Abstract
OBJECTIVE
Frailty is one of the important factors in predicting the outcomes of surgery. Many surgical specialties have adopted a frailty assessment in the preoperative period for prognostication; however, there are limited data on the effects of frailty on the outcomes of cerebral aneurysms. The object of this study was to find the effect of frailty on the surgical outcomes of anterior circulation unruptured intracranial aneurysms (UIAs) and compare the frailty index with other comorbidity indexes.
METHODS
A retrospective study was performed utilizing the National Inpatient Sample (NIS) database (2016–2018). The Hospital Frailty Risk Score (HFRS) was used to assess frailty. On the basis of the HFRS, the whole cohort was divided into low-risk (0–5), intermediate-risk (> 5 to 15), and high-risk (> 15) frailty groups. The analyzed outcomes were nonhome discharge, complication rate, extended length of stay, and in-hospital mortality.
RESULTS
In total, 37,685 patients were included in the analysis, 5820 of whom had undergone open surgical clipping and 31,865 of whom had undergone endovascular management. Mean age was higher in the high-risk frailty group than in the low-risk group for both clipping (63 vs 55.4 years) and coiling (64.6 vs 57.9 years). The complication rate for open surgical clipping in the high-risk frailty group was 56.1% compared to 0.8% in the low-risk group. Similarly, for endovascular management, the complication rate was 60.6% in the high-risk group compared to 0.3% in the low-risk group. Nonhome discharges were more common in the high-risk group than in the low-risk group for both open clipping (87.8% vs 19.7%) and endovascular management (73.1% vs 4.4%). Mean hospital charges for clipping were $341,379 in the high-risk group compared to $116,892 in the low-risk group. Mean hospital charges for coiling were $392,861 in the high-risk frailty group and $125,336 in the low-risk group. Extended length of stay occurred more frequently in the high-risk frailty group than in the low-risk group for both clipping (82.9% vs 10.7%) and coiling (94.2% vs 12.7%). Frailty had higher area under the receiver operating characteristic curve values than those for other comorbidity indexes and age in predicting outcomes.
CONCLUSIONS
Frailty affects surgical outcomes significantly and outperforms age and other comorbidity indexes in predicting outcome. It is imperative to include frailty assessment in preoperative planning.
Keywords: frailty, Hospital Frailty Risk Score, aneurysms, microsurgical clipping, vascular disorders
THE incidence of unruptured intracranial aneurysms (UIAs) has been approximately 0.2% to 9.9% of the population.1 Those aneurysms are often detected incidentally or as a result of symptoms ranging from headaches to neurological deficits related to neurovascular compression. There is scant literature on how patient age and comorbidities influence outcomes following the treatment of UIAs. Evidence has shown that factors like increasing age and the presence of comorbidities are associated with worse functional outcomes following surgical and endovascular treatment of UIAs.2–6 With the changing global demographics and improved life expectancy worldwide, diseases requiring interventions in elderly people have increased significantly, and predictors of outcomes like a frailty index have gained popularity in the last 2 decades.
Frailty has been defined as a state of reduced physiological reserve associated with increased susceptibility to disability.2,7,8 Frailty occurs from the continuous decline of multiple physiological systems, creating a limited reserve to withstand stressors and leading to an increased susceptibility to poor outcomes. Several factors constitute a frail status, including advanced age, impaired cognitive function, chronic malnutrition, unexplained falls, depression, and anemia.9 Frailty is not considered as a convergent function of age, although frailty is more common with an increase in age. Frailty has been reported to be a better predictor of patient outcomes across multiple surgical specialties.10–13
Although some studies have investigated the effect of frailty on the outcomes of cerebral aneurysms, a quantified frailty score has not been applied. In the present study, we used the Hospital Frailty Risk Score (HFRS)17 in patients from a national representative administrative database to identify the effects of frailty on outcomes and to compare the frailty score with age and comorbidities using standardized indexes.
Methods
Data Extraction
We queried the latest National Inpatient Sample (NIS) database (2016–2018, i.e., 3 years) for patients with anterior circulation UIAs who had undergone microsurgical clipping or endovascular coiling. The NIS database is the largest publicly available database, containing more than 7 million hospitalizations each year, and covers more than 97% of the United States population. The NIS adapted ICD-10 diagnostic codes from the end of 2015. We used only ICD-10 diagnostic codes (i.e., latest NIS) to prevent heterogeneity and the loss of data during the transition from ICD-9 to ICD-10 codes. We extracted the data using the ICD-10 diagnostic codes and procedural codes for filtering anterior circulation unruptured aneurysm patients (Supplementary e-Table 1). Our study inclusion criteria were as follows: age > 18 years, patients admitted with a primary diagnosis of anterior circulation UIA, and patients who had undergone craniotomy and clipping or endovascular management. Our exclusion criteria were as follows: the unruptured aneurysm was not the primary cause of admission, and data on primary outcome variables were missing.
Selection of the Frailty Index
Different frailty indexes are available.14–16 We used the HFRS as our frailty index, which was recently developed on the basis of ICD-10 diagnostic codes in a large administrative health database and externally validated, making it highly suitable for the NIS database (Supplementary e-Table 2).17 The HFRS contains 110 diagnostic clusters as domains and is calculated using individual weights for each positive variable, creating a numerical score whose severity is indicated by higher scores.17–20 Each variable has a specific weight assigned, with the summation of all variables creating a composite score. All details of the variables with individual weights are provided in Supplementary e-Table 2. A total score of 0–5 was categorized as a low frailty risk, > 5 to 15 as an intermediate frailty risk, and > 15 as a high frailty risk. Most available frailty indexes only define frailty as a measure of binary outcome (yes/no).14 By using the HFRS, frailty was quantified in our study.
Comorbidities
For assessing comorbidities, we used two different comorbidity indexes for quantification. The Elixhauser Comorbidity Index (ECI) was developed on the basis of 31 different comorbidities, each with individual weights.21 Another comorbidity index called the Neurovascular Comorbidity Index (NCI) was used, which was explicitly developed for neurovascular pathologies like aneurysms and arteriovenous malformations (Supplementary e-Table 3).22 Both of these indexes were validated in multiple studies for predicting outcomes.23
Outcomes
The following clinical endpoints were considered as outcome variables in our study: extended length of stay (ELOS), nonhome discharge, complications, and death. ELOS was defined as any LOS greater than the 75th percentile of the median stay of the respective groups (open surgery/endovascular management). Patients discharged to home were considered to have a good outcome or minimal disability. Any discharge other than the home was considered a poor outcome. Most studies have validated the concept of discharge dispositions as a marker of functional independence. Although it may not be as accurate as an objective scale, discharge disposition serves as a surrogate marker of outcome.
A complication was defined as the presence of any complication during the hospital course. The complications considered were cardiac complications such as postoperative myocardial infarction cardiac failure, etc.; neurological complications such as postoperative deficits, seizures, ischemic stroke, etc.; wound complications; gastrointestinal complications; respiratory complications along with pneumonia; renal complications; deep venous thrombosis; and embolism. The details of the ICD-10 codes used are provided in Supplementary e-Table 1. Discussing these individual complications rates in the main text of the paper would be beyond the scope of the current study; however, we intend to grossly show the complication rates across the frailty groups.
Death in the hospital was defined as in-hospital mortality occurring after either intervention.
Statistical Analysis
The whole study cohort with unruptured aneurysms was analyzed on the basis of the three frailty categories for both open surgery and endovascularly treated patients. The normality of the data was assessed using quantile-quantile plots and the Shapiro-Wilk test. For all analyses, we used the discharge weight variable provided by the NIS database. Using the discharge weight provided an accurate national-level estimate. The Agency for Health-care Research and Quality highly recommends using the discharge weight variable in all forms of the analysis based on the survey design. The categorical variables were described as proportions, and continuous variables were expressed as the mean with standard deviation or median with interquartile range, as appropriate. Categorical variables across the groups were compared using the chi-square test, and continuous variables were compared using the one-way ANOVA test. Binary logistic regression analysis was performed to compute the odds ratios and 95% confidence intervals after adjusting for age, gender, insurance, race, and comorbidities. The Hosmer-Lemeshow goodness of fit test was used for the model. The receiver operating characteristic (ROC) with the area under the curve (AUC) function was used to compare the accuracy of age, frailty score, and comorbidities in predicting the outcome variables. For the ROC analysis, the HFRS (continuous variable) was used instead of the frailty categories. All variables were assessed for colinearity for the ROC analysis. A p value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics 27 software (IBM Corp.). The graphs were made using R software (ggplot2 plugin, R Foundation for Statistical Computing) as well as Microsoft Excel and SPSS (IBM).
Results
Open Surgery
A total of 5820 patients underwent clipping during the study period, 54.8% of whom (n = 3190) were in the low-risk frailty group, 41.7% of whom (n = 2425) were in the intermediate-risk group, and 3.5% of whom (n = 205) were in the high-risk group (Table 1). The mean age was highest in the high-risk frailty group (63 vs 55.4 years in the low-risk group and 58.9 years in the intermediate-risk group, p < 0.001). Females were the predominant gender across all groups (p = 0.703). Whites were the predominant population across all the groups (p = 0.154). The mean ECI score was highest in the high-risk frailty group (23.07 vs 8.4 low risk and 14.62 intermediate risk, p < 0.001). The most common insurance in the high-risk group was Medicare (46.3%), whereas private insurance was the most common in the low-risk group. The mean LOS was higher in the high-risk group (16.29 ± 12.1 days) than in the other groups (3.75 days in low risk and 6.62 days in intermediate risk). The mean hospital charges were significantly higher in the high-risk frailty group ($341,379) than in the low-risk frailty group ($116,892).
TABLE 1.
Demographics and discharge dispositions among 5820 patients who underwent open surgical clipping of UIAs
| Variable | Low-Risk Frailty (HFRS 0–5) | Intermediate-Risk Frailty (HFRS >5–15) | High-Risk Frailty (HFRS >15) | p Value |
|---|---|---|---|---|
| No. of patients | 3190 | 2425 | 205 | |
| Mean age in yrs (SD) | 55.42 (10.6) | 58.87 (10.45) | 63 (11.24) | <0.001 |
| Female gender | 74.8% | 74.6% | 80.5% | 0.703 |
| Race | 0.154 | |||
| White | 64.3% | 63.1% | 56.1% | |
| Black | 11.1% | 15.3% | 12.2% | |
| Hispanic | 12.4% | 10.7% | 19.5% | |
| Asian/Pacific Islander | 3.6% | 2.3% | 2.4% | |
| Native American | 0.5% | 1.4% | 2.4% | |
| Other | 4.1% | 2.5% | 2.4% | |
| ECI score (SD) | 8.40 (3.9) | 14.62 (5.5) | 23.07 (5.3) | <0.001 |
| NCI score (SD) | 0.967 (2.47) | 4.99 (4.55) | 11.82 (5.97) | <0.001 |
| Insurance | <0.001 | |||
| Medicare | 24.8% | 41.6% | 46.3% | |
| Medicaid | 17.7% | 16.1 % | 7.3% | |
| Private | 50.3% | 36.1% | 39% | |
| Self-pay | 2.7% | 2.7% | 7.3% | |
| No charge | 0.3% | 0.4% | NA | |
| Other | 4.2% | 2.9% | NA | |
| Discharge disposition | ||||
| Discharge to home | 79.9% | 54.2% | 7.3% | <0.001 |
| Discharge to other facilities | 19.7% | 45.4% | 87.8% | <0.001 |
| Death in hospital | 0.3% | 0.4% | 4.9% | <0.001 |
| Mean LOS (SD) | 3.75 (2.4) | 6.62 (6.04) | 16.29 (12.1) | <0.001 |
| Any complication | 0.8% | 11.3% | 56.1% | <0.001 |
| ELOS | 10.7% | 36.5% | 82.9% | <0.001 |
| Total charges in $US (SD) | 116,892 (72,242) | 166,750 (178,444) | 341,379 (254,472) | <0.001 |
NA = not any.
Boldface type indicates statistical significance.
Endovascular Management
A total of 31,865 patients underwent coiling during the study period, 68.9% (n = 21,940) of whom were in the low-risk frailty group, 29.5% (n = 9405) of whom were in the intermediate-risk group, and 1.6% (n = 520) of whom were in the high-risk frailty group (Table 2). Mean age was highest in the high-risk group (64.6 vs 57.9 years in the low-risk group and 62.0 years in the intermediate-risk group). Females were the predominant gender across all the groups. Whites were the predominant population across all the groups. The mean ECI and NCI scores increased as the frailty score increased. The most common insurance in the low-risk frailty group was private insurance (42.5%), whereas the most common insurance in the high-risk group was Medicare (51.9%). The mean charges and hospital LOS (1.64 days in the low-risk vs 3.47 in the intermediate-risk and 17.8 days in the high-risk groups) increased as the frailty score increased.
TABLE 2.
Demographics and discharge dispositions among 31,865 patients who underwent endovascular management of UIAs
| Variable | Low-Risk Frailty (HFRS 0–5) | Intermediate-Risk Frailty (HFRS >5–15) | High-Risk Frailty (HFRS >15) | p Value |
|---|---|---|---|---|
| No. of patients | 21,940 | 9405 | 520 | |
| Mean age in yrs (SD) | 57.91 (12.7) | 61.99 (12.1) | 64.57 (12.87) | <0.001 |
| Female gender | 77.4% | 75% | 78.8% | 0.101 |
| Race | <0.001 | |||
| White | 65.5% | 66.4% | 67.3% | |
| Black | 11.6% | 15.3% | 13.5% | |
| Hispanic | 11.9% | 10.3% | 8.7% | |
| Asian/Pacific Islander | 3.2% | 2.2% | 3.8% | |
| Native American | 0.6% | 0.4% | NA | |
| Other | 3.7% | 2.3% | 2.9% | |
| Mean ECI score (SD) | 8.32 (3.92) | 14 (5.35) | 23.56 (6.13) | <0.001 |
| Mean NCI score (SD) | 0.539 (1.92) | 3.68 (5.3) | 10.5 (5.61) | <0.001 |
| Insurance | <0.001 | |||
| Medicare | 37.1% | 52.0% | 51.9% | |
| Medicaid | 14.4% | 13.3% | 15.4% | |
| Private | 42.5% | 29.1% | 27.9% | |
| Self-pay | 2.5% | 2.1% | NA | |
| No charge | 0.3% | 0.2% | NA | |
| Other | 3.2% | 3.2% | 4.8% | |
| Discharge disposition | ||||
| Discharge to home | 95.4% | 79.4% | 22.1% | <0.001 |
| Discharge to other facilities | 4.4% | 19.3% | 73.1% | <0.001 |
| Death in hospital | 0.1% | 1.3% | 4.8% | <0.001 |
| Mean LOS (SD) | 1.64 (1.84) | 3.47 (7.25) | 17.87 (28.11) | <0.001 |
| Any complication | 0.3% | 10.2% | 60.6% | <0.001 |
| ELOS | 12.7% | 36% | 94.2% | <0.001 |
| Total charges in $US (SD) | 125,336 (84,925) | 153,996 (128,980) | 392,861 (377,820) | <0.001 |
Boldface type indicates statistical significance.
Outcomes
Open Surgery
Nonhome discharges were higher in the high-risk frailty group (87.8%) than in the intermediate- (45.4%) and low-risk (19.7%) frailty groups (p < 0.001). The complication rate was highest in the high-risk frailty group (56.1% vs 0.8% low and 11.3% intermediate). Moreover, ELOS was highest in the high-risk frailty group (82.9%; Fig. 1). The death rate was highest in the high-risk frailty group (4.9% vs 0.3% low and 0.4% intermediate, p < 0.001).
FIG. 1.
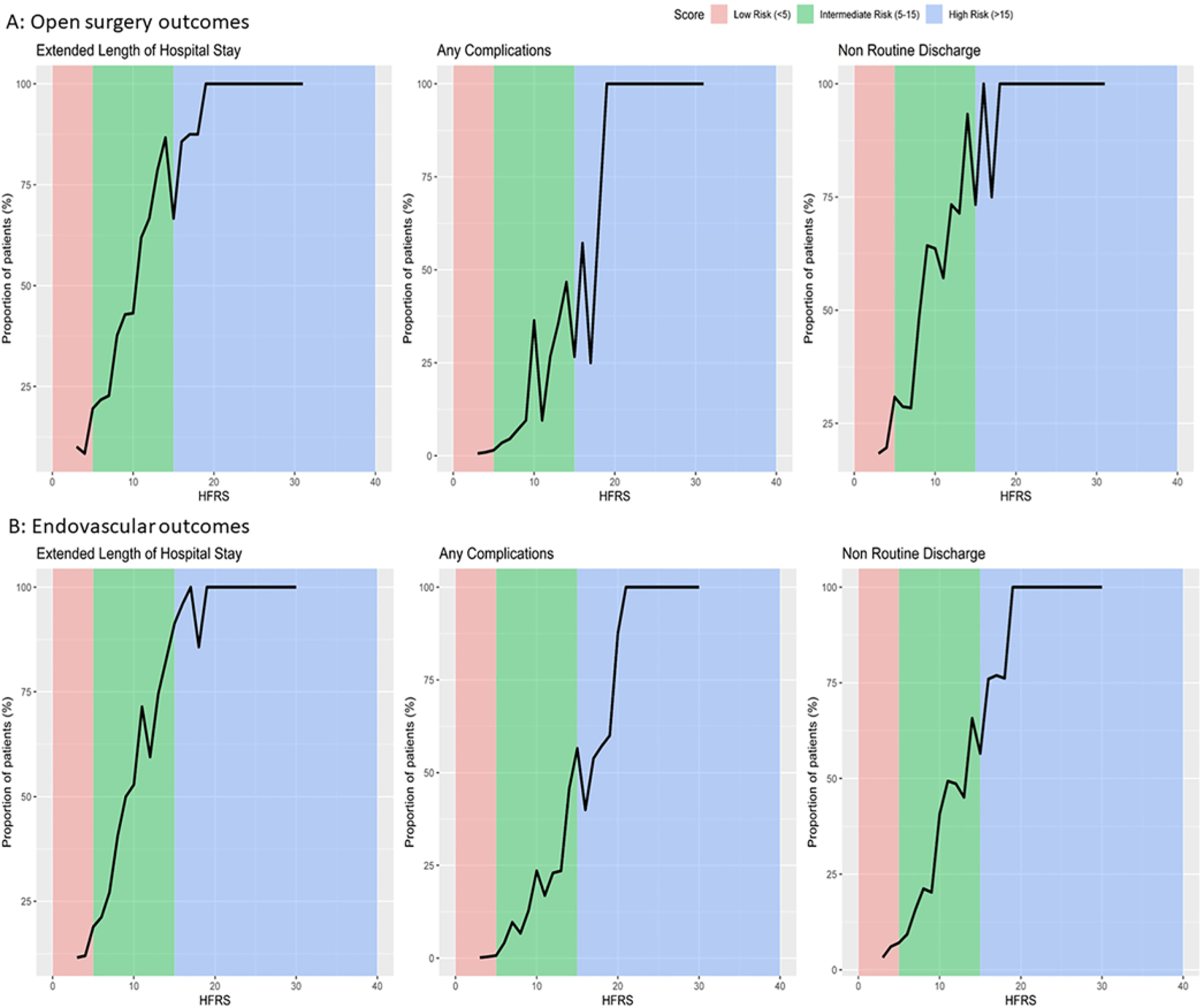
A: Line diagrams representing different outcomes in open surgery. B: Line diagrams representing different outcomes in endovascular management.
Endovascular Management
Nonhome discharges were higher in the high-risk frailty group (73.1%) compared to the 4.4% in the low-risk group. The complication rate increased as the frailty score increased (0.3% low risk, 10.2% intermediate risk, and 60.6% high risk). ELOS also increased as the frailty score increased. The death rate was highest in the high-risk frailty group (4.8% vs 0.1% low risk and 1.3% intermediate risk).
Key Differences Between Clipping and Endovascular Groups
The mean HFRS and ECI score were higher in the open surgery group than in the endovascular group. The NCI was higher in the coiling group. Mean LOS was lower in the coiling group (2.44 vs 5.39 days). Nonhome discharge was higher in the clipping group (32.8% vs 10%; Table 3).
TABLE 3.
Demographics and outcomes for open surgery compared to endovascular management for anterior circulation UIAs
| Variable | Clipping | Coiling | p Value |
|---|---|---|---|
| No. of patients | 5820 | 31,865 | |
| Mean age in yrs (SD) | 57.12 (10.77) | 59.22 (12.75) | <0.001 |
| Female gender | 74.9% | 76.7% | 0.003 |
| Race | |||
| White | 63.5% | 65.8% | <0.001 |
| Black | 12.9% | 12.7% | |
| Hispanic | 11.9% | 11.4% | |
| Asian/Pacific Islander | 3% | 2.9% | |
| Native American | 0.9% | 0.5% | |
| Other | 3.4% | 3.3% | |
| Mean HFRS (SD) | 5.95 (3.73) | 4.91 (2.996) | <0.001 |
| Low-risk frailty | 54.8% | 68.9% | |
| Intermediate-risk frailty | 41.7% | 29.5% | |
| High-risk frailty | 3.5% | 1.6% | |
| Mean ECI score (SD) | 11.51 (6.06) | 10.24 (5.41) | <0.001 |
| Mean NCI score (SD) | 1.63 (3.45) | 3.02 (4.46) | <0.001 |
| Insurance | <0.001 | ||
| Medicare | 32.6% | 41.7% | |
| Medicaid | 16.7% | 14.1% | |
| Private | 44% | 38.3% | |
| Self-pay | 2.8% | 2.3% | |
| No charge | 0.3% | 0.3% | |
| Other | 3.5% | 3.2% | |
| Discharge disposition | |||
| Discharge to home | 66.7% | 89.5% | <0.001 |
| Discharge to other facilities | 32.8% | 10% | <0.001 |
| Death in hospital | 0.5% | 0.5% | 0.977 |
| Mean LOS (SD) | 5.39 (5.46) | 2.44 (5.9) | <0.001 |
| Any complication | 7.1% | 4.2% | <0.001 |
| ELOS | 24% | 20.9% | |
| Total charges in $US (SD) | 145,561 (142,774) | 138,106 (115,819) | <0.001 |
Boldface type indicates statistical significance.
A subgroup analysis was performed in the high-risk frailty group to compare the outcomes between open surgery and endovascular management. Nonhome discharge was higher in the clipping group (92.7% vs 77.9%, p < 0.001). There was no significant difference in complications (p = 0.269) or mean LOS between the two groups (p = 0.431; Fig. 2B).
FIG. 2.
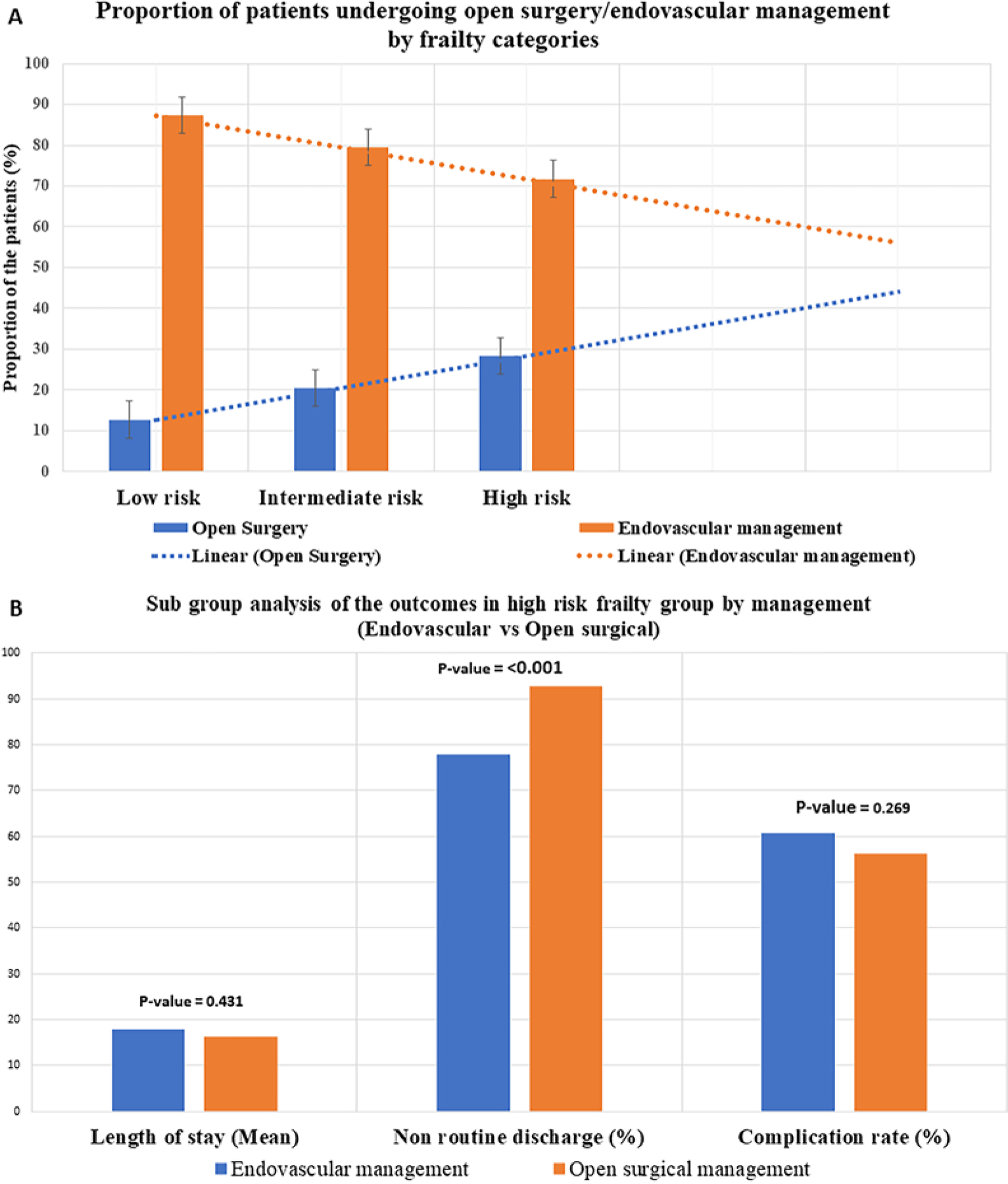
A: Bar graph showing the proportion of the patients who underwent open surgery and endovascular management with an increase in frailty score. B: Subgroup analysis of the outcomes in the high-risk frailty group (open surgery vs endovascular management).
Regression Analysis
Binary regression analysis was performed after adjusting for age, gender, race, insurance, and comorbidities.
Open Surgery
Death was predicted only by age (OR 1.204, 95% CI 1.031–1.406, p = 0.01). LOS was predicted by ECI (OR 1.059, 95% CI 1.023–1.096, p = 0.001), NCI (OR 1.105, 95% CI 1.060–1.152, p < 0.001), and HFRS (OR 1.185, 95% CI 1.113–1.261, p < 0.001). Nonhome discharge (poor outcome) was predicted by age, ECI, NCI, and HFRS. Complications were predicted only by ECI (OR 1.082, 95% CI 1.026–1.142, p = 0.04) and HFRS (OR 1.313, 95% CI 1.208–1.426, p < 0.001; Supplementary e-Table 4).
Endovascular Management
Death was predicted by (female) gender (OR 2.17, 95% CI 1.34–3.51, p = 0.002), ECI (OR 1.173, 95% CI 1.14–1.20, p < 0.001), and NCI (OR 1.05, 95% CI 1.01–1.09, p = 0.01). The HFRS did not predict death. ELOS, poor outcome, and complication were predicted by ECI, NCI, and HFRS (Supplementary e-Table 5).
ROC Curves
Open Surgery
The ROC analysis for death comparing age, frailty status, and comorbidities revealed that ECI (AUC 0.793, 95% CI 0.636–0.950) and NCI (AUC 0.822, 95% CI 0.678–0.966) had greater AUC values. With complications as the outcome variable, the HFRS had the highest AUC value (0.88, 95% CI 0.851–0.925, p < 0.001). ROC analysis for nonhome discharge revealed that HFRS had the highest AUC value (0.722, 95% CI 0.689–0.755, p < 0.001). ROC analysis for ELOS revealed HFRS had the highest AUC value (0.773, 95% CI 0.739–0.807, p < 0.001; Fig. 3 and Table 4).
FIG. 3.
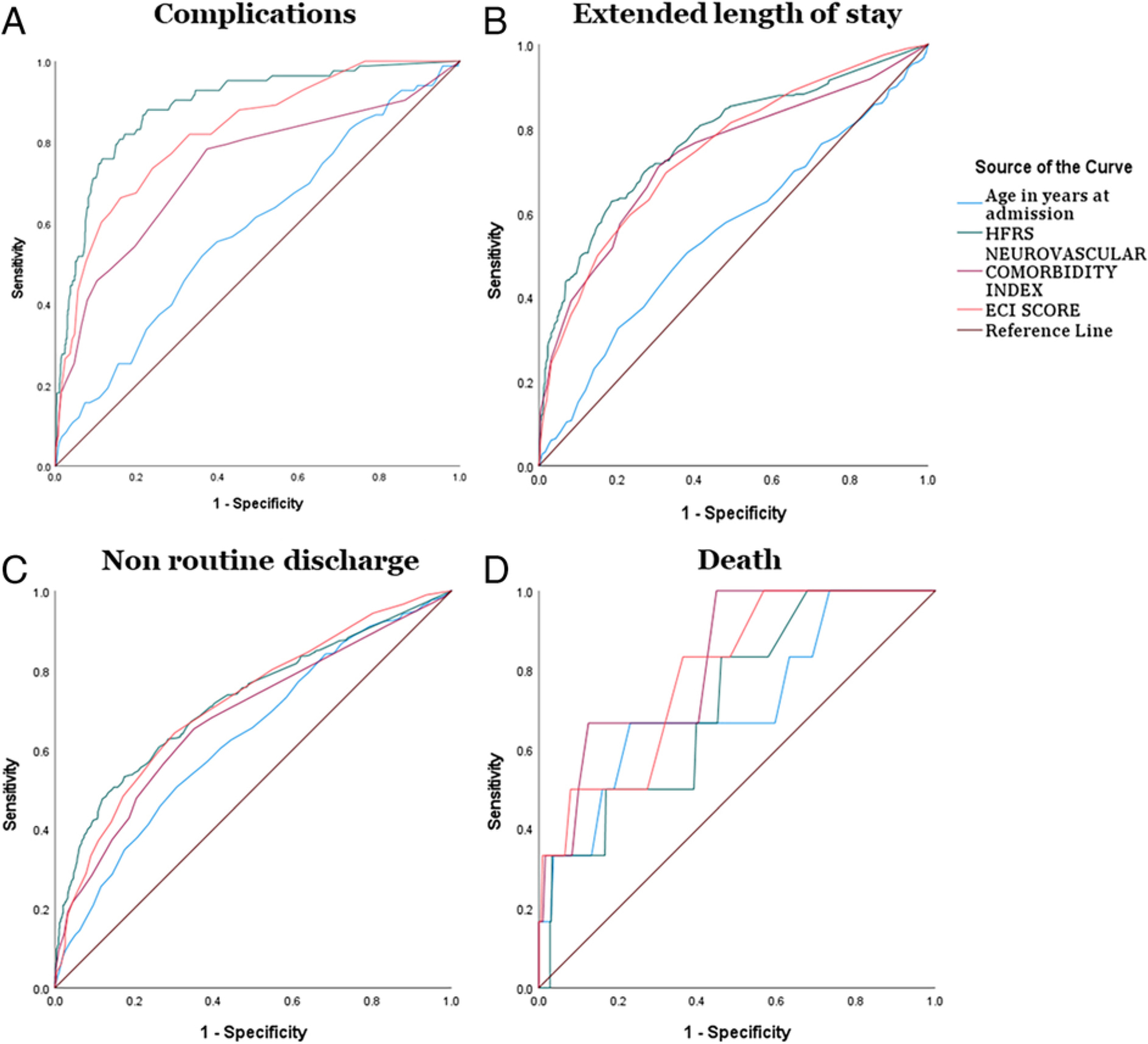
ROC curves for outcomes comparing different indexes in open surgery.
TABLE 4.
ROC analysis for open surgery and endovascular management comparing different indexes and age
| Outcome | AUC | 95% CI | p Value |
| Open surgery | |||
| Any complication | |||
| HFRS | 0.888 | 0.851–0.925 | <0.001 |
| ECI | 0.825 | 0.779–0.871 | <0.001 |
| NCI | 0.737 | 0.673–0.801 | <0.001 |
| Age | 0.587 | 0.523–0.652 | 0.008 |
| ELOS | |||
| HFRS | 0.773 | 0.739–0.807 | <0.001 |
| ECI | 0.744 | 0.710–0.778 | <0.001 |
| NCI | 0.735 | 0.698–0.772 | <0.001 |
| Age | 0.555 | 0.514–0.595 | <0.001 |
| Nonhome discharge | |||
| HFRS | 0.722 | 0.689–0.755 | <0.001 |
| ECI | 0.715 | 0.683–0.747 | <0.001 |
| NCI | 0.680 | 0.646–0.713 | <0.001 |
| Age | 0.632 | 0.598–0.666 | 0.006 |
| Death | |||
| HFRS | 0.716 | 0.535–0.896 | 0.06 |
| ECI | 0.793 | 0.636–0.950 | 0.01 |
| NCI | 0.822 | 0.678–0.966 | 0.006 |
| Age | 0.714 | 0.492–0.936 | 0.07 |
| Endovascular surgery | |||
| Any complication | |||
| HFRS | 0.918 | 0.903–0.933 | <0.001 |
| ECI | 0.821 | 0.794–0.849 | <0.001 |
| NCI | 0.805 | 0.775–0.836 | <0.001 |
| Age | 0.600 | 0.564–0.636 | <0.001 |
| ELOS | |||
| HFRS | 0.824 | 0.800–0.848 | <0.001 |
| ECI | 0.806 | 0.783–0.829 | <0.001 |
| NCI | 0.793 | 0.767–0.818 | <0.001 |
| Age | 0.566 | 0.537–0.595 | <0.001 |
| Nonhome discharge | |||
| HFRS | 0.791 | 0.771–0.811 | <0.001 |
| ECI | 0.763 | 0.743–0.783 | <0.001 |
| NCI | 0.715 | 0.692–0.738 | <0.001 |
| Age | 0.641 | 0.618–0.664 | <0.001 |
| Death | |||
| HFRS | 0.870 | 0.819–0.921 | <0.001 |
| ECI | 0.889 | 0.841–0.936 | <0.001 |
| NCI | 0.768 | 0.680–0.856 | <0.001 |
| Age | 0.615 | 0.520–0.709 | 0.023 |
Boldface type indicates statistical significance.
Endovascular Management
The ROC analysis revealed higher AUC values for HFRS in nonhome discharge (0.791, 95% CI 0.771–0.811), ELOS (0.824, 95% CI 0.800–0.848), and complications (0.918, 95% CI 0.903–0.933). ECI had a higher AUC function (0.889, 95% CI 0.841–0.936) in death (Fig. 4 and Table 4).
FIG. 4.
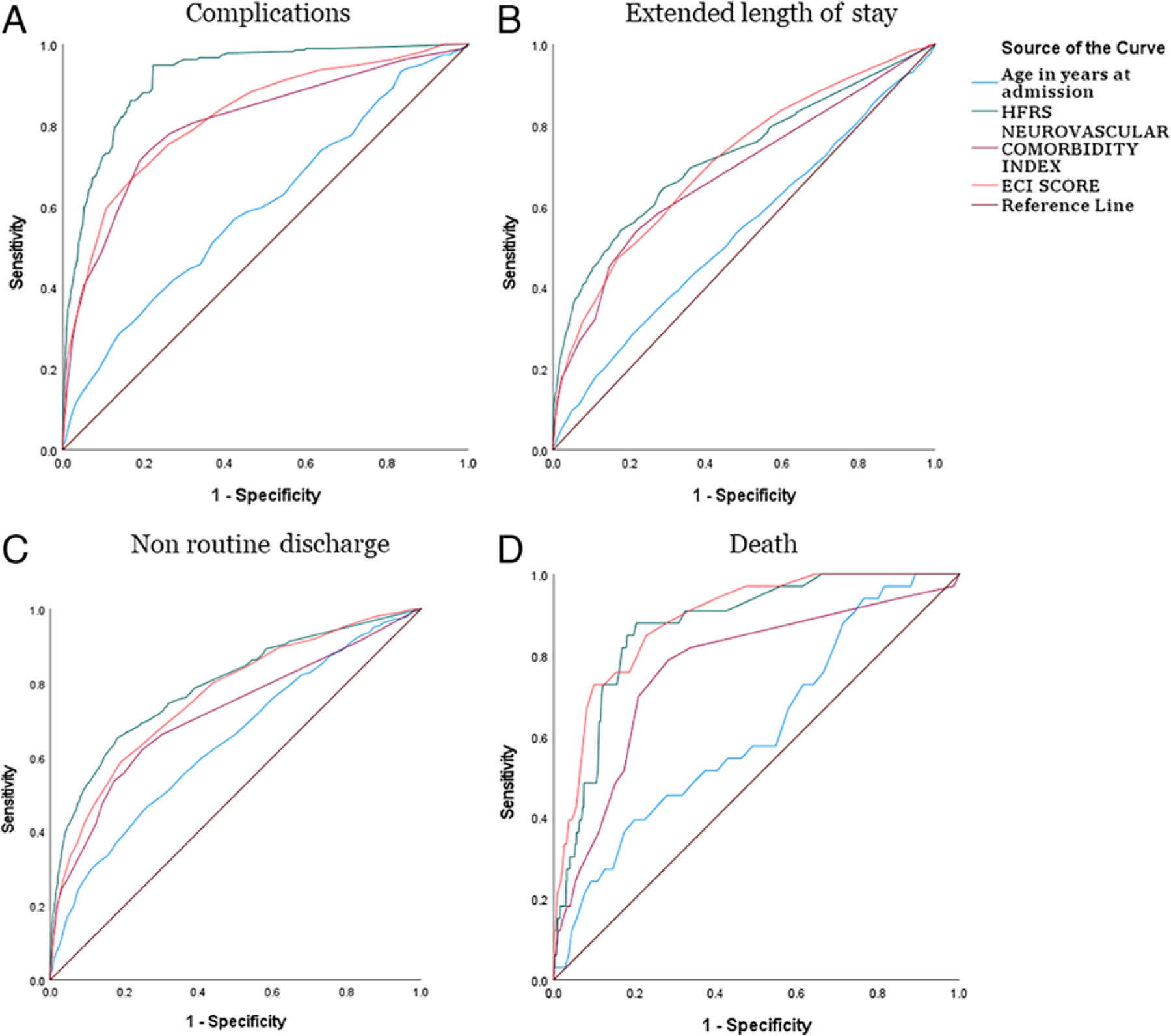
ROC curves for outcomes comparing different indexes in endovascular management.
Discussion
We limited our selection in the NIS database to anterior circulation IAs to reduce the treatment heterogeneity and to UIAs to negate the confounding effects of clinical subarachnoid hemorrhage (SAH) admission grades (Hunt and Hess or World Federation of Neurosurgical Societies). Based on the ROC curves from our analysis, frailty and comorbidity scores have better discriminative ability than age in predicting all outcome variables. Overall, the prevalence of high-risk frailty in UIAs was 3.5% for clipping and 1.6% for endovascular management. In the clipping group, the high-risk frailty population had a 50-times-greater risk of complications than the low-risk frailty population. Similarly, in the coiling group, the high-risk frailty population had 60 times more complications than the low-risk population. Along with these complications, hospital charges, LOS, and death in the hospital increased with an increase in the frailty score (Fig. 1). Based on the regression analyses, LOS, nonhome discharge, and complications were predicted by the frailty and comorbidity scores.
The role of frailty in preoperative assessment and patient selection has been established in other specialties like cardiac surgery and general surgery.24,25 However, neurosurgery has lagged behind in preoperative prognostication and outcome prediction scales, although there are disease-specific indexes. Our study emphasizes the importance of prognostic indexes like those for frailty in predicting postintervention outcomes and hospital costs. Our results clearly showed that for patients with anterior circulation UIAs undergoing any form of intervention (coiling or clipping), frailty has a better discriminating ability than age and other comorbidity indexes (NCI and ECI) in predicting ELOS, complications, nonhome discharge, and hospital charges. NCI and ECI had better discriminating ability than frailty and age in predicting mortality.
These findings agree with most studies, in which frailty had the better discriminating ability compared to other factors in predicting complications.26,27 Another interesting factor was the correlation of treatment with frailty score. With an increase in the frailty score, there was a gradual decline in the proportion of patients undergoing endovascular management and a gradual increase in patients undergoing open surgery (Fig. 2A). This finding contradicts the traditional thinking that clipping is a more morbid procedure than coiling in the frail population. One potential explanation for this contradiction would be the confounding effect of the aneurysm morphology, which led to the selection of the approach. However, in larger data sets, these factors would assume a normal bell curve, decreasing the confounding effect.
Also, in the subgroup analysis of the high-risk frailty group, LOS and complication rates were similar for open surgery and endovascular management. This was an interesting finding; that is, that regardless of the approach, frail patients fare worse than their nonfrail counterparts. This finding raises the basic question, do the outcomes justify the intervention? In the era of minimally invasive surgery, the benefit of intervention for an unruptured aneurysm in high-risk patients is still debatable. The outcomes did not differ with endovascular treatment, which is deemed to be less invasive than open surgery. This suggests that shared decision-making should take place while selecting frail patients for the treatment of unruptured aneurysms. Evaluation by a multidisciplinary team and a clear discussion should take place to anticipate the outcomes realistically. The risk of rupture and the benefits of treatment should be carefully reviewed in these select cases.
Brinjikji et al. studied the effect of age on unruptured aneurysms and reported poorer treatment (clipping and coiling) outcomes in patients with an increase in age.2 In their study, these authors mainly compared the outcomes of clipping and coiling with respect to age. The outcomes used in their study were discharge to a long-term facility, LOS, and mortality. However, complications were not studied. Coiling had lower morbidity rates than clipping in the elderly population. Similarly, Silva et al. reported poorer outcomes in elderly patients in comparison to those in younger populations.28 Elderly patients (age > 65 years) had a postoperative stroke rate of 10.8% versus 5.8% in nonelderly patients. However, these studies had groups based on age and cannot be compared directly with our study.
Several studies across multiple specialties have examined the effects of age and frailty on surgical outcomes. Most studies have shown that frailty is an independent and better predictor than age alone. Feghali et al. studied the effect of frailty and age in 275 unruptured aneurysms (clipping and coiling) from a prospective institutional database.26 In their study, the frailty index (5-factor modified frailty index) was significantly associated with complications (OR 2.3, 95% CI 1.3–4.1, p = 0.004). Also, when age along with frailty index was evaluated, the combination discriminated better than the frailty index alone (AUC = 0.600 vs 0.610). However, few studies have reported age as a better predictor. McIntyre et al. compared the effect of age and frailty in aneurysmal SAH and reported that frailty was associated with worse SAH grades, increased complication rates, and higher mortality.13 Only age and Hunt and Hess grades were independent predictors in their multivariate analysis, but not frailty.
The frail patients (intermediate-risk and high-risk groups) in our cohort had a significantly higher percentage of Medicare coverage than the low-risk group. The high-risk frailty group had three times higher hospital charges than the low-risk frailty patients, reflecting higher complication rates and longer LOSs. Bock et al. analyzed healthcare costs in frail patients. They reported higher hospital charges, with a mean increase of 44% in inpatient costs, 31% in outpatient costs, and 19% in pharmaceutical costs.29 Given the common diagnosis of an aneurysm in the elderly, with a dramatic increase in hospital charges in frail patients, it is imperative to include frailty assessment in the preoperative screening and prognostication. Different frailty scales are available for implementation in the hospital systems. The frailty scales like the HFRS, Johns Hopkins Adjusted Clinical Groups, electronic frailty index, etc., developed using administrative health databases, can be integrated into the electronic medical records of the patients. Various algorithms for the calculation of scores were publicly available free of cost. Slight modifications of the algorithms suiting the individual hospital needs can be made for implementation. Also, frailty scales like the Edmonton Frail Scale, Fried Frailty Index, etc., can be implemented bedside or on an outpatient basis. Discussing various frailty scores in detail is beyond the scope of this study; however, we provide a literature review table with various resources and URLs for frailty score calculators in Supplementary e-Table 6.
Study Limitations
There are several limitations to our study. The data are from single hospitalizations, undermining actual complication rates and readmissions. The NIS database does not provide objective functional outcomes like the modified Rankin Scale score, hence the necessity of using discharge disposition as a surrogate marker. We have reduced the heterogeneity and selection bias by selecting a homogeneous population (UIAs). The aneurysm morphology and location were other important factors impacting outcomes. Unfortunately, the NIS database does not provide details regarding morphology; thus, we reduced this bias by selecting only anterior circulation aneurysms. Scores like the PHASES score (population, hypertension, age, size of the aneurysm, earlier SAH, site) and Unruptured Intracranial Aneurysm Treatment Score (UIATS) indicate the risk of rupture in unruptured aneurysms. Integrating these scores with frailty preoperatively helps to realistically assess the risk-benefit ratio. Using these scores, vulnerable patients can be identified and a true shared decision can be made with patients. However, given the limitations of the current data sets, we could not integrate the frailty score with the UIATS and PHASES score. We intend to use the frailty score together with the PHASES score and UIATS in our future institutional studies. We also acknowledge some of the limitations of the frailty index we selected, that is, HFRS. Most frailty scores in the current era have been criticized for their low discrimination abilities and complex scores, making it challenging to apply them at individual patient levels. However, our focus was to show that a frailty index, regardless of the index selected, is an independent predictor of outcome and should be applied as a routine screening tool in preoperative risk stratification. However, as the algorithm of the HFRS can be easily applied to large national databases, it has the potential to identify the prevalence of frailty in different diseases and shape health policies. The HFRS was constructed on the basis of ICD-10 diagnostic codes, which precluded us from using the score on earlier years, limiting our data to the last 3 years.
Conclusions
In summary, frailty (HFRS) and comorbidity (NCI and ECI) are independently associated with a higher risk of complications after treatment for anterior circulation UIAs. These prognosticators predicted outcomes better than age. This study signifies the importance of including frailty in the preoperative assessments for prognostication and prehabilitation of patients.
Supplementary Material
Acknowledgments
We acknowledge the help of Ms. Eilis Keeble, statistician (Nuffield Trust, United Kingdom), for providing the ICD codes for calculating the HFRS.
This work was supported in part by the National Institute of General Medical Sciences of the National Institutes of Health (grant no. U54 GM104940), which funds the Louisiana Clinical and Translational Science Center.
ABBREVIATIONS
- AUC
area under the curve
- ECI
Elixhauser Comorbidity Index
- ELOS
extended LOS
- HFRS
Hospital Frailty Risk Score
- LOS
length of stay
- NCI
Neurovascular Comorbidity Index
- NIS
National Inpatient Sample
- ROC
receiver operating characteristic
- SAH
subarachnoid hemorrhage
- UIA
unruptured intracranial aneurysm
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Supplemental Information
Online-Only Content
Supplemental material is available with the online version of the article.
Supplementary e-Tables 1–6. https://thejns.org/doi/suppl/10.3171/2022.8.JNS22372.
Previous Presentations
Part of the abstract was presented as a poster at the 2022 American Association of Neurological Surgeons Annual Scientific Meeting held in Philadelphia, Pennsylvania, on April 29–May 2, 2022.
References
- 1.International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339(24):1725–1733. [DOI] [PubMed] [Google Scholar]
- 2.Brinjikji W, Rabinstein AA, Lanzino G, Kallmes DF, Cloft HJ. Effect of age on outcomes of treatment of unruptured cerebral aneurysms: a study of the National Inpatient Sample 2001–2008. Stroke. 2011;42(5):1320–1324. [DOI] [PubMed] [Google Scholar]
- 3.Hishikawa T, Date I. Unruptured cerebral aneurysms in elderly patients. Neurol Med Chir (Tokyo). 2017;57(6):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neill AH, Chandra RV, Slater LA, et al. Influence of comorbidities on treatment of unruptured intracranial aneurysms in the elderly. J Clin Neurosci. 2019;62:38–45. [DOI] [PubMed] [Google Scholar]
- 5.Abuelem T, Dornbos D III, Arthur A. Editorial. Unruptured aneurysms in the elderly: handle with care. Neurosurg Focus. 2018;44(5):E5. [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji W, Lanzino G, Rabinstein AA, Kallmes DF, Cloft HJ. Age-related trends in the treatment and outcomes of ruptured cerebral aneurysms: a study of the Nationwide Inpatient Sample 2001–2009. AJNR Am J Neuroradiol. 2013; 34(5):1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thillainadesan J, Scott IA, Le Couteur DG. Frailty, a multisystem ageing syndrome. Age Ageing. 2020;49(5):758–763. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. [DOI] [PubMed] [Google Scholar]
- 10.Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2020;155(1):e194620–e194620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel AN, Lee JT, Gurrola JG II, Wang MB, Suh JD. The impact of frailty on perioperative outcomes and resource utilization in sinonasal cancer surgery. Laryngoscope. 2020; 130(2):290–296. [DOI] [PubMed] [Google Scholar]
- 12.Mori K, Wada K, Otani N, et al. Validation of effectiveness of keyhole clipping in nonfrail elderly patients with unruptured intracranial aneurysms. J Neurosurg. 2017;127(6):1307–1314. [DOI] [PubMed] [Google Scholar]
- 13.McIntyre MK, Gandhi C, Long A, et al. Age predicts out comes better than frailty following aneurysmal subarachnoid hemorrhage: a retrospective cohort analysis. Clin Neurol Neurosurg. 2019;187:105558. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman R, Abrams C, Weiner J. Development and Evaluation of the Johns Hopkins University Risk Adjustment Models for Medicare+ Choice Plan Payment. Johns Hopkins University; 2003. Accessed August 10, 2022. https://www.hopkinsacg.org/document/development-and-evaluation-of-the-johns-hopkins-university-risk-adjustment-models-for-medicarechoice-plan-payment/ [Google Scholar]
- 15.Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. New 5-factor modified frailty index using American College of Surgeons NSQIP data. J Am Coll Surg. 2018;226(2):173–181.e8. [DOI] [PubMed] [Google Scholar]
- 16.Sternberg SA, Wershof Schwartz A, Karunananthan S, Bergman H, Mark Clarfield A. The identification of frailty: a systematic literature review. J Am Geriatr Soc. 2011;59(11): 2129–2138. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132): 1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert T, Cordier Q, Polazzi S, et al. External validation of the Hospital Frailty Risk Score in France. Age Ageing. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAlister F, van Walraven C. External validation of the Hospital Frailty Risk Score and comparison with the Hospital-patient One-year Mortality Risk Score to predict outcomes in elderly hospitalised patients: a retrospective cohort study. BMJ Qual Saf. 2019;28(4):284–288. [DOI] [PubMed] [Google Scholar]
- 20.Eckart A, Hauser SI, Haubitz S, et al. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: results of a prospective, observational study. BMJ Open. 2019;9(1):e026923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 22.Newman WC, Neal DW, Hoh BL. A new comorbidities index for risk stratification for treatment of unruptured cerebral aneurysms. J Neurosurg. 2016;125(3):713–719. [DOI] [PubMed] [Google Scholar]
- 23.Newman WC, Kubilis PS, Hoh BL. Validation of a neurovascular comorbidities index for retrospective database analysis. J Neurosurg. 2018;130(1):273–277. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252–289. [DOI] [PubMed] [Google Scholar]
- 25.Mrdutt MM, Papaconstantinou HT, Robinson BD, Bird ET, Isbell CL. Preoperative frailty and surgical outcomes across diverse surgical subspecialties in a large health care system. J Am Coll Surg. 2019;228(4):482–490. [DOI] [PubMed] [Google Scholar]
- 26.Feghali J, Gami A, Rapaport S, et al. Adapting the 5-factor modified frailty index for prediction of postprocedural outcome in patients with unruptured aneurysms. J Neurosurg 2022;136(2):456–463.. [DOI] [PubMed] [Google Scholar]
- 27.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. [DOI] [PubMed] [Google Scholar]
- 28.Silva NA, Shao B, Sylvester MJ, Eloy JA, Gandhi CD. Unruptured aneurysms in the elderly: perioperative outcomes and cost analysis of endovascular coiling and surgical clipping. Neurosurg Focus. 2018;44(5):E4. [DOI] [PubMed] [Google Scholar]
- 29.Bock JO, König HH, Brenner H, et al. Associations of frailty with health care costs – results of the ESTHER cohort study. BMC Health Serv Res. 2016;16(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


