Abstract
New concepts:
Ideas about the causes of lower urinary tract signs (LUTS) in cats have changed significantly in the past 40 years. Recent research is challenging the conventional view that the bladder is always the perpetrator of LUTS, and suggests that the bladder can also be one victim of a systemic process associated with a sensitized central stress response system.
Aim:
In this article the authors provide their perspective on the implications of these findings for the diagnosis and treatment of cats with LUTS, provide some historical context, and suggest ways that the veterinary profession might work together to better understand the disorders underlying these signs, and possibly reduce their prevalence.
Where we are
Clinical signs referable to the lower urinary tract – dysuria, hematuria, periuria (behavioral inappropriate urination), pollakiuria and stranguria – are a common reason that pet cats are brought in for evaluation and treatment to primary care, feline focused, and secondary and tertiary veterinary practices (Figure 1). According to US-based insurer Veterinary Pet Insurance (VPI), the most common insurance claim submitted for cats in 2012 was (unfortunately described as) ‘bladder infection’, although most cases actually were diagnosed as ‘idiopathic LUTS’ (C McConnell, 2013, personal communication). In the same year, ‘cystitis’ was one of the most common diagnoses made by veterinarians in the US-based Banfield veterinary hospital group (and the most common urinary system disorder). 2
Figure 1.
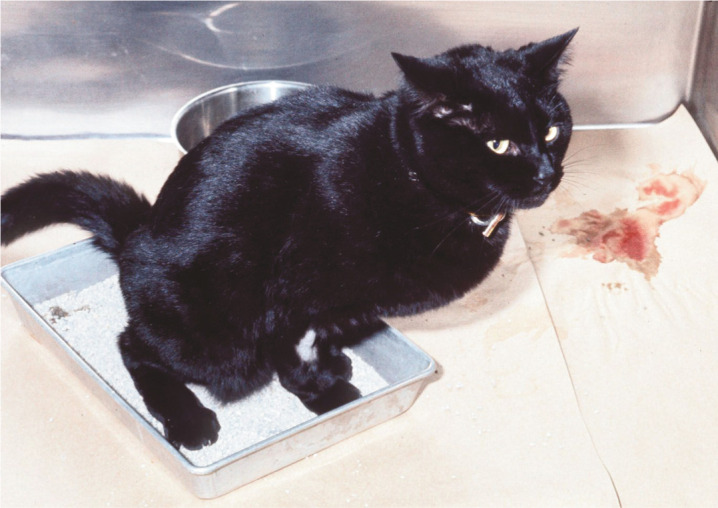
Hematuria is one of the common lower urinary tract signs that prompt clients to seek veterinary attention for their cat. In evaluating such patients, the veterinarian needs to look beyond the bladder; there may be additional health problems and/or the environment may be playing a role

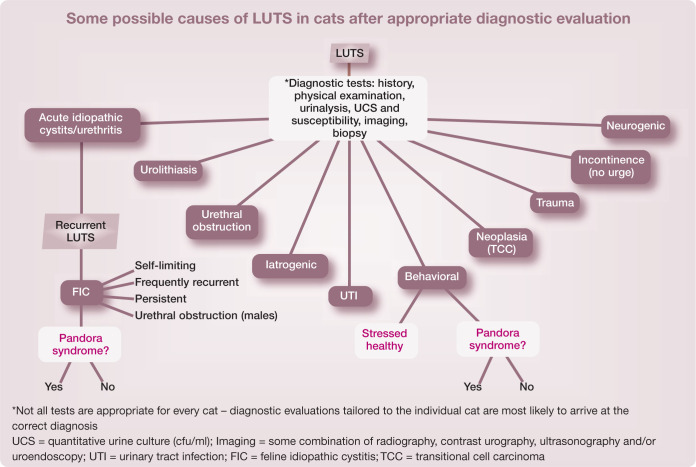
While recommendations for diagnosis and treatment are widely available for the most common causes of lower urinary tract signs (LUTS) currently identified in unobstructed cats, which include ‘idiopathic’, urolithiasis, bacterial infection and problematic voiding behaviors (see box on page 386), some 30 distinct causes of LUTS have been described. 3 When presented with a cat with LUTS, clinicians also need to consider whether they are seeing the cat’s initial episode, or whether the cat has chronic, recurrent disease, and what other health problems the cat may have. 4 This information permits judicious utilization of resources by helping to identify appropriate diagnostic tests to tailor treatment protocols to each individual cat.
Recent research further complicates the diagnostic challenge: cats may have multiple reasons for their clinical signs as well as other medical conditions and environmental requirements that need to be addressed. For example, we have presented evidence that some cats with severe, chronic LUTS seem to have a functional rather than a structural lower urinary tract disorder, 5 and have found that periuria can occur in apparently healthy cats exposed to stressful circumstances. 6 There currently is significant overlap among treatment recommendations for some LUT disorders, particularly with regard to ensuring that the patient’s environmental needs are met.7–9
How we got here
Clinical veterinary medicine has been informed for some time by a diagnostic approach that might be called the ‘brick paradigm’. That is, when a cat (or any patient for that matter) is presented for investigation of some clinical sign(s), the common underlying assumption is that the patient was healthy and happy until the ‘brick’ – injury, infection, etc – struck it. It has only been quite recently that our understanding of the etiopathogenesis of clinical signs for some disorders has expanded to recognize the variety of vulnerability factors that might result in a ‘susceptible individual’, and the range of features that might constitute a ‘provocative environment’.
A familiar example of a susceptible individual in a provocative environment is that of lactose intolerance, wherein an individual lacks the lactase gene, so cannot digest lactose. If not exposed to lactose, that individual can grow, reproduce and live a long and healthy life, oblivious to the absence of this gene. Within hours of exposure to lactose, however, it will become clear that something is seriously amiss. One can imagine how difficult an accurate diagnosis of this condition was before the genetic defect was identified; and, while we still cannot replace the lactase gene, we can help these individuals avoid such ‘provocative environments’ in the future.
Evolving terminology for cats with LUTS
Feline urologic syndrome (FUS)
In 1970, Osbaldiston and Taussig coined the term ‘feline urologic syndrome’ (FUS) to describe ‘the feline disease syndrome characterized by dysuria, urethral obstruction, urolithiasis and hematuria ….’ 10 They reported on 46 cats presenting with LUTS. Of these, 41 were male (32 of which were found to have bladder distension due to urethral obstruction by crystalline [23] or organic [9] material). No previous episodes of LUTS were identified in 20 of the cats, which were fed a variety of foods and housed in an assortment of environments. Urine was collected for bacterial culture by cystocentesis from 31 of the 46 cats, and bacteria were cultured in 17 cases (quantitative urinalysis [cfu/ml] was not performed). No cases of bladder calculi, periuria or transitional cell carcinoma were reported. Nineteen of the 46 cats died as a result of uropathy (sic), one with pneumonia, one in shock following cystotomy, two of unknown causes after release from the hospital, and 15 reportedly were free of signs at the time the paper was written, although all had experienced one or more recurrences. The authors concluded by declaring ‘the need for further investigation of FUS is emphasized by observations in this study which indicate that the condition may not be a single disease entity, but rather a group of separate urologic problems.’
Feline lower urinary tract disease (FLUTD) with heterogeneous causes
This concept was aired again in 1984, when Osborne and colleagues recommended replacement of the term ‘feline urologic syndrome’. 11 They advocated that replacement ‘would be of considerable value because it would help to eliminate the stereotypical approach to treatment and prevention of feline urological syndrome that is currently in vogue’. Echoing and expanding Osbaldiston and Taussig’s appeal, they suggested that the term FUS be substituted with ‘descriptive terms pertaining to the site (urethra, bladder, and so on), causes (bacteria, parasites, neoplasms, metabolic disturbances, idiopathic forms, and so on), morphologic changes (inflammation, neoplasia, and so on), and pathophysiologic mechanisms (obstructive uropathy, reflex dyssnergia, and so on) whenever possible. In this fashion, the sameterminology and approach to diagnosis and treatment used for other species (eg, dogs and humans) will more likely be used for cats.’
Unfortunately, Osborne et al’s chapter was subtitled, ‘Feline lower urinary tract disease with heterogeneous causes’, which resulted in the (presumably) unintended consequence of replacement of one acronym, ‘FUS’, with another ‘FLUTD’. The ‘heterogeneous causes’ concept for LUTS was lost again, which continued to promote an incomplete understanding of the etiopathogenesis of various lower urinary tract disorders.
Feline interstitial cystitis/feline idiopathic cystitis (FIC)
We coined the terms ‘feline interstitial cystitis’ and ‘feline idiopathic cystitis’ (FIC) in 1999, largely as a result of our investigations of cats with severe, chronic idiopathic LUTS as a naturally occurring model of interstitial cystitis (IC) in women. 12 We proposed that feline interstitial cystitis be defined as a disease of chronic irritative voiding signs, sterile and cytologically negative urine, and cystoscopic observation of submucosal petechial hemorrhages, to be diagnosed only when all three of these factors were documented and attempts to find a more objective cause for the signs were negative. We proposed the term feline idiopathic cystitis to describe cats with chronic irritative voiding signs that had sterile and cytologically negative urine in which cystoscopy was not performed, but in which other appropriate diagnostic procedures, such as imaging of the lower urinary tract, did not identify a cause. This suggestion led to a series of exchanges in JAVMA that reflected the thinking of the time regarding LUTS and IC.13–15
Limitations to ‘FUS’, ‘FLUTD’ and ‘FIC’
The limitations of these definitions became apparent when we subsequently identified a variety of other abnormalities in the cats donated to us.
Concurrent physiologic and neuroanatomic abnormalities
In addition to epithelial abnormalities identified in the bladder of cats with FIC,16–18 we also found significant alterations in components of acetylcholine synthesis and release in the esophageal mucosa from cats with FIC. This suggested that changes in the non-neuronal cholinergic system may contribute to alterations in cell–cell contacts and possibly communication with underlying cells that may, in turn, contribute to changes in sensory function and visceral hyperalgesia. 19
Differences in sensory neuron anatomy and physiology are also present in cats with FIC. For example, dorsal root ganglion cell bodies of both bladder-identified and non-bladder neurons from cats with FIC were 30% larger, expressed altered neuropeptide profiles, and exhibited slowly desensitizing, capsaicin-induced currents related to increased protein kinase C-mediated phosphorylation of the transient receptor potential vanilloid 1 (TRP-V1) receptor. Similar findings were observed in dorsal root ganglion cells throughout the lumbosacral (L4–S3) spinal cord, suggesting a more widespread abnormality of sensory neuron function.20,21 We also found the acoustic startle response, a brainstem reflex response to unexpected auditory sensory stimuli, to be increased in cats with FIC. The acoustic startle response in cats with FIC was greatest and most different from that of healthy cats during stressful situations, but was still greater in cats with FIC than in healthy cats, even after they had become acclimated to enriched housing conditions. 22
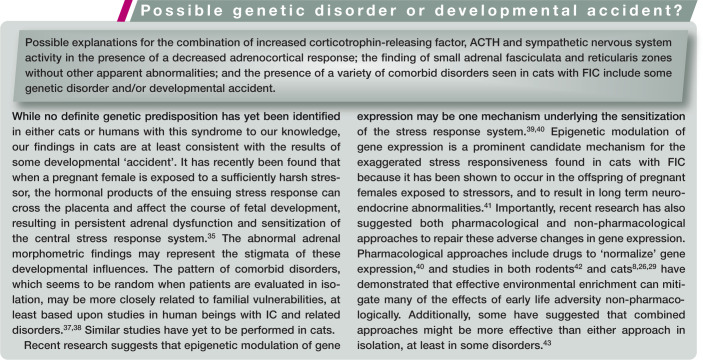
Differences in sympathetic nervous system function have been identified in cats with FIC, including increases in tyrosine hydroxylase (the rate-limiting enzyme of catecholamine synthesis) immunoreactivity in the pontine locus coeruleus 23 and the paraventricular nucleus of the hypothalamus. 24 The locus coeruleus contains the largest number of nor-adrenergic neurons, and is the most important source of norepinephrine in the feline (and human) central nervous system. It is involved in such global brain functions as vigilance, arousal and analgesia, and appears to mediate visceral responses to stress. 25 We also observed increased immunoreactivity for corticotrophin-releasing factor in the locus coeruleus and paraventricular nucleus. 24 A functional desensitization of alpha-2 adrenergic receptors in affected cats was additionally identified by evaluating cats’ responses to the selective alpha-2 adrenergic receptor agonist, medetomidine, in both in vivo and in vitro studies.26,27 In the brainstem (particularly the area of the locus coeruleus), alpha-2 agonists inhibit norepinephrine release, whereas in the spinal cord they inhibit transmission of nociceptive input to the brain. 28 These findings provided clues to explain the observations that clinical symptoms of FIC in cats follow a waxing and waning course, and can be aggravated by environmental stressors.29,30
In addition to the sympathetic nervous system, some cats with FIC also appear to have abnormalities in the hypothalamic–pituitary– adrenal (HPA) axis. Administration of ovine corticotrophin-releasing factor resulted in significant increases in adrenocorticotrophic hormone (ACTH), but not cortisol, 31 and administration of synthetic ACTH resulted in significantly decreased serum cortisol responses in cats with FIC as compared with healthy cats. 32 Although no obvious adrenal abnormalities were identified by histopathology, morphometric analysis revealed that the zona fasciculata and reticularis were significantly smaller in sections of glands from cats with FIC than in glands from healthy cats. Therefore, it appeared that the sympathoneural system was activated in these cats, but the adrenocortical component of the HPA axis was not.
Comorbid disorders
Furthermore, cats with FIC often have variable combinations of comorbid disorders such as behavioral, endocrine, cardiovascular and gastrointestinal (GI) problems.7,8,33,34 In a recent study of 12 healthy cats and 20 cats with FIC donated to us, 6 we investigated sickness behaviors referable to the GI and urinary tracts, the skin and behavior problems for 77 weeks in response to instances of unusual external events (ie, stressors). These events included changes in personnel caring for the cats, disruptions in normal animal facility routine, and lack of interaction with the investigators. We found that increases in age and exposure to stressors, but not disease status, significantly increased the total number of sickness behaviors when results were controlled for other factors. Increasing age was associated with increases in relative risk for upper GI tract signs (1.2) and avoidance behavior (1.7), whereas exposure to unusual external events was associated with much greater increases in risk for decreases in food intake (9.3) and eliminations (6.4). Exposure to stressors was also associated with significantly increased risk for perichezia (9.8) and periuria (1.6).
These findings suggest that some of the most commonly reported abnormalities in client-owned cats were observed after exposure of cats in both groups to external stressors. Many of the sickness behaviors observed in the donated cats were described in the medical records of the cats that we obtained at the time of donation. These clinical signs appeared to be exacerbated during changes in their environment, but were rarely recorded during periods of consistent care.
Many human beings with IC also suffer from variable combinations of comorbid disorders that affect a variety of other body systems.35,36 The observation that patients with FIC and IC have variable combinations of other comorbid disorders raises the question of the extent to which a different etiology affects each organ versus the extent to which some common disorder affects all organs, which then respond in their own characteristic ways.
We and others also have observed that comorbid disorders can precede, accompany or follow the diagnosis of (F)IC, and there seems to be no consistent pattern of onset. This suggests that comorbid disorders are not likely to be the result of the LUTS, and we need to look for alternative explanations (see box on page 389).
The nosology of LUTS
Nosology is defined as the classification of diseases. The names of disorders influence our thinking about their underlying biology. As many investigators have found, presenting signs often initially lead to descriptive names that can take on a life of their own once introduced, and frequently become harder to change than one might imagine at the time proposed. In the case of cats presented for treatment of LUTS, ‘feline urological syndrome’, 10 ‘feline lower urinary tract disease’ 11 and ‘feline interstitial cystitis’ 12 initially appeared to fairly accurately capture the signs cats presented with; they all focused on the lower urinary tract, reflected the prominent presenting signs, and often resulted in lower urinary tract-focused diagnostic testing and treatment modalities. As subsequent studies have shown, however, these names (naturally) were coined with an incomplete understanding of the etiopathogenesis of the various causes of LUTS in all cats.
Feinstein 44 has suggested that ‘an important principle in naming apparently new ailments is to avoid etiologic titles until the etiologic agent has been suitably demonstrated. A premature causal name can impair a patient’s recovery from the syndrome, and impede research that might find the true cause.’ Ongoing research in both human beings and cats with chronic LUTS has begun to include a more comprehensive evaluation of the entire patient. In humans, this has resulted in the suggestion of names such as ‘medically unexplained syndrome’, 45 ‘functional somatic syndrome’ 46 or ‘central sensitivity syndrome’ 47 to describe the multiple abnormalities observed in these patients. The list of chronic disorders subsumed by these names is long, and includes problems addressed by most of the human medical subspecialties.
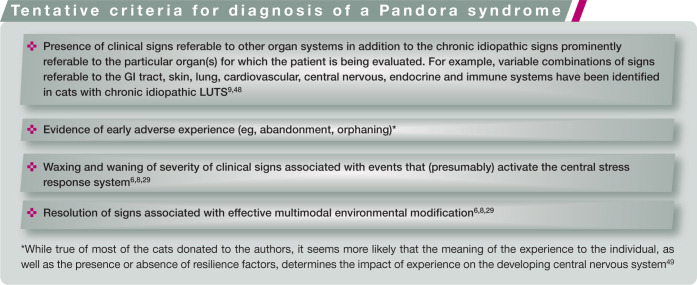
For cats, one of us (CATB) proposed the provisional term ‘Pandora syndrome’ to describe (among others) cats with chronic recurrent LUTS in the presence of comorbid disorders (behavioral, cardiovascular, dermatological, endocrine, GI, etc) until the most biologically appropriate nosological term is identified. 5 A name like Pandora syndrome seems appropriate for at least two reasons. First, it does not identify any specific cause or organ, and, secondly, it seems to capture the dismay and dispute associated with the identification of so many problems (‘evils’) outside the organ of interest of any particular subspecialty. Some tentative criteria for diagnosis of Pandora syndrome, together with a description of the sequence of events that might lead to development of the syndrome, are presented in the accompanying boxes.
Regardless of the names eventually chosen to describe cats with chronic idiopathic LUTS and other clinical signs, restricting the description of these patients to their LUTS does not capture all of the currently recognized features of the syndrome.6,8,48 Notwithstanding the current academic debate on the most accurate descriptive term for this syndrome, we encourage clinicians to conduct a more comprehensive evaluation of cats presented with these and other chronic idiopathic signs to determine whether only signs referable to a single organ occur, or whether variable combinations of comorbid somatic and behavioral abnormalities (Figure 2) also are present, and to include these findings in reports of studies of these cats. Such an evaluation (see later) may result in a more complete diagnosis, which could lead to implementation of additional approaches to treatment for some patients that may be associated with better outcomes. 8
Figure 2.
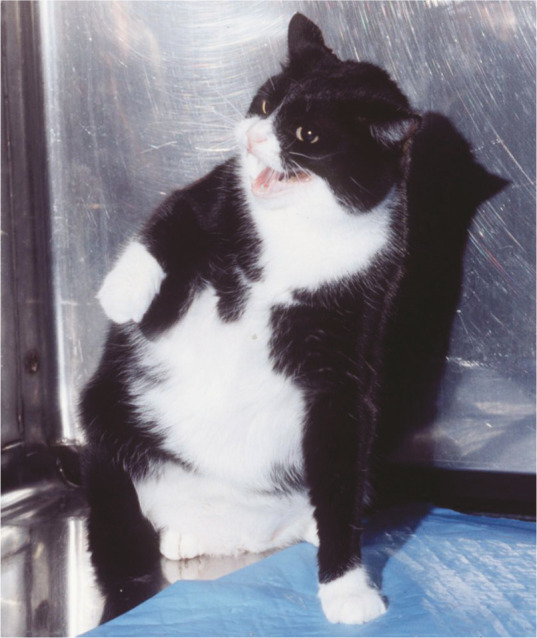
Problematic behaviors may be an entirely relevant finding in cats with LUTS, and may point to a ‘sensitive’ individual experiencing a ‘provocative’ environment
Where to now?
Interdisciplinary approaches to benefit future generations of cats
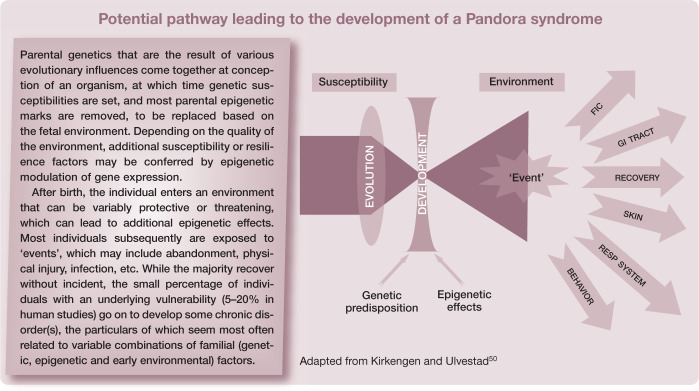
The ‘developmental origins of health and disease’ (DOHaD) paradigm, which has emerged only within the past decade or so, offers a potentially helpful companion to the ‘brick paradigm’ for cats with chronic, poorly understood medical disorders. 51 DOHaD is a rapidly expanding area of medical investigation that combines clinical, epidemiological, experimental and public health research to understand how events in early life shape later morbidity risk, especially of non-communicable chronic diseases. 52 DOHaD research seeks to understand the evolutionarily conserved mechanisms of biological plasticity that permit organisms to adapt their phenotype in response to external cues such as nutrients and hormones. These responses may be divided into those that immediately benefit the organism and those intended to improve fitness in the environment from which the signals emanated. When the responses are appropriate to the environment the individual is exposed to, the match is successful. When there is a mismatch between individual and environment, disease can occur. Importantly, the underlying mechanisms that enable such phenotypic alterations also include epigenetic modulation of gene expression. 53 Additionally, and significantly, epigenetically marked genes can be inherited, and so may contribute to non-genomic heritable disease risk and resistance. 54
A number of opportunities to better understand the etiopathogenesis and treatment of LUTS in cats present themselves. As mentioned, investigators could report, and journals could require reporting of, a more comprehensive description of the animals studied. In addition to signalment, urinary parameters and initial or recurrent nature of the signs, the presence or absence of other, potentially comorbid disorders could be detailed, along with their temporal order of onset, possibly as supplementary material. Longer term follow-up, preferably at least a year, could be provided for treatment studies to better determine the durability of treatments and the frequency of recurrences of all comorbid signs, since in our experience all do not recur together. 6
Even more ambitious, difficult, and potentially rewarding, a patient registry could be developed. Patient registries represent a powerful method for collecting large amounts of patient data, and are becoming more widely adopted in human academic and private healthcare settings.55,56 Registries offer the possibility of improving awareness of patient outcomes, and providing a clearer prospective understanding of the natural history of susceptibility to and resilience from disease using a standardized reporting format. An additional refinement might be storage of DNA to permit eventual investigation of genetic contributions to the various lower urinary tract and other disorders. Such an undertaking would not be trivial. A relatively large group of stakeholders – clinicians, clinical scientists, commercial entities (eg, pharmacology, insurance and pet food companies) – would have to come together to agree on a standardized reporting format to ensure a common language to describe the history, physical and environmental findings, and to follow the cats prospectively through time.
Instruments would have to be developed and validated for use in a variety of settings. For example, a ‘central sensitization inventory’ (CSI) to assess somatic and emotional complaints often associated with central sensitivity syndrome in humans was recently validated. 57 This self-report inventory consists of two parts: part A, which has 25 statements about physical, psychological and sensory symptoms experienced by the individual; and part B, which asks if a doctor has diagnosed the person with any of 10 central sensitivity disorders, and the year of diagnosis. A subsequent report investigated use of the CSI to assess 121 patients referred to a multidisciplinary pain center. A CSI score of 40/100 was found to best distinguish patients with a central sensitivity syndrome from a non-patient comparison sample (n = 129). 58
An inventory conceptually similar to this could be developed and tested to determine its ability to aid in the differential diagnosis of cats with chronic idiopathic disorders, and might offer a more positive diagnosis of Pandora syndrome.
In-clinic approaches to benefit the individual cat
Questions we consider while obtaining a history, comprehensive physical examination and environmental assessment during an initial encounter with a patient (usually with chronic) LUTS have been developed into a ‘cat and client history form’ (see right). Questions in the history are designed to elucidate the effect on risk for Pandora syndrome, and cover:
Where the cat was obtained;
Any other health or behavior problems that may be present;
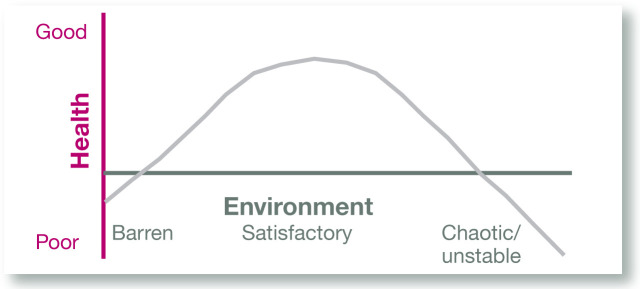
The general structure of the cat’s environment – amount of time indoors, activity level, availability and management of resources (eg, food, water, litter boxes, resting areas, opportunities for activity), other cats and people in the home, etc (Figure 3). (As illustrated in Figure 4, the relationship between the environment and health is quadratic rather than linear, with both deficient and threatening environments increasing the risk of poor health outcomes);
Presence of signs referable to other organ systems (eg, skin, lung or GI tract – more increases risk);
Perceived allergic responses of the skin, lung or GI tract (more increases risk);
Any unusual or problematic behaviors (more increases risk).
Figure 3.

Availability of resources (a) and opportunities for activity (b) are elements of the environment that need to be probed during history taking for any cat with LUTS
Figure 4.
Quadratic relationship between environmental quality and health. Cats with FIC (and those with Pandora syndrome) appear to tolerate a narrower range of environmental conditions than do healthy cats. For example, they may be more threatened by other cats, their owners, or features of their environment that would not adversely affect an otherwise healthy cat

We perform the physical examination by evaluating the lower urinary tract last, to avoid being distracted and missing other abnormalities, such as over-grooming, obesity, acne, cardiac arrhythmia/abnormal heart sounds, etc.
For an initial episode in an apparently healthy, young, unobstructed patient, the most likely explanation for the signs is either a sickness behavior in an otherwise healthy cat, or acute idiopathic LUTS. Accordingly, we may tell the client that this is most likely to be something similar to a ‘headache in the bladder’ that will pass soon, and can be made less likely to recur following implementation of individually tailored multimodal environmental modifications to be certain the cat’s environmental needs are met; recommendations are widely available.7,59–63 A stone in the urinary system and a bacterial urinary tract infection also are possible, so we may discuss with the client the option of obtaining additional positive diagnostic information from imaging or urinalysis as an alternative to ‘watchful waiting’, based on their preference.
The situation changes in the event that the cat has had previous episodes of LUTS, has a history or the presence of other health problems, or is older (>8 years of age) or obstructed. If the cat has had previous episodes, we encourage additional diagnostic evaluation to rule out the presence of other disorders related to the lower urinary tract, including stones, infection, anatomic anomalies and cancer (see box on page 386).
Key Points
Recent research challenges the conventional view that the bladder is always the perpetrator of LUTS, and suggests that it can also be one victim of a systemic process associated with a sensitized central stress response system.
An overarching principle is that LUTS and a variety of other signs can occur as a consequence of placing a ‘sensitive’ cat into a ‘provocative’ environment.
Even presumably healthy cats can develop sickness behaviors when exposed to sufficiently provocative environments, so meeting the environmental needs of pet cats to ensure their health and well-being is an animal husbandry responsibility that falls on all pet owners.
While the cats that have been studied by the authors appear to be more sensitive to their surroundings than healthy cats are, they just seem to be further along a continuum that likely includes all pet cats.
With current technology, we have the opportunity to develop collaborative strategies to learn far more about ‘FUS’, ‘FLUTD’, ‘FIC’ and ‘Pandora syndrome’; potentially enough to come up with a truly evidence-based nosology, and appropriate treatments based on this understanding.
Supplemental Material
Where are we, how did we get here, and where to now?
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors declare that there is no conflict of interest.
References
- 1. Yeates J, Everitt S, Innes JF, Day MJ. Ethical and evidential considerations on the use of novel therapies in veterinary practice. J Small Anim Pract 2013; 54: 119–123. [DOI] [PubMed] [Google Scholar]
- 2. Banfield Pet Hospital. State of pet health 2013 report. www.stateofpethealth.com (accessed January 15, 2014).
- 3. Osborne CA, Kruger JM, Lulich JP. Feline lower urinary tract disorders. Definition of terms and concepts. Vet Clin North Am Small Anim Pract 1996; 26: 169–179. [DOI] [PubMed] [Google Scholar]
- 4. Westropp J, Buffington CAT. Lower urinary tract disorders in cats. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 7th ed. St Louis: Elsevier Saunders, 2010, pp 2069–2086. [Google Scholar]
- 5. Buffington CA. Idiopathic cystitis in domestic cats – beyond the lower urinary tract. J Vet Intern Med 2011; 25: 784–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stella JL, Lord LK, Buffington CAT. Sickness behaviors in response to unusual external events in healthy cats and cats with feline interstitial cystitis. J Am Vet Med Assoc 2011; 238: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buffington CAT. External and internal influences on disease risk in cats. J Am Vet Med Assoc 2002; 220: 994–1002. [DOI] [PubMed] [Google Scholar]
- 8. Buffington CAT, Westropp JL, Chew DJ, Bolus RR. Clinical evaluation of multimodal environmental modification (MEMO) in the management of cats with idiopathic cystitis. J Feline Med Surg 2006; 8: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stepita ME. Animal behavior case of the month. Urine marking. J Am Vet Med Assoc 2013; 242: 1491–1493. [DOI] [PubMed] [Google Scholar]
- 10. Osbaldiston GW, Taussig RA. Clinical report on 46 cases of feline urological syndrome. Vet Med Small Anim Clin 1970; 65: 461–468. [PubMed] [Google Scholar]
- 11. Osborne CA, Johnston GR, Polzin DJ, Kruger JM, Poffenbarger EM, Bell FW, et al. Redefinition of the feline urologic syndrome: feline lower urinary tract disease with heterogeneous causes. Vet Clin North Am Small Anim Pract 1984; 14: 409–438. [DOI] [PubMed] [Google Scholar]
- 12. Buffington CAT, Chew DJ, Woodworth BE. Feline interstitial cystitis. J Am Vet Med Assoc 1999; 215: 682–687. [PubMed] [Google Scholar]
- 13. Osborne CA, Kruger JM, Lulich JP, Polzin DJ. Feline urolog-ic syndrome, feline lower urinary tract disease, feline interstitial cystitis: what’s in a name? J Am Vet Med Assoc 1999; 214: 1470–1480. [PubMed] [Google Scholar]
- 14. Buffington CA, Chew DJ, DiBartola SP. On the definition of feline interstitial cystitis. J Am Vet Med Assoc 1999; 215: 186–188. [PubMed] [Google Scholar]
- 15. Feaga WP. Thoughts on the causes of FUS. J Am Vet Med Assoc 1999; 215: 316. [PubMed] [Google Scholar]
- 16. Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, et al. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol 2004; 287: F1084–1091. [DOI] [PubMed] [Google Scholar]
- 17. Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol 2003; 285: F423–F429. [DOI] [PubMed] [Google Scholar]
- 18. Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol 2000; 278: F540–553. [DOI] [PubMed] [Google Scholar]
- 19. Wolf-Johnston AS, Hanna-Mitchell AT, Buffington CA, Shinde S, Roppolo JR, Mayer E, et al. Alterations in the non-neuronal acetylcholine synthesis and release machinery in esophageal epithelium. Life Sci 2012; 91: 1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Abnormal excitability in capsaicin-responsive DRG neurons from cats with feline interstitial cystitis. Exp Neurol 2005; 193: 437–443. [DOI] [PubMed] [Google Scholar]
- 21. Sculptoreanu A, de Groat WC, Buffington CA, Birder LA. Protein kinase C contributes to abnormal capsaicin responses in DRG neurons from cats with feline interstitial cystitis. Neurosci Lett 2005; 381: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hague DW, Stella JL, Buffington CA. Effects of interstitial cystitis on the acoustic startle reflex in cats. Am J Vet Res 2013; 74: 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reche AJ, Buffington CAT. Increased tyrosine hydroxylase immunoreactivity in the locus coeruleus of cats with interstitial cystitis. J Urol 1998; 159: 1045–1048. [DOI] [PubMed] [Google Scholar]
- 24. Welk KA, Buffington CAT. Effect of interstitial cystitis on central neuropeptide and receptor immunoreactivity in cats [Abstract]. Research Insights into Interstitial Cystitis, 30 October to 1 November 2003, Alexandria, VA; p 74. [Google Scholar]
- 25. Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 2008; 583: 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Westropp JL, Kass PH, Buffington CA. In vivo evaluation of alpha(2)-adrenoceptors in cats with idiopathic cystitis. Am J Vet Res 2007; 68: 203–207. [DOI] [PubMed] [Google Scholar]
- 27. Buffington CAT, Teng BY, Somogyi GT. Norepinephrine content and adrenoceptor function in the bladder of cats with feline interstitial cystitis. J Urology 2002; 167: 1876–1880. [PubMed] [Google Scholar]
- 28. Zhang X, Bai X. New therapeutic uses for an alpha adrenergic receptor agonist – Dexmedetomidine in pain management. Neurosci Lett 2014; 561: 7–12. [DOI] [PubMed] [Google Scholar]
- 29. Westropp JL, Kass PH, Buffington CA. Evaluation of the effects of stress in cats with idiopathic cystitis. Am J Vet Res 2006; 67: 731–736. [DOI] [PubMed] [Google Scholar]
- 30. Buffington CA, Pacak K. Increased plasma norepinephrine concentration in cats with interstitial cystitis. J Urol 2001; 165: 2051–2054. [DOI] [PubMed] [Google Scholar]
- 31. Westropp J, Welk K, Buffington T. Adrenal abnormalities in cats with feline interstitial cystitis. J Urol 2003; 169: 258. [DOI] [PubMed] [Google Scholar]
- 32. Westropp JL, Buffington CAT. Effect of a corticotropin releasing factor (crf) antagonist on hypothalamic-pituitary-adrenal activation in response to crf in cats with interstitial cystitis [Abstract]. Research Insights into Interstitial Cystitis, 30 October to 1 November 2003, Alexandria, VA; p 74. [Google Scholar]
- 33. Buffington CA. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol 2004; 172: 1242–1248. [DOI] [PubMed] [Google Scholar]
- 34. Freeman LM, Brown DJ, Smith FW, Rush JE. Magnesium status and the effect of magnesium supplementation in feline hypertrophic cardiomyopathy. Can J Vet Res 1997; 61: 227–231. [PMC free article] [PubMed] [Google Scholar]
- 35. Buffington CAT. Comorbidity of interstitial cystitis with other unexplained clinical conditions. J Urol 2004; 172: 1242–1248. [DOI] [PubMed] [Google Scholar]
- 36. Warren JW, Wesselmann U, Morozov V, Langenberg PW. Numbers and types of nonbladder syndromes as risk factors for interstitial cystitis/painful bladder syndrome. Urology 2011; 77: 313–319. [DOI] [PubMed] [Google Scholar]
- 37. Warren J, Jackson T, Meyers D, Xu J. Fishbein/interstitial cystitis association (ICA) survey of interstitial cystitis among family members of ICA members: preliminary analysis. Urology 2001; 57: 126–127. [DOI] [PubMed] [Google Scholar]
- 38. Altman D, Lundholm C, Milsom I, Peeker R, Fall M, Iliadou AN, et al. The genetic and environmental contribution to the occurrence of bladder pain syndrome: an empirical approach in a nationwide population sample. Eur Urol 2011; 59: 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jensen P. Transgenerational epigenetic effects on animal behav-iour. Prog Biophys Mol Bio 2013; 113: 447–454. [DOI] [PubMed] [Google Scholar]
- 40. Buchheit T, Van de Ven T, Shaw A. Epigenetics and the transition from acute to chronic pain. Pain Med 2012; 13: 1474–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reynolds RM, Labad J, Buss C, Ghaemmaghami P, Rälkkönen K. Transmitting biological effects of stress in utero: implications for mother and offspring. Psychoneuroendocrinology 2013; 38: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 42. Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci 2012; 15: 1475–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Castren E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry 2013; 70: 983–989. [DOI] [PubMed] [Google Scholar]
- 44. Feinstein AR. The Blame-X syndrome: problems and lessons in nosology, spectrum, and etiology. J Clin Epidemiol 2001; 54: 433–439. [DOI] [PubMed] [Google Scholar]
- 45. Schur EA, Afari N, Furberg H, Olarte M, Goldberg J, Sullivan PF, et al. Feeling bad in more ways than one: comorbidity patterns of medically unexplained and psychiatric conditions. J Gen Intern Med 2007; 22: 818–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ablin K, Clauw DJ. From fibrositis to functional somatic syndromes to a bell-shaped curve of pain and sensory sensitivity: evolution of a clinical construct. Rheum Dis Clin North Am 2009; 35: 233–251. [DOI] [PubMed] [Google Scholar]
- 47. Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum 2008; 37: 339–352. [DOI] [PubMed] [Google Scholar]
- 48. Buffington CAT, Westropp JL, Chew DJ, Bolus RR. A case-control study of indoor-housed cats with lower urinary tract signs. J Am Vet Med Assoc 2006; 228: 722–725. [DOI] [PubMed] [Google Scholar]
- 49. Shonkoff JP, Garner AS; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care and Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012; 129: e232–246. [DOI] [PubMed] [Google Scholar]
- 50. Kirkengen AL, Ulvestad E. Heavy burdens and complex disease – an integrated perspective. Tidsskr Nor Laegeforen 2007; 127: 3228–3231. [PubMed] [Google Scholar]
- 51. Gluckman PD, Hanson MA. The conceptual basis for the developmental origins of health and disease. In: Gluckman PD, Hanson MA. (eds). Developmental origins of health and disease. Cambridge: Cambridge University Press, 2006; pp 33–50. [Google Scholar]
- 52. Gluckman PD, Hanson MA, Buklijas T. A conceptual framework for the developmental origins of health and disease. JDoHAD 2010; 1: 6–18. [DOI] [PubMed] [Google Scholar]
- 53. Feinberg AP. Epigenetics at the epicenter of modern medicine. JAMA 2008; 299: 1345–1350. [DOI] [PubMed] [Google Scholar]
- 54. Jablonka E, Lamb MJ. Evolution in four dimensions: genetic, epigenetic, behavioral, and symbolic variation in the history of life. Cambridge, MA: MIT Press, 2005. [Google Scholar]
- 55. Natter MD, Quan J, Ortiz DM, Bousvaros A, Ilowite NT, Inman CJ, et al. An i2b2-based, generalizable, open source, self-scaling chronic disease registry. J Am Med Inform Assoc 2013; 20: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnson KJ, Hussain I, Williams K, Sanrens R, Mueller NL, Gutmann DH. Development of an international internet-based neurofibromatosis Type 1 patient registry. Contemp Clin Trials 2013; 34: 305–311. [DOI] [PubMed] [Google Scholar]
- 57. Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract 2012; 12: 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Neblett R, Cohen H, Choi Y, Hartzell MM, Williams M, Mayer TG, et al. The Central Sensitization Inventory (CSI): establishing clinically significant values for identifying central sensitivity syndromes in an outpatient chronic pain sample. J Pain 2013; 14: 438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Herron ME, Buffington CA. Environmental enrichment for indoor cats: implementing enrichment. Compend Contin Educ Vet 2012; 34: E1–5. [PMC free article] [PubMed] [Google Scholar]
- 60. Herron ME, Buffington CAT. Environmental enrichment for indoor cats. Compend Contin Educ Pract Vet 2010; 32: E1–E5. [PMC free article] [PubMed] [Google Scholar]
- 61. Ellis SL. Environmental enrichment: practical strategies for improving feline welfare. J Feline Med Surg 2009; 11: 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Buffington CA, Westropp JL, Chew DJ, Bolus RR. Clinical evaluation of multimodal environmental modification (MEMO) in the management of cats with idiopathic cystitis. J Feline Med Surg 2006; 8: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ellis SL, Rodan I, Carney HC, Heath S, Rochlitz I, Shearburn LD, et al. AAFP and ISFM feline environmental needs guidelines. J Feline Med Surg 2013; 15: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Where are we, how did we get here, and where to now?