP-1/GIP analogs: potential impact in the landscape of obesity pharmacotherapy. Expert Opinion on Pharmacotherapy 24587–597. ( 10.1080/14656566.2023.2192865) [DOI] [PubMed] [Google Scholar]
- Lagou V, Jiang L, Ulrich A, Zudina L, González KSG, Balkhiyarova Z, Faggian A, Maina JG, Chen S, Todorov PV, et al.2023GWAS of random glucose in 476,326 individuals provide insights into diabetes pathophysiology, complications and treatment stratification. Nature Genetics 551448–1461. ( 10.1038/s41588-023-01462-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorraca NR Venkatakrishnan AJ & Dror RO. 2017GPCR dynamics: structures in motion. Chemical Reviews 117139–155. ( 10.1021/acs.chemrev.6b00177) [DOI] [PubMed] [Google Scholar]
- Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, et al.2015Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. Journal of Medicinal Chemistry 587370–7380. ( 10.1021/acs.jmedchem.5b00726) [DOI] [PubMed] [Google Scholar]
- Levental I Lingwood D Grzybek M Coskun U & Simons K. 2010Palmitoylation regulates raft affinity for the majority of integral raft proteins. PNAS 10722050–22054. ( 10.1073/pnas.1016184107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y Zhou Q Dai A Zhao F Chang R Ying T Wu B Yang D Wang MW & Cong Z. 2023Structural analysis of the dual agonism at GLP-1R and GCGR. PNAS 120e2303696120. ( 10.1073/pnas.2303696120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L & Astruc D. 2011The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications: an overview. Coordination Chemistry Reviews 2552933–2945. ( 10.1016/j.ccr.2011.06.028) [DOI] [Google Scholar]
- Lim CY Davis OB Shin HR Zhang J Berdan CA Jiang X Counihan JL Ory DS Nomura DK & Zoncu R. 2019ER-lysosome contacts enable cholesterol sensing by mTORC1 and drive aberrant growth signalling in Niemann-Pick type C. Nature Cell Biology 211206–1218. ( 10.1038/s41556-019-0391-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, IJzerman AP, et al.2012Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337232–236. ( 10.1126/science.1219218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longuet C Sinclair EM Maida A Baggio LL Maziarz M Charron MJ & Drucker DJ. 2008The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metabolism 8359–371. ( 10.1016/j.cmet.2008.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorizate M, Terrones O, Nieto-Garai JA, Rojo-Bartolomé I, Ciceri D, Morana O, Olazar-Intxausti J, Arboleya A, Martin A, Szynkiewicz M, et al.2021Super-resolution microscopy using a bioorthogonal-based cholesterol probe provides unprecedented capabilities for imaging nanoscale lipid heterogeneity in living cells. Small Methods 5e2100430. ( 10.1002/smtd.202100430) [DOI] [PubMed] [Google Scholar]
- Lucey M Pickford P Bitsi S Minnion J Ungewiss J Schoeneberg K Rutter GA Bloom SR Tomas A & Jones B. 2020Disconnect between signalling potency and in vivo efficacy of pharmacokinetically optimised biased glucagon-like peptide-1 receptor agonists. Molecular Metabolism 37100991. ( 10.1016/j.molmet.2020.100991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey M, Ashik T, Marzook A, Wang Y, Goulding J, Oishi A, Broichhagen J, Hodson DJ, Minnion J, Elani Y, et al.2021Acylation of the incretin peptide exendin-4 directly impacts glucagon-like peptide-1 receptor signaling and trafficking. Molecular Pharmacology 100319–334. ( 10.1124/molpharm.121.000270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchetti G Sircar R Kong JH Nachtergaele S Sagner A Byrne EFX Covey DF Siebold C & Rohatgi R. 2016Cholesterol activates the G-protein coupled receptor smoothened to promote Hedgehog signaling. eLife 5e20304. ( 10.7554/eLife.20304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Shen Q, Zhao LH, Mao C, Zhou XE, Shen DD, de Waal PW, Bi P, Li C, Jiang Y, et al.2020Molecular basis for hormone recognition and activation of corticotropin-releasing factor receptors. Molecular Cell 77669–680.. ( 10.1016/j.molcel.2020.01.013) [DOI] [PubMed] [Google Scholar]
- Manchanda Y Bitsi S Chen S Broichhagen J Bernardino de la Serna J Jones B & Tomas A. 2023Enhanced endosomal signaling and desensitization of GLP-1R vs GIPR in pancreatic beta cells. Endocrinology 16419–27. ( 10.1210/endocr/bqad028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchanda Y Bitsi S Kang Y Jones B & Tomas A. 2021Spatiotemporal control of GLP-1 receptor activity. Current Opinion in Endocrine and Metabolic Research 1619–27. ( 10.1016/j.coemr.2020.07.003) [DOI] [Google Scholar]
- Manna M Niemelä M Tynkkynen J Javanainen M Kulig W Müller DJ Rog T & Vattulainen I. 2016Mechanism of allosteric regulation of β2-adrenergic receptor by cholesterol. eLife 5e18432. ( 10.7554/eLife.18432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrink SJ, Corradi V, Souza PCT, Ingólfsson HI, Tieleman DP, Sansom MSP.2019Computational modeling of realistic cell membranes. Chemical Reviews 1196184–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrink SJ Monticelli L Melo MN Alessandri R Tieleman DP & Souza PCT. 2023Two decades of Martini: better beads, broader scope. WIREs Computational Molecular Science 13e1620. ( 10.1002/wcms.1620) [DOI] [Google Scholar]
- Marroqui L Alonso-Magdalena P Merino B Fuentes E Nadal A & Quesada I. 2014Nutrient regulation of glucagon secretion: involvement in metabolism and diabetes. Nutrition Research Reviews 2748–62. ( 10.1017/S0954422414000031) [DOI] [PubMed] [Google Scholar]
- Mayendraraj A Rosenkilde MM & Gasbjerg LS. 2022GLP-1 and GIP receptor signaling in beta cells – a review of receptor interactions and co-stimulation. Peptides 151170749. ( 10.1016/j.peptides.2022.170749) [DOI] [PubMed] [Google Scholar]
- McGlone ER Manchanda Y Jones B Pickford P Inoue A Carling D Bloom SR Tan T & Tomas A. 2021Receptor activity-modifying protein 2 (RAMP2) alters glucagon receptor trafficking in hepatocytes with functional effects on receptor signalling. Molecular Metabolism 53101296. ( 10.1016/j.molmet.2021.101296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlone ER Ansell TB Dunsterville C Song W Carling D Tomas A Bloom SR Sansom MSP Tan T & Jones B. 2022Hepatocyte cholesterol content modulates glucagon receptor signalling. Molecular Metabolism 63101530. ( 10.1016/j.molmet.2022.101530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw C Koretz KS Oseid D Lyman E & Robinson AS. 2022Cholesterol dependent activity of the adenosine A2a receptor is modulated via the cholesterol consensus motif. Molecules 273529. ( 10.3390/molecules27113529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michałowska J Miller-Kasprzak E Seraszek-Jaros A Mostowska A & Bogdański P. 2022The link between three single nucleotide variants of the GIPR gene and metabolic health. Genes (Basel) 131534. ( 10.3390/genes13091534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mroz PA Finan B Gelfanov V Yang B Tschöp MH DiMarchi RD & Perez-Tilve D. 2019Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Molecular Metabolism 2051–62. ( 10.1016/j.molmet.2018.12.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TD, Finan B, Bloom SR, D'Alessio D, Drucker DJ, Flatt PR, Fritsche A, Gribble F, Grill HJ, Habener JF, et al.2019Glucagon-like peptide 1 (GLP-1). Molecular Metabolism 3072–130. ( 10.1016/j.molmet.2019.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TD Finan B Clemmensen C DiMarchi RD & Tschöp MH. 2017The new biology and pharmacology of glucagon. Physiological Reviews 97721–766. ( 10.1152/physrev.00025.2016) [DOI] [PubMed] [Google Scholar]
- Müller WA Faloona GR Aguilar-Parada E & Unger RH. 1970Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. New England Journal of Medicine 283109–115. ( 10.1056/NEJM197007162830301) [DOI] [PubMed] [Google Scholar]
- Mulvihill EE.2018Regulation of intestinal lipid and lipoprotein metabolism by the proglucagon-derived peptides glucagon like peptide 1 and glucagon like peptide 2. Current Opinion in Lipidology 2995–103. ( 10.1097/MOL.0000000000000495) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro E Atilla-Gokcumen GE & Eggert US. 2014Lipids in cell biology: how can we understand them better? Molecular Biology of the Cell 251819–1823. ( 10.1091/mbc.E13-09-0516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA Baller B & Meier JJ. 2004Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 53(Supplement 3) S190–S196. ( 10.2337/diabetes.53.suppl_3.s190) [DOI] [PubMed] [Google Scholar]
- Nauck MA & Müller TD. 2023Incretin hormones and type 2 diabetes. Diabetologia 661780–1795. ( 10.1007/s00125-023-05956-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale C Herce HD Pomès R & García AE. 2015Can specific protein-lipid interactions stabilize an active state of the beta 2 adrenergic receptor? Biophysical Journal 1091652–1662. ( 10.1016/j.bpj.2015.08.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicovich PR Kwiatek JM Ma Y Benda A & Gaus K. 2018FSCS reveals the complexity of lipid domain dynamics in the plasma membrane of live cells. Biophysical Journal 1142855–2864. ( 10.1016/j.bpj.2018.04.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oates J & Watts A. 2011Uncovering the intimate relationship between lipids, cholesterol and GPCR activation. Current Opinion in Structural Biology 21802–807. ( 10.1016/j.sbi.2011.09.007) [DOI] [PubMed] [Google Scholar]
- Oddi S Dainese E Fezza F Lanuti M Barcaroli D De Laurenzi V Centonze D & Maccarrone M. 2011Functional characterization of putative cholesterol binding sequence (CRAC) in human type-1 cannabinoid receptor. Journal of Neurochemistry 116858–865. ( 10.1111/j.1471-4159.2010.07041.x) [DOI] [PubMed] [Google Scholar]
- Pándy-Szekeres G Caroli J Mamyrbekov A Kermani AA Keserű GM Kooistra AJ & Gloriam DE. 2023GPCRdb in 2023: state-specific structure models using AlphaFold2 and new ligand resources. Nucleic Acids Research 51D395–D402. ( 10.1093/nar/gkac1013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternoster S & Falasca M. 2018Dissecting the physiology and pathophysiology of glucagon-like peptide-1. Frontiers in Endocrinology 9584. ( 10.3389/fendo.2018.00584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BP & Miller EA. 2021Membrane protein folding and quality control. Current Opinion in Structural Biology 6950–54. ( 10.1016/j.sbi.2021.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P Baillie RA Wiest MM Mirshahi F Choudhury J Cheung O Sargeant C Contos MJ & Sanyal AJ. 2007A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology 461081–1090. ( 10.1002/hep.21763) [DOI] [PubMed] [Google Scholar]
- Rahman SMK Uyama T Hussain Z & Ueda N. 2021Roles of endocannabinoids and endocannabinoid-like molecules in energy homeostasis and metabolic regulation: a nutritional perspective. Annual Review of Nutrition 41177–202. ( 10.1146/annurev-nutr-043020-090216) [DOI] [PubMed] [Google Scholar]
- Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O’Donnell CJ, et al.2011Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. Journal of Clinical Investigation 1211402–1411. ( 10.1172/JCI44442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoda A Harada N Kato T Ikeguchi E Iwasaki K Yamane S Murata Y Hirasawa A & Inagaki N. 2019Free fatty acid receptors, G protein-coupled receptor 120 and G protein-coupled receptor 40, are essential for oil-induced gastric inhibitory polypeptide secretion. Journal of Diabetes Investigation 101430–1437. ( 10.1111/jdi.13059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R Shrivastava S & Chattopadhyay A. 2015Cholesterol-induced changes in hippocampal membranes utilizing a phase-sensitive fluorescence probe. Biochimica et Biophysica Acta 18481699–1705. ( 10.1016/j.bbamem.2015.05.001) [DOI] [PubMed] [Google Scholar]
- Schneider F Waithe D Galiani S de la Serna JB Sezgin E & Eggeling C. 2018Nanoscale spatiotemporal diffusion modes measured by simultaneous confocal and stimulated emission depletion nanoscopy imaging. Nano Letters 184233––4240.. ( 10.1021/acs.nanolett.8b01190) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghieri M Christensen AS Andersen A Solini A Knop FK & Vilsbøll T. 2018Future perspectives on GLP-1 receptor agonists and GLP-1/glucagon receptor co-agonists in the treatment of NAFLD. Frontiers in Endocrinology 9649. ( 10.3389/fendo.2018.00649) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejdiu BI & Tieleman DP. 2020Lipid-protein interactions are a unique property and defining feature of G protein-coupled receptors. Biophysical Journal 1181887–1900. ( 10.1016/j.bpj.2020.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A & Simons K. 2010Lipidomics: coming to grips with lipid diversity. Nature Reviews. Molecular Cell Biology 11593–598. ( 10.1038/nrm2934) [DOI] [PubMed] [Google Scholar]
- Siebold C & Rohatgi R. 2023The inseparable relationship between cholesterol and hedgehog signaling. Annual Review of Biochemistry 92273–298. ( 10.1146/annurev-biochem-052521-040313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva LLSE Fernandes MSS Lima EA Stefano JT Oliveira CP & Jukemura J. 2021Fatty pancreas: disease or finding? Clinics (Sao Paulo) 76e2439. ( 10.6061/clinics/2021/e2439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skočaj M, Resnik N, Grundner M, Ota K, Rojko N, Hodnik V, Anderluh G, Sobota A, Maček P, Veranič P, et al.2014Tracking cholesterol/sphingomyelin-rich membrane domains with the ostreolysin A-mCherry protein. PLoS One 9e92783.. ( 10.1371/journal.pone.0092783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W Corey RA Ansell TB Cassidy CK Horrell MR Duncan AL Stansfeld PJ & Sansom MSP. 2022PyLipID: A python package for analysis of protein–lipid interactions from molecular dynamics simulations. Journal of Chemical Theory and Computation 181188–1201. ( 10.1021/acs.jctc.1c00708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza PCT, Alessandri R, Barnoud J, Thallmair S, Faustino I, Grünewald F, Patmanidis I, Abdizadeh H, Bruininks BMH, Wassenaar TA, et al.2021Martini 3: a general purpose force field for coarse-grained molecular dynamics. Nature Methods 18382–388. ( 10.1038/s41592-021-01098-3) [DOI] [PubMed] [Google Scholar]
- Suppli MP, Bagger JI, Lund A, Demant M, van Hall G, Strandberg C, Kønig MJ, Rigbolt K, Langhoff JL, Wewer Albrechtsen NJ, et al.2020Glucagon resistance at the level of amino acid turnover in obese subjects with hepatic steatosis. Diabetes 691090–1099. ( 10.2337/db19-0715) [DOI] [PubMed] [Google Scholar]
- Suppli MP Lund A Bagger JI Vilsbøll T & Knop FK. 2016Involvement of steatosis-induced glucagon resistance in hyperglucagonaemia. Medical Hypotheses 86100–103. ( 10.1016/j.mehy.2015.10.029) [DOI] [PubMed] [Google Scholar]
- Tanabe A, Kaneto H, Kamei S, Hirukawa H, Shimoda M, Kimura T, Obata A, Okauchi S, Tatsumi F, Kohara K, et al.2016Clinical effects of liraglutide are possibly influenced by hypertriglyceridemia and remaining pancreatic β-cell function in subjects with type 2 diabetes mellitus. Journal of Diabetes and Its Complications 301201–1203. ( 10.1016/j.jdiacomp.2016.04.005) [DOI] [PubMed] [Google Scholar]
- Tappenden KA Albin DM Bartholome AL & Mangian HF. 2003Glucagon-like peptide-2 and short-chain fatty acids: a new twist to an old story. Journal of Nutrition 1333717–3720. ( 10.1093/jn/133.11.3717) [DOI] [PubMed] [Google Scholar]
- Trier S Linderoth L Bjerregaard S Andresen TL & Rahbek UL. 2014Acylation of glucagon-like peptide-2: interaction with lipid membranes and in vitro intestinal permeability. PLOS ONE 9e109939. ( 10.1371/journal.pone.0109939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aalst E & Wylie BJ. 2021Cholesterol is a dose-dependent positive allosteric modulator of CCR3 ligand affinity and G protein coupling. Frontiers in Molecular Biosciences 8724603. ( 10.3389/fmolb.2021.724603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden WJC Lindquist P Madsen JS Stassen RHMJ Wewer Albrechtsen NJ Holst JJ Hauser AS & Rosenkilde MM. 2022Molecular and in vivo phenotyping of missense variants of the human glucagon receptor. Journal of Biological Chemistry 298101413. ( 10.1016/j.jbc.2021.101413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G & de Kroon AIPM. 2011Lipid map of the mammalian cell. Journal of Cell Science 1245–8. ( 10.1242/jcs.071233) [DOI] [PubMed] [Google Scholar]
- Vázquez P Roncero I Blázquez E & Alvarez E. 2005Substitution of the cysteine 438 residue in the cytoplasmic tail of the glucagon-like peptide-1 receptor alters signal transduction activity. Journal of Endocrinology 18535–44. ( 10.1677/joe.1.06031) [DOI] [PubMed] [Google Scholar]
- Wewer Albrechtsen NJ, Færch K, Jensen TM, Witte DR, Pedersen J, Mahendran Y, Jonsson AE, Galsgaard KD, Winther-Sørensen M, Torekov SS, et al.2018Evidence of a liver–alpha cell axis in humans: hepatic insulin resistance attenuates relationship between fasting plasma glucagon and glucagonotropic amino acids. Diabetologia 61671–680. ( 10.1007/s00125-017-4535-5) [DOI] [PubMed] [Google Scholar]
- Wootten D & Miller LJ. 2020Structural basis for allosteric modulation of class b g protein-coupled receptors. Annual Review of Pharmacology and Toxicology 6089–107. ( 10.1146/annurev-pharmtox-010919-023301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Wang C, Gregory KJ, Han GW, Cho HP, Xia Y, Niswender CM, Katritch V, Meiler J, Cherezov V, et al.2014Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science 34458–64. ( 10.1126/science.1249489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Tang JJ, Peng C, Wang Y, Fu L, Qiu ZP, Xiong Y, Yang LF, Cui HW, He XL, et al.2017Cholesterol modification of smoothened is required for hedgehog signaling. Molecular Cell 66154–162.. ( 10.1016/j.molcel.2017.02.015) [DOI] [PubMed] [Google Scholar]
- Xu P, Huang S, Zhang H, Mao C, Zhou XE, Cheng X, Simon IA, Shen DD, Yen HY, Robinson CV, et al.2021Structural insights into the lipid and ligand regulation of serotonin receptors. Nature 592469–473. ( 10.1038/s41586-021-03376-8) [DOI] [PubMed] [Google Scholar]
- Yang D, Zhou Q, Labroska V, Qin S, Darbalaei S, Wu Y, Yuliantie E, Xie L, Tao H, Cheng J, et al.2021G protein-coupled receptors: structure- and function-based drug discovery. Signal Transduction and Targeted Therapy 67. ( 10.1038/s41392-020-00435-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaribeygi H Maleki M Butler AE Jamialahmadi T & Sahebkar A. 2021The impact of incretin-based medications on lipid metabolism. Journal of Diabetes Research 20211815178. ( 10.1155/2021/1815178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeliseev A Iyer MR Joseph TT Coffey NJ Cinar R Zoubak L Kunos G & Gawrisch K. 2021Cholesterol as a modulator of cannabinoid receptor CB2 signaling. Scientific Reports 113706. ( 10.1038/s41598-021-83245-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip RG Boylan MO Kieffer TJ & Wolfe MM. 1998Functional GIP receptors are present on adipocytes. Endocrinology 1394004–4007. ( 10.1210/endo.139.9.6288) [DOI] [PubMed] [Google Scholar]
- Zarghamravanbakhsh P Frenkel M & Poretsky L. 2021Metabolic causes and consequences of nonalcoholic fatty liver disease (NAFLD). Metabolism Open 12100149. ( 10.1016/j.metop.2021.100149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL Wang JJ Li JN & Yang Y. 2021Nonalcoholic fatty pancreas disease: an emerging clinical challenge. World Journal of Clinical Cases 96624–6638. ( 10.12998/wjcc.v9.i23.6624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Qiao A, Yang D, Yang L, Dai A, de Graaf C, Reedtz-Runge S, Dharmarajan V, Zhang H, Han GW, et al.2017Structure of the full-length glucagon class B G-protein-coupled receptor. Nature 546259–264. ( 10.1038/nature22363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LH, Ma S, Sutkeviciute I, Shen DD, Zhou XE, de Waal PW, Li CY, Kang Y, Clark LJ, Jean-Alphonse FG, et al.2019Structure and dynamics of the active human parathyroid hormone receptor-1. Science 364148–153. ( 10.1126/science.aav7942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P Truong TT Merlin J Sexton PM & Wootten D. 2022Implications of ligand-receptor binding kinetics on GLP-1R signalling. Biochemical Pharmacology 199114985. ( 10.1016/j.bcp.2022.114985) [DOI] [PubMed] [Google Scholar]
- Zheng H Pearsall EA Hurst DP Zhang Y Chu J Zhou Y Reggio PH Loh HH & Law PY. 2012Palmitoylation and membrane cholesterol stabilize μ-opioid receptor homodimerization and G protein coupling. BMC Cell Biology 136. ( 10.1186/1471-2121-13-6) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a