The ministry of Health and Family welfare, Government of India reported six laboratory confirmed Nipah virus cases during the period from 12 to 15 September 2023 in Kozhikode district, Kerala. Out of these reported cases, two succumbed to the disease. Aside from the first case, whose source of infection is unknown, other cases were family members and hospital contacts of the first case. Till 27th September 2023, about 1288 possible contacts of these confirmed cases have been traced and are put under quarantine and monitoring for 21 days. This is not the first outbreak, as since 2001, around six outbreaks of Nipah virus have been reported in India. The first outbreak was dated 2001 in Siliguri, West Bengal (66 cases, CFR: 68 %). Subsequently, in 2007, five outbreaks were reported in Nadia district, West Bengal (5 cases; CFR: 100 %). Later after almost a decade, in 2018, Nipah virus outbreak was reported in Kozhikode and Malappuram, Kerala (23 cases, CFR: 91 %), In 2019, a single case was reported in Ernakulum, Kerala which recovered fully (1 case, CFR:0). This was followed by another outbreak in 2021 involving a 12-year-old boy who died of infection in Kozhikode, Kerala (1case, CFR: 100 %). ((https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON490).
Nipah virus (NiV) belongs to the family Paramyxoviridae and genus Henipavirus. It has a non-segmented negative strand RNA genome which codes for six structural proteins and three non-structural proteins. Structural proteins include nucleocapsid protein (N), phosphoprotein (P), matrix protein (M), fusion protein (F), glycoprotein (G) and large protein (L) or RNA polymerase. While the three non-structural proteins, C, V and W are encoded by the P gene. NiV has been genetically categorized into two distinct genotypes: NiV-Malaysia (NiV-MY) identified in Malaysia and Cambodia and NiV-Bangladesh (NiV-BD) identified in Bangladesh and India [1]. NiV was first reported in Malaysia in 1998-99 affecting 265 individuals with a CFR of 40 % including 105 deaths [2]. The majority of NiV infections are transmitted to humans through infected animals (like pigs or bats) or food contaminated with saliva, urine, and excreta of infected animals. It can also be transmitted directly from person to person through close contact with an infected person (although this represents a less common transmission route). Human to human transmission, although uncommon, may be possible through exposure to the body fluids of infected patients. NiV can remain viable on surfaces, so a fomite mode of transmission cannot be ruled out [3].
Based on serological and virological evidence, large fruit bats from the genus Pteropus were identified as the natural reservoir for NiV. They shed the NiV in their secretions and excretions and humans acquire the virus by encountering these bats [4]. Epidemiological data further suggests that NiV has the potential to infect a wide range of mammals, including other animals like pigs, dogs, cats, goats, and rodents [4]. NiV infected patients initially show symptoms similar to acute respiratory infections like fever, headache, dizziness and vomiting which later develops to severe encephalitis. A peculiar and interesting feature of NiV infection is the development of relapse and late onset encephalitis, which can occur months or years after the acute illness. The MRI of brain shows multifocal and confluent lesion in cortex and white matter [5].
As NiV disease is highly contagious and fatal, an early and prompt diagnosis is critical for the containment of an outbreak and appropriate management of infected patients. NiV is diagnosed by nucleic acid amplifications testing (NAAT) like RT-PCR, IgG/IgM/antigen ELISA, Immunofluorescence assay, histopathology and virus isolation and neutralization assays. NAAT is regarded as the most sensitive method for diagnosis of acute NiV infection, while IgM ELISA can be used as an alternative approach in settings where PCR is not available. Histopathology is used as a post-mortem diagnosis method to confirm NiV in fatal cases. Virus isolation and neutralization assays must be carried out in BSL-4 facilities under strict safety precautions [4]. Human specimens, preferably collected during the early stages of disease, from throat or nasal swabs, cerebrospinal fluid, urine, and blood can be subjected for viral detection [4].
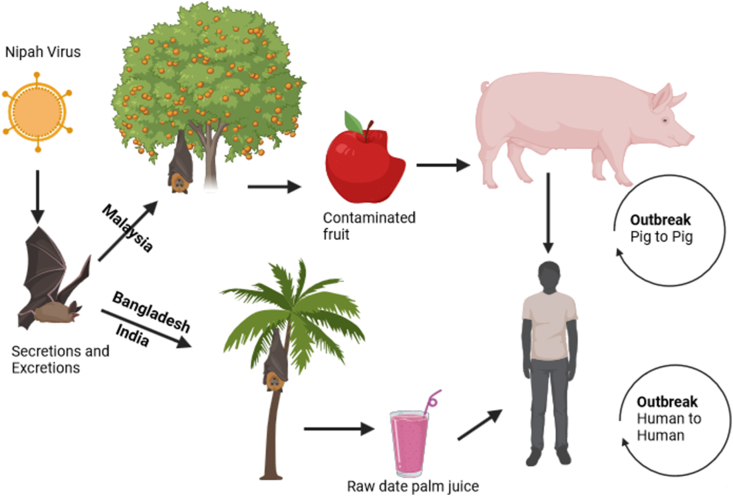
Being a zoonotic disease, an effective strategy to control the transmission of NiV to humans should be focused on reducing the contact with infected animals, mainly bats and pigs (Fig. 1). Raw date palm tree juice is a delicacy in Bengali culture. This is traditionally harvested by overnight collection which can be contaminated by urine or saliva from infected bats. Human NiV outbreaks show a seasonal pattern during winter and spring months, which shows a strong association with the Pteropus breeding season and the date palm sap harvesting season [5]. As there is a possibility of human-to-human transmission, unprotected physical contact with NiV infected people should be avoided. High risk individuals like health care workers, or close contact of suspected or confirmed cases should always take necessary precautions to avoid physical contact as well as contact with virus laden droplets.
Fig. 1.
Schematic representation of different Nipah virus (NiV) transmission routes in humans and animals (Modified with permission from Gazal et al., Pathogens, 2022; 11(12):1419).
As of now, there are no available effective therapies or vaccines for NiV disease. Therefore, the case management must focus on both supportive care and intensive care for febrile cases with neurological complications. Epidemiological surveillance data shows that NiV outbreak in Bangladesh coincide with outbreak in west Bengal, India. Bangladesh shares about 94 % of its land border areas with India having unrestricted animal movements. This is further supported by the fact that the Nipah virus isolated from Kerala and west Bengal shows high degree of similarity (96.15 %) to the Nipah virus strain found in Bangladesh. Active community-based surveillance activities like door-to-door surveillance and declaration of containment zones have their own importance in controlling the spread of the disease. Emergency departments should be revamped with isolation rooms and ICUs to handle suspected cases. The first responders that are the health care workers should be trained on infection prevention and control and adequate stocks of PPE, drugs and other logistics should be provided to them. Adequate information should be disseminated to the public via audio and video mode to educate the public and to reduce the spread of unnecessary rumors about the disease.
CRediT authorship contribution statement
Subrat Kumar: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing, Formal analysis, Resources, Software, Visualization. Ritesh Pattnaik: Conceptualization, Writing – original draft, Writing – review & editing. Basavaraj Mathapati: Methodology, Writing – review & editing. Priyadarshi Soumyaranjan Sahu: Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Patricia Schlagenhauf
Contributor Information
Subrat Kumar, Email: Subrat_kumar@yahoo.com.
Ritesh Pattnaik, Email: rpattnaik@kiitbiotech.ac.in.
Basavaraj Mathapati, Email: basavaraj.mathapati@gmail.com.
Priyadarshi Soumyaranjan Sahu, Email: priyadarshi_sahu@yahoo.com.
References
- 1.Harcourt B.H., Tamin A., Ksiazek T.G., Rollin P.E., Anderson L.J., Bellini W.J., Rota P.A. Molecular characterization of Nipah virus, a newly emergent paramyxovirus. Virology. 2000 Jun 5;271(2):334–349. doi: 10.1006/viro.2000.0340. [DOI] [PubMed] [Google Scholar]
- 2.Chua K.B. Nipah virus outbreak in Malaysia. J Clin Virol. 2003;26:265–275. doi: 10.1016/s1386-6532(02)00268-8. [DOI] [PubMed] [Google Scholar]
- 3.Clayton B.A. Nipah virus: transmission of a zoonotic paramyxovirus. Curr Opin Virol. 2017 Feb;22:97–104. doi: 10.1016/j.coviro.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Gazal S., Sharma N., Gazal S., Tikoo M., Shikha D., Badroo G.A., Rashid M., Lee S.-J. Nipah and Hendra Viruses: Deadly zoonotic Paramyxoviruses with the potential to cause the next pandemic. Pathogens. 2022;11(12):1419. doi: 10.3390/pathogens11121419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno L., Nappo M.A., Ferrari L., Di Lecce R., Guarnieri C., Cantoni A.M., Corradi A. Nipah virus disease: epidemiological, clinical, diagnostic and legislative aspects of this unpredictable emerging zoonosis. Animals (Basel) 2022 Dec 31;13(1):159. doi: 10.3390/ani13010159. [DOI] [PMC free article] [PubMed] [Google Scholar]