Abstract
It is a challenge to keep abreast of all the clinical and scientific advances in the field of respiratory medicine. This article contains an overview of laboratory-based science, clinical trials and qualitative research that were presented during the 2023 European Respiratory Society International Congress within the sessions from the five groups of Assembly 1 (Respiratory Clinical Care and Physiology). Selected presentations are summarised from a wide range of topics: clinical problems, rehabilitation and chronic care, general practice and primary care, electronic/mobile health (e-health/m-health), clinical respiratory physiology, exercise and functional imaging.
Shareable abstract
This article provides an overview of some of the highlights from @ERSAssembly1 (Respiratory Clinical Care and Physiology) presented during #ERSCongress 2023 https://bit.ly/3UVpMbS
Introduction
The 2023 edition of the European Respiratory Society (ERS) International Congress was held in hybrid format. It provided a much-valued occasion to meet in person in Milan, Italy, and an important opportunity to hear about the latest developments in research and clinical practice in the world's largest scientific and educational conference in the field of respiratory medicine. For the 2023 ERS Congress, 4127 abstracts were accepted for presentation and 20 608 delegates attended some of the 401 sessions.
Assembly 1 (Respiratory Clinical Care and Physiology) is the largest of the 14 ERS assemblies, comprising 8555 members, 39% of them being under 40 years old (early-career members). Among the 609 abstracts submitted across the five groups within the assembly, 461 were accepted for presentation. Although the virtual platform allows presentations to be replayed, it can be challenging to keep up to date with all the scientific and clinical advances. That's why, every year, the Early Career Members Committee coordinates reports summarising the most significant presentations from each assembly [1–14]. This article, therefore, aims to share some of the highlights from the Respiratory Clinical Care and Physiology Assembly.
Group 1.01: clinical problems
In this section, we summarise the sessions “Best abstracts in clinical problems” and “ALERT (abstracts leading to evolution in respiratory medicine trials) 2: organisation of care”. In these sessions, updates on coronavirus disease 2019 (COVID-19) pathophysiology and the efficacy of available treatment regimens were highlighted. The prevalence and clinical implications of post-COVID-19 syndrome were also discussed. The ALERT 2 session focused on enhancing the standard of care in diagnostics, monitoring, and treatment of respiratory diseases by implementing use of new digital solutions or alternative imaging techniques.
Impact of COVID-19 pathophysiology on the cardiovascular system
COVID-19 has affected millions of people worldwide but few studies have analysed the impact of the disease on the cardiovascular system by systematic cardiac imaging [15]. In a large Tunisian prospective study, echocardiography and capillaroscopy were performed in 158 patients 1 month after infection from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in an attempt to investigate the link between endothelial dysfunction and left ventricular global strain (LVGS), and their effect on disease prognosis. The authors concluded that there was a strong correlation between endothelial function and LVGS which affects patients both in the acute phase of the disease and also during recovery [16].
Risk factors for the development of post-COVID-19 syndrome and effects of corticosteroids on the course of the disease
Many patients infected by SARS-CoV-2 present with persistent symptoms even months after acute COVID-19. Hence, the long-term consequences of the disease need to be identified and treated accordingly [17]. In a retrospective, single-centre study, Sesé et al. [18] (Bobigny, France) aimed to estimate the prevalence of functional respiratory complaints (FRCs) identified by the Nijmegen questionnaire, and associated risk factors. It was observed that COVID-19 patients presented more frequently with FRCs than the general population [19], and that females were more affected than males. Furthermore, the prevalence of FRCs was strongly associated with dyspnoea intensity and inversely correlated with the initial COVID-19 severity, but not pulmonary function tests [18]. Very close conclusions were drawn in two other monocentric French studies (figure 1) [20, 21].
FIGURE 1.
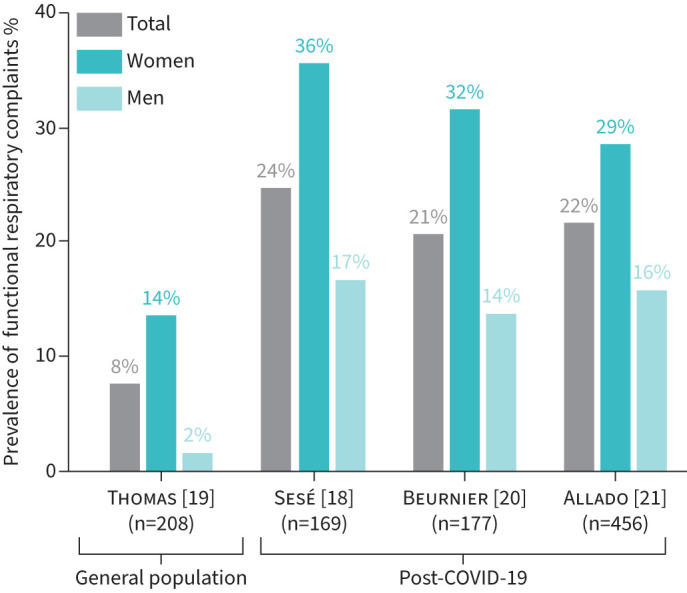
Estimated prevalence of functional respiratory complaints (Nijmegen score ≥23/64) in the general population and in three French post-COVID-19 cohorts.
A previous UK multicentre, prospective, cohort study assessing physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID), found that factors associated with worse recovery were female sex, older age, comorbidities, and illness severity [22]. Even though corticosteroid treatment in hospitalised COVID-19 patients with respiratory failure was shown to improve survival [23], the long-term effect on health outcomes is not completely clear yet. Russell et al. [24] aimed to investigate the effect of corticosteroid treatment on health-related quality of life (HR-QoL) and functional capacity, at 1 year after hospitalisation, in 1226 patients from the PHOSP-COVID cohort: the prevalence of persistent health issues was not different between COVID-19 patients who had received corticosteroids during the acute phase, and those who had not.
Effect of granulocyte–macrophage colony-stimulating factor inhalation therapy for autoimmune pulmonary alveolar proteinosis
Autoimmune pulmonary alveolar proteinosis is a rare disease characterised by abnormalities in myeloid cells’ function, surfactant accumulation and innate immunity [25]. Even though whole lung-lavage is still the indicated treatment, the application of inhaled granulocyte–macrophage colony-stimulating factor (GM-CSF) as a regulatory mediator for surfactant homeostasis, is being tested in clinical trials. In a phase III investigator-initiated clinical trial performed in Japan, inhaled therapy with GM-CSF was administered in 61 patients and the efficacy and safety of the drug was previously reported [26]. At 2-year follow-up, the authors observed that the improvement in oxygenation by GM-CSF therapy was transient and that the period without additional treatment was affected by the value of vital capacity (VC) during the initial evaluation. In addition, no side-effects were reported and the investigators concluded that it was important to maintain VC above 80% of predicted value to improve disease prognosis [27].
Automated oxygen titration to adjust supplementation during acute hypoxaemic events
Currently, the titration of oxygen to adjust supplementation based on peripheral oxygen saturation (SpO2) measurements during hospitalisation is manually carried out by nursing staff. A multicentre study by Sandau et al. [28] (Hvidovre, Denmark), having included 157 patients with acute exacerbation of COPD (AE-COPD), investigated the safety and effects of an automated oxygen titration device using continuous measurements to adjust the oxygen flow based on varying oxygen needs. The study did demonstrate that using automated oxygen titration significantly improved the time that SpO2 levels were within the target intervals, while manually controlled oxygen flow resulted in more time with hypoxaemia or hyperoxaemia. There were no differences in the safety outcomes between the two study groups, highlighting a superior and beneficial effect of automated control versus manual control of oxygen supplementation. However, no differences were found in the number of patients successfully weaned off oxygen, nor in duration of hospitalisation [28]. In the same cohort of patients, the authors had previously published that automated oxygen titration contributed to reduced breathing discomfort and dyspnoea [29]. Another study of patients with acute hypoxaemic respiratory failure also compared manual to automatic oxygen control, and found improved time in SpO2 optimum as well as reduced workload for staff [30].
Implementation of multiorgan ultrasound in the diagnosis of pulmonary embolism
D-dimer measurement is a cornerstone to rule out pulmonary embolism (PE), given its high sensitivity. Unfortunately, the specificity is much lower, further reduced with advancing age and comorbidities. Consequently, the high false positive rate of D-dimer measurement results in numerous referrals for diagnostic imaging, with small proportions of individuals actually receiving a confirmed diagnosis of PE [31]. A randomised controlled trial (RCT) conducted by Falster et al. [32] (Odense, Denmark), having included 150 patients with suspected PE, compared multiorgan ultrasound (lung, cardiac and deep venous ultrasound) as a part of diagnostics, to a control group receiving usual care. This approach led to a 45% reduction in referrals to diagnostic imaging. Evaluating the 3-month failure rate, no missed PEs were noted in the control group, while the ultrasound group experienced two missed PEs amongst 30 initially dismissed by the ultrasound approach (failure rate 6.6%, 95% CI 1.8–21) [32]. This study explores the potential of an expanded role for ultrasound in PE diagnostics, but further studies with greater statistical power are essential to conclusively determine its precision and safety. A systematic review covering studies on the possible role of ultrasound in PE diagnostics showed that cardiopulmonary ultrasound could supplement existing diagnostic imaging as a non-invasive method and play a role in PE diagnosis, but further research is needed to determine the ideal setup and how ultrasound will play a role within the context of PE [33].
Take-home messages
Endothelial dysfunction and LVGS were prominent features in a subgroup of COVID-19 patients and affect disease course both during the acute phase and recovery.
Corticosteroid administration during hospitalisation for COVID-19 might not alter the prevalence of persistent health problems after 1 year.
COVID-19 patients present more frequently with FRCs than the general population, with a female preponderance. Dyspnoea intensity and milder initial disease severity seem to be associated with higher prevalence of FRCs.
GM-CSF inhalation therapy for autoimmune pulmonary proteinosis appears to transiently improve oxygenation levels, and changes in VC may be used as a guide for therapeutic intervention.
Automated control of oxygen administration in patients with acute hypoxaemic respiratory failure seems efficient and safe.
Regarding suspected PEs, an expanded role for ultrasound might lessen the need for referral for more invasive diagnostic imaging, but its precise role in diagnostic algorithms is yet to be determined.
Group 1.02: rehabilitation and chronic care
Sessions from Group 1.02 covered various topics, including the different modalities to deliver pulmonary rehabilitation (PR), and factors predicting survival in chronic respiratory diseases (CRDs) (figure 2). In this section, we summarise the sessions “Best abstracts in pulmonary rehabilitation and chronic care”, “Updating pulmonary rehabilitation outcomes and chronic management”, “Respiratory muscle function and rehabilitation”, “Best abstracts in respiratory physiotherapy” and “Rehabilitation of chronic respiratory diseases”.
FIGURE 2.
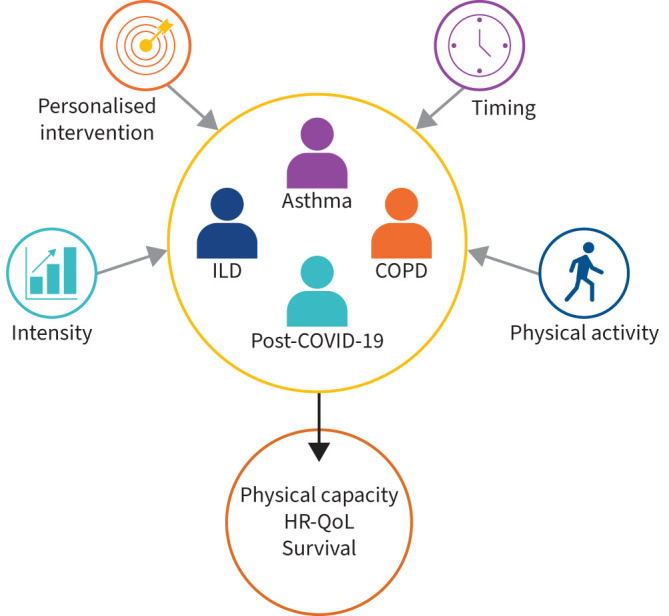
Topics covered in sessions from Group 1.02. ILD: interstitial lung disease; HR-QoL: health-related quality of life.
Post-COVID-19 rehabilitation
Interim analysis of the German ReLoAd study analysed the benefit of tailored symptom-based rehabilitation versus usual care (no intervention) in 87 patients with post-COVID-19 syndrome, 40% of whom were unable to return to work due to their post-COVID-19 syndrome. 3 weeks' rehabilitation targeting the primary symptom (fatigue, cognition or somatic) significantly improved HR-QoL (as assessed by the Physical and Mental Health components of the SF-36 questionnaire), as well as symptoms of anxiety (Patient Health Questionnaire (PHQ)-9) and depression (Generalised Anxiety Disorder (GAD)-7) [34].
On a similar topic, Evans et al. [35] (Leicester, UK) presented preliminary results from the PHOSP-COVID study [22, 36] comparing outcomes from patients after severe COVID-19 requiring hospitalisation who had received complex (high-intensity) clinical assessment and/or frequent rehabilitation sessions versus no or light-touch follow-up. Data from 1013 participants across 45 sites in the UK revealed that only 28% of patients felt fully recovered 12 months post-discharge. HR-QoL measured with EQ5D-5L utility index was 0.82 pre-COVID and 0.69 post-COVID. High-intensity clinical assessment combined with high-intensity rehabilitation was associated with the largest increase in health status over 12 months. Together, these studies highlight the ongoing clinical need amongst this population and the importance of high-quality follow-up and intervention. More data from the PHOSP-COVID study monitoring recovery from COVID-19 infection, in 1096 patients classified with respect to their Muscle Quality Index (MQI), showed that individuals with higher skeletal muscle quality were more likely to feel recovered at 12 months and have higher HR-QoL [37], strongly suggesting that the impact of a targeted intervention for individuals with low muscle quality should be examined.
Exercise modalities
A recurring topic at the 2023 ERS Congress was the importance of exercise intensity and the need to tailor exercise interventions to meet the needs of different populations. Latimer et al. [38] (Leicester, UK) showed that patients with COPD have a similar muscle mRNA response to aerobic training compared with healthy controls, but blunted adaptation in mitochondrial function. These differences, not explained by protein expression patterns, were related to low absolute training intensities in patients with ventilatory limitation.
A novel intervention targeting skeletal muscle in people with severe dyspnoea or ventilatory limitation to exercise is blood-flow restricted (BFR) exercise. Kuhn et al. [39] (Zurich, Switzerland) described lower ventilation (−3.05 L·min−1, interquartile range (IQR) −4.38– −1.72) and subjective dyspnoea (−0.62, IQR −1.09– −0.14, on the Borg scale), but increased subjective leg effort (1.57, IQR 1.2–1.94), during BFR cycling exercise compared to cycling exercise without BFR in 24 healthy individuals. In a pilot study of high-load strength training versus low-load strength training with BFR, the same team found BFR to be acceptable to 30 patients with COPD and showed encouraging trends towards strength improvements that warrant investigation in a larger trial [40].
Exercise in asthma
Two studies addressed the characteristics and intensity of exercise training in asthma patients. Aparecido Da Silva and co-workers [41, 42] (São Paulo, Brazil) randomised 55 patients with moderate and severe asthma to high-intensity interval training (HIIT) or constant load exercise during a 12-week programme. Consumption of short-acting β-agonists was reduced and aerobic fitness increased in both groups, but peak expiratory flow (PEF) and asthma control were significantly improved in the HIIT group.
Elsewhere, Kim et al. [43] (São Paulo, Brazil) showed that aerobic exercise training improved asthma control (Asthma Control Questionnaire (ACQ)) and increased walking performance (incremental shuttle walk distance (ISWD)) in 44 patients with moderate-to-severe asthma. The addition of breathing retraining improved walking performance and reduced the proportion of patients with FRCs, suggesting that there may be a subset of patients for whom breathing retraining is particularly beneficial.
Delivering pulmonary rehabilitation
An RCT of home rehabilitation versus usual care (medication) during an AE-COPD not requiring hospitalisation reported no adverse events amongst the 50 randomised patients (24 receiving rehabilitation). Evidence of significantly improved symptoms, muscle strength and functional capacity at 3 weeks in the rehabilitation group highlighted the need for larger trials with longer follow-up [44].
Géphine et al. [45] (Pérenchies, France) presented a retrospective analysis of PR data collected between 2011 and 2022 comparing the effects of once- versus twice-weekly supervised home-based PR on symptoms (COPD Assessment Test (CAT)), HR-QoL (Visual Simplified Respiratory Questionnaire (VSRQ)), exercise tolerance (6-Minute Stepper Test (6MST)), anxiety and depression (Hospital Anxiety and Depression Scale (HADS)) in COPD. Similar responses were shown for 809 patients who received eight sessions and 294 patients who received 16 sessions over 8 weeks. Reduced supervision may increase capacity in clinical services; therefore, future prospective studies should be conducted to ensure that quality is maintained. Following results of a previous non-randomised study [46], in a non-inferiority RCT 436 patients (63% COPD) were randomised to perform an 8-week outpatient supervised programme with minimal (walking circuits, exercise in body weight, portable weights, etc.) or specialist exercise equipment (gym apparatus). No differences were observed between groups for exercise capacity improvement (ISWD) or the number of adverse events [47].
The above three studies addressed potential ways to increase access to PR, highlighting potential effectiveness of delivering programmes with low supervision and using low-cost equipment. The efficacy in promoting physical activity (PA) through PR was explored in a network meta-analysis of different PR interventions on a range of PA variables. From 48 articles (4178 patients), the authors concluded that addition of a PA promotion intervention to centre-based PR improved the volume of PA levels (step count). No PR-related intervention was superior to centre-based PR alone in increasing intensity of PA performed [48].
Another meta-analysis (99 studies; 5138 patients) examining interventions to increase fat-free mass in COPD emphasised that exercise-induced increases in fat-free mass are localised to the trained region (not whole-body), and highlighted potential benefits of nutritional supplementation and anabolic steroids in addition to exercise to maximise benefit [49]. Future research in this area should consider a personalised medicine approach to tailor interventions for individuals.
Mortality prediction
Two studies highlighted the predictive value of exercise capacity and daily PA on survival. Vaes et al. [50] (Horn, the Netherlands) validated previous data from a Dutch cohort [51] in 261 COPD patients from Switzerland, according to their physical capacity (6-minute walk distance (6MWD)) and habitual PA (number of daily steps). Survival data adjusted for age, forced expiratory volume in the first second (FEV1), dyspnoea grade on the modified Medical Research Council (mMRC) scale, and body mass index (BMI), showed that 401 m and 4028 steps per day were the best thresholds to predict 6-year survival for male patients, and 394 m and 3457 steps per day for women. People with COPD and relatively preserved exercise capacity (the “can do, do do” and “can do, don't do” groups) had significantly greater probability of survival at 6 years. Trends towards additional survival benefit of higher PA levels in both high and low exercise capacity groups were not significant in this cohort [50]. Similarly, in a cohort of 441 patients with CRD, Björklund et al. [52] (Lund, Sweden) showed that exercise capacity (30-s sit-to-stand test) but not dyspnoea symptoms (Dyspnoea Exertion Scale) was associated with overall mortality, with comparable results between COPD (n=271) and interstitial lung disease (ILD; n=90) subgroups.
In a cohort from England and Wales, survival was assessed in 3721 COPD patients depending on whether they did or did not reach the minimal clinically important difference (MCID) for walking distance (63 m for ISWD, or 57 m for 6MWD) following PR. Survival data corrected for age, sex, smoking, FEV1, BMI, mMRC dyspnoea grade, home oxygen use, number of comorbidities and hospital admission during the PR course showed a 43% (95% CI 28–55) mortality reduction for patients who had achieved the walking distance MCID compared to those who did not [53]. Although they were observational and cannot prove causation, these data support the hypothesis that effective PR might directly affect survival.
Take-home messages
There is a clinical need for individualised high-quality, high-intensity follow-up and interventions for people who experience ongoing post-COVID-19 symptoms.
Intensity is important: exercise training and other interventions targeting skeletal muscle in a rehabilitation context should tailor the needs of the individual.
Aerobic exercise training in moderate-to-severe asthma not only increases exercise capacity, but also asthma control.
Further work is required to confirm the benefit of PR in patients experiencing an exacerbation of COPD at home, to determine the number of sessions required to optimise patient benefit and maximise clinical resources.
PA interventions added to centre-based PR increase the volume of PA undertaken by individuals, but increasing intensity of PA is harder, and may be constrained by limited physical capacity.
Exercise capacity and daily PA are an important predictor of survival in CRDs, as is the response to PR. The influence of the volume of habitual activity needs to be more studied.
Group 1.03: general practice and primary care
Improving respiratory care in primary settings is not only crucial for alleviating the clinical and economic burdens associated with CRDs, but also fundamental for improving patients' HR-QoL and long-term health outcomes. This section aims to shed light on the strategies and interventions that could be employed to improve respiratory care from a primary care perspective. The session summarised here was entitled “Improving respiratory care from a primary care perspective”.
Spirometry and breathlessness evaluation in primary care
Following a systematic literature review, Sunjaya et al. [54] (Sydney, Australia) previously proposed a stepwise algorithm to assess patients coming to their general practitioner (GP) with breathlessness as their primary complaint, with spirometry as a first-line diagnostic test. They estimated that up to 55% of patients could be diagnosed with anamnesis, physical examination, and only spirometry, pulse oximetry and ECG as initial diagnostic tests [54]. In a new work, they evaluated the acceptability and feasibility of their algorithm through ascertaining its alignment with GPs views and current practice. When GPs were asked to classify diagnostic tests by estimated frequency of use, the median rank of spirometry was only 9. But when analysing de-identified electronic health record of 78 912 patients complaining from breathlessness from a primary care database of 405 GP practices, the underuse of spirometry appeared even larger, as it was undertaken in only 10.3% of these patients [55]. This issue might eventually be addressed, at least in some settings, by ready-to-use solutions as the “Breathlessness Diagnostics in a Box” (BiaB) system presented by Kocks et al. [56] (Groningen, the Netherlands). This tool includes a pulse oximeter, a portable spirometer/oscillometer, a 4-patch 12-lead ECG, a point-of-care N-terminal pro-brain natriuretic peptide (NT-proBNP) test, and an iPad with an application integrating test results into a working diagnosis. Preliminary results of a feasibility study suggested that running BiaB in primary care was feasible and fast (20 min). Next steps will include follow-up data with referrals and comparison of suspected and confirmed diagnoses.
Artificial intelligence (AI) might also help GPs to perform spirometry, by increased their confidence in quality assessment and interpretation of the results. In a Belgian study, structured interviews were conducted with 30 GPs from 18 practices to assess spirometry services in primary care and the potential usefulness of ArtiQ.Spiro, an AI-powered decision support tool. Physicians agreed with the AI-suggested diagnosis in 85% of cases. They found ArtiQ.Spiro useful for automatic estimation of the quality and for interpretation of the results: 4.13 and 4.01 respectively, on a Likert scale ranging from 1 to 5, versus an evaluated confidence of 3.65 and 3.85 in their own judgement without help from AI. From a practical perspective, 28.6% of patients with high risk for COPD in this study (smokers older than 35 years old with at least one respiratory complaint) were flagged as probable COPD [57, 58]. This suggests that AI could support the detection of previously undiagnosed COPD.
Clinical pathways for chronic respiratory diseases
Licskai et al. [59] (London, ON, Canada) showed a changing trend of health service utilisation in COPD patients after primary care intervention. They included all the 2451 COPD patients managed by the “Best Care” integrated disease management programme in Ontario, with 3-year retrospective data before the enrolment in the programme, and 3-year data from the intervention period. At 1-year follow-up, the monthly rates of COPD-related emergency department (ED) visits and COPD-related hospital admissions per 1000 individuals were respectively reduced by 19 (95% CI 12.5–25.5) and 9.1 (5.4–12.7), representing a relative reduction of 46% and 56%. After 3 years, rate reductions almost doubled. The same trends were observed for all cause ED visits and hospitalisations.
Minshall et al. [60] (Stoke-on-Trent, UK) presented some results from a programme using an asthma nurse educator (ANE) as a bridge between primary and secondary care. They incorporated a 4-step referral pathway to severe asthma centres based on confirmed diagnosis, treatment adherence, technique check, and ED visit and/or frequent need of oral steroids in the 12 previous months. During 9 months, 711 at risk patients over 33 practices were identified and reviewed by the ANE, generating 160 new patient referrals into the local severe asthma centre, and shortening the time to access to biologic therapy when needed. The ANE also delivered asthma education to primary care clinicians in a variety of formats, resulting in significantly increased confidence across many aspects of asthma care (diagnosis, management, treatment modification, referral, etc.).
Assessing and optimising inhaler technique
Given the lack of a validated and routinely used scoring system to assess inhaler device technique (IT) in clinical practice, De Vos et al. [61] (Portsmouth, UK) performed a systematic review to collate and evaluate methods of scoring IT in research literature. 77 studies were analysed, assessing 18 different inhaler devices. The authors observed a lack of consistency in the number of steps and content in published inhaler technique checklists across all device types. They concluded that an ideal scoring system should include a standardised and content-valid IT checklist, robust validation processes, and a meaningful score outcome that could facilitate IT optimisation. This is easier for a metered-dose inhaler where crucial steps are identified (e.g. remove the cap; shake well; breathe out fully; place the mouthpiece in the mouth; press down while inhaling deeply and slowly; hold breath for 10 s; exhale slowly) but will possess serious challenge in dry powder inhalers. Nonetheless, the availability of a validated measurement tool applicable to all inhaler device types would be an asset in clinical practice to guide the assessment, measurement and optimisation of IT, not only in primary care but also in all settings.
Online questions and answers service for primary clinicians
In 2020, the COVID-19 pandemic spurred Fitch et al. [62] (Hasselt, Belgium) to use the International Primary Care Respiratory Group (IPCRG) sentinel network, comprising over 120 physicians from every continent, to identify unanswered primary care questions, reflecting on the ground needs. As a result, an iQ&A service was launched in 2021, providing concise and updated evidence-based responses. By June 2023, 210 questions were raised, 57 were prioritised and answers provided. Over 17 000 views and 18 000 downloads of the iQ&A webpages have been recorded to June 2023 from all the world, half of these for the top 10 answers, mainly concerning COVID-19 treatments, comorbidities, vaccines (strategy, efficacy, safety), and long-term consequences. In the near future, this service will also include information about other respiratory conditions, supporting clinical practice and communication to patients.
Take-home messages
Primary-care-based disease management could significantly improve disease trajectory and reduce morbidity and mortality.
Three main topics for primary care improvement were highlighted: patient and clinician education, decision support, and improving diagnostics.
Primary care spirometry is profoundly underutilised in clinical practice.
Decision support frameworks with validated checklists and algorithms, sometimes supported by AI systems, are rapidly expanding and could significantly boost the confidence and effectiveness of physicians.
Group 1.04: m-health/e-health
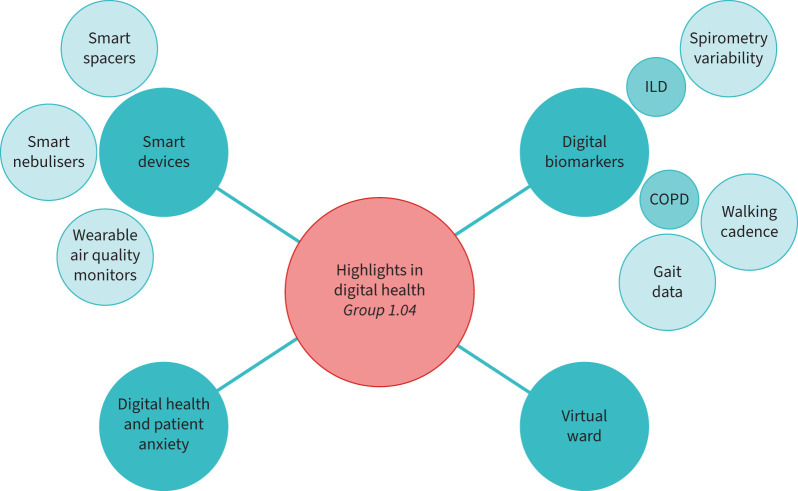
Digital health is an ERS priority, as reflected by the launch of the ERS-funded Clinical Research Collaboration “CONNECT” (Moving multiple digital innovations towards connected respiratory care: addressing the over-arching challenges of whole systems implementation) [63]. Several advancements were presented, compared to previous ERS Congresses [1, 64–67], on a great diversity of topics (figure 3). The sessions summarised in this section are “Digital solutions for respiratory medicine: a new way of working”, “Remote monitoring of respiratory patients” and “Digital health innovations in respiratory healthcare”.
FIGURE 3.
Highlights in digital health from Group 1.04. ILD: interstitial lung disease.
Smart devices
Smart devices are essential to allow the remote monitoring of patients and their environment. Fiebig et al. [68] (Starnberg, Germany) presented the results of a smart nebuliser (eTrack Controller with the eFlow nebuliser), together with an app (PARI Connect app) used by 70 patients with cystic fibrosis over a total period of 2 years. The average adherence was 52%, higher than in the literature (around 35%), but it declined after the first 3 months of use. Dierick et al. [69] (Groningen, the Netherlands) presented a smart spacer to monitor inhaler use and technique for patients with asthma. The device was evaluated in a 2-month randomised controlled feasibility trial in 42 asthma patients, resulting in a statistically significant reduction of inhaler errors by 26.2% while in the usual care group errors increased by 14.6%.
Using a cross-over trial, the impact of a tablet app monitoring disease-related variables was evaluated on the HR-QoL of 59 individuals with Global Initiative for Chronic Obstructive Lung Disease (GOLD) D COPD. The system was proven to be feasible and safe, but had no significant impact on the score of different scales and questionnaires (SF-12, CAT, mMRC, EQ5D, HADS) [70].
Finally, two teams focused on measuring personal exposure to pollution. Bernasconi et al. [71] (Milan, Italy) developed a wearable system that consisted of a miniaturised air quality sensor mounted on a wristband. The system collected data such as respiratory and heart rates, particulate matter (PM) of different sizes (PM1, PM2.5, PM10), carbon oxides, total volatile organic compounds, and nitrogen dioxide. Data were processed by a smartphone app. The app provided real-time feedback on patients' level of exposure to the air pollutants. Similarly, Atzeni et al. [72] (Padua, Italy) used a portable air quality monitor (Atmotube PRO, Atmotech Inc.) and a wrist activity monitor (Charge 5, Fitbit Inc), to measure the individual level of inhaled PM.
Digital biomarkers
Digital biomarkers are objective quantifiable measurements collected by digital devices to explain and/or predict the health-related outcomes. A dataset of 6276 home spirometry results from 101 ILD patients was used to assess whether 3-month functional variability associated with worsened breathlessness. Low variability over time in PEF and forced expiratory flow at 25–75% of the VC was associated with a higher risk of increased dyspnoea at 3 months, but not variability in FEV1 or forced VC [73].
In COPD, Delgado-Ortiz et al. [74] (Barcelona, Spain) analysed the data from 603 patients during 12 consecutive months and found that higher real-world walking cadence (in steps per min) was significantly associated with lower functional severity of the disease, better HR-QoL, and lower number of severe AE-COPD (adjusted incidence rate ratio 0.95, 95% CI 0.91–0.99). Similarly, Buekers et al. [75] (Barcelona, Spain) collected the gait data from 17 mild-severe COPD patients and 20 healthy participants, and found that the gait impairment in the patient group mainly manifested during relatively long walking bouts (>30 s).
Virtual ward
Ampikaipakan et al. [76] (Norwich, UK) presented the data from their virtual ward at the Norfolk and Norwich University Hospital. This virtual ward was launched during the COVID-19 pandemic for stable patients with COPD, bronchiectasis, pneumonia, empyema and COVID-19, and has continued ever since. Composed of 40 beds, it provides a 24/7 real-time home monitoring service to patients who are followed by a nursing team with daily consultant review. Since 2021, 1988 medical and surgical patients had been through the service with 15 604 bed days saved, an average length of stay of 7.8 days, and a re-admission rate limited to 10%.
Digital health and patient anxiety
A survey was conducted among 10 500 adults in the UK regarding their use of technology to manage their asthma. Patients who overused their reliever treatment were found to be more anxious and more adherent to monitoring. However, too frequent monitoring may further reinforce their anxiety [77]. This indicates that digital health interventions could be more easily adopted by anxious patients who feel the need to be monitored regularly.
Take-home messages
The field of smart devices continues to expand, with smart nebulisers, smart spacers, and wearable/portable sensors allowing patients to monitor their respiratory conditions and personal exposure to air pollution in real time.
Digital biomarkers including home spirometry variability in ILDs, walking cadence and gait patterns in COPD, are increasingly being studied and validated by real-world data.
Virtual wards, which involve 24/7 in-home monitoring of patients by a dedicated team of nurses and doctors, have become a reality with convincing results.
Digital health interventions could be more easily adopted by anxious patients who feel the need to be monitored regularly, with a caveat of the risk of reinforcing their anxiety with “too frequent” monitoring.
Group 1.05: clinical respiratory physiology, exercise and functional imaging
Imaging lung function in respiratory diseases
The “Imaging lung function in respiratory diseases” oral session focused on the role of functional imaging to detect, quantify, and monitor early lung changes in different respiratory diseases. The future role of functional imaging as a unique tool to personalise and optimise therapy was highlighted.
New developments in thoracic magnetic resonance imaging
To aid the clinical translation of hyperpolarised (HP) xenon-129 magnetic resonance imaging (129Xe-MRI), healthy-reference values for gas exchange metrics must be derived to better match demographics and improve diagnostic accuracy of early lung disease [78]. Collier et al. [79] (Sheffield, UK) addressed this practical challenge in 62 healthy subjects: they confirmed that 129Xe-MRI metrics depend on age and gender, HP gas doses, and breathing manoeuvres, which should be controlled for better diagnostic accuracy [80].
HP 129Xe-MRI was used to describe the longitudinal changes in lung function and microstructure in 136 patients with asthma and/or COPD taking part in the NOVELTY study [81]. MRI ventilation defect percent and gas transfer (129Xe red blood cell/membrane) worsened over 1 year, as well as diffusing capacity of the lung for carbon monoxide, whereas no changes in FEV1, residual volume/total lung capacity or alveolar microstructure were appreciable [82].
Foo et al. [83] (Victoria, Australia) used HP helium-3 MRI to assess the regional ventilation differences following direct and indirect inhalational challenges in eight patients with mild asthma. The results highlighted the heterogeneity of the disease as, despite a similar fall in FEV1, the distribution of ventilation defects was different and certain airways were more susceptible to bronchial provocation, regardless of the challenge method.
Functional computed tomography and micro-computed tomography imaging
Functional computed tomography (CT) imaging, which derives ventilation biomarkers from the analysis of images acquired at two different volumes, was investigated in two studies. Using a methodology developed in an earlier study [84], Shekarnabi et al. [85] (Grenoble, France) investigated the association of quantitative CT imaging phenotypes with clinical outcomes in a prospective cohort of 97 mechanically ventilated COVID-19 acute respiratory distress syndrome (ARDS) patients. With a clustering model, they found that those with less positive end-expiratory pressure-induced recruitment and more caudal ventilation had a significantly lower mortality (hazard ratio 0.30, 95% CI 0.13–0.69), suggesting that local lung behaviour may be a promising approach for patient stratification in clinical trials and for personalised mechanical ventilation in ARDS.
In a preclinical setting, Pennati et al. [86] (Milan, Italy) derived ventilation biomarkers from free-breathing micro-CT. In a longitudinal study using a bleomycin-induced murine model of lung fibrosis, they assessed the antifibrotic effect of nintedanib. They found that the bleomycin-induced alterations of the lung caused a progressive decrease in ventilation, whereas nintedanib resulted in a reduced accumulation of fibrotic lung tissue and restored ventilation. In the context of preclinical research, functional micro-CT provided relevant biomarkers to monitor disease progression, insights into the mechanism of action of candidate drugs, and may support clinical translation.
Innovative technologies for functional imaging
Innovative technologies for functional imaging were also reported during the congress. X-ray velocimetry, an emerging imaging technique that infers regional ventilation from the analysis of lung tissue motion from fluoroscopy during tidal breathing [87], was performed in 28 COPD patients and 22 healthy controls. Velocimetry-derived measures of ventilation were associated with COPD severity, symptoms, and lung function [88].
Minsuok Kim and colleagues (Seoul, Republic of Korea) investigated the CT-based full-scale airway network (FAN) flow model [89, 90] to simulate pulmonary ventilation in patients with persistent post-COVID-19 dyspnoea. The technique showed a good correlation of lobar ventilation with HP 129Xe-MRI and a higher fractal dimension in regions with low ventilation, which may be associated with breathlessness [91].
Long-term effects of COVID-19
Based on standard CT, long-term effects of COVID-19-associated ARDS (CARDS) were quantified, at 6- and 12-month follow-up after discharge from intensive care unit. In a study including 70 patients, Sfar et al. [92] (Kairouan, Tunisia) showed that residual lung abnormalities were present on CT scans in 69% of them after 6 months. Age, Charlson score, oxygen arterial partial pressure/inspired fraction ratio, and respiratory support duration, were found as predictors factors on univariate analysis. Milano et al. [93] (Vimercate, Italy) presented CT results of 34 patients with CARDS, during the acute phase and at 1-year follow-up, compared to a group of 20 healthy subjects. They found that median normo-aerated lung volume, extremely compromised during the acute phase in the patient group (1746 mL), significantly improved after 1 year (3974 mL), but remained significantly lower than in controls (5324 mL). The same team also presented consistent results from a larger study, supporting the need for long-term radiological and functional follow-up post-CARDS [94].
The organ-wide reach of exercise in chronic respiratory disease
The symposium “The organ-wide reach of exercise in chronic respiratory disease” was jointly organised by Assemblies 1 and 9. Its aims were to inform attendees on the role of inter-organ cross-talk during exercise, describe the beneficial effects of exercise training on skeletal muscles and identify patients that may need additional support to derive optimised benefits. The session concluded on the emerging idea that exercise may regenerate the lungs in patients with CRD.
Inter-organ cross-talk and cellular communication
Ronan Berg (Copenhagen, Denmark) presented recent evidence suggesting that skeletal muscles produce signalling molecules in response to exercise, called myokines, allowing for cross-talk between muscles and other organs [95]. In this context, myokines have potential roles in improving cardiovascular, metabolic, immune, and neurological function [96]. For instance, exercise may contribute to the downregulation of central mechanisms of feeding through the release of interleukin-6, while cathespsin B and irisin release may upregulate the expression of brain-derived neurotrophic factor, thus favouring hippocampal neurogenesis [97]. However, whether myokines mediate such beneficial effects of exercise in CRDs remains to be further investigated, as studies on inter-organ cross-talk and myokines signalling are scarce [97].
Effects of exercise training on muscle quality… and on lung regeneration?
One thing is certain: skeletal muscles represent a key target for rehabilitative exercise training in CRDs [98, 99]. Lorna Latimer (Leicester, UK) presented the latest evidence that muscle mRNA responses of genes linked to fat and carbohydrate oxidation were similar in COPD versus healthy controls after aerobic exercise training. Changes in whole-body exercise capacity and mitochondrial function were, however, absent in COPD, suggesting that the plasticity of skeletal muscles to exercise-induced stress is diminished at the post-transcriptional level [100]. Strategies to enhance muscle-level training intensity (e.g. interval training) may thus be valuable to derive appropriate muscle-level adaptation in COPD [101].
Could HIIT have hitherto unsuspected benefits? In that way, Ulrik Iepsen (Copenhagen, Denmark) offered perspectives regarding the regeneration potential of the lungs in COPD: their research team proposed that this exercise training modality may improve gas exchange during exercise in COPD patients by interrupting mesenchymal senescence, thus re-establishing adaptive angiogenesis [102]. This, however, will need to be experimentally tested.
Beneficial effects of ambulatory oxygen supplementation
Exertional hypoxaemia and dyspnoea are cardinal features of fibrotic ILDs (f-ILDs). Ambulatory oxygen supplementation may improve exercise capacity in this patient population despite no clear beneficial effects on dyspnoea [103, 104]. In this context, Mathieu Marillier (Grenoble, France) presented evidence that oxygen supplementation may have beneficial effects beyond the cardiopulmonary axis during exercise in f-ILDs, such as improved skeletal muscle [105, 106] and brain functioning [107, 108]. These studies thus offer a strong rationale for the use of oxygen during exercise in ILDs, regardless of which system or symptom is improved, in order to assist patients doing more daily physical exercise.
Take-home messages
The assessment of regional ventilation may facilitate treatment, monitoring and surveillance of patients with respiratory dysfunction.
In longitudinal studies, functional imaging was sensitive to early changes in different respiratory diseases.
The distribution of local ventilation is promising to predict clinical outcome and stratify patients.
Skeletal muscles produce myokines in response to exercise, allowing for cross-talk with other organs and promoting cardiovascular, metabolic, immune and neurological health.
Rehabilitative exercise training can improve skeletal muscle function in COPD, but alternative exercise modalities may be required in given patient subpopulations to derive such benefits.
Emerging hypotheses suggest that HIIT may “regenerate the lungs” in COPD by stopping mesenchymal senescence and re-establishing adaptive angiogenesis.
Positive effects of ambulatory oxygen supplementation in f-ILDs are not restricted to “the lungs”: this intervention can also benefit skeletal muscle and brain function in these patients.
Conclusion
In addition to the reports from the other assemblies [109–121], we hope that the highlights summarised in this article will help update readers with the impressive amount of respiratory research and advances in pulmonary clinical care presented through the sessions from ERS Assembly 1 (Respiratory Clinical Care and Physiology), alongside suggestions for further investigations. We also hope to have encouraged the readership to contribute to Assembly 1 activities, and to take part in the 2024 ERS Congress to be held in Vienna in September, where further scientific novelties and clinical developments on these topics will be discussed.
Footnotes
Provenance: Commissioned article, peer reviewed.
Conflict of interest: C-Y. Hui is a visitor at the University of Edinburgh and is a senior consultant in healthcare sustainability at Turner and Townsend. Her research with the University of Edinburgh is independent from, and not financially supported by, Turner and Townsend. Her views in this publication and the ERS Congress are her own, and not those of Turner and Townsend. Neither she, nor Turner and Townsend, stand to gain financially from work reported in this publication.
Conflict of interest: C. Vicente reports personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Mylan, outside the submitted work.
Conflict of interest: V. Poberezhets reports personal fees from AstraZeneca outside the submitted work.
Conflict of interest: F.M.E. Franssen reports grants and personal fees from AstraZeneca, Chiesi, GlaxoSmithKline and Sanofi, and personal fees from Pieris, outside the submitted work.
Conflict of interest: I. Vogiatzis is an associate editor of this journal.
Conflict of interest: T. Gille reports personal fees from Roche SAS, outside the submitted work.
Conflict of interest: The other authors have nothing to disclose.
References
- 1.Vontetsianos A, Karadeniz Güven D, Betka S, et al. ERS International Congress 2022: highlights from the Respiratory Clinical Care and Physiology Assembly. ERJ Open Res 2023; 9: 00194-2023. doi: 10.1183/23120541.00194-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valentin S, Lopez Padilla D, Nolasco S, et al. ERS International Congress 2022: highlights from the Respiratory Intensive Care Assembly. ERJ Open Res 2023; 9: 00532-2022. doi: 10.1183/23120541.00532-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuevas Ocaña S, El-Merhie N, Kuipers ME, et al. ERS International Congress 2022: highlights from the Basic and Translational Science Assembly. ERJ Open Res 2023; 9: 00561-2022. doi: 10.1183/23120541.00561-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradicich M, Siciliano M, Schiavi E, et al. ERS International Congress 2022: highlights from the Sleep Disordered Breathing Assembly. ERJ Open Res 2023; 9: 00582-2022. doi: 10.1183/23120541.00582-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beech A, Portacci A, Herrero-Cortina B, et al. ERS International Congress 2022: highlights from the Airway Diseases Assembly. ERJ Open Res 2023; 9: 00034-2023. doi: 10.1183/23120541.00034-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkin JGH, Delgado-Ortiz L, Delvert R, et al. ERS International Congress 2022: highlights from the Epidemiology and Environment Assembly. ERJ Open Res 2023; 9: 00574-2022. doi: 10.1183/23120541.00574-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardura-Garcia C, Kainz K, Mallet MC, et al. ERS International Congress 2022: highlights from the Paediatrics Assembly. ERJ Open Res 2023; 9: 00653-2022. doi: 10.1183/23120541.00653-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magouliotis DE, Bos S, Esendagli D, et al. ERS International Congress 2022: highlights from the Thoracic Surgery and Lung Transplantation Assembly. ERJ Open Res 2023; 9: 00671-2022. doi: 10.1183/23120541.00671-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price OJ, Paixão C, Poddighe D, et al. ERS International Congress 2022: highlights from the Allied Respiratory Professionals Assembly. ERJ Open Res 2023; 9: 00013-2023. doi: 10.1183/23120541.00013-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banka R, Chichirelo-Konstantynovych K, Horton KL, et al. ERS International Congress 2022: highlights from the Respiratory Infections Assembly. ERJ Open Res 2023; 9: 00628-2022. doi: 10.1183/23120541.00628-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catarata MJ, Van Geffen WH, Banka R, et al. ERS International Congress 2022: highlights from the Thoracic Oncology Assembly. ERJ Open Res 2023; 9: 00579-2022. doi: 10.1183/23120541.00579-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karampitsakos T, Diep PP, Loth DW, et al. ERS International Congress 2022: highlights from the Interstitial Lung Diseases Assembly. ERJ Open Res 2023; 9: 00584-2022. doi: 10.1183/23120541.00584-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtblau M, Piccari L, Ramjug S, et al. ERS International Congress 2021: highlights from the Pulmonary Vascular Diseases Assembly. ERJ Open Res 2022; 8: 00665-2021. doi: 10.1183/23120541.00665-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bal C, Falster C, Carvalho A, et al. ERS International Congress 2021: highlights from the Clinical Techniques, Imaging and Endoscopy Assembly. ERJ Open Res 2022; 8: 00116-2022. doi: 10.1183/23120541.00116-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation 2020; 142: 342–353. doi: 10.1161/CIRCULATIONAHA.120.047971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touil I, Hassen IHA, Salma C, et al. Correlation between endothelial dysfunction and left ventricular global strain in patients with COVID-19 (from the TUN ENDCOV Study). Eur Respir J 2023; 62: Suppl. 67, OA4323. doi: 10.1183/13993003.congress-2023.OA4323 [DOI] [Google Scholar]
- 17.Yelin D, Wirtheim E, Vetter P, et al. Long-term consequences of COVID-19: research needs. Lancet Infect Dis 2020; 20: 1115–1117. doi: 10.1016/S1473-3099(20)30701-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sesé L, Chauveau S, Giroux-Leprieur B, et al. Prevalence and associated factors to post-COVID-19 functional respiratory complaints (FRCs) identified by the Nijmegen questionnaire (NQ). Eur Respir J 2023; 62: Suppl. 67, OA4324. doi: 10.1183/13993003.congress-2023.OA4324 [DOI] [Google Scholar]
- 19.Thomas M, McKinley RK, Freeman E, et al. The prevalence of dysfunctional breathing in adults in the community with and without asthma. Prim Care Respir J 2005; 14: 78–82. doi: 10.1016/j.pcrj.2004.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beurnier A, Savale L, Jaïs X, et al. Functional respiratory complaints among COVID-19 survivors: a prospective cohort study. ERJ Open Res 2023; 9: 00063-2023. doi: 10.1183/23120541.00063-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allado E, Poussel M, Hamroun A, et al. Is there a relationship between hyperventilation syndrome and history of acute SARS-CoV-2 infection? A cross-sectional study. Healthcare (Basel) 2022; 10: 2154. doi: 10.3390/healthcare10112154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans RA, McAuley H, Harrison EM, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med 2021; 9: 1275–1287. doi: 10.1016/S2213-2600(21)00383-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Yan B, Gao R, et al. Effectiveness of corticosteroids to treat severe COVID-19: a systematic review and meta-analysis of prospective studies. Int Immunopharmacol 2021; 100: 108121. doi: 10.1016/j.intimp.2021.108121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russell R, Leavy O, Kerr S, et al. One year health-outcomes in adults hospitalised with COVID-19 who received systemic corticosteroid treatment: prospective cohort study. Eur Respir J 2023; 62: Suppl. 67, OA4325. doi: 10.1183/13993003.congress-2023.OA4325 [DOI] [Google Scholar]
- 25.McCarthy C, Carey BC, Trapnell BC. Autoimmune pulmonary alveolar proteinosis. Am J Respir Crit Care Med 2022; 205: 1016–1035. doi: 10.1164/rccm.202112-2742SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tazawa R, Ueda T, Abe M, et al. Inhaled GM-CSF for pulmonary alveolar proteinosis. N Engl J Med 2019; 381: 923–932. doi: 10.1056/NEJMoa1816216 [DOI] [PubMed] [Google Scholar]
- 27.Nakata K, Tazawa R, Page Research Group, et al. Two-year cohort study after GM-CSF inhalation therapy for autoimmune pulmonary alveolar proteinosis. Eur Respir J 2023; 62: Suppl. 67, OA4322. doi: 10.1183/13993003.congress-2023.OA4322 [DOI] [Google Scholar]
- 28.Sandau C, Jensen J-U, Pedersen L, et al. Automatic oxygen control during admission with exacerbation of COPD - a multicenter randomised controlled trial. Eur Respir J 2023; 62: Suppl. 67, RCT3213. doi: 10.1183/13993003.congress-2023.RCT3213 [DOI] [Google Scholar]
- 29.Sandau C, Hansen EF, Ringbæk TJ, et al. Automated oxygen administration alleviates dyspnea in patients admitted with acute exacerbation of COPD: a randomized controlled trial. Int J Chron Obstruct Pulmon Dis 2023; 18: 599–614. doi: 10.2147/COPD.S397782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roca O, Caritg O, Santafé M, et al. Closed-loop oxygen control improves oxygen therapy in acute hypoxemic respiratory failure patients under high flow nasal oxygen: a randomized cross-over study (the HILOOP study). Crit Care 2022; 26: 108. doi: 10.1186/s13054-022-03970-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dronkers CEA, van der Hulle T, Le Gal G, et al. Towards a tailored diagnostic standard for future diagnostic studies in pulmonary embolism: communication from the SSC of the ISTH. J Thromb Haemost 2017; 15: 1040–1043. doi: 10.1111/jth.13654 [DOI] [PubMed] [Google Scholar]
- 32.Falster C, Nielsen RW, Møller JE, et al. Does ultrasound in suspected pulmonary embolism safely reduce referral to diagnostic imaging? – a randomized controlled trial. Eur Respir J 2023; 62: Suppl. 67, RCT3216. doi: 10.1183/13993003.congress-2023.RCT3216 [DOI] [Google Scholar]
- 33.Cao J, Sun J, Wang Y, et al. Diagnostic accuracy of cardiopulmonary ultrasound for pulmonary embolism: a systematic review and meta-analysis. Echocardiography 2022; 39: 185–193. doi: 10.1111/echo.15280 [DOI] [PubMed] [Google Scholar]
- 34.Leitl D, Schneeberger T, Jarosch I, et al. Effects of a symptom-based rehabilitation on cardiopulmonary exercise capacity in post COVID-19: a preliminary subgroup analysis of the ReLoAd cohort. Eur Respir J 2023; 62: Suppl. 67, PA5052. doi: 10.1183/13993003.congress-2023.PA5052 [DOI] [Google Scholar]
- 35.Evans RA, Ibbetson A, Houchen-Wolloff L, et al. Post-hospitalisation COVID-19 healthcare pathways and 1 year health-related quality of life (HRQoL): effect of clinical assessment and rehabilitation. Eur Respir J 2023; 62: Suppl. 67, OA4224. doi: 10.1183/13993003.congress-2023.OA4224 [DOI] [Google Scholar]
- 36.Elneima O, McAuley HJC, Leavy OC, et al. Post-hospitalisation COVID-19 (PHOSP-COVID): a prospective multi-centre UK cohort study. Eur Respir J 2023; 62: Suppl. 67, OA2491. doi: 10.1183/13993003.congress-2023.OA2491 [DOI] [Google Scholar]
- 37.McAuley H, Flynn C, Latimer L, et al. High muscle quality index in early follow up predicts 1 year recovery following hospitalisation for COVID-19. Eur Respir J 2023; 62: Suppl. 67, PA1797. doi: 10.1183/13993003.congress-2023.PA1797 [DOI] [Google Scholar]
- 38.Latimer L, Constantin D, Houchen-Wolloff L, et al. Muscle mRNA and protein responses to aerobic exercise training (AET) in COPD and health. Eur Respir J 2023; 62: Suppl. 67, OA3256. doi: 10.1183/13993003.congress-2023.OA3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn M, Clarenbach CF, Kläy A, et al. Blood-flow restricted cycling maximizes leg muscle effort with less ventilatory work compared to work-matched free-flow exercise: a randomized crossover study. Eur Respir J 2023; 62: Suppl. 67, OA3253. doi: 10.1183/13993003.congress-2023.OA3253 [DOI] [Google Scholar]
- 40.Kohlbrenner D, Kuhn M, Manettas A, et al. Low-load blood flow restriction strength training in patients with COPD: a randomised single-blind pilot study. Thorax 2024; 79: 340–348. doi: 10.1136/thorax-2023-220546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aparecido da Silva R, Leite Rocco PG, Stelmach R, et al. Constant-load exercise versus high-intensity interval training on aerobic fitness in moderate-to-severe asthma: a randomized controlled trial. J Allergy Clin Immunol Pract 2022; 10: 2596–2604.e7. doi: 10.1016/j.jaip.2022.05.023 [DOI] [PubMed] [Google Scholar]
- 42.Aparecido da Silva R, Fernandes T, Stelmach R, et al. High-intensity interval training (HIIT) versus constant-load exercise (CLE) on the short-acute beta agonist (SABA) consumption and peak-expiratory flow (PEF) in subjects with moderate to severe asthma. Eur Respir J 2023; 62: Suppl. 67, OA4219. doi: 10.1183/13993003.congress-2023.OA4219 [DOI] [Google Scholar]
- 43.Kim F, Rocha J, Lunardi AC, et al. Effects of association of aerobic and breathing exercises on clinical controls, physical capacity, and psychosocial status in patients with moderate to severe asthma: an RCT. Eur Respir J 2023; 62: Suppl. 67, OA4222. doi: 10.1183/13993003.congress-2023.OA4222 [DOI] [Google Scholar]
- 44.Machado AF, Dias C, Gonçalves AP, et al. Effects of pulmonary rehabilitation during acute exacerbations of COPD. Eur Respir J 2023; 62: Suppl. 67, OA2619. doi: 10.1183/13993003.congress-2023.OA2619 [DOI] [Google Scholar]
- 45.Géphine S, Rouzic OL, Chenivesse C, et al. Is longer really better? Results of a real-life study comparing once- versus twice-weekly home-based pulmonary rehabilitation session in people with COPD. Eur Respir J 2023; 62: Suppl. 67, PA966. doi: 10.1183/13993003.congress-2023.PA966 [DOI] [Google Scholar]
- 46.Patel S, Palmer MD, Nolan CM, et al. Supervised pulmonary rehabilitation using minimal or specialist exercise equipment in COPD: a propensity-matched analysis. Thorax 2021; 76: 264–271. doi: 10.1136/thoraxjnl-2020-215281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nolan CM, Glen C, Patel S, et al. Minimal versus specialist equipment in the delivery of pulmonary rehabilitation: a non-inferiority RCT. Eur Respir J 2023; 62: Suppl. 67, RCT3215. doi: 10.1183/13993003.congress-2023.RCT3215 [DOI] [Google Scholar]
- 48.Manifield J, Chaudry YA, Singh SJ, et al. Pulmonary rehabilitation and physical activity: a step in the right direction? A network meta-analysis. Eur Respir J 2023; 62: Suppl. 67, OA3260. doi: 10.1183/13993003.congress-2023.OA3260 [DOI] [Google Scholar]
- 49.Jenkins AR, Gaynor-Sodeifi K, Lewthwaite H, et al. Efficacy of interventions to alter measures of fat-free mass in people with COPD: a systematic review and meta-analysis. ERJ Open Res 2023; 9: 00102-2023. doi: 10.1183/23120541.00102-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaes AW, Sievi NA, Clarenbach CF, et al. Mortality in COPD: physical capacity of physical activity? Eur Respir J 2023; 62: Suppl. 67, OA3259. doi: 10.1183/13993003.congress-2023.OA3259 [DOI] [Google Scholar]
- 51.Vaes AW, Spruit MA, Koolen EH, et al. “Can do, do do” quadrants and 6-year all-cause mortality in patients with COPD. Chest 2022; 161: 1494–1504. doi: 10.1016/j.chest.2021.12.657 [DOI] [PubMed] [Google Scholar]
- 52.Björklund F, Palm A, Gorani JA, et al. Breathlessness and exercise performance to predict mortality in long-term oxygen therapy - the population-based DISCOVERY study. Respir Med 2023; 216: 107306. doi: 10.1016/j.rmed.2023.107306 [DOI] [PubMed] [Google Scholar]
- 53.Ward T, Greening NJ, Bolton CE, et al. Survival following pulmonary rehabilitation (PR): the effect of achieving the minimal important difference (MID) in walking distance. Eur Respir J 2023; 62: Suppl. 67, OA3254. doi: 10.1183/13993003.congress-2023.OA3254 [DOI] [Google Scholar]
- 54.Sunjaya AP, Homaira N, Corcoran K, et al. Assessment and diagnosis of chronic dyspnoea: a literature review. NPJ Prim Care Respir Med 2022; 32: 10. doi: 10.1038/s41533-022-00271-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sunjaya AP, Martin A, Tanna GLD, et al. Developing a clinical pathway for breathlessness in primary care based on analysis of routine primary care data. Eur Respir J 2023; 62: Suppl. 67, OA762. doi: 10.1183/13993003.congress-2023.OA762 [DOI] [Google Scholar]
- 56.Kocks JWH, Arling C, Rütte TAL, et al. Improving the diagnosis of breathlessness in primary care: development and implementation of the “Breathlessness Diagnostics in a Box” (BiaB). Eur Respir J 2023; 62: Suppl. 67, OA761. doi: 10.1183/13993003.congress-2023.OA761 [DOI] [Google Scholar]
- 57.de Vos M, Maes J, Orshoven KV, et al. Insights into spirometry usage and the potential for AI-based decision support software in Belgian primary care. Eur Respir J 2023; 62: Suppl. 67, OA764. doi: 10.1183/13993003.congress-2023.OA764 [DOI] [Google Scholar]
- 58.Willaert S, Maes J, Topalovic M, et al. Belgian General Practitioners’ perspectives on the use of spirometry in primary care: a qualitative study. Eur Respir J 2023; 62: Suppl. 67, PA536. doi: 10.1183/13993003.congress-2023.PA536 [DOI] [Google Scholar]
- 59.Licskai C, Hussey A, Rowley V, et al. Sustained health system benefits of primary care based integrated disease management for COPD: an interrupted time series. Eur Respir J 2023; 62: Suppl. 67, OA768. doi: 10.1183/13993003.congress-2023.OA768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minshall H, Cooper A, Idris E. The role of the asthma nurse educator in identifying severe asthma in primary care. Eur Respir J 2023; 62: Suppl. 67, OA767. doi: 10.1183/13993003.congress-2023.OA767 [DOI] [Google Scholar]
- 61.de Vos R, Hicks A, Lomax M, et al. A systematic review of methods of scoring inhaler technique. Respir Med 2023; 219: 107430. doi: 10.1016/j.rmed.2023.107430 [DOI] [PubMed] [Google Scholar]
- 62.Fitch N, Kaplan A, Mosgrove F, et al. Providing evidence based answers to first hand COVID and respiratory questions from the IPCRG Sentinel Network of practising primary care clinicians: a novel service report. Eur Respir J 2023; 62: Suppl. 67, OA770. doi: 10.1183/13993003.congress-2023.OA770 [DOI] [Google Scholar]
- 63.van Boven JFM, Drummond D, Chan AHY, et al. ERS “CONNECT” Clinical Research Collaboration – moving multiple digital innovations towards connected respiratory care: addressing the over-arching challenges of whole systems implementation. Eur Respir J 2023; 62: 2301680. doi: 10.1183/13993003.01680-2023 [DOI] [PubMed] [Google Scholar]
- 64.Gille T, Sivapalan P, Kaltsakas G, et al. ERS International Congress 2021: highlights from the Respiratory Clinical Care and Physiology Assembly. ERJ Open Res 2022; 8: 00710-2021. doi: 10.1183/23120541.00710-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daines L, Buekers J, Bolado BA, et al. ERS International Congress 2020: highlights from the General Pneumology Assembly. ERJ Open Res 2021; 7: 00841-2020. doi: 10.1183/23120541.00841-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vanfleteren LEGW, Blervaque L, Franssen FME, et al. ERS International Congress, Madrid, 2019: highlights from the General Pneumology Assembly. ERJ Open Res 2020; 6: 00323-2019. doi: 10.1183/23120541.00323-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vanfleteren LEGW, Ojanguren I, Nolan CM, et al. European Respiratory Society International Congress, Paris, 2018: highlights from the Clinical Assembly. ERJ Open Res 2019; 5: 00176-2018. doi: 10.1183/23120541.00176-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fiebig D, Reimpell P, Mautone S, et al. Making therapy behaviour transparent by using an app and a Bluetooth enabled electronic nebulizer, was found to increase therapy adherence. Eur Respir J 2023; 62: Suppl. 67, OA3177. doi: 10.1183/13993003.congress-2023.OA3177 [DOI] [Google Scholar]
- 69.Dierick BJH, Achterbosch M, Eikholt AA, et al. Electronic monitoring with a digital smart spacer to support personalized inhaler use education in patients with asthma: the randomized controlled OUTERSPACE trial. Respir Med 2023; 218: 107376. doi: 10.1016/j.rmed.2023.107376 [DOI] [PubMed] [Google Scholar]
- 70.Frerichs M, Andelid K, Andersson A, et al. Remote MONITORing of patients with chronic obstructive pulmonary disease using a tablet system. A randomized crossover study on quality of life measurements. Eur Respir J 2023; 62: Suppl. 67, OA3179. doi: 10.1183/13993003.congress-2023.OA3179 [DOI] [Google Scholar]
- 71.Bernasconi S, Angelucci A, Rossi A, et al. A wearable system for personal air pollution exposure: a walk-about in Milan. Eur Respir J 2023; 62: Suppl. 67, PA2908. doi: 10.1183/13993003.congress-2023.PA2908 [DOI] [Google Scholar]
- 72.Atzeni M, Cossu L, Grisi C, et al. AirPredict - a mobile app for monitoring personal exposure to particulate matter and respiratory outcomes in asthma patients. Eur Respir J 2023; 62: Suppl. 67, PA2894. doi: 10.1183/13993003.congress-2023.PA2894 [DOI] [Google Scholar]
- 73.Gandhi A, Wen J, Edwards C, et al. Variability in home spirometry is associated with dyspnoea in interstitial lung disease. Eur Respir J 2023; 62: Suppl. 67, OA3182. doi: 10.1183/13993003.congress-2023.OA3182 [DOI] [Google Scholar]
- 74.Delgado-Ortiz L, Ranciati S, Arbillaga-Etxarri A, et al. Clinical validation of real-world walking cadence in patients with COPD. Eur Respir J 2023; 62: Suppl. 67, OA3138. doi: 10.1183/13993003.congress-2023.OA3138 [DOI] [Google Scholar]
- 75.Buekers J, Megaritis D, Koch S, et al. Laboratory and free-living gait performance in adults with COPD and healthy controls. ERJ Open Res 2023; 9: 00159-2023. doi: 10.1183/23120541.00159-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ampikaipakan SN, Beard C, Cooper K, et al. Virtual ward (VW) in respiratory medicine - a fleeting premise or a sustainable enterprise? Eur Respir J 2023; 62: Suppl. 67, OA3180. doi: 10.1183/13993003.congress-2023.OA3180 [DOI] [Google Scholar]
- 77.Bennett-Steele N, Cumella A, Francis A, et al. Exploring technology preferences and reliever inhaler use in adults with asthma – survey results with behavioural segmentation analysis. Eur Respir J 2023; 62: Suppl. 67, OA3176. doi: 10.1183/13993003.congress-2023.OA3176 [DOI] [Google Scholar]
- 78.Plummer JW, Willmering MM, Cleveland ZI, et al. Childhood to adulthood: accounting for age dependence in healthy-reference distributions in 129Xe gas-exchange MRI. Magn Reson Med 2023; 89: 1117–1133. doi: 10.1002/mrm.29501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Collier G, Saunders L, Smith LJ, et al. Age, sex and lung volume dependence of 129Xe MRI gas exchange metrics. Eur Respir J 2023; 62: Suppl. 67, OA4952. doi: 10.1183/13993003.congress-2023.OA4952 [DOI] [PubMed] [Google Scholar]
- 80.Niedbalski PJ, Hall CS, Castro M, et al. Protocols for multi-site trials using hyperpolarized 129Xe MRI for imaging of ventilation, alveolar-airspace size, and gas exchange: a position paper from the 129Xe MRI clinical trials consortium. Magn Reson Med 2021; 86: 2966–2986. doi: 10.1002/mrm.28985 [DOI] [PubMed] [Google Scholar]
- 81.Marshall H, Wild JM, Smith LJ, et al. Functional imaging in asthma and COPD: design of the NOVELTY ADPro substudy. ERJ Open Res 2023; 9: 00344-2022. doi: 10.1183/23120541.00344-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marshall H, Smith L, Biancardi A, et al. Longitudinal change in lung physiology and 129Xe MRI over 1 year in patients with asthma and/or COPD. Eur Respir J 2023; 62: Suppl. 67, OA4964. doi: 10.1183/13993003.congress-2023.OA4964 [DOI] [Google Scholar]
- 83.Foo CT, Nilsen K, Cowin G, et al. Hyperpolarised helium MRI in direct and indirect asthmatic airway challenge. Eur Respir J 2023; 62: Suppl. 67, OA4956. doi: 10.1183/13993003.congress-2023.OA4956 [DOI] [Google Scholar]
- 84.Shekarnabi M, Ahaouari T, Richard J-C, et al. CT registration-derived biomarkers of recruitability in ARDS. In: IEEE 20th International Symposium on Biomedical Imaging (ISBI). Cartagena, Colombia; 2023; pp. 1–5. [Google Scholar]
- 85.Shekarnabi M, Sigaud F, Gaillet M, et al. Phenotypes of functional CT imagining predict clinical outcome in ARDS patients. Eur Respir J 2023; 62: Suppl. 67, OA4955. doi: 10.1183/13993003.congress-2023.OA4955 [DOI] [Google Scholar]
- 86.Pennati F, Leo L, Ferrini E, et al. Micro-CT-derived ventilation biomarkers for the longitudinal assessment of pathology and response to therapy in a mouse model of lung fibrosis. Sci Rep 2023; 13: 4462. doi: 10.1038/s41598-023-30402-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siddharthan T, Grealis K, Kirkness JP, et al. Quantifying ventilation by X-ray velocimetry in healthy adults. Respir Res 2023; 24: 215. doi: 10.1186/s12931-023-02517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raju S, Grealis K, Gonzalez E, et al. Ventilation heterogeneity from X-ray velocimetry predicts COPD severity. Eur Respir J 2023; 62: Suppl. 67, OA4953. doi: 10.1183/13993003.congress-2023.OA4953 [DOI] [Google Scholar]
- 89.Kim M, Doganay O, Hwang HJ, et al. Lobar ventilation in patients with COPD assessed with the full-scale airway network flow model and xenon-enhanced dual-energy CT. Radiology 2021; 298: 201–209. doi: 10.1148/radiol.2020202485 [DOI] [PubMed] [Google Scholar]
- 90.Inui S, Yoon SH, Doganay O, et al. Impaired pulmonary ventilation beyond pneumonia in COVID-19: a preliminary observation. PLoS One 2022; 17: e0263158. doi: 10.1371/journal.pone.0263158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim M, Hwang J, Grist JT, et al. Functional impairment in small airways associated with the breathlessness symptoms in long-coronavirus disease. J Thorac Imaging 2024; 39: 79–85. doi: 10.1097/RTI.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 92.Sfar H, Mansour SB, Ali YB, et al. Chest CT scan pulmonary sequelae in ICU COVID-19 survivors. Eur Respir J 2023; 62: Suppl. 67, OA4960. doi: 10.1183/13993003.congress-2023.OA4960 [DOI] [Google Scholar]
- 93.Milano C, Corrò B, Pagani S, et al. COVID-19 associated ARDS: quantitative CT study from acute phase to 1-year follow-up. Eur Respir J 2023; 62: Suppl. 67, OA4959. doi: 10.1183/13993003.congress-2023.OA4959 [DOI] [Google Scholar]
- 94.Ripamonti R, Corrò B, Pagani S, et al. Quantitative CT lung alterations and lung function in COVID-19 associated ARDS: 1-year follow-up. Eur Respir J 2023; 62: Suppl. 67, PA3528. doi: 10.1183/13993003.congress-2023.PA3528 [DOI] [Google Scholar]
- 95.Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev 2020; 41: 594–609. doi: 10.1210/endrev/bnaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chow LS, Gerszten RE, Taylor JM, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol 2022; 18: 273–289. doi: 10.1038/s41574-022-00641-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol 2019; 15: 383–392. doi: 10.1038/s41574-019-0174-x [DOI] [PubMed] [Google Scholar]
- 98.Marillier M, Bernard A-C, Vergès S, et al. Locomotor muscles in COPD: the rationale for rehabilitative exercise training. Front Physiol 2019; 10: 1590. doi: 10.3389/fphys.2019.01590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rochester CL, Alison JA, Carlin B, et al. Pulmonary rehabilitation for adults with chronic respiratory disease: an official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2023; 208: e7–e26. doi: 10.1164/rccm.202306-1066ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Latimer LE, Constantin-Teodosiu D, Popat B, et al. Whole-body and muscle responses to aerobic exercise training and withdrawal in ageing and COPD. Eur Respir J 2022; 59: 2101507. doi: 10.1183/13993003.01507-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Louvaris Z, Chynkiamis N, Spetsioti S, et al. Greater exercise tolerance in COPD during acute interval, compared to equivalent constant-load, cycle exercise: physiological mechanisms. J Physiol 2020; 598: 3613–3629. doi: 10.1113/JP279531 [DOI] [PubMed] [Google Scholar]
- 102.Nymand SB, Hartmann JP, Ryrsø CK, et al. Exercise adaptations in COPD: the pulmonary perspective. Am J Physiol Lung Cell Mol Physiol 2022; 323: L659–L666. doi: 10.1152/ajplung.00549.2020 [DOI] [PubMed] [Google Scholar]
- 103.Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 2020; 202: e121–e141. doi: 10.1164/rccm.202009-3608ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bell EC, Cox NS, Goh N, et al. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev 2017; 26: 160080. doi: 10.1183/16000617.0080-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marillier M, Bernard A-C, Verges S, et al. Oxygen supplementation during exercise improves leg muscle fatigue in chronic fibrotic interstitial lung disease. Thorax 2021; 76: 672–680. doi: 10.1136/thoraxjnl-2020-215135 [DOI] [PubMed] [Google Scholar]
- 106.Marillier M, Bernard A-C, Verges S, et al. Quantifying leg muscle deoxygenation during incremental cycling in hypoxemic patients with fibrotic interstitial lung disease. Clin Physiol Funct Imaging 2023; 43: 192–200. doi: 10.1111/cpf.12809 [DOI] [PubMed] [Google Scholar]
- 107.Marillier M, Bernard A-C, Verges S, et al. Influence of exertional hypoxemia on cerebral oxygenation in fibrotic interstitial lung disease. Respir Physiol Neurobiol 2021; 285: 103601. doi: 10.1016/j.resp.2020.103601 [DOI] [PubMed] [Google Scholar]
- 108.Marillier M, Gruet M, Bernard A-C, et al. Beyond the lungs: O2 supplementation improves cerebral oxygenation and fatigue during exercise in interstitial lung disease. Med Sci Sports Exerc 2023; 55: 1735–1744. doi: 10.1249/MSS.0000000000003208 [DOI] [PubMed] [Google Scholar]
- 109.Bianquis C, Leiva Agüero S, Cantero C, et al. ERS International Congress 2023: highlights from the Respiratory Intensive Care Assembly. ERJ Open Res 2024; 10: 00886-2023. doi: 10.1183/23120541.00886-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reddy KD, Bizymi N, Schweikert A, et al. ERS International Congress 2023: highlights from the Basic and Translational Sciences Assembly. ERJ Open Res 2024; 10: 00875-2023. doi: 10.1183/23120541.00875-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Siciliano M, Bradicich M, Tondo P, et al. ERS International Congress 2023: highlights from the Sleep Disordered Breathing Assembly. ERJ Open Res 2024; 10: 00823-2023. doi: 10.1183/23120541.00823-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bergantini L, Baker J, Bossios A, et al. ERS International Congress 2023: highlights from the Airway Diseases Assembly. ERJ Open Res 2024; 10: 00891-2023. doi: 10.1183/23120541.00891-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Delgado-Ortiz L, Çakmakcı Karakaya S, Williams PJ, et al. ERS International Congress 2023: highlights from the Epidemiology and Environment Assembly. ERJ Open Res 2024; 10: 00134-2024. doi: 10.1183/23120541.00134-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vijverberg SJH, Kampouras A, Nayir Büyükşahin H, et al. ERS International Congress 2023: highlights from the Paediatrics Assembly. ERJ Open Res 2024; 10: 00853-2023. doi: 10.1183/23120541.00853-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zajacova A, Scaramozzino MU, Bellini A, et al. ERS International Congress 2023: highlights from the Thoracic Surgery and Lung Transplantation Assembly. ERJ Open Res 2024; 10: 00854-2023. doi: 10.1183/23120541.00854-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jacinto T, Smith E, Diciolla NS, et al. ERS International Congress 2023: highlights from the Allied Respiratory Professionals Assembly. ERJ Open Res 2024; 10: 00889-2023. doi: 10.1183/23120541.00889-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bindo F, Fumagalli G, Myroniuk-Konstantynovych K, et al. ERS International Congress 2023: highlights from the Respiratory Infections Assembly. ERJ Open Res 2024; 10: 00880-2023. doi: 10.1183/23120541.00880-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Catarata MJ, Creamer AW, Dias M, et al. ERS International Congress 2023: highlights from the Thoracic Oncology Assembly. ERJ Open Res 2024; 10: 00860-2023. doi: 10.1183/23120541.00860-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fabbri L, Guiot J, Vermant M, et al. ERS International Congress 2023: highlights from the Interstitial Lung Diseases Assembly. ERJ Open Res 2024; 10: 00839-2023. doi: 10.1183/23120541.00839-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cullivan S, Boucly A, Jevnikar M, et al. ERS International Congress 2023: highlights from the Pulmonary Vascular Diseases Assembly. ERJ Open Res 2024; 10: 00847-2023. doi: 10.1183/23120541.00847-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moretti A, Pietersen PI, Hassan M, et al. ERS International Congress 2023: highlights from the Clinical Techniques, Imaging and Endoscopy Assembly. ERJ Open Res 2024; 10: 00836-2023. doi: 10.1183/23120541.00836-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]