Abstract
Non-vesicular lipid transport via lipid transfer proteins (LTPs) at membrane contact sites (MCSs) is critical for the maintenance of the lipid composition of biological membranes. The ability to measure lipid transfer activity of diverse LTPs in live cells without interrupting the fine structural organization is essential to better understand the contribution of non-vesicular lipid transport to membrane organization. Here, we describe a semi-quantitative method to analyze phosphatidylinositol 4-phosphate (PI4P) and phosphatidylserine (PS) changes at the plasma membrane (PM) as they relate to LTP functions. This live cell-method is based on bioluminescence resonance energy transfer (BRET) measurements between a luciferase-tagged lipid recognizing module and a PM-targeted fluorescent acceptor. Oxysterol-binding protein related protein (ORP) 5 is a PI4P/PS lipid transfer protein which is stably tethered to the ER and also dynamically interacts with PM PI4P/PI(4,5)P2 via its pleckstrin homology (PH) domain. We show that this method is able to detect PI4P and PS changes in the PM upon acute recruitment of an ORP5 construct to the PM. This method is convenient and robust, utilizing population of cells in 96-well plates analyzed in a plate reader. We will also highlight potential further applications extending the method for other LTPs and other lipid cargoes.
Keywords: Lipid transfer protein, Membrane contact sites, Plasma membrane, ORP5, Phosphatidylinositol 4-phosphate, Phosphatidylserine, Bioluminescence resonance energy transfer
1. Introduction
Membrane contact sites (MCSs) are specialized regions of biological membranes where different organelles are juxtapositioned with their membranes forming tight contacts separated by only 30 nm. MCSs are highly conserved in eukaryotes from yeasts to mammals [1,2]}. MCSs are important as sites where intracellular organelles dynamically exchange various molecules. Lipids, in particular have a highly unfavorable energetics when crossing from one membrane to another via the aqueous phase due to their hydrophobicity. Lipids can be transported between membranes in a number of ways, but mostly use either vesicular lipid transport or lipid transfer proteins (non-vesicular lipid transport) [3]. Non-vesicular lipid transport utilizes numerous lipid transfer proteins (LTPs) to deliver lipid molecules at MCSs with a wide variety of cargo specificity and intracellular localization. LTPs are classified by their structural features. For instance, Oxysterol binding protein (OSBP) family or OSBP-related proteins (ORPs), which are found in both mammals (12 homologs) and yeast (7 homologs) possess OSBP-related domain (ORD) as the site for lipid transfer [4]. Some ORPs also possess FFAT domains to interact with the ER-protein VAP-A and VAP-B, and N-terminal PH domains to recognize phosphatidylinositol 4-phosphate (PI4P) or other phosphoinositides [4]. The ORD domain of OSBP and the yeast protein Osh4 can transport both PI4P and cholesterol [5,6]}while the ORD domain of ORP5 and its yeast homolog Osh6p are capable of transporting PI4P from the PM to the ER and phosphatidylserine (PS) in the reverse direction [7,8]. In the following sections we will describe how to examine the lipid transfer function of LTPs using ORP5 as an example. Most commonly, lipid transfer activities of LTPs have been examined using recombinant LTPs and liposomes of various lipid composition applied to in vitro lipid transfer assays. This method measures the ability of an LTP to exchange lipids between donor and acceptor liposomes of specific lipid compositions. While these assays have been and remain extremely useful to study these processes under well-defined in vitro conditions, they have several limitations. First, they require highly purified recombinant proteins that may work differently in isolation than they do inside the cells where some other proteins or lipids will affect their functions. Second, the lipid composition of the liposomes is only a best estimate of what is found inside the cell in the real membranes. Third, the ratio of aqueous to lipid phase in the cell is vastly different from that used in the in vitro lipid transfer reactions. An additional problem arises if a fluorescent lipid analogue is used, which may behave very differently from its endogenous counterparts.
Some of these caveats are overcome when studying LTPs (usually as GFP-tagged constructs) in live cells and study their distribution and movements. This can be combined with fluorescent lipid sensors to monitor changes in lipid distribution using confocal microscopes [9]. However, changes observed in these microscopy studies are not easy to quantitate and the individual cell-to-cell variations make it difficult to make firm conclusions without analyzing a large number of cells with proper statistics and without bias.
Here we describe in detail a method that we developed to analyze the lipid transfer function of ORP5 based on bioluminescence resonance energy transfer (BRET) analysis. This method combines the power of quantitation of changes in specific lipid species at the PM. We utilized chimeric ORP5 by replacing its pleckstrin homology (PH) domain which binds the PM via PI4P and PI(4,5)P2 with an FK506-binding protein12 (FKBP12) module. Combined with the expression of a PM-targeted FRB module, this system allows an acute establishment of an ER-PM contact upon addition of rapamycin and hence acutely turn on the lipid transfer process. In this present protocol, we combine this recruitable system with BRET analysis to measure PM PI4P or PS changes upon activation of the ORP5-mediated lipid transfer process. The original design of the BRET system was published by the Varnai group [10] and they describe the basic principles in a separate Chapter in this Volume. Our method monitors PI4P and PS changes in a 96-well format read by a simple fluorescence plate reader. It is a perfect complement of microscopy studies where similar lipid changes can be monitored in single cells, but BRET takes out the bias from the measurement and immediately gives a cell population average. This method should be quite useful in cellular knockdown or knock-out studies where population measurements are more informative. Lastly, this method can be extended to various FKBP-fused LTPs and to various membrane contacts in addition to the ER-PM contact sites.
2. Materials
Cell culture and transfection: The experiments described in this chapter were performed in a HEK-AT1 cell line, which is a HEK293 cell stably expressing the rat AT1a angiotensin receptor [11], the parent HEK293 cells available from ATCC are equally useable with this protocol. HEK-AT1 cells were cultivated in Dulbecco’s Modified Eagle Medium with high glucose (4500 mg/L) containing 10% (vol/vol) Fetal Bovine Serum (FBS) supplemented with 1% Penicillin/Streptomycin and 5 μg/mL of Plasmocin™ Prophylactic (InvivoGen, San Diego, CA) which is used to ensure mycoplasma-free condition (see Note 1). Plasmocin™ Prophylactic was used at higher concentration (25 μg/mL) only for one week after thawing the cells. Cells were grown in T80 tissue culture flasks with filter caps and incubated in 5% humidified CO2 incubator at 37 °C. All experiments were performed in cell passages from 4 to 17 after thawing (see Note 1). Cells were plated onto the poly-L-Lysine coated non-transparent white 96-well plates (Costar 3917). For coating 96-well plates, poly-L-lysine (P8920) was purchased from Sigma, 100x diluted in sterilized phosphate buffer saline (PBS), filtered (0.22 μm pore size), and stored in 4°C refrigerator. The cells were transfected with Effectene Transfection Reagent Kit (Qiagen) following the manufacturer’s instructions as described in the Method section.
DNA constructs: For BRET analysis, L10-mVenus-T2A-sLuc-P4M(2x) was obtained from Varnai group and used to monitor PM PI4P [10]. L10-mVenus-T2A-sLuc-Lact-C2 was generated by utilizing the PS reporter Lact-C2 [12] as described previously and used to measure PM PS [13]. As a PM-anchoring recruiter, Lyn-targeted FRB (PM2-FRB) was used, which was generated in the Meyer lab [14]. mCherry-tagged FKBP-ORP5 was created by replacing the PH domain with an FKBP12 module as described in our recent publication [15](see Note 2). These plasmids are available upon request from either the Varnai group in Budapest, Hungary or from the Balla group at the NIH, USA.
Modified Krebs-Ringer buffer: 120mM NaCl, 4.7mM KCl, 0.7mM MgSO4, 10mM glucose, and 10mM Na-HEPES, (pH adjusted with NaOH to 7.4) (see Note 3).
Coelenterazine h: As a substrate of luciferase, coelenterazine h (1–361301-200) was purchased from Regis Technologies Inc. (Morton Grove, IL). Coelenterazine h was dissolved in 100% Ethanol (500 μM stock concentration) and stored unfrozen in a −20°C freezer with no light (see Note 4).
Rapamycin: Rapamycin (553210) was purchased from MilliporeSigma. Rapamycin was dissolved in DMSO (100 μM stock concentration) and stored at −20°C (see Note 5).
Tristar2 LB 942 Multimode Microplate Reader (Berthold Technologies, Bad Wildbad, Germany) was used for luminescence measurements. The plate reader was equipped with 540/40 nm (Venus fluorescent measurement) and 475/20 nm (Luciferase measurement) emission filters.
3. Methods
3.1. Cell seeding (Day 1)
Coat non-transparent white 96-well plates with 200 μl/well diluted poly-L-lysine in tissue culture hood (room temperature) for 0.5 to 1 h.
Remove poly-L-lysine and wash the plate once with 200 μl/well 1x PBS.
Seed 30,000 cells per well in triplicates in 200 μL of medium (see Note 6).
Incubate the cells for one day before transfection.
3.2. Transfection (Day 2)
Dilute plasmids to 100 ng/μL concentration each (see Note 7).
Add Enhancer (part of the Effectene Reagent Kit from Qiagen) 14.4 μL total (2.4 μL/well × 6 wells).
Vortex for 2−−3 sec and incubate for 5 min in room temperature.
Add Effectene 18 μL total (3 μL/well × 6 wells).
Vortex for 2−−3 sec and incubate for 10 min in room temperature.
Add 1/6 of transfection mixture to each well.
Incubate at 37 °C for one day in a tissue culture incubator.
Table 2.
Transfection mixture. Total 1.8 μg/transfection groups* (300ng/well x 6 wells) of DNA mixture is prepared according to the following table.
| Control | FKBP-ORP5 | |
|---|---|---|
| EC buffer (part of the Quiagen Effectene Kit) | 180 μL (30 μL/well x 6 wells) | 180 μL (30 μL/well x 6 wells) |
| PM2-FRB | 600 ng (100 ng/well x 6 wells) | 600 ng (100 ng/well x 6 wells) |
| FKBP-ORPs | mCherry 600 ng (100 ng/well x 6 wells) | mCherry-FKBP-ORP5 600 ng (100 ng/well x 6 wells) |
| BRET sensor | L10-mVenus-T2A-sLuc-P4M2× 600 ng (100 ng/well x 6 wells, for PI4P measurement) or L10-mVenus-T2A-sLuc-Lact-C2 600 ng (100 ng/well x 6 wells, for PS measurement) | |
Since we plan to use control and treatment (both in triplicates), our transfection groups are calculated for 6 plates.
3.1. BRET measurement (Day 3)
- Make following preparation:
- Modified Krebs-Ringer buffer with CaCl2 (final 2 mM concentration) (see Note 3).
- 40x-diluted Coelenterazine h (final 12.5 μM concentration) in the Ca2+-containing Krebs-Ringer buffer (see Note 4).
- 100x-diluted DMSO or Rapamycin (final 1 μM concentration) in the Ca2+-containing Krebs-Ringer buffer (see Note 5).
Set up the plate reader (depends on the brand of reader used). In the Berthold Tristar2 LB 942 reader we set up measurements at every 15sec, and in each cycle, mVenus and Luciferase signals are measured for 0.25sec, each. (see note 10)
Remove medium from the 96-well plate and wash the cells once with 200 μL of Krebs-Ringer buffer.
Incubate the cells in 50 μL of Krebs-Ringer buffer at room temperature for 30 min (see Note 9).
Pipette 40 μL of Coelenterazine h mixture (final 5 μM Coelenterazine h) to all wells and immediately measure basal level BRET signals with emission wavelengths (λem) for both Renilla luciferase (465−−485 nm) and mVenus (520−−560 nm) for 4−−5 min.
Pipette 10 μL of DMSO or Rapamycin mixture (final 100 nM concentration) and immediately start BRET measurements for 30 min (see Note 10).
3.4. Data analysis
Calculate mVenus/luciferase from each emission intensity at each time point of the measurement (see Note 8).
Calculate mean values of triplicates (see Note 10).
Analyze relative change in lipid levels upon PM recruitment of FKBP-ORP5 by calculating Rapamycin/DMSO values at each time point of measurement.
For an absolute comparison between control and FKBP-ORP5, normalize each value (Rapamycin/DMSO) to the mean BRET values obtained before Rapamycin addition. (see Note 10 and 11).
4. Notes
Testing mycoplasma contamination regularly and maintaining mycoplasma-free condition is essential for obtaining reliable and reproducible data. In addition, culturing and passaging cells under consistent conditions (e.g. confluency, passages used in experiments, etc) will ensure reproducibility of experiments.
When recruiting proteins to the PM in BRET experiments one has to consider that an mRFP or mCherry-tagged protein recruited to the PM can start “stealing” energy from the Luciferase-excited Venus, thereby decreasing the BRET signal. This may appear as if the PM lipid measured by BRET is decreasing in response to the recruited protein. To test for this “FRET contamination” of the BRET signal, one has to perform some controls. The best is to use a LTP construct mutated in its lipid cargo binding site to make it functionally dead. We found that in the case of the mCherry-FKBP12-tagged ORP5, which occupies only a small fraction of the PM after PM recruitment because of its ER-anchoring, this FRET contamination is negligible. However, when a cytosolic mCherry-FKBP12 protein was used as a control, this FRET contamination was significant. Therefore, for most of our BRET applications, we used a “dark” (non-fluorescent) recruiter and an iRFP-tagged recruitable construct to avoid these FRET-based artifacts.
Modified Krebs-Ringer buffer was stored at room temperature and freshly prepared with CaCl2 addition (final 2 mM concentration) prior to individual experiment.
Coelenterazine h is light-sensitive. Keeping Coelenterazine h in darkness is important for reproducibility of BRET analysis. In addition, it is also recommended that the stock solution is kept in screw-capped tubes to minimize evaporation of ethanol.
By making aliquots (10 μL each), freezing and thawing cycle of Rapamycin was minimized.
Although it is impossible to check with microscope if cells are seeded evenly in the non-transparent 96-well plate, even cell seeding is a critical to ensure good efficiency and consistency of transfection.
Using midiprep quality DNA is highly recommended to maintain high transfection efficiency. Due to low DNA amount used in 96-well scale transfections, plasmid DNA was diluted to 100 ng/μL concentration with ultra-pure water. This procedure improves accuracy of pipetting during transfection. Meanwhile, minimizing freeze-thawing cycles of diluted DNA is important to prevent DNA denaturation.
The BRET value can be influenced by co-transfection efficiency of the additional individual DNAs. Especially, expression levels of mVenus and luciferase in the BRET sensor can affect the absolute BRET values. Thus, it is recommended that the emission intensity of luciferase (475/20 nm) is checked for each transfection groups to make sure they are comparable within the various groups to be compared.
It may be required to repeat the same experiment in 37 °C and to check the temperature dependency of the process in question.
Although we set the plate reader to measure for 15 s per cycle and 0.25 s per each emission, each cycle will ran with a delay while the plate is moved from well to well (less than 15 s) and therefore the time stamp for each measurement could be different from the set cycle time. This is an important consideration when setting up too many wells in one plate. This time delay is reflected in the final figures presented as real running time including delays which resulted in longer measurement time than 30 min.
The raw counts in the Luc channel in our plate reader are between 250,000–350,000. These number decline with time as the coelenterazine substrate is consumed. The counts in the Venus channel are also between 250,000–350,000. The ratio of Venus/Luc is expected to be in the range of 0.8–1.0 in case of a strong BRET signal. This BRET ratio (used as the raw BRET value) also changes in time, therefore, it is always important to have a control group that receives only solvent treatment. This control serves as a reference for any treatment regime. The result then is expressed as a BRET ratio of treated/control. Occasionally these final ratios show a slight difference even before any treatment (even if they should not), in which case the data can be adjusted to correct this difference. However, caution should be used, because the expression level of the BRET constructs has an impact on the absolute BRET ratios. When using expression of other plasmids along with the BRET construct, they can have an effect on the expression level of the latter. It is important to use appropriate control DNAs to ensure equal BRET construct expression. Our practice is to check if the counts in the Luc channel are comparable between different transfections or knock-downs groups. In some cases it may be even better to run the plates through in fluorescent mode determining the Venus expression level to evaluate whether the BRET components are equally expressed among treatment groups.
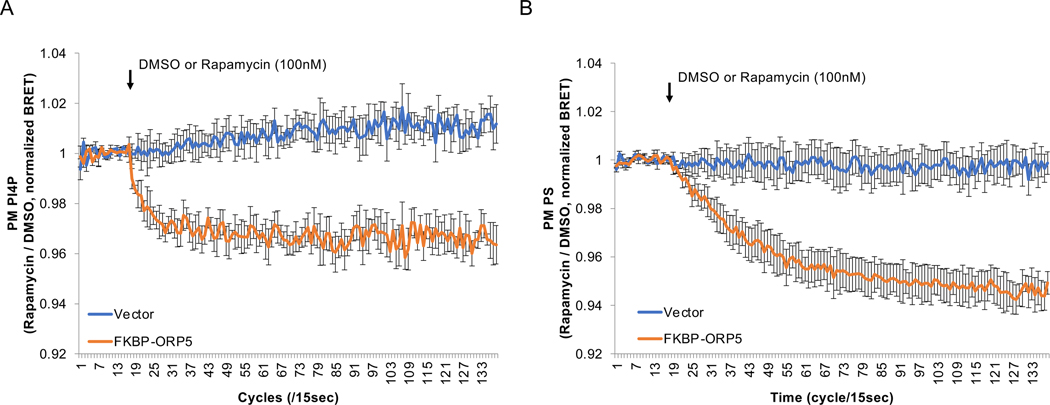
Figure 1.
Quantitation of plasma membrane (PM) PI4P (A) and PS (B) changes using BRET analysis under acute recruitment of ORP5 to the PM. Representative raw BRET ratios from a single experiment are shown. One day after transfection, BRET analysis was performed before and after PM recruitment of ORP5 using Rapamycin (100 nM) treatment for the indicated number of cycles (15 sec each). The values plotted are means of triplicate measurements where the BRET ratio was calculated by dividing the Venus counts by the luciferase counts at each time points. Note the differences in the initial BRET values justifying the needs for appropriate controls. The ratios of Rapa/DMSO values in each group are close to 1.
Figure 2.
Final results from three independent BRET experiments showing changes in plasma membrane (PM) PI4P (A) and PS (B) after acute recruitment of ORP5 to the PM. In this plot Rapamycin/DMSO ratio values were calculated and normalized to the mean ratio value obtained before Rapamycin addition in each of the three experiments. Grand means ± S.E.M are shown from the three experiments, each performed in triplicates.
Table 1.
Cell seeding in a 96-well plate. Cells were seeded in triplicates as displayed below. The cells are transfected with the indicated construct plus the non-coloured plasma membrane-targeted FRB and the PM-BRET sensor for the particular lipid in question. Group ① and ② are subjected to DMSO treatment while ③ and ④ are to Rapamycin treatment. It is highly recommended that this grouping and relative positioning is changed from experiment-to-experiment to avoid possible systemic errors biasing the final conclusion.
| ① mCherry |
① mCherry |
① mCherry |
② mCherry-FKBP-ORP5 |
② mCherry-FKBP-ORP5 |
② mCherry-FKBP-ORP5 |
| ③ mCherry |
③ mCherry |
③ mCherry |
④ mCherry-FKBP-ORP5 |
④ mCherry-FKBP-ORP5 |
④ mCherry-FKBP-ORP5 |
Acknowledgments
We are grateful for DNA constructs provided by the Grinstein, Meyer, Yin and Varnai laboratories. This work was supported by the intramural research program of the Eunice Kennedy Shriver NICHD, at the National Institutes of Health.
References
- 1.Gatta AT, Levine TP (2017) Piecing Together the Patchwork of Contact Sites. Trends Cell Biol 27 (3):214–229. doi: 10.1016/j.tcb.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 2.Saheki Y, De Camilli P (2017) Endoplasmic Reticulum-Plasma Membrane Contact Sites. Annu Rev Biochem. doi: 10.1146/annurev-biochem-061516-044932 [DOI] [PubMed] [Google Scholar]
- 3.Lahiri S, Toulmay A, Prinz WA (2015) Membrane contact sites, gateways for lipid homeostasis. Curr Opin Cell Biol 33:82–87. doi: 10.1016/j.ceb.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kentala H, Weber-Boyvat M, Olkkonen VM (2016) OSBP-Related Protein Family: Mediators of Lipid Transport and Signaling at Membrane Contact Sites. Int Rev Cell Mol Biol 321:299–340. doi: 10.1016/bs.ircmb.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 5.de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, Antonny B, Drin G (2011) Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol 195 (6):965–978. doi:jcb.201104062 [pii] 10.1083/jcb.201104062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesmin B, Bigay J, Moser von Filseck J, Lacas-Gervais S, Drin G, Antonny B (2013) A four-step cycle driven by PI(4)P hydrolysis directs sterol/PI(4)P exchange by the ER-Golgi tether OSBP. Cell 155 (4):830–843. doi:S0092–8674(13)01232–4 [pii] 10.1016/j.cell.2013.09.056 [DOI] [PubMed] [Google Scholar]
- 7.Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P (2015) INTRACELLULAR TRANSPORT. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science 349 (6246):428–432. doi: 10.1126/science.aab1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moser von Filseck J, Copic A, Delfosse V, Vanni S, Jackson CL, Bourguet W, Drin G (2015) INTRACELLULAR TRANSPORT. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science 349 (6246):432–436. doi: 10.1126/science.aab1346 [DOI] [PubMed] [Google Scholar]
- 9.Hammond GR, Balla T (2015) Polyphosphoinositide binding domains: Key to inositol lipid biology. Biochim Biophys Acta 1851 (6):746–758. doi: 10.1016/j.bbalip.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth JT, Gulyas G, Toth DJ, Balla A, Hammond GR, Hunyady L, Balla T, Varnai P (2016) BRET-monitoring of the dynamic changes of inositol lipid pools in living cells reveals a PKC-dependent PtdIns4P increase upon EGF and M3 receptor activation. Biochim Biophys Acta 1861 (3):177–187. doi: 10.1016/j.bbalip.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunyady L, Baukal AJ, Gaborik Z, Olivares-Reyes JA, Bor M, Szaszak M, Lodge R, Catt KJ, Balla T (2002) Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. JCell Biol 157:1211–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S (2008) Membrane phosphatidylserine regulates surface charge and protein localization. Science 319 (5860):210–213. doi:319/5860/210 [pii] 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- 13.Sohn M, Ivanova P, Brown HA, Toth DJ, Varnai P, Kim YJ, Balla T (2016) Lenz-Majewski mutations in PTDSS1 affect phosphatidylinositol 4-phosphate metabolism at ER-PM and ER-Golgi junctions. Proc Natl Acad Sci U S A 113 (16):4314–4319. doi: 10.1073/pnas.1525719113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T (2005) An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat Methods 2 (6):415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohn M, Korzeniowski M, Zewe JP, Wills RC, Hammond GRV, Humpolickova J, Vrzal L, Chalupska D, Veverka V, Fairn GD, Boura E, Balla T (2018) PI(4,5)P2 controls plasma membrane PI4P and PS levels via ORP5/8 recruitment to ER-PM contact sites. J Cell Biol. doi: 10.1083/jcb.201710095 [DOI] [PMC free article] [PubMed] [Google Scholar]