Abstract
Objective:
Sexual HIV transmission is more likely to occur when plasma HIV RNA level (viral load) exceeds 1500 copies/ml. We assessed the percentage of person-time spent with viral load above 1500 copies/ml (pPT >1500) among adults with HIV in care.
Design:
Observational cohort in eight United States HIV clinics.
Methods:
Participants had at least one HIV Outpatient Study (HOPS) clinic visit and at least two viral loads during 2000–2014. We assessed pPT above 1500 in time intervals between consecutive viral load pairs, overall and by ART status. Trends in pPT above 1500 and associations between pPT above 1500 and chosen baseline demographics and clinical characteristics were analyzed using generalized estimating equations.
Results:
There were 5873 patients contributing 37 794 person-years; 86.0% person-years had prescribed ART, with increasing coverage over time. Over 2000–2014 pPT above 1500 was 24.2%, decreasing from 38.3% in 2000–2002 to 11.3% in 2012–2014. During observation time with ART prescribed, pPT above 1500 was 16.4% overall, decreasing from 29.9% in 2000–2002 to 8.0% in 2012–2014. pPT above 1500 was higher in patients less than 35 vs. at least 50 years old (31.5 vs. 15.6%), women vs. men (30.8 vs. 22.3%), and black vs. white and Latino/Hispanic patients (32.7 vs. 19.9 and 23.7%, respectively). Multivariable correlates of higher pPT above 1500 included no prescribed ART, being younger, non-Hispanic black vs. white, baseline viral load above 1500 copies/ml or lower CD4+ count, and baseline public vs. private insurance.
Conclusion:
pPT above 1500 declined during 2000–2014. Results support decreasing HIV transmission risk from persons in HIV care over the last decade, and the need to focus interventions on patient groups more consistently viremic.
Keywords: cohort studies, HIV infections, integrase inhibitors, medication adherence, outpatients, protease inhibitors, viral load
Introduction
In the United States, as the population of HIV-diagnosed persons in care has grown in part because of improvements in HIV testing, linkage to HIV care, and improved survival on antiretroviral therapy (ART) [1,2], HIV transmission from persons unaware of their HIV serostatus has proportionally declined, whereas that attributed to persons diagnosed but not retained in HIV care has increased [3–5]. ART can effectively lower the virus to levels that prevent transmission [6], yet because of poor medication adherence or HIV drug resistance, some patients do not maintain consistent viral load suppression [7,8] and may continue to transmit HIV through higher-risk sexual practices or sharing of injection drug use equipment.
There is effectively no risk of sexual transmission when HIV viral load is durably suppressed with prescribed ART. Data from early studies of serodiscordant heterosexual couples can be used to establish the viral load threshold at which transmission begins to occur. In one large study of heterosexual serodiscordant couples, Quinn et al. [9] found that a plasma viral load of 1500 copies/ml may represent a threshold above which transmission risk begins to increase; no transmissions were observed beneath this viral load level. In another study of serodiscordant couples by Tovanabutra et al. [10] no HIV transmissions occurred when plasma viral load was below 1094 copies/ml. Although few cases of HIV transmission have been documented at lower viral load levels [11], for the vast majority of heterosexual serodiscordant couples in which HIV transmissions occurred, the infected partner’s viral load exceeded 1500 copies/ml.
There is considerable interest in understanding how ongoing advances in HIV care and ‘treatment as prevention’ may affect transmission potential and HIV incidence [12–14]. Evolution in HIV treatment guidelines toward earlier initiation of ART [15,16] and the advent of new drug classes (e.g. integrase inhibitors) that are more potent and tolerable, may reduce ‘spikes’ in viral load that exceed 1500 copies/ml. Analyses of longitudinal HIV cohort data with information on ART prescriptions can improve understanding of temporal changes in the proportion of time patients are at risk of transmitting HIV to others, and identify patient subsets at the highest risk. Here, we examined the percentage of person-time spent with viral load above 1500 copies/ml (pPT>1500) over 15 years in a clinical cohort of 5873 persons living with HIV (PLWH).
Methods
Study population
HOPS is an open, longitudinal cohort of PLWH individuals, 18 years or older, receiving care in HIV specialty clinics in the United States [17]. Patient data, including demographic and social characteristics, diagnoses, prescribed medications, and laboratory values, are abstracted from medical records and entered by trained staff into a single electronic database (Discovere; Cerner Corporation, Kansas City, Missouri, USA). The abstracted data are reviewed for quality and analyzed centrally. The HOPS protocol has been reviewed and approved annually by the institutional review boards of the Centers for Disease Control and Prevention (Atlanta, Georgia) and each site. The study protocol conforms to the guidelines of the U.S. Department of Health and Human Services for the protection of human participants in research. All participants have provided written, informed consent.
For the present analyses, from among 10 436 participants seen at eight clinic sites as of 31 June 2015, we excluded 1924 patients from inactive HOPS sites, 421 patients with only 1 HOPS visit, and 1815 patients with no clinic visits during 2000–2014, to obtain 6465 participants who had at least two HOPS clinic visits, with at least one occurring between 2000 and 2014. We excluded 592 patients lacking at least two viral loads at least 30 days apart during 2000–2014, to arrive at the analytic sample of 5873 patients (Supplemental Figure 1, http://links.lww.com/QAD/B315).
Outcome measure
The outcome variable was pPT above 1500 and is a rate that reflects the number of days above a viral load of 1500 copies/ml, relative to the total number of person-days on observation. We computed this outcome using consecutive viral load pairs, from the method of Marks et al. [14] (see Fig. 1). If both viral load measurements of a pair were above 1500 copies/ml, then all intervening person-time was considered to have been above 1500 copies/ml (time between 0 days and 110 days in the figure). If both viral load measurements of a pair were less than or equal to 1500 copies/ml, then all intervening person-time was considered to have been less than or equal to 1500 copies/ml (time between 234 and 362 days in the figure). If the first viral load in the pair was above 1500 copies/ml, and the second was less than or equal to 1500 copies/ml (time between 110 and 234 days in the figure), or vice versa (time between 362 and 492 days in the figure), we used a straight-line approximation to estimate viral loads between measurements. In this scenario, the estimation method entailed calculating the difference in magnitude between viral load results, calculating the difference in magnitude between the viral load exceeding the threshold and 1500 copies/ml, dividing the latter by the former, and then multiplying this fraction by the length of the time interval. For example, in the last viral load pair of the figure, the first viral load measured 750 copies/ml and the second viral load measured 2300 copies/ml. The fraction would be (2300 – 1500)/(2300 – 750) = 800/1550 = 0.516. These viral loads are 130 days apart, so 67.1 days in that period (0.516 × 130) have viral load above 1500 copies/ml. For all consecutive viral load pairs in our patient population, we summed the number of days above 1500 copies/ml in the numerator, divided by the total days of observation in the denominator, and multiplied by 100 to derive pPT above 1500.
Fig. 1.
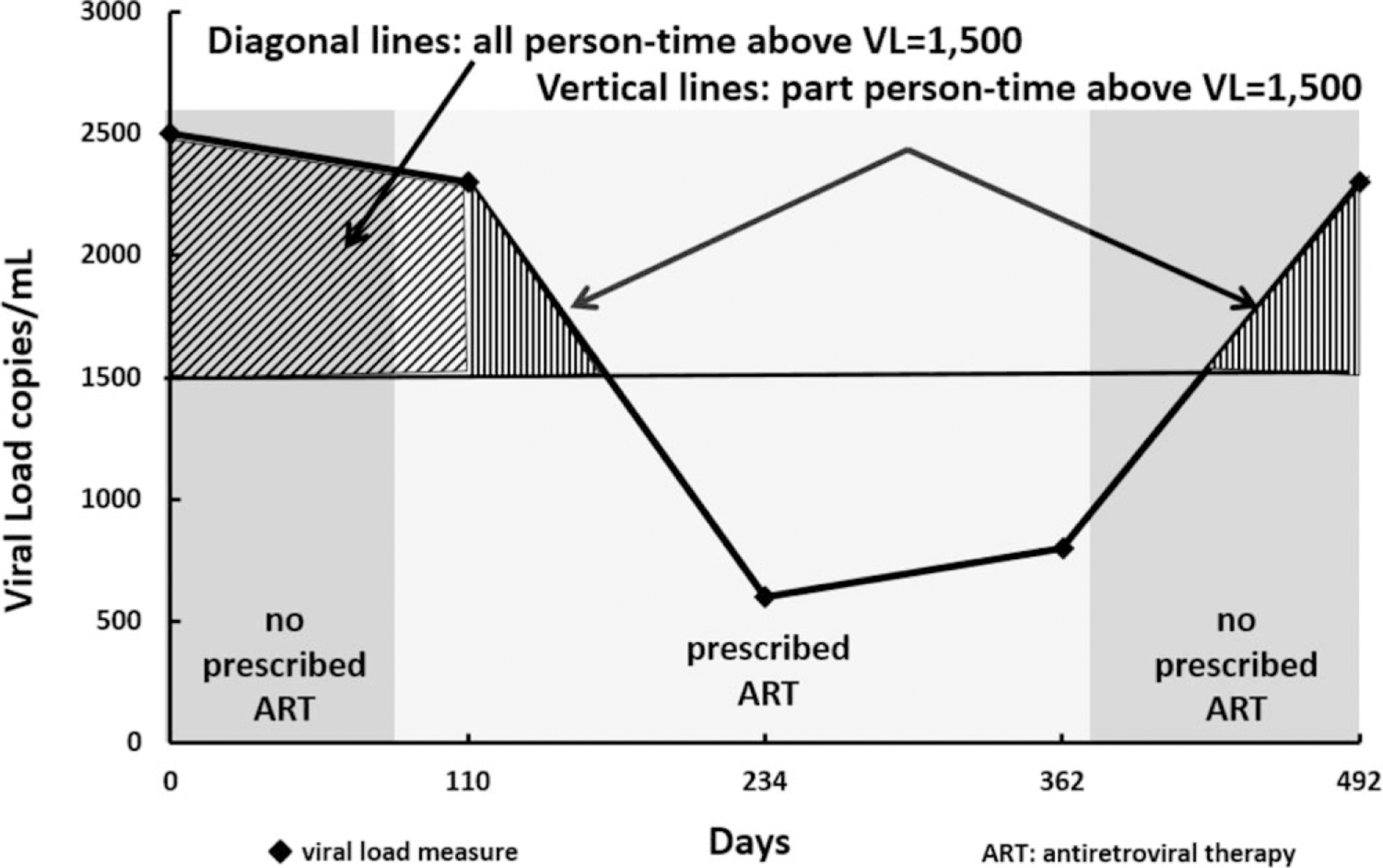
Estimating person-time above a transmission threshold of 1500 copies/ml (illustrative example).
Predictor variables
Start of observation (baseline) for the analysis was defined as the time of the participant’s first viral load on or after 1 January 2000. We analyzed the following demographic variables: baseline age, sex, race/ethnicity, sexual orientation (heterosexual female, heterosexual male, or MSM), injection drug use (IDU), and insurance type. Abstracted clinical information included AIDS diagnosis, plasma HIV viral loads, CD4+ cell counts, and ART regimens with their start and stop dates. Prescribed or not prescribed ART (where those not prescribed ART have either not yet started ART, or have interrupted or discontinued ART) was defined as a time-dependent variable. We categorized regimens into the following main classes: protease inhibitor-containing, nonnucleoside reverse transcriptase inhibitor (NNRTI)-containing, integrase strand transfer-inhibitor (INSTI)-containing, and all other regimens [nucleoside reverse transcriptase inhibitors (NRTIs) only, or entry inhibitors]. Regimens that contained INSTI were classified in the INSTI category regardless of other agents. Regimens that contained both a protease inhibitor and an NNRTI were classified in the protease inhibitor category. We also assessed regimen dosing (once daily, twice, thrice or more) and intervals between adjacent viral load measures over time.
Statistical analysis
We summarized the outcome variable for patients by demographic and clinical subgroups, calendar time period, and ART prescription status. Calendar time period was formed by grouping the 15 years of study observation time into five consecutive 3-year time periods. Some patients contributed observation time to both ‘prescribed ART’ and ‘not prescribed ART’ strata. Generalized estimating equations (GEE), assuming a Poisson model with a log link and a robust variance estimator [18] were used to control for an individual’s correlated viral loads, and to obtain point estimates and 95% confidence intervals for both pPT above 1500 and rate ratios, in both univariable and multivariable models. Use of both backward and forward selection methods yielded the same multivariable model. Inclusion criteria for forward selection was based on a univariable model P less than 0.20 using an a priori chosen set of characteristics: calendar time period, time-dependent ART status, baseline age, race/ethnicity, sexual orientation, CD4+ cell count, baseline viral load, IDU, and baseline insurance. All variables included in the final multivariable model using both forward and backward selection methods had a P < 0.05. Multivariable models were adjusted for the main effects of calendar time period, baseline age, race/ethnicity, baseline viral load above 1500 copies/ml, baseline CD4+ cell count, time-dependent ART status (prescribed or not), and site. Trend analyses in demographic and clinical subgroups were conducted by stratifying on subgroup categories and modeling pPT above 1500 against calendar time period for each stratum. Analyses were carried out using SAS (version 9.4; SAS Institute Inc., Cary, North Carolina, USA). Statistical results with P less than 0.05 were considered significant.
Results
Demographic and clinical characteristics at baseline
The 5873 individuals in our study population had a median age of 40.7 years at baseline, 78.0% were men, 50.8% were white, 33.6% black, and 12.0% Hispanic/Latino (Table 1). Most patients were classified as MSM (58.7%); 9.8% reported IDU.
Table 1.
Demographic and clinical characteristics of study participants and percentage of time above 1500 copies/ml during observation, HIV outpatient study, 2000–2014 (N = 5873).
| N (%) or median (IQR) | Percentage (95% confidence interval) of person-time with viral load above 1500 copies/ml during observation | |
|---|---|---|
| By characteristics at baseline | ||
| All participants | 5873 (100.0) | 24.2 (23.4, 25.1) |
| Age (years) | ||
| <35 | 1546 (26.3) | 31.5 (29.6, 33.5) |
| 35–49 | 3318 (56.5) | 23.6 (22.5, 24.7) |
| 50+ | 1009 (17.2) | 15.6 (14.1, 17.3) |
| Median age (IQR) | 40.7 (34.6–47.2) | n/a |
| Sex | ||
| Female | 1289 (22.0) | 30.8 (28.9, 32.8) |
| Male | 4584 (78.0) | 22.3 (21.4, 23.3) |
| Race/ethnicity | ||
| Non-Hispanic/Latino white | 2981 (50.8) | 19.9 (18.9, 21.0) |
| Non-Hispanic/Latino black | 1974 (33.6) | 32.7 (31.0, 34.4) |
| Hispanic/Latino | 705 (12.0) | 23.7 (21.5, 26.3) |
| Other/unknown/missing | 213 (3.6) | 18.6 (15.2, 22.6) |
| Sexual orientation | ||
| Heterosexual female | 1289 (22.0) | 30.8 (28.9, 32.8) |
| Heterosexual male | 1134 (19.3) | 25.9 (23.9, 28.0) |
| MSM | 3450 (58.7) | 21.3 (20.2, 22.3) |
| Injection drug-use risk history | ||
| Yes | 577 (9.8) | 26.9 (24.3, 29.9) |
| No | 5296 (90.2) | 23.9 (23.0, 24.8) |
| Insurance | ||
| Private | 3184 (54.2) | 20.7 (19.6, 21.7) |
| Public | 2113 (36.0) | 30.7 (29.1, 32.3) |
| Self pay/none | 576 (9.8) | 23.4 (20.7, 26.5) |
| CD4+ cell count (cells/μl) | ||
| <50 | 422 (7.2) | 36.1 (32.5, 40.2) |
| 50–199 | 888 (15.1) | 29.0 (26.7, 31.4) |
| 200–349 | 1211 (20.6) | 25.2 (23.4, 27.2) |
| 350–499 | 1115 (19.0) | 21.3 (19.6, 23.2) |
| 500+ | 2014 (34.3) | 21.2 (19.9, 22.5) |
| Unknown | 223 (3.8) | 27.8 (23.1, 33.6) |
| Median (IQR) | 388 (213–597) | n/a |
| Viral load (copies/ml) | ||
| ≤1500 | 3142 (53.5) | 13.8 (12.9, 14.7) |
| >1500 | 2731 (46.5) | 38.2 (36.9, 39.6) |
| Antiretroviral exposure history | ||
| Experienced | 4233 (72.1) | 22.9 (21.9, 23.9) |
| Naïve | 1430 (24.4) | 28.5 (26.8, 30.4) |
| Unknown | 210 (3.6) | 27.5 (22.8, 33.1) |
| Years since HIV diagnosis | ||
| ≥5 | 2863 (48.8) | 25.1 (24.0, 26.3) |
| <5 | 3010 (51.2) | 23.2 (22.0, 24.4) |
| Years since HIV diagnosis, median (IQR) | 5.21 (0.88–10.31) | n/a |
| Prior AIDS diagnosis | ||
| Yes | 3020 (51.4) | 24.6 (23.5, 25.8) |
| No | 2853 (48.6) | 23.8 (22.6, 25.0) |
| By characteristics during observation | ||
| Calendar time period | ||
| 2000–2002 | 3146 (53.6) | 38.3 (36.8, 39.8) |
| 2003–2005 | 3470 (59.1) | 34.4 (33.0, 35.8) |
| 2006–2008 | 3568 (60.8) | 23.2 (22.0, 24.5) |
| 2009–2011 | 3553 (60.5) | 14.4 (13.5, 15.4) |
| 2012–2014 | 3133 (53.3) | 11.3 (10.3, 12.3) |
| Type of ART regimen prescribeda: | ||
| PI-containing | 9048 (38.4) | 21.4 (20.3, 22.7) |
| NNRTI-containing | 5322 (22.6) | 10.0 (9.2, 10.8) |
| INSTI-containing | 2061 (8.8) | 11.1 (9.8, 12.5) |
| All other | 3679 (15.6) | 22.6 (20.7, 24.8) |
| Not prescribed any ART | 3432 (14.6) | 72.4 (70.0, 74.8) |
| Viral load spacing | ||
| 0–<6 months | 79 438 (80.1) | 21.4 (20.6, 22.2) |
| 6–<9 months | 11 930 (12.0) | 20.4 (19.4, 21.5) |
| ≥9 months | 7826 (7.9) | 32.8 (31.2, 34.4) |
| Dosing of regimen | ||
| Once per day | 6383 (32.1) | 11.6 (10.7, 12.6) |
| Twice per day | 12 564 (63.3) | 19.5 (18.5, 20.5) |
| Thrice or more per day | 915 (4.6) | 17.6 (15.0, 20.7) |
| Years of follow-up, median (IQR) | 5.31 (2.14–10.66) | n/a |
ART, antiretroviral therapy; IQR, interquartile range; regimen categories: protease inhibitor (PI)-containing, nonnucleoside reverse transcriptase inhibitor (NNRTI)-containing, integrase strand transfer-inhibitor (INSTI)-containing, and all other [nucleoside reverse transcriptase inhibitors [NRTIs] only, or entry inhibitors].
Regimens that had INSTI were classified in the INSTI category regardless of other agents, and regimens that had both a PI and an NNRTI were classified in the PI category.
Most patients had health insurance (54.2% private, 36.0% public). At baseline, median CD4+ cell count was 388 cells/μl with 34.3% of patients having CD4+ cell count above 500 cells/μl. Less than half (46.5%) the patients had a baseline viral load above 1500 copies/ml, 48.8% had been diagnosed with HIV infection 5 years or longer, and 72.1% were ART-experienced (Table 1).
Longitudinal profile of viral loads and antiretroviral therapy use
Collectively, among the 5873 persons studied, 86.0% were not prescribed ART for some time during observation. There were 4050 (69.0%) who had at least one viral load above 1500 copies/ml. Of the 3142 (53.5%) patients who had viral load 1500 copies/ml or less at baseline, 1319 (42.0%) had at least one viral load above 1500 copies/ml during follow-up. The total number of viral loads measured during the analytic period was 105 068, with a median of 15 viral loads per person (interquartile range 7–27). Of these viral load measurements, 74.0% were 1500 copies/ml or less and obtained when a patient was prescribed ART, 3.7% were 1500 copies/ml or less and obtained when a patient was not prescribed ART, 13.1% were above 1500 copies/ml and obtained when a patient was prescribed ART, and 9.2% were above 1500 copies/ml and obtained when a patient was not prescribed ART. Of consecutive viral load pairs, 15 982 (16.1%) pairs had both measurements above 1500 copies/ml; 72 214 (72.8%) pairs had both measurements 1500 copies/ml or less; 6313 (6.4%) pairs had the first measurement above 1500 copies/ml (2136 of which coincided with times of ART introduction) and the second measurement at or below that level; and 4686 (4.7%) pairs had the first measurement at or below 1500 copies/ml (1132 of which coincided with ART interruption or discontinuation) and the second measurement above that level. The majority of viral load pairs were separated by less than 6 months. Over time, however, a smaller percentage of pairs were separated by less than 6 months, whereas pairs spaced six to less than 9 months became more common. Percentages of other between-pair viral load time categories were relatively stable (Supplemental Figure 2, http://links.lww.com/QAD/B315).
Of the 37 794 person-years, 86.0% was time when ART was prescribed. Prescribed ART use per year increased during 2000–2014, and the number of patients not prescribed ART proportionately decreased. The number of patients prescribed integrase inhibitors increased markedly relative to the short time they have been available (Fig. 2). The percentage of observation time when ART was not prescribed increased from 12.1% in 2000 to 20.6% in 2003, and subsequently diminished to 6.6% in 2014.
Fig. 2. Number of HOPS patients prescribed a given type of antiretroviral therapy regimen, 2000–2014 (N = 5873).
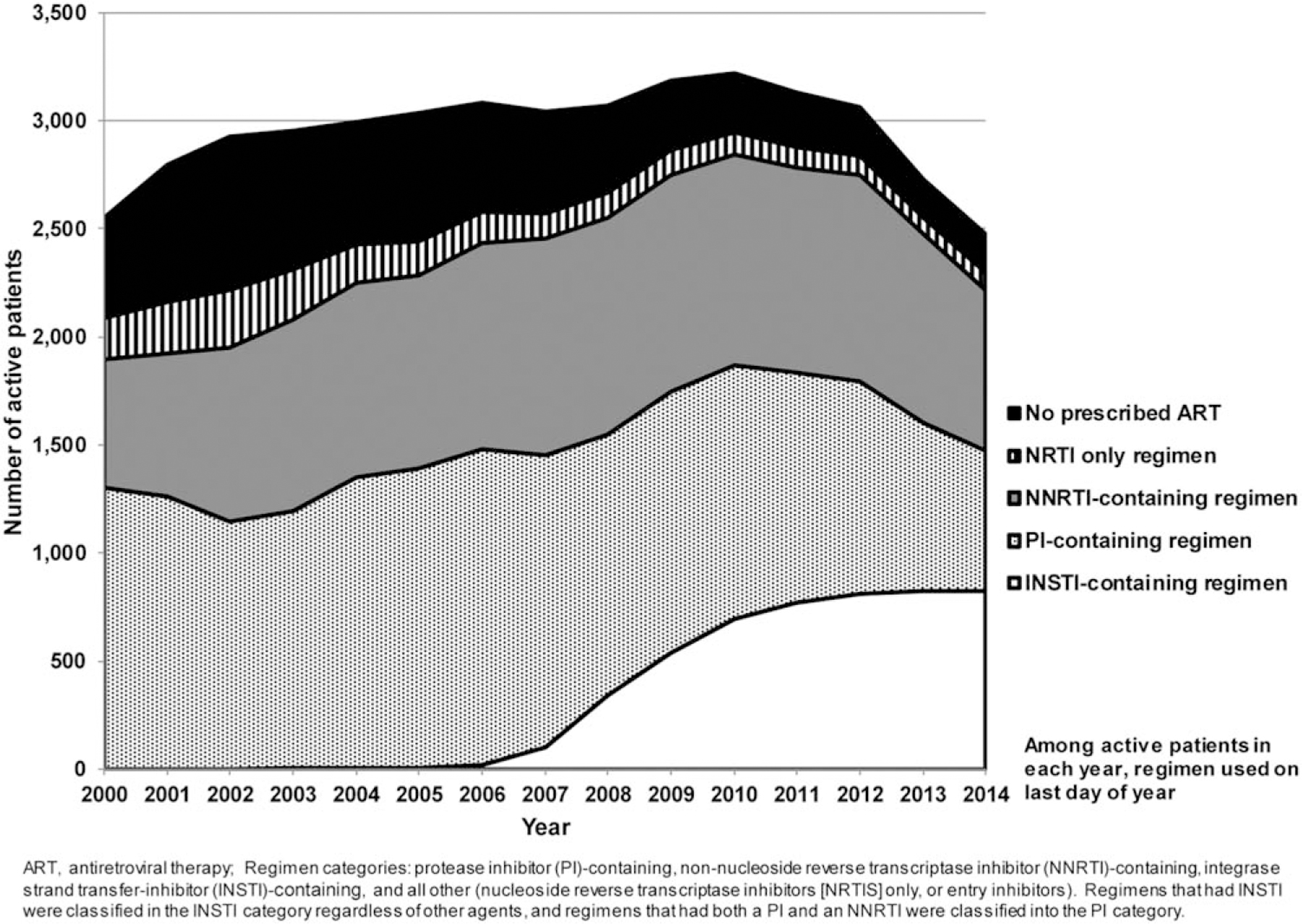
HOPS, HIV Outpatient Study.
Correlates of percentage of time with viral load above 1500 copies/ml
Among the 5873 study participants, the overall amount of person-time spent with viral load above 1500 copies/ml was 24.2% (Table 1). The youngest patients (less than 35 years of age at baseline) spent 31.5% of their observation time above 1500 copies/ml, the greatest proportion of all age groups. Other patient subgroups with higher pPT above 1500 were women (30.8%), blacks (32.7%), persons with a history of IDU (26.9%), and those with public insurance at baseline (30.7%). Stratified by clinical variables, subgroups with higher pPT above1500 were patients with baseline CD4+ cell count less than 50 cells/μl (36.1%), those with baseline viral load above 1500 copies/ml (38.2%), persons who were ART-naive at baseline (28.5%), and those with a prior AIDS diagnosis (24.6%). In addition, being prescribed a regimen other than NNRTI-based or INSTI-based, having a regimen that was dosed twice or more daily compared with once daily, and having 9 months or more between consecutive HIV viral loads (compared with a shorter time period) were all associated with greater pPT above 1500 (Table 1).
In univariable regression (Table 2), during time periods when not prescribed ART, patients had 4.4 times the pPT above 1500 than during time periods when prescribed ART. In addition to time-dependent ART, baseline factors significantly associated with greater pPT above 1500 included age less than 50 years compared with at least 50 years, black or Hispanic/Latino race/ethnicity compared with white, being heterosexual compared with MSM, using injection drugs, public insurance compared with private, a baseline CD4+ cell count less than 350 compared with at least 500 cells/μl, and a baseline viral load above 1500 copies/ml compared with a lower level. In the final multivariable GEE model (Table 2), factors most strongly associated with greater pPTabove 1500 included a baseline viral load above the 1500 copies threshold (rate ratio = 2.2) and time-dependent prescribed ART (not prescribed vs. prescribed, rate ratio = 3.4); younger age, black race, public insurance, and lower CD4+ cell count were associated to a lesser extent.
Table 2.
Factors associated with greater percentage of person-time above 1500 copies/ml, HIV Outpatient Study, 2000–2014 (N = 5873).
| Characteristic | Univariable model rate ratio (95% CI) | Multivariable model rate ratio (95% CI)b |
|---|---|---|
| Calendar time period | ||
| 2000–2002 | Referent | Referent |
| 2003–2005 | 0.73 (0.71, 0.74) | 0.75 (0.74, 0.77) |
| 2006–2008 | 0.53 (0.51, 0.55) | 0.57 (0.55, 0.59) |
| 2009–2011 | 0.38 (0.36, 0.40) | 0.43 (0.40, 0.45) |
| 2012–2014 | 0.28 (0.26, 0.30) | 0.32 (0.30, 0.34) |
| Antiretroviral therapya | ||
| Not prescribed | 4.41 (4.18, 4.65) | 3.44 (3.26, 3.63) |
| Prescribed | Referent | Referent |
| Age (years) | ||
| <35 | 2.02 (1.79, 2.27) | 1.49 (1.35, 1.64) |
| 35–49 | 1.51 (1.35, 1.69) | 1.34 (1.23, 1.47) |
| 50+ | Referent | Referent |
| Race/ethnicity | ||
| non-Hispanic/Latino white | Referent | Referent |
| non-Hispanic/Latino black | 1.64 (1.53, 1.77) | 1.19 (1.10, 1.28) |
| Hispanic/Latino | 1.19 (1.06, 1.34) | 1.02 (0.93, 1.13) |
| Other/unknown/missing | 0.93 (0.76, 1.14) | 0.92 (0.78, 1.07) |
| Sexual orientation | ||
| Heterosexual female | 1.45 (1.34, 1.57) | Not in final model |
| Heterosexual male | 1.22 (1.12, 1.33) | |
| MSM | Referent | |
| Injection drug use risk history | ||
| Yes | 1.13 (1.01, 1.26) | Not in final model |
| No | Referent | |
| Insurance | ||
| Private | Referent | Referent |
| Public | 1.49 (1.38, 1.60) | 1.23 (1.14, 1.32) |
| Self pay/none | 1.13 (0.99, 1.30) | 0.97 (0.86, 1.09) |
| CD4+ cell count (cells/μl) | ||
| unknown | 1.32 (1.08, 1.61) | 1.05 (0.90, 1.23) |
| <50 | 1.71 (1.51, 1.93) | 1.20 (1.09, 1.33) |
| 50–199 | 1.37 (1.23, 1.52) | 1.17 (1.08, 1.28) |
| 200–349 | 1.19 (1.08, 1.32) | 1.14 (1.05, 1.23) |
| 350–499 | 1.01 (0.91, 1.12) | 0.94 (0.87, 1.02) |
| 500+ | Referent | Referent |
| Viral load (copies/ml) | ||
| ≤1500 | Referent | Referent |
| >1500 | 2.77 (2.57, 2.99) | 2.24 (2.08, 2.40) |
ART, antiretroviral therapy.
Time-dependent ART status; all other variables measured at baseline.
Multivariable models adjusted for the main effects of site.
Temporal trends in percentage of time with viral load above 1500 copies/ml
Overall, pPT above 1500 decreased from 38.3% in 2000–2002 to 11.3% in 2012–2014 (trend P < 0.001, Fig. 3). When patients were prescribed ART (86.0% of total observation time in the analysis), pPT above 1500 was 16.4% overall, decreasing from 29.9% in 2000–2002 to 8.0% in 2012–2014 (trend P < 0.001). During times when patients were not prescribed ART, the pPT above 1500 was 72.4% overall, decreasing from 80.5% in 2000–2002 to 53.0% by 2012–2014 (trend P < 0.001). The pPT above 1500 when not prescribed ART was further stratified into two strata (1) not prescribed ART and confirmed ART-naive and (2) not prescribed ART when already ART-experienced or ART history incomplete/unknown (i.e. during confirmed and possible ART interruptions). The pPT>1500 among ART-naive patients decreased from 74.8% in 2000–2002 to 47.7% in 2012–2014 (trend P < 0.001) for possible reasons explored in the discussion. The pPT above 1500 when not prescribed ART among patients already ART-experienced or with unknown ART history decreased from 82.2% in 2000–2002 to 55.5% in 2012–2014 (trend P < 0.001).
Fig. 3.
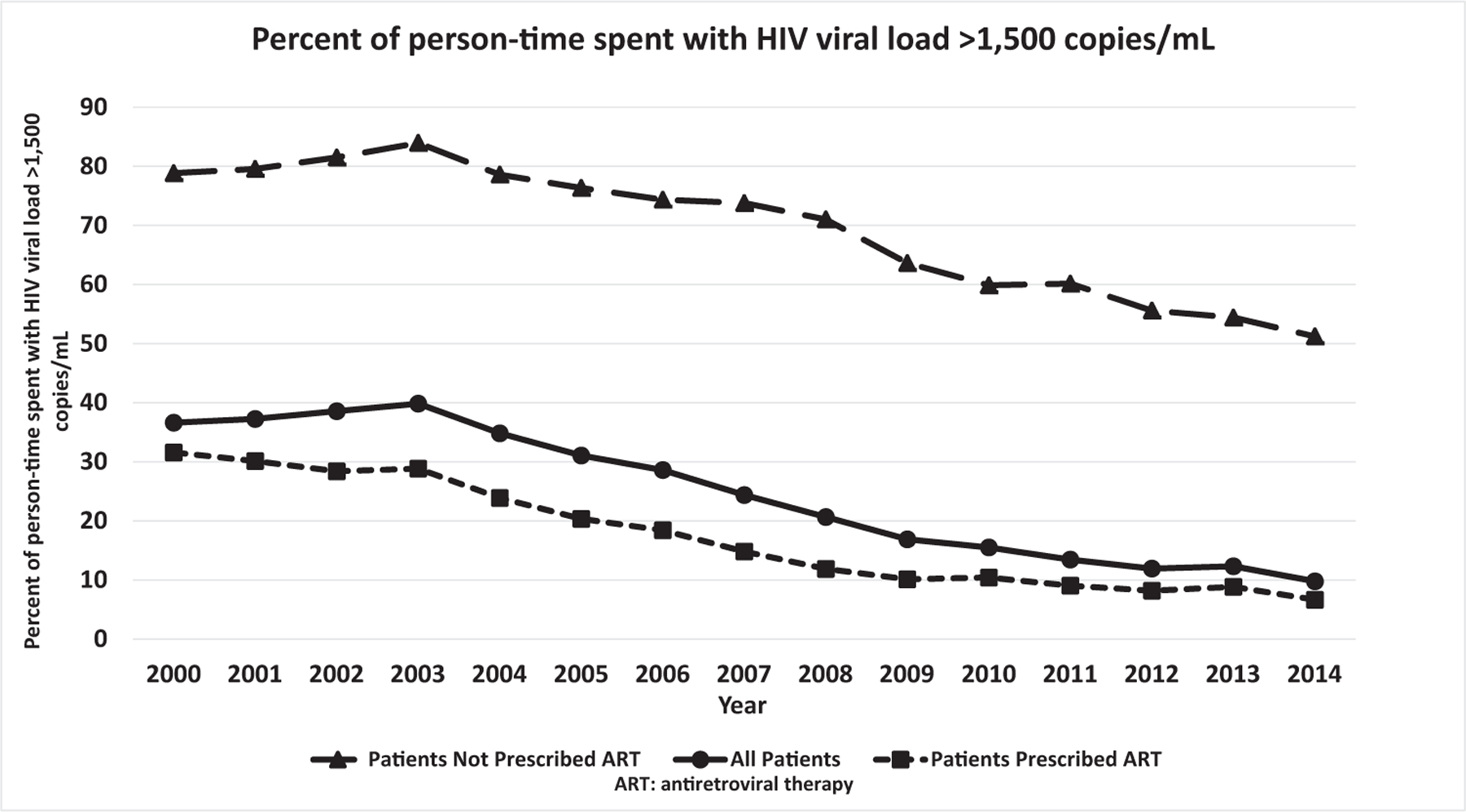
Percentage of person-time spent with HIV viral load above 1500 copies/ml.
Decreases in pPT above 1500 during 2000–2014 were documented in all demographic patient subgroups (all P < 0.001): men (36.0–9.6%) and women (46.7–16.1%); persons who started observation aged less than 35 years (41.9–19.6%), 35–49 years (39.4–9.3%), and 50 years and older (28.9–4.9%); persons who were white (33.0–5.9%), black (49.9–18.2%), and Hispanic/Latino (39.6–12.5%); heterosexual men (41.2–13.8%) and MSM (34.5–8.1%).
Discussion
In this large, diverse, prospectively followed cohort of United States adult PLWH in care, we observed that from 2000 to 2014, the pPT above 1500 decreased markedly from 38.3 to 11.3%. Reasons for this finding likely include: the evolving standard of care during this calendar time period mandated progressively earlier initiation of ART; recommended ART combination regimens became simpler and more adherable (more tolerable, once-daily dosing, and use of fixed-dose combination and single-tablet regimens), more potent, and, with some regimens, less likely to result in development of resistance or virologic failure in patients with suboptimal adherence. Our central finding of decreased pPT above 1500 in our patient population over time implies not only improved health outcomes in the population but also a reduced opportunity for HIV transmission when engaging in condomless sex. Although some demographic subgroups of patients had an overall higher pPT above 1500, including patients who were younger, women, or black, the decreases in pPT above 1500 were observed consistently in all major demographic subgroups.
Crepaz et al. (2012–2013) used United States national surveillance data to estimate that PLWH spent a mean of 17% person-time with a viral load above 1500 copies/ml [19]. In that study, women, racial/ethnic groups other than white, persons in an HIV risk category other than MSM, younger people, and those with a gap in care had significantly more person-time above 1500 copies/ml compared with their respective counterparts. In the Marks et al. [14] study (2009–2013) of patients from six HIV clinics, the estimated pPT above 1500 was 26.3%, higher than in the Crepaz et al. study (2012–2013), possibly because of an earlier calendar time frame and selection of clinics. ART use information was available at only two time points in Marks et al., and was not available for the Crepaz et al. analysis. By comparison, the pPT greater than 1500 in the current eight-clinic HOPS analysis was 14.4% during 2009–2011 and 11.3% during 2012–2014. Such reductions in viral load have been associated with fewer short-term and long-term adverse clinical outcomes [20].
The decreases in pPT above 1500 over the 15 years we observed should be interpreted in the context of a multitude of changes in HIV treatments and patient management practices during this period [21,22]. First, there was a shift in recommendations toward ART at higher CD4+ cell counts, and ultimately universal prescribed ART at HIV diagnosis, affecting the underlying population characteristics of patients prescribed ART quickly vs. remaining untreated (i.e. sicker patients treated preferentially, leaving generally healthier patients in the untreated pool). There were significant advances in ART, including the introduction of simpler combination regimens and the advent of more potent and tolerable agents, including integrase inhibitors. Notably, the pPT above 1500 decreased across all regimen categories over time among HOPS patients, with the lowest estimates for persons prescribed NNRTI-containing and INSTI-containing ART (data not shown). These observational findings suggest a higher likelihood of virologic suppression on newer and simpler (e.g. fixed dose combinations) NNRTI-containing and INSTI-containing ART than older types of protease inhibitor-containing and other ART regimens [21,22]. Second, during 2000–2014, participants in our cohort spent progressively less observation time in prescribed ART interruptions, likely affected by the report that CD4+ cell count-guided interruptions were inferior [23,24], and also because of the clinical consensus emerging since 2007 that continuous retention in HIV care and continuous virological suppression has clear-cut health benefits, in terms of both AIDS-defining and non-AIDS defining events, as well as mortality [15,16,25,26]. Third, the demographics of our open PLWH cohort have evolved over time, with increasing representation of patients who are older, women, nonwhite, and publically insured (data not shown). With the exception of older age, these patient characteristics have been associated with poorer adherence (in other studies) and with greater time spent with viral load above 1500 copies/ml in this and prior HOPS work.
The temporal decreases in pPT above 1500 during periods when patients were not prescribed ART are noteworthy. For ART-naive time, this may be because the remaining ART-naive patients may have had higher CD4+ cell counts and lower viral loads (than those treated) and this shrinking untreated group could have been progressively more enriched over time with elite or viremic controllers [27,28]. During time periods spent not prescribed ART because of interruptions or discontinuation, the observed population-level decrease in pPT above 1500 could be because of two additional factors, which we cannot ascertain from our data: greater likelihood of ongoing HIV viral load suppression for some days/weeks after ART discontinuation with use of increasingly persistent (longer half-life) agents or overall better control of viral reservoirs [28], and a potential shift in types of and reasons for breaks in ART prescription (e.g. dropout from care vs. physician-supervised drug holidays vs. waiting periods for insurance approvals when switching ART) in increasingly healthy patients.
Our study is not without limitations. Interpretation of the baseline age effect is somewhat ambiguous as baseline age was defined to be the patient’s age at study start time, which may or may not be correlated with more recent HIV diagnosis. Additionally, as HIV viral load changes often occur rapidly, the linear interpolation of the trajectory between viral loads could lead to some inaccuracy in the estimation of the pPT above 1500. We did not have information on important social determinants of health (poverty, educational status, stigma) and clinic level/structural variables (availability of adherence support interventions) that could be associated with virologic outcomes and may confound some of the associations observed (e.g. black race/ethnicity having a higher pPT above 1500). Finally, the overall reduction in pPT above 1500 could be in part because of our aging patient population (the median age of our population increased from 40.4 to 48.5 years from the first 3-year to the last 3-year calendar period) surviving longer on potent ART, as older age is generally associated with better adherence to ART [29] and medical treatments, in general. However, we would expect aging to be counteracted by greater representation over time of nonwhite and female persons in our study population, subsets which had higher pPT above 1500. Strengths of our study lie in the ability to assess viral load trends in an open, longitudinal cohort over a 15-year period. The routine documentation of ART prescriptions, not available in other data sources such as the United States national HIV surveillance data, allowed us to investigate granular associations of prescribed ART use, as well as that of major types of ART regimens, with our study outcome.
In conclusion, over a 15-year time period during which routine and progressively earlier use of more modern (effective and tolerable) ART provided improved viral load control, we observed decreasing pPT above 1500, suggesting reduced risk of HIV transmission from treated patients in care over time. Reassuringly, decreases in pPT above 1500 were observed in all major demographic subgroups. Continued efforts are needed to address social, behavioral, and structural factors, focusing on patient subgroups more likely to experience suboptimal viral suppression.
Supplementary Material
Financial support:
Centers for Disease Control and Prevention (contract nos. 200-2001-00133, 200-2006-18797, and 200-2011-41872).
The HIV Outpatient Study (HOPS) Investigators currently include the following persons and sites: Kate Buchacz, Marcus D. Durham, Division of HIV/AIDS Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, Georgia; Stacey Purinton, Kate Shelton, Nabil Rayeed, Carl Armon, Thilakavathy Subramanian, Cheryl Akridge, Linda Battalora, Cerner Corporation, Kansas City, Missouri; Frank J. Palella, Saira Jahangir, Conor Daniel Flaherty, Patricia Bustamante, Feinberg School of Medicine, Northwestern University, Chicago, Illinois; John Hammer, Kenneth S. Greenberg, Barbara Widick, Rosa Franklin, Rose Medical Center, Denver, Colorado; Bienvenido G. Yangco, Kalliope Chagaris, Infectious Disease Research Institute, Tampa, Florida; Douglas J. Ward, Troy Thomas, Cheryl Stewart, Dupont Circle Physicians Group, Washington, DC; Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, Jane Esteves, State University of New York (SUNY), Stony Brook, NY; Ellen M. Tedaldi, Ramona A. Christian, Faye Ruley, Dania Beadle, Princess Davenport, Lewis Katz School of Medicine at Temple University, Philadelphia, Pennsylvania; Richard M. Novak, Andrea Wendrow, University of Illinois at Chicago, Chicago, Illinois; Benjamin Young, Mia Scott, Barbara Widick, Billie Thomas, APEX Family Medicine, Denver, Colorado.
Footnotes
Disclaimers: The findings and conclusion in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2015; vol. 27. Department of Health and Human Services. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html. Published November 2016. [Accessed 12 April 2017]. [Google Scholar]
- 2.Siddiqi AE, Hall HI, Hu X, Song R. Population-based estimates of life expectancy after HIV diagnosis: United States 2008–2011. J Acquir Immune Defic Syndr 2016; 72:230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 2006; 20:1447–1450. [DOI] [PubMed] [Google Scholar]
- 4.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS 2012; 26:893–896. [DOI] [PubMed] [Google Scholar]
- 5.Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med 2015; 175:588–596. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. , HPTN 052 Study Team. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data–—United States and 6 dependent areas, 2014. HIV Surveillance Supplemental Report 2016; 21(No. 4). Available at: http://www.cdc.gov/hiv/library/reports/surveillance/. Published July 2016. [Accessed 12 April 2017]. [Google Scholar]
- 8.Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL. Trends in racial and ethnic disparities in antiretroviral therapy prescription and viral suppression in the United States, 2009–2013. J Acquir Immune Defic Syndr 2016; 73:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342:921–929. [DOI] [PubMed] [Google Scholar]
- 10.Tovanabutra S, Robison V, Wongtrakul J, Sennum S, Suriyanon V, Kingkeow D, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr 2002; 29:275–283. [DOI] [PubMed] [Google Scholar]
- 11.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23:1397–1404. [DOI] [PubMed] [Google Scholar]
- 12.Lasry A, Sansom SL, Wolitski RJ, Green TA, Borkowf CB, Patel P, et al. HIV sexual transmission risk among serodiscordant couples: assessing the effects of combining prevention strategies. AIDS 2014; 28:1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walensky RP, Paltiel AD, Losina E, Morris BL, Scott CA, Rhode ER, et al. , CEPAC Investigators. Test and treat DC: forecasting the impact of a comprehensive HIV strategy in Washington DC. Clin Infect Dis 2010; 51:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks G, Gardner LI, Rose CE, Zinski A, Moore RD, Holman S, et al. Time above 1500 copies: a viral load measure for assessing transmission risk of HIV-positive patients in care. AIDS 2015; 29:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at: http://www.aidsinfo.nih.gov/Content-Files/AdultandAdolescentGL.pdf. [Accessed 12 April 2017]. [Google Scholar]
- 16.Gunthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA 2016; 316:191–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moorman AC, Holmberg SD, Marlowe SI, Von Bargen JC, Yangco BG, Palella FJ, et al. Changing conditions and treatments in a dynamic cohort of ambulatory HIV patients: the HIV outpatient study (HOPS). Ann Epidemiol 1999; 9:349–357. [DOI] [PubMed] [Google Scholar]
- 18.Hardin JW, Hilbe JM. Generalized estimating equations. 2nd ed. Boca Raton, FL: CRC Press, Taylor & Francis Group, LLC; 2013. [Google Scholar]
- 19.Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012–2013. Clin Infect Dis 2016; 63:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laut KG, Shepherd LC, Pedersen C, Rockstroh JK, Sambatakou H, Paduta D, et al. Associations between HIV-RNA-based indicators and virological and clinical outcomes. Aids 2016; 30:1961–1972. [DOI] [PubMed] [Google Scholar]
- 21.Krentz HB, Cosman I, Lee K, Ming JM, Gill MJ. Pill burden in HIV infection: 20 years of experience. Antivir Ther 2012; 17:833–840. [DOI] [PubMed] [Google Scholar]
- 22.Sheth AN, Ofotokun I, Buchacz K, Armon C, Chmiel JS, Hart RL, et al. Antiretroviral regimen durability and success in treatment-naive and treatment-experienced patients by year of treatment initiation, United States, 1996–2011. J Acquir Immune Defic Syndr 2016; 71:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of anti-retroviral treatment. N Engl J Med 2006; 355:2283–2296. [DOI] [PubMed] [Google Scholar]
- 24.Lundgren JD, Babiker A, El-Sadr W, Emery S, Grund B, Neaton JD, et al. Inferior clinical outcome of the CD4+ cell count-guided antiretroviral treatment interruption strategy in the SMART study: role of CD4+ Cell counts and HIV RNA levels during follow-up. J Infect Dis 2008; 197:1145–1155. [DOI] [PubMed] [Google Scholar]
- 25.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mugavero MJ, Westfall AO, Cole SR, Geng EH, Crane HM, Kitahata MM, et al. , Centers for AIDS Research Network of Integrated Clinical Systems (CNICS). Beyond core indicators of retention in HIV care: missed clinic visits are independently associated with all-cause mortality. Clin Infect Dis 2014; 59:1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okulicz JF, Lambotte O. Epidemiology and clinical characteristics of elite controllers. Curr Opin HIV AIDS 2011; 6:163–168. [DOI] [PubMed] [Google Scholar]
- 28.Perkins M, Bradley W, Lalani T, Agan B, Whitman T, Ferguson T, et al. Prevalence of posttreatment controller phenotype is rare in HIV-infected persons after stopping antiretroviral therapy. J Acquir Immune Defic Syndr 2017; 75:364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beer L, Skarbinski J. Adherence to antiretroviral therapy among HIV-infected adults in the United States. AIDS Educ Prev 2014; 26:521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


