Abstract
Norwegian Forest cats (NFCs) are often listed as a breed predisposed to cardiomyopathy, but the characteristics of cardiomyopathy in this breed have not been described. The aim of this preliminary study was to report the features of NFC cardiomyopathy based on prospective echocardiographic screening of affected family groups; necropsy findings; and open-source breed screening databases. Prospective examination of 53 NFCs revealed no murmur or left ventricular (LV) outflow tract obstruction in any screened cat, though mild LV hypertrophy (defined as diastolic LV wall thickness ≥5.5mm) was present in 13/53 cats (25%). Gross pathology results and histopathological sections were analysed in eight NFCs, six of which had died of a cardiac cause. Myocyte hypertrophy, myofibre disarray and interstitial fibrosis typical of hypertrophic cardiomyopathy were present in 7/8 cats, but endomyocardial fibrosis suggestive of restrictive cardiomyopathy was also present in the same cats. Pedigree data analysis from 871 NFCs was supportive of a familial cardiomyopathy in this breed.
Introduction
Cardiomyopathies are a heterogeneous group of diseases that affect the myocardium, and parallels have been drawn between feline and human cardiomyo-pthies.1–4 In cats, the most common type of cardiomyopathy is hypertrophic cardiomyopathy (HCM),5–8 but other forms have been described, including restrictive cardiomyopathy (RCM), dilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy (ARVC).
HCM is defined as inappropriate hypertrophy of a non-dilated left ventricle in the absence of a plausible cardiovascular or systemic cause for the degree of hypertrophy observed.2,9,10 In both humans and cats, the clinical diagnosis is principally based on an increased left ventricular (LV) diastolic maximal wall thickness on echocardiography (echo), but there is wide phenotypic variability in both species. RCM is a less common form of cardiomyopathy in cats, often morphologically subclassified into two forms: myocardial and endomyocardial.3,11 Echocardiographically, both forms of RCM are characterised by atrial enlargement in the absence of LV hypertrophy.8,12 In the endomyocardial form, thick hyperechoic tissue bridges the LV lumen.
Histopathologically, myofibre disarray has been considered to be the hallmark feature of HCM, and is characterised by adjacent groups of muscle cells that are aligned obliquely or perpendicularly to one another.2,4,9,13,14 Other histopathological features of HCM include myocardial fibrosis (either interstitial or replacement), small vessel disease characterised by medial hypertrophy of intramural coronary arteries (intramural arteriosclerosis) and myocyte hypertrophy characterised by enlarged cells with large, rectangular, hyperchromic nuclei.13–16 The characteristic necropsy findings in RCM are an increase in cardiac mass and, commonly, biatrial dilation. 17 LV cavity dimensions are usually normal or mildly dilated, and the thickness of the LV walls is normal or only minimally increased. Histopathologically, endomyocardial fibrosis is characteristic.11,17–20 Recently, findings thought to be typical of HCM, such as myocyte hypertrophy, interstitial fibrosis and intramural arteriosclerosis, have also been found in cats classed as having RCM. 3 It has been suggested that HCM and RCM may represent different phenotypic expressions of the same disease state. 3
HCM is the most common inherited cardiac disorder in humans, and has been associated with over 1400 different mutations in sarcomeric genes. 10 It is usually inherited as an autosomal dominant trait with incomplete penetrance.21–23 Mutations affecting the myosin binding protein C gene have been reported in Maine Coon and Ragdoll cats.24,25 In Maine Coons, HCM is inherited as an autosomal dominant trait, and has been described as a progressive disease that is not apparent during the first years of life. 4 The mode of inheritance in Ragdoll cats has not yet been identified, but homozygous-affected cats have reduced survival times.25,26 HCM has been described as a familial disease in other breeds, as well as in non-pedigree cats,27,28 and genetic causes are suspected in other breeds but have not been identified. 29 The phenotypic characteristics and heritability of cardiomyopathy in Norwegian Forest cats (NFCs) have not been reported.
Anecdotal reports of HCM in one family of NFCs in the UK led us to hypothesise that cardiomyopathy is familial in this breed. In order to identify the inheritance pattern of cardiomyopathy in NFCs, the aims of this study were as follows: (1) to screen NFCs in the UK prospectively for cardiomyopathy using auscultation and echocardiography (echo); (2) to obtain pathological and histopathological data from cases where a NFC was suspected of being affected with cardiomyopathy; (3) to analyse NFC pedigrees using a combination of screening data and necropsy data to assess the inheritance pattern in this breed.
Materials and methods
Prospective screening for HCM
Ethical approval was obtained from the Ethics and Welfare Committee of the Royal Veterinary College (URN 2010 1009). Owners of cats related to the index case (cat A) were contacted, as well as breeders and owners of unrelated NFCs, to encourage echo screening for cardiomyopathy. The pedigree of each cat was analysed for kinship with other scanned cats. If an affected cat was identified, attempts were made to contact the owners of related cats.
Cats younger than 12 months of age were excluded, as were pregnant or lactating queens. Each cat underwent a physical examination including cardiac auscultation. Systolic blood pressure (SBP) was obtained non-invasively by Doppler sphygmomanometry, as previously described. 30 Cats were considered hypertensive when the mean of five consecutive SBP measurements was ≥180 mmHg. 31 Standard transthoracic echo was performed by the same trained observer (IM) using a Vivid 7 ultrasonographic system (GE Medical Systems) with a 7.5 MHz phased array transducer. Echo examination was performed in right and left lateral recumbency from underneath, using a table with a cut-out area and continuous echo monitoring. The echo data were analysed offline using a computer workstation (EchoPac PC; General Electric Medical Systems).
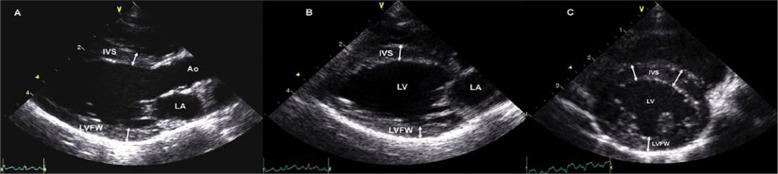
The end-diastolic thickness of the left ventricular free wall (LVFWd) and interventricular septum (IVSd) was measured from both two-dimensional echo (2DE) and M-mode images. For 2D imaging, measurements were obtained from three different 2DE views: a right parasternal long axis (RPLAx) LV outflow view (Figure 1a); a RPLAx LV inflow view (Figure 1b); and a right parasternal LV short axis view at the level of the papillary muscles (Figure 1c). The wall thickness in each view was measured at end-diastole, defined as either the frame immediately after closure of the mitral valve or the frame of maximal LV diameter. 7 The thickness of the IVS was measured in all cats from the leading edge of the right ventricular side of the septum to the trailing edge of the LV side of the septum, including the endocardium but excluding right and LV papillary muscles and insertion of false tendons.7,32 The LVFWd was assessed between the mitral annulus and the posterior papillary muscle, including the endocardium, but excluding the pericardium. For each 2DE view, the thickest region was measured in two to three cardiac cycles and the maximal consistent measurement was used.
Figure 1.
Two-dimensional echocardiographic measurements of end-diastolic left ventricular wall thickness in an unaffected cat. (a) Right parasternal long axis outflow (five chamber) view; (b) right parasternal long axis inflow (four chamber) view; (c) short axis view at the papillary muscle level. IVS = interventricular septum; LA = left atrium; Ao = aorta; LV = left ventricle; LVFW = left ventricular free wall
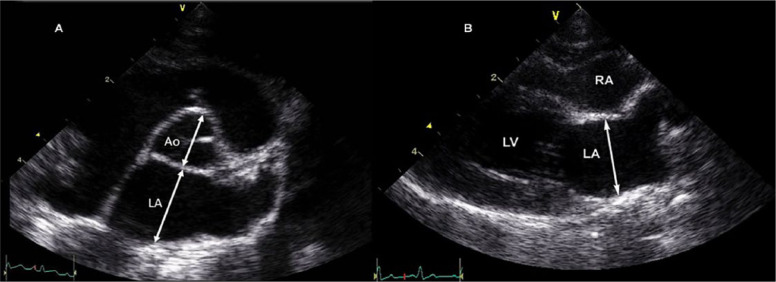
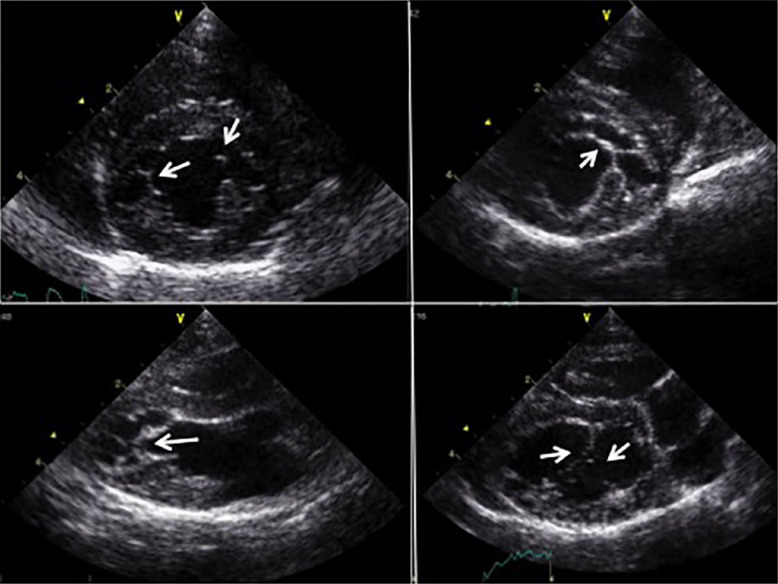
For LV M-mode measurements, 2D-guided M-mode images were recorded from a right parasternal short axis view at the level of the papillary muscles. End-diastolic measurements of the LV inner diameter were made at the beginning of the QRS complex, and end-systolic LV diameter measured at the nadir of septal motion, from leading edge to leading edge. 33 The left atrial to aortic ratio (LA/Ao) was obtained from a right parasternal short axis view in diastole (Figure 2a). The left atrial diameter (LAD) was obtained from the RPLAx inflow view in systole just before opening of the mitral valve measuring from inner edge to inner edge (Figure 2b).35–37 For each left atrial size measurement, the mean value of three cardiac cycles was used. Left atrial enlargement was defined as LA/Ao >1.5 and LAD ≥16 mm.35,37 2D images of the LV outflow tract were used to identify the presence of systolic anterior motion (SAM) of the mitral valve, colour Doppler echo was used to interrogate for LV outflow tract obstruction, and spectral Doppler was used to assess maximal velocities of the left and right ventricular outflow tracts. False tendons were recorded by 2DE and defined as present or absent (Figure 3).
Figure 2.
Two-dimensional echocardiographic measurements of left atrial diameter in an unaffected cat. (a) Right parasternal short axis view at the level of the aortic valve. The left atrial to aortic ratio was measured in the first frame after aortic ejection, where the aortic leaflets are visible using an inner-edge to inner-edge method. 34 (b) Right parasternal long axis inflow view. The maximal left atrial diameter was measured at end-systole. LA = left atrium; Ao = aorta; LV = left ventricle; PV = pulmonary vein; RA = right atrium
Figure 3.
False tendons (arrows) were described as absent or present, based on right parasternal short and long axis views. Most connected the free wall with the septum or the papillary muscles with the septum
To determine inter-observer variability, five cats were scanned independently by two examiners, one resident (IM) and one board-certified cardiologist (VLF). Each examiner measured the images from their own scan to evaluate inter-echocardiographer agreement in obtaining and measuring IVS and LVFW thickness. Inter-measurer agreement was evaluated by comparing the agreement in diagnosis and measurement of wall thickness between the two examiners following offline measurements in the examined cats (n = 53). The final classification of each cat into one of the three groups (unaffected, equivocal or affected [HCM or RCM]) was based on the consensus of both investigators.
In order to correspond as far as possible with the HCM criteria used in the open databases, cats were classified as affected with HCM when either the mean M-mode or maximal 2DE IVSd or LVFWd measured ≥5.5 mm.28,34,39 Cats were classified as equivocal if the wall thickness measured ≥5.0 but <5.5 mm or if SAM of the mitral valve was detected despite a maximal IVS or LVFW thickness of <5 mm. Cats with a maximal LV wall thickness of <5.5 mm and either left atrial (LA/Ao >1.5) or biatrial enlargement were classified as RCM.
Pathological examination
Following diagnosis of cardiac disease in the index case, the pathology archives at the Royal Veterinary College were searched for details of NFCs undergoing full necropsy. Information on the study was disseminated via breed clubs to notify NFC breeders of our interest in any NFC that had undergone necropsy and was suspected of having heart disease. Each contact was followed up, and external pathology institutions and laboratories were contacted directly. For cases from the Royal Veterinary College, original slides were reviewed of standardised sections from paraffin blocks of tissue preserved in 4% neutral buffered formalin. For other institutions, original cardiac histopathology slides and original paraffin blocks were requested where possible, but sections were not standardised. All slides were stained with haematoxylin and eosin. Using an Olympus BX51TF microscope, section(s) of myocardium on each slide were initially scanned at low power (× 10 and × 20 magnifications). Representative areas were subsequently examined at × 40 and × 100 magnifications.
Interpretation of the histopathological sections was carried out by two trained observers (KS and LW), and included semiquantitative assessment of myocyte hypertrophy, myofibre disarray, interstitial fibrosis, replacement fibrosis, intramural arteriosclerosis and endomyocardial fibrosis, each scored from 0 (absent) to 3 (most severe).13–16 No attempt was made to quantify the percentage of myofibre disarray according to site, as sections were not standardised for all samples. Lesions were recorded based on the consensus of both investigators. A classification of HCM was based on the presence of myofibre disarray with or without interstitial fibrosis or arteriosclerosis. A classification of RCM was based on the presence of endomyocardial fibrosis. If features of both phenotypes were noted, a classification of HCM/RCM was made.
Pedigree analysis using open-source databases
The pedigrees obtained in the prospective study were used as the starting point for investigation of related cats by using open sources available on the internet. PawPeds (www.pawpeds.com) is a global database of pedigrees and health information for purebred cats and dogs. Echo examinations for this database are performed by a selected group of veterinarians. Approximately two to 10 generations were reported in the NFC database, but health information was not available for all cats. At the time of writing, PawPeds defines cats as affected with HCM when a subjective impression of hypertrophy (eg, regional or global and/or papillary muscle hypertrophy) is supported by either a mean M-mode or maximal 2DE IVSd, or LVFWd measurement ≥5.5 mm. Cats are classified as equivocal if the wall thickness is between 5.0 and 5.5 mm, and as unaffected if wall thickness is <5.0 mm. These definitions are valid only in cats with a body weight between 2.5 and 6.0 kg. This weight range is valid for most of the screened feline population, but cats outside this weight range are evaluated on a case-by-case basis.28,34,39 Cats with normal wall thickness and SAM are classified as equivocal, and cats with normal wall thickness and left atrial or biatrial enlargement are classed as having RCM. 39 Information was available on diagnosis, age of diagnosis and repeat examinations for some cats, but not all.
The second open-source database searched for details of NFC health status was a private webpage operated by Winterfyre (www.winterfyre.com/testing/). Only a qualitative cardiac assessment was provided for some cats without any detailed echo measurements. Age and cause of death were listed for cats that had died. In this database, cats were also included if HCM was diagnosed by post-mortem examination, so information in this database was used to complement the information obtained by the prospective study and PawPeds, as well as to identify further cats with HCM.
The pedigrees of cats included in the prospective study were cross-referenced with data from the open-source databases. A list was thereby generated including name, sex, date of birth, name of sire and dam, cardiac diagnosis, age of diagnosis, and survival status (if applicable). The identity of all cats was kept confidential. Cats were classified into three groups: (1) unaffected, (2) equivocal and (3) affected (HCM or RCM).
Statistical methods
Data for the prospective study were analysed using commercial software (SPSS for windows v. 18 and v. 20; IBM). Normality of distribution was visually assessed and tested using the Kolmogorov–Smirnov test, defined as normally distributed if the P value was >0.05. Normally distributed results were reported as mean ± SD and non-normally distributed results as median (range). Comparison of continuous data between groups (unaffected, equivocal and affected) was performed using a one-way ANOVA test if normally distributed and Kruskal–Wallis test if non-normally distributed. If there was a significant difference, post-hoc-tests (Student’s t-test if normally distributed and Mann–Whitney U-test if non-normally distributed) were performed. Association between sex and HCM was assessed using a χ2 test. A P value <0.05 was described as significant. Inter-observer agreement was assessed with intra-class correlation coefficients for continuous variables and Cohen’s Kappa for categorical variables.
For the pedigree analysis, pedigree trees of all cats included in the prospective study, as well as of all cats diagnosed with HCM recognised by open-source databases, were constructed using GenoPro Genology Software (http://www.genopro.com). Parent–offspring regression was attempted to estimate heritability.
Results
Prospective screening for HCM
The prospective study comprised 55 NFCs. No sedation was used for echo in any cat, and two cats were excluded because of uncooperative behaviour; therefore, data from 53 NFCs were used for the analysis. The study group consisted of more female cats (70%) than male cats (30%), with a sex distribution of 13 female spayed, 24 female entire, 11 male neutered and five male entire cats. There was no significant difference (P = 0.50) in sex between diagnostic groups (unaffected, equivocal, affected). Mean age at echo examination was 55.1 ± 38.4 months. The mean age of cats diagnosed with HCM was 57.8 ± 35.7 months, equivocal cats was 70.0 ± 27.7 months and unaffected cats was 39.0 ± 136.0 months. No significant difference between groups (Table 1) was found for age, blood pressure or heart rate. Body weight was lower in the unaffected group (unaffected compared with equivocal P <0.01; unaffected compared with affected P <0.01), but there was no difference in body weight between the equivocal and affected groups (P = 0.98).
Table 1.
Basic data and echocardiographic (echo) results obtained from cats in the prospective echo study (n = 53)
| Unaffected | Equivocal | HCM | P value | |
|---|---|---|---|---|
| Number of cats | 35 | 5 | 13 | |
| Male, n (%) | 9 (26) | 1 (20) | 6 (46) | 0.34 |
| Age (months) | 48 ± 37 | 70 ± 27 | 58 ± 35 | 0.41 |
| Body weight (kg) | 4.2 ± 0.9 | 5.6 ± 0.8 | 5.6 ± 1.0 | <0.01 |
| Blood pressure (mmHg) | 137 ± 19 | 143 ± 20 | 149 ± 16 | 0.29 |
| Heart rate (bpm) | 164 (136–200) | 168 (160–188) | 180 (140–200) | 0.90 |
| LA/Ao | 1.39 ± 0.14 | 1.44 ± 0.08 | 1.33 ± 0.13 | 0.26 |
| LAD (cm) | 1.40 ± 0.11 | 1.52 ± 0.15 | 1.45 ± 0.12 | 0.09 |
| IVSd (mm) | 4.3 (3.3–4.9) | 5.3 (4.8–5.5) | 5.9 (5.7–7.1) | – |
| LVFWd (mm) | 4.1 (3.5–4.9) | 5.1 (4.7–5.4) | 5.3 (4.3–6.6) | – |
| LVIDd (mm) | 15.0 ± 1.8 | 16.2 ± 1.4 | 15.7 ± 2.8 | 0.32 |
| LVIDs (mm) | 9.0 ± 1.5 | 9.3 ± 1.3 | 8.7 ± 2.5 | 0.77 |
HCM = hypertrophic cardiomyopathy; bpm = beats per minute; LA/Ao = left atrial to aortic ratio; LAD = left atrial diameter in systole; IVSd = interventricular septum in end-diastole (two-dimensional); LVFWd = left ventricular free wall in end-diastole; LVIDd = left ventricular internal diameter in diastole (M-mode); LVIDs = left ventricular internal diameter in systole (M-mode)
Cardiac auscultation was unremarkable, and no heart murmur or gallop was detected in any cat of the prospective study population. One cat (cat B) had an arrhythmia during auscultation, but no arrhythmia was identified on a concurrent echo during echo examination. This cat was diagnosed with HCM by echo, which was subsequently confirmed by histopathology.
Inter-echocardiographer variability was obtained in five cats, with good intraclass correlation coefficients (ICC; ICC for IVS 0.98; ICC for LVFW 0.98). Results of classification of all screened cats showed very good agreement (k 0.96), 36 with the classification differing between observers in only 1/53 cats. Inter-measurer agreement for wall thickness values in all screened cats was also very good (ICC for IVSd 0.96; ICC for LVFWd 0.93). On the basis of echo examination, 13 (25%) cats were classified as affected with HCM, five (9%) cats were classified as equivocal and 35 (66%) cats as unaffected. One cat had SAM on 2DE; however, LV outflow tract velocities and spectral Doppler velocity profiles were normal. Although LV wall thickness was <5 mm, this cat was classified as equivocal because of the presence of SAM. Asymmetric hypertrophy affecting the septum was found in 9/13 cats with HCM, but was not associated with LV outflow tract obstruction or SAM in any case. The IVS was hypertrophied (≥5.5 mm) in all cats diagnosed with HCM, whilst the LVFW exceeded 5.5 mm in only 6/13 cats. Two cats showed symmetric LV hypertrophy.
False tendons were found in all cats, independent of group classification. These extended from the papillary muscles to the septum or from the free wall to the septum. They were noted as single or multiple strands, and in some cases formed a ‘spider’s web’-like network (Figure 3). In the majority of cats, the endocardium was observed to be particularly thick, prominent and hyperechogenic. Aortic regurgitation was found in four cats, none of which were classified as having HCM. In each case, the aorta was morphologically normal, with three leaflets, no significant thickening and a normal outflow velocity. No difference between the groups was found for left atrial size or LV diameter. Echo results are summarised in Table 1.
Pathological examination
Results of histopathology were available for eight cats. Four cats were male (three neutered and one entire) and four were female (two neutered and two entire), with a median age of 4.3 years. In three cats (cats A, B and G) the necropsy examination was performed at the Royal Veterinary College. In the other five cats (cats C, D, E, F and H) histology slides were provided for examination from external sources. A cardiac cause of death was identified in six cats; two died of heart failure and three cats died suddenly, all at less than 4 years of age. The final cat developed arterial thromboembolism and was euthanased at the age of 10 years − 7 years after an initial echo diagnosis of HCM and previous episodes of congestive heart failure. The cause of death of the two remaining cats was non-cardiac, and the owners did not observe clinical signs of heart disease at any time. These cats were over 7 years of age; HCM was diagnosed in one of them by echo prior to death. The relationship between cats in the post-mortem analysis is displayed in Figure 4.
Figure 4.
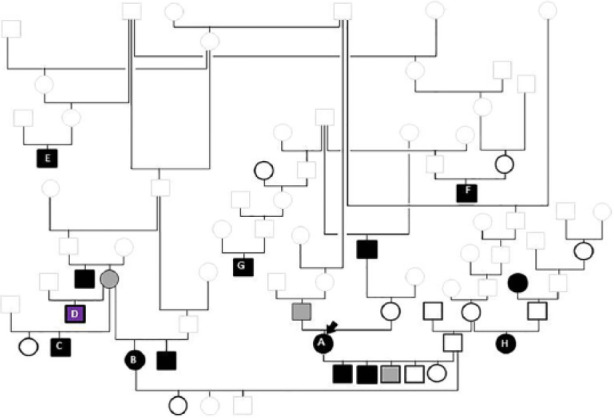
Pedigree tree of the cats that underwent necropsy. Squares denote male cats; circles denote female cats. Solid black symbols denote affected cats; solid grey symbols cats with equivocal results and black open symbols unaffected cats. Grey open symbols indicate the cats for which cardiac health status was unknown. Cat D died suddenly and was diagnosed with myocarditis on histopathology. The index case (A, arrow) has an equivocal sire. She was mated to an unaffected sire, producing affected kittens. An equivocal female was mated to two different males with unknown health status; both litters included affected offspring (B and C). The sibling of this dam was diagnosed with hypertrophic cardiomyopathy in the prospective study
Macroscopic and microscopic examination of the heart revealed gross LV hypertrophy in 5/8 cats, myofibre disarray (Figure 5) to a varying extent in all cats, myocyte hypertrophy in 7/8 cats, interstitial fibrosis (Figure 6) in 7/8 cats and a variable extent of intramural arteriosclerosis in 3/8 cats. Endomyocardial fibrosis was seen in 7/8 cats, but no cat had LV ‘bridging scar’. Histopathological examination of the heart from cat D revealed diffuse inflammatory cell infiltration. Occasional myofibre disarray was present within areas of inflammation, but myocyte hypertrophy and intramural arteriosclerosis were absent.
Figure 5.
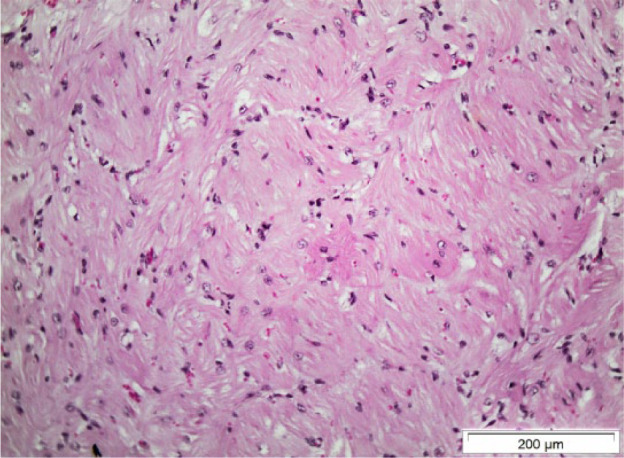
Histological section from the interventricular septum of cat F. Myofibre disarray is present. There is distorted arrangement of the myocytes with adjacent cells arranged at perpendicular and oblique angles. Some myocytes show signs of hypertrophy with nuclear enlargement (haematoxylin and eosin stain)
Figure 6.
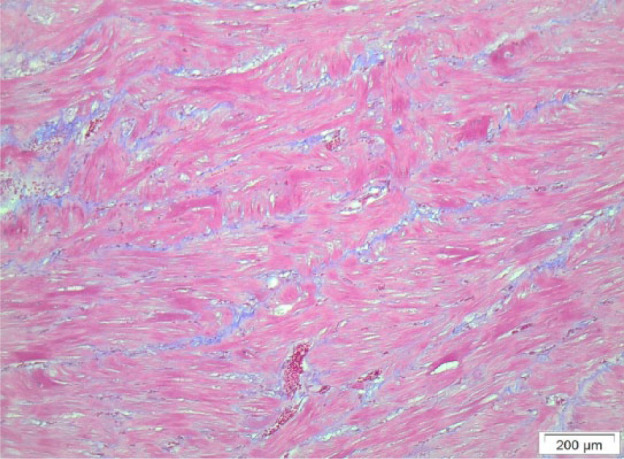
Histological section from the interventricular septum of cat F, showing diffuse interstitial fibrosis and myofibre disarray. Masson trichrome stain demonstrates bands of fibrous tissue (stained blue) between myocytes
Cat B was diagnosed with HCM as part of the prospective study 7 months prior to euthanasia for non-cardiac disease. Echo evaluation using 2DE revealed symmetric hypertrophy with IVSd thickness of 5.8 mm and LVFWd thickness of 5.9 mm. Myofibre disorganisation was present in the IVS and LVFWd, with loss of normal parallel fibre orientation contributing to bizarre cellular architecture, characteristic of myofibre disarray. Endomyocardial, interstitial and replacement fibrosis was confirmed with Masson trichrome stain. Myocytes were hypertrophied and had abundant eosinophilic fibrillar cytoplasm, and large, rectangular, hyperchromic nuclei. The main histopathological findings are summarised in Table 2.
Table 2.
Details of the cats submitted for necropsy
| Cat A* | Cat B | Cat C | Cat D | Cat E | Cat F | Cat G | Cat H | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 3.4 | 7.0 | 2.4 | 1.4 | 7.8 | 10.0 | 4.3 | 4.0 |
| Sex | FE | FN | MN | ME | MN | MN | FE | FN |
| Cause of death | Euthanasia (ATE & CHF) | Euthanasia (cholangiohepatitis) | Euthanasia (CHF) | SD | Euthanasia (FIP) | Euthanasia (ATE) | SD | SD |
| Method of diagnosis | Echo, necropsy | Echo, necropsy | Necropsy | Necropsy | Necropsy | Echo, necropsy | Necropsy | Necropsy |
| Heart weight (g) | 27 | – | – | 25.0 | 22.0 | 52.2 | 15.1 | – |
| RVFW thickness (mm) | – | – | – | – | 1.5 | 3.0 | 5.0 | – |
| IVS thickness (mm) | – | – | – | – | 8.0 | 4.5 | 2.0 | – |
| LVFW thickness (mm) | – | – | – | – | 10.0 | 9.0 | 8.0 | – |
| Gross ventricular changes | Focal LVH | Concentric LVH | Concentric LVH | – | Concentric LVH | Asymmetric LVH | Pale, opaque endocardium | – |
| Left atrial enlargement | Yes | No | Yes | – | No | Yes | No | – |
| Intracardiac thrombus | Yes | No | No | – | No | Yes | No | – |
| Myocyte hypertrophy | + | ++ | + | 0 | + | +++ | ++ | ++ |
| Myofibre disarray | ++ | ++ | ++ | + | ++ | +++ | ++ | ++ |
| Subendocardial fibrosis | +++ | + | +++ | 0 | +++ | ++ | ++ | + |
| Interstitial fibrosis | +++ | ++ | +++ | 0 | ++ | + | ++ | ++ |
| Replacement fibrosis | + | + | 0 | 0 | 0 | 0 | 0 | + |
| Intramural arteriosclerosis | + | 0 | + | 0 | 0 | +++ | 0 | 0 |
The index case (Cat A). Histopathological changes are graded from 0 (absent) to +++ (most severe). RVFW = right ventricular free wall; IVS = interventricular septum; LVFW = left ventricular free wall; FE = female entire; FN = female neutered; MN = male neutered; ME = male entire; ATE = arterial thromboembolism; CHF = congestive heart failure; SD = sudden death; FIP = feline infectious peritonitis; Echo = echocardiography; LVH = left ventricular hypertrophy
Pedigree analysis using open-source databases
Of the cats screened as part of the prospective study, three affected cats had at least one affected parent, and two affected cats had at least one parent classified as equivocal. Further information was then sought from open-source databases describing the results of HCM screening in NFCs.
Cardiac health information was obtained for 871 cats by combining the prospective echo study (n = 53), pathological examination (n = 8) and open-source data (n = 810). Of cats included in the pedigree analysis, 97 (11%) were classified as affected with cardiomyopathy, 41 (5%) were classified as equivocal and 733 (84%) as unaffected. An association between HCM and sex was found, with male cats being more likely to be diagnosed with HCM than female cats (P <0.01). Age distribution showed that 42/97 (43%) affected cats with HCM were <3 years of age compared with 428/733 (58%) cats classified as unaffected. Information about cardiac death was available for 18 cases listed in the open-source database used for the pedigree analysis. Seven of these cats were diagnosed at necropsy, without prior echo. The median age of these cats was 5.5 years (range 1.0–14.0 years). The phenotype of cats related to the index cat is shown in Figure 4.
Thirteen cats with HCM had cardiac health information available for both sire and dam. None of these matings identified both parents as affected with HCM. Matings resulting in HCM-affected cats included normal × normal (n = 2), normal × equivocal (n = 2), normal × HCM (n = 7), equivocal × HCM (n = 1) and equivocal × equivocal (n = 1). At least one of the parents in each of the normal × normal matings was scanned at <2 years of age. Calculation of parent–offspring regression was attempted but could not be determined owing to insufficient data regarding the health of both parents for the affected cats.
Discussion
Characterisation of an abnormal phenotype is essential in any familial disease before further genetic studies are possible. Although it is widely believed that NFCs are predisposed to HCM, to our knowledge the characteristics of NFC cardiomyopathy have not been reported. In this study we evaluated echo and pathological data from multiple sources in order to describe the features of cardiomyopathy in this breed. We found several distinctive features in affected NFCs.
Firstly, murmurs and dynamic LV outflow tract obstruction were uncommon. Functional murmurs and murmurs associated with outflow tract obstruction are both common in non-pedigree cats (affecting up to 34%), 7 but neither form of murmur was found in our prospective echo study. Although SAM was documented in one cat, it was not associated with elevated LV outflow tract velocities. False tendons were present in all cats in our prospective study but were not associated with LV outflow tract obstruction, despite a previous report in cats suggesting a link. 40 False tendons are increasingly being recognised as a normal finding in humans, 41 and we suggest they are also a normal finding in NFCs. The low prevalence of murmurs has important diagnostic implications for NFCs as detection of a heart murmur is a common indication for echo, and affected cats without a heart murmur are less likely to be diagnosed before clinical signs develop.
Secondly, LV hypertrophy was modest. Although we used a low cut-off value (5.5 mm) for maximum LV wall thickness as a criterion for LV hypertrophy, and this may have contributed to low median values for IVSd and LVFWd in affected cats, it does not explain the relatively low values recorded for the upper limits of range (7.1 mm and 6.6 mm, respectively). Mild hypertrophy might be expected when screening a generally healthy population with a mean age of <6 years for a disease with age-related penetrance, but hypertrophy was also relatively mild in the cats examined at necropsy, including 5/6 cats dying of their heart disease. In cat B, although echo LV wall thicknesses were below the commonly used cut-off value of 6 mm, HCM was confirmed on histopathology. This suggests a lower cut-off than 6.0 mm may be justified when screening, at least in NFCs and Maine Coons. 34 In our study, we wanted to use the same criteria for diagnosing HCM as used in open-source databases such as PawPeds (5.5 mm cut-off), accepting that lower cut-off values will increase the risk of false positive results for HCM. LV hypertrophy is the principal criterion for a diagnosis of HCM when screening for affected cats, but diagnosis can be challenging in cats with mild LV hypertrophy. It is recognised that some people with a pathogenic HCM mutation will exhibit a normal cardiac phenotype, so that there is no lower limit of LV wall thickness that rules out inherited HCM in humans. Diagnosis of preclinical RCM in cats also presents challenges, as LV dimensions are generally normal. The PawPeds criterion for diagnosing RCM is left atrial enlargement with normal LV dimensions. In other cardiac conditions, left atrial enlargement will occur only when left heart filling pressures are increased, suggesting the screening criteria for RCM may identify only cats with relatively advanced disease, depending on the cut-off used for left atrial enlargement.
Thirdly, endomyocardial fibrosis was commonly found on necropsy, in addition to histopathological changes typically considered characteristic of HCM (myofibre disarray, interstitial fibrosis, myocyte hypertrophy). Many pathologists consider endomyocardial fibrosis to be a typical feature of RCM, 19 although there is little general consensus on the histopathological criteria for classifying RCM in cats. None of the cats examined at necropsy showed bridging scar, as considered typical for the endomyocardial form of RCM. 11 A recent report of the myocardial form of RCM suggested that some histopathological features of HCM may also be present in cats with RCM, 3 leaving the distinction between HCM and the myocardial form of RCM still less clear. Furthermore, similarities between end-stage HCM (ES-HCM) and RCM have been recognised. The only study describing pathological changes in cats with ES-HCM found myofibre disarray and endomyocardial fibrosis, similar to that described in our study. However, in contrast to the NFCs reported here, the cats with ES-HCM also had large regions of myocardial replacement fibrosis, which is considered to be a histopathologic hallmark of ES-HCM. In humans, some sarcomeric mutations have been associated with a mixed HCM/RCM phenotype; troponin-T mutations in humans can result in a phenotype with minimal LV hypertrophy and potentially life-threatening disease.42–44 It is possible that NFC cardiomyopathy may represent a mixed HCM/RCM phenotype. Myocarditis is not normally associated with sarcomeric mutations, so it remains unclear whether cat D had a completely unrelated form of cardiac disease compared with related cats, or whether myocarditis is also part of the phenotypic spectrum of NFC cardiomyopathy.
Pedigree analysis showed that at least five cats classed as affected in the prospective echo study had an affected or equivocal parent. Extending the analysis to the open-source databases identified, 13 affected cats with screened parents were found, 11 of which had one affected or equivocal parent, suggestive of an autosomal dominant trait. Although two affected cats had unaffected parents, in both matings at least one parent was <2 years of age. In humans, penetrance has been described to be age-dependent, so that periodic evaluation of ‘unaffected’ family members is necessary as some may not express the phenotype until later in life.45,46 While it is possible that one of these apparently unaffected cats may have subsequently developed HCM later in life, an autosomal recessive pattern or incomplete penetrance cannot be excluded. Males were more commonly affected but both males and females were represented, making an X-linked inheritance pattern less likely. A male predisposition has been widely recognised in studies of HCM.7,28,47,48 Neither the prospective screening nor the evaluation of open-source data was complete enough to calculate parent–offspring regression.
This preliminary study had a number of limitations. Sample size was small in both the prospective echo study and the necropsy/histopathology study, and both were potentially subject to selection bias. Although the open-source data sampled a large number of cats, selection bias could still have influenced results, and with no standardisation of training in those submitting echo or pathological data, classification may not have been consistent. Some examiners may have classified HCM using M-mode and others using 2DE measurements, which can yield different results. 7 Standardisation of the technique used for acquisition of echo images and measurement of dimensions would ensure greater consistency in screening results. The age at diagnosis may also have influenced the results, as age-related penetrance is likely with cardiomyopathies. Most unaffected cats used in the pedigree analysis were examined at <3 years of age, and most affected cats were >3 years old. Most cats were only examined once.
On the whole, the subjects evaluated for the prospective echo study and in the open-source databases were only mildly affected or normal, whereas the majority of the cats submitted for necropsy died as a result of their heart disease. It is impossible to determine whether these different populations had the same cardiomyopathy of differing severity, or whether they represent different conditions. A cross-sectional study with unbiased selection, longitudinal follow-up and necropsy confirmation would be necessary to clarify this. Furthermore, classification of cardiomyopathies is based on different criteria for echo and histopathology, and further work is needed to evaluate the correlation between the two techniques in cats. Ideally, the echo and pathological criteria should also be standardised for defining cardiomyopathy phenotypes.
Conclusions
Cardiomyopathy was diagnosed in NFCs based on echo and histopathological findings. Affected cats typically had no murmur or LV outflow tract obstruction, mild LV hypertrophy and histopathological features of HCM (myofibre disarray, interstitial fibrosis) and also endomyocardial fibrosis without bridging scar. Pedigree analysis was suggestive of an autosomal dominant inheritance pattern with incomplete penetrance, but other modes of inheritance should not be excluded. We suggest that cardiomyopathy in NFCs has characteristics of both HCM and RCM.
Acknowledgments
We would like to thank Yve Hamilton-Bruce for helping to initiate this study and for providing consent for post-mortem examination of two cats; the owners of all the cats participating in the study; first-opinion vets; cooperation of UK NFC Breed Council; Silke Schmitz for helping to restrain the cats for echo examinations; Cheryl Sarges for allowing the use of data from Winterfyre; Ulrika Olsson and Anne Dajm at PawPeds for allowing use of the PawPeds database; and Dr Michael Ashworth (Great Ormond Street Hospital) for advice on the histopathology.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: Part of this study was funded by the Winn Feline Foundation.
Accepted: 8 September 2014
References
- 1. Fox PR, Maron BJ, Basso C, et al. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: a new animal model similar to the human disease. Circulation 2000; 102: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 2. Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation 1995; 92: 2645–2651. [DOI] [PubMed] [Google Scholar]
- 3. Fox PR, Basso C, Thiene G, et al. Spontaneously occurring restrictive nonhypertrophied cardiomyopathy in domestic cats: a new animal model of human disease. Cardiovasc Pathol 2014; 23: 28–34. [DOI] [PubMed] [Google Scholar]
- 4. Kittleson MD, Meurs KM, Munro MJ, et al. Familial hypertrophic cardiomyopathy in maine coon cats: an animal model of human disease. Circulation 1999; 99: 3172–3180. [DOI] [PubMed] [Google Scholar]
- 5. Liu SK. Acquired cardiac lesions leading to congestive heart failure in the cat. Am J Vet Res 1970; 31: 2071–2088. [PubMed] [Google Scholar]
- 6. Paige CF, Abbott JA, Elvinger F, et al. Prevalence of cardiomyopathy in apparently healthy cats. J Am Vet Med Assoc 2009; 234: 1398–1403. [DOI] [PubMed] [Google Scholar]
- 7. Wagner T, Fuentes VL, Payne JR, et al. Comparison of auscultatory and echocardiographic findings in healthy adult cats. J Vet Cardiol 2010; 12: 171–182. [DOI] [PubMed] [Google Scholar]
- 8. Ferasin L, Sturgess CP, Cannon MJ, et al. Feline idiopathic cardiomyopathy: a retrospective study of 106 cats (1994–2001). J Feline Med Surg 2003; 5: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu SK, Maron BJ, Tilley LP. Feline hypertrophic cardiomyopathy: gross anatomic and quantitative histologic features. Am J Pathol 1981; 102: 388–395. [PMC free article] [PubMed] [Google Scholar]
- 10. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy. Circulation 2011; 124: e783–e831. [DOI] [PubMed] [Google Scholar]
- 11. Fox PR. Endomyocardial fibrosis and restrictive cardiomyopathy: pathologic and clinical features. J Vet Cardiol 2004; 6: 25–31. [DOI] [PubMed] [Google Scholar]
- 12. Fox PRS, Sisson DD, Moise SN. Textbook of canine and feline cardiology: principles and clinical practice. 2nd ed. St Louis, MO: Saunders, 1999. [Google Scholar]
- 13. Liu SK, Roberts WC, Maron BJ. Comparison of morphologic findings in spontaneously occurring hypertrophic cardiomyopathy in humans, cats and dogs. Am J Cardiol 1993; 72: 944–951. [DOI] [PubMed] [Google Scholar]
- 14. Tilley LP, Liu SK, Gilbertson SR, et al. Primary myocardial disease in the cat. A model for human cardiomyopathy. Am J Pathol 1977; 86: 493–522. [PMC free article] [PubMed] [Google Scholar]
- 15. Fox PR. Hypertrophic cardiomyopathy. Clinical and pathologic correlates. J Vet Cardiol 2003; 5: 39–45. [DOI] [PubMed] [Google Scholar]
- 16. Vanvleet JF, Ferrans VJ, Weirich WE. Pathologic alterations in hypertrophic and congestive cardiomyopathy of cats. Am J Vet Res 1980; 41: 2037–2048. [PubMed] [Google Scholar]
- 17. Liu SK, Tilley LP. Animal-models of primary myocardial diseases. Yale J Biol Med 1980; 53: 191–211. [PMC free article] [PubMed] [Google Scholar]
- 18. Liu SK. Pathology of feline heart diseases. Vet Clin North Am 1977; 7: 323–339. [DOI] [PubMed] [Google Scholar]
- 19. Stalis IH, Bossbaly MJ, Van Winkle TJ. Feline endomyocarditis and left ventricular endocardial fibrosis. Vet Pathol 1995; 32: 122–126. [DOI] [PubMed] [Google Scholar]
- 20. Palmer JK. Pathology of domestic animals. 5th ed. St Louis, MO: Saunders, 2007. [Google Scholar]
- 21. Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol 2008; 19: 104–110. [DOI] [PubMed] [Google Scholar]
- 22. Bos JM, Ommen SR, Ackerman MJ. Genetics of hypertrophic cardiomyopathy: one, two, or more diseases? Curr Opin Cardiol 2007; 22: 193–199. [DOI] [PubMed] [Google Scholar]
- 23. Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol 2009; 54: 201–211. [DOI] [PubMed] [Google Scholar]
- 24. Meurs KM, Sanchez X, David RM, et al. A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy. Hum Mol Genet 2005; 14: 3587–3593. [DOI] [PubMed] [Google Scholar]
- 25. Meurs KM, Norgard MM, Ederer MM, et al. A substitution mutation in the myosin binding protein C gene in ragdoll hypertrophic cardiomyopathy. Genomics 2007; 90: 261–264. [DOI] [PubMed] [Google Scholar]
- 26. Borgeat K, Casamian-Sorrosal D, Helps C, et al. Association of the myosin binding protein C3 mutation (MYBPC3 R820W) with cardiac death in a survey of 236 Ragdoll cats. J Vet Cardiol 2014; 16: 73–80. [DOI] [PubMed] [Google Scholar]
- 27. Nakagawa K, Takemura N, Machida N, et al. Hypertrophic cardiomyopathy in a mixed breed cat family. J Vet Med Sci 2002; 64: 619–621. [DOI] [PubMed] [Google Scholar]
- 28. Granström S, Nyberg Godiksen MT, Christiansen M, et al. Prevalence of hypertrophic cardiomyopathy in a cohort of british shorthair cats in Denmark. J Vet Intern Med 2011; 25: 866–871. [DOI] [PubMed] [Google Scholar]
- 29. Meurs KM, Norgard MM, Kuan M, et al. Analysis of 8 sarcomeric candidate genes for feline hypertrophic cardiomyopathy mutations in cats with hypertrophic cardiomyopathy. J Vet Intern Med 2009; 23: 840–843. [DOI] [PubMed] [Google Scholar]
- 30. Henik RA, Dolson MK, Wenholz LJ. How to obtain a blood pressure measurement. Clin Tech Small Anim Pract 2005; 20: 144–150. [DOI] [PubMed] [Google Scholar]
- 31. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007; 21: 542–558. [DOI] [PubMed] [Google Scholar]
- 32. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 33. Sahn DJ, Demaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978; 58: 1072–1083. [DOI] [PubMed] [Google Scholar]
- 34. Gundler S, Tidholm A, Häggström J. Prevalence of myocardial hypertrophy in a population of asymptomatic Swedish Maine coon cats. Acta Vet Scand 2008; 50: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hansson K, Haggstrom J, Kvart C, et al. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002; 43: 568–575. [DOI] [PubMed] [Google Scholar]
- 36. Maerz I, Schober K, Oechtering G. Echocardiographic measurement of left atrial dimension in healthy cats and cats with left ventricular hypertrophy. Tieraerztl Prax K H 2006; 34: 331–340. [Google Scholar]
- 37. Schober KE, Maerz I. Assessment of left atrial appendage flow velocity and its relation to spontaneous echocardiographic contrast in 89 cats with myocardial disease. J Vet Intern Med 2006; 20: 120–130. [DOI] [PubMed] [Google Scholar]
- 38. Côté E, Harpster NK, Laste NJ, et al. Atrial fibrillation in cats: 50 cases (1979–2002). J Am Vet Med Assoc 2004; 225: 256–260. [DOI] [PubMed] [Google Scholar]
- 39. Peersmans M, Haggstrom J, Olsen U. HCM screening form. http://pawpeds.com/healthprogrammes/HCM-form.pdf (accessed 2010).
- 40. Schober K, Todd A. Echocardiographic assessment of left ventricular geometry and the mitral valve apparatus in cats with hypertrophic cardiomyopathy. J Vet Cardiol 2010; 12: 1–16. [DOI] [PubMed] [Google Scholar]
- 41. Loukas M, Louis RG, Jr, Black B, et al. False tendons: an endoscopic cadaveric approach. Clin Anat 2007; 20: 163–169. [DOI] [PubMed] [Google Scholar]
- 42. Menon SC, Michels VV, Pellikka PA, et al. Cardiac troponin T mutation in familial cardiomyopathy with variable remodeling and restrictive physiology. Clin Genet 2008; 74: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Webber SA, Lipshultz SE, Sleeper LA, et al. Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: a report from the Pediatric Cardiomyopathy Registry. Circulation 2012; 126: 1237–1244. [DOI] [PubMed] [Google Scholar]
- 44. Kubo T, Gimeno JR, Bahl A, et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardio-myopathy with restrictive phenotype. J Am Coll Cardiol 2007; 49: 2419–2426. [DOI] [PubMed] [Google Scholar]
- 45. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002; 287: 1308–1320. [DOI] [PubMed] [Google Scholar]
- 46. Marian AJ. Hypertrophic cardiomyopathy: from genetics to treatment. Eur J Clin Invest 2010; 40: 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Payne J, Luis Fuentes V, Boswood A, et al. Population characteristics and survival in 127 referred cats with hypertrophic cardiomyopathy (1997 to 2005). J Small Anim Pract 2010; 51: 540–547. [DOI] [PubMed] [Google Scholar]
- 48. Harpster NK. Feline cardiomyopathy. Vet Clin North Am 1977; 7: 355–371. [DOI] [PubMed] [Google Scholar]