Abstract
Objectives
The objective of this study was to describe seasonality, demographics, presentations, treatments, complications and outcomes for cats with Ixodes holocyclus causing tick paralysis, and to identify risk factors for mortality.
Methods
This was a retrospective single cohort study with 2077 cases occurring between 2008 and 2016, and presenting to one of four emergency clinics in south-eastern Queensland, Australia. Case mortality at 5 days post-presentation could be determined for 1742 cases, and potential risk factors for mortality were assessed using random-effects logistic regression.
Results
Cases occurred all year round, but there was a marked seasonal pattern with more cases presenting in spring than any other season. Overall, 54/1742 cases (3%) died by 5 days after presentation. Five day mortality incidence for cases that received polyclonal canine tick antitoxin serum (TAS) and recommended treatment was 28/1410 (2%) vs 4/52 (8%) for cases that did not receive TAS (P <0.001). Mechanical ventilation was recommended for 131/2077 cases (6%). Where mechanical ventilation was recommended but not implemented, mortality incidence was 15/17 (88%), whereas 4/22 cases (18%) that received mechanical ventilation died by day 5. From multivariable analyses, initial gait score (overall P = 0.047) and body temperature on presentation (overall P <0.001) were independently associated with mortality; cases with higher gait scores and those with body temperatures <35°C were at greater risk of death. Cases that had an adverse reaction to TAS were also more likely to die (P = 0.002). Additional ticks were detected at coat clipping for 80/872 (9%) the cases that were clipped, and coat clipping was associated with a reduced risk of mortality (P = 0.020). Risk of mortality did not differ significantly by time of year, clinic location, breed, sex, neuter status, age, weight, coat length or number of ticks found.
Conclusions and relevance
The overall mortality risk for cats treated for tick paralysis caused by I holocyclus is low. Risk factors for mortality include advanced gait and respiratory scores, and hypothermia at presentation. Coat clipping and TAS reduce the risk of mortality, whereas the occurrence of a TAS reaction increases the risk. Mechanical ventilation reduces mortality risk in cats with respiratory failure due to tick paralysis.
Introduction
Tick paralysis in Australia is predominantly caused by the paralysis tick, Ixodes holocyclus. This tick is endemic to a narrow coastal area along the eastern coast of Australia.1,2 Tick paralysis is an acute, progressive, ascending, lower motor neuron paralysis caused by neurotoxins in tick saliva, which enter the host during feeding. 3 The majority of peer-reviewed publications regarding tick paralysis in domestic species concern canine patients. Thus, there is a need to describe the seasonality, demographics, clinical presentations, treatments, complications and outcomes for cases of tick paralysis in cats. Furthermore, there is a need to understand the pathophysiology and to identify risk factors for mortality and optimal treatment in affected cats.
The most recent published review of tick paralysis in cats highlighted the need for further research on this condition, 4 but little progress has been made, particularly in describing the differences in disease expression between cats and dogs, and variation within cats. The pathogenesis and clinical presentation(s) of tick paralysis in cats differ from dogs; therefore, research findings from canine tick paralysis cases should not automatically be extrapolated to cats.5–7 Direct cardiotoxicity and concurrent gastrointestinal effects (megaoesophagus, regurgitation and vomiting) have been identified in dogs. These changes are often the cause of lower airway disease, including aspiration pneumonia and pulmonary oedema. Little is known about the exact pathophysiology in cats.8–10 Treatment of feline cases typically involves administration of tick antitoxin serum (TAS), sedatives and provision of a low-stress environment. Monitoring for laryngeal obstruction and/or respiratory muscle paralysis and subsequent respiratory failure is carried out. 11 In the canine patient, lower motor neuron signs predominate in the early stages of tick paralysis. 12 In contrast, in the cat, respiratory signs may predominate over gait changes. Stress in cats can dramatically increase respiratory effort and oxygen demand associated with upper respiratory tract dysfunction, resulting in acute decompensation and respiratory or cardiac arrest. 5
There have been very few studies on mortality in cats with tick paralysis. Westwood et al 13 documented the clinical presentations, treatments and durations of recovery for 98 affected cats in the Sydney area but did not report mortality incidence. Eppleston et al 14 used voluntarily reported data to describe distribution, seasonality and risk factors for tick paralysis in 745 cats, but treatments and mortality incidence were not described. Musca and Gunew 15 described treatments and outcomes for 158 cats with tick paralysis, and the only death was in a cat that had previously been administered TAS. Atwell and Campbell 16 surveyed veterinarians about adverse reactions to TAS in dogs and cats, but 40% of respondents provided estimates rather than precise results based on analyses of practice data. As there is limited published information about tick paralysis in cats, the aims of the current study were to describe the seasonality, demographics, clinical presentations, treatments, complications and outcomes for a large cohort of cases of tick paralysis in cats, and to identify risk factors for mortality.
Materials and methods
Study overview
A retrospective single cohort study was conducted using cases of tick paralysis in cats presented to one of four geographically dispersed veterinary clinics in south-eastern Queensland, Australia, from 2008–2016. Data were extracted from the medical records database. Each disease episode constituted one medical record or ‘case’. Any given cat could contribute one or more cases (ie, cats affected on more than one occasion).
Case selection and data collection
Clinical records for all cases of cats presenting with tick paralysis at any of four Animal Emergency Service (AES) clinics from 1 April 2008 to 30 November 2016 were retrieved by searching the practice database (RX Works; Henry Schein Veterinary Solutions) for the term ‘tick paralysis’. AES offers after-hours clinical services for primary care and referral cases; clinics are closed every weekday morning; cases are either discharged (1) home to owners or (2) back to the referring clinics, or (3) transferred to Veterinary Specialist Services (VSS) (Underwood and Gold Coast clinics). VSS is a referral hospital operating within the same premises as AES. All cases that remained critical or mechanically ventilated were offered referral to this specialist service to avoid transportation. In total, 2213 cases were identified for review. From those cases, all those meeting the study inclusion criteria were enrolled and relevant data from their records were extracted.
Selection criteria
Cases had to meet two criteria to be classified as having tick paralysis. One or more tick craters and/or one or more adult or nymph I holocyclus tick(s) must have been recorded. In addition, the cat must have had gait changes consistent with lower motor neuron weakness and/or changes in respiratory rate, pattern or effort.
Seasonal distribution, demographics and clinical presentations
For each case, date of presentation, clinic location, signalment, body weight, number of tick(s), location(s) of ticks attached to the patient, rectal body temperature on presentation, and initial gait and respiratory scores were extracted. Gait and respiratory scores were recorded to reflect the severity of each cat’s neuromuscular paralysis and respiratory signs, respectively, using a predefined scoring system (Table 1).
Table 1.
Criteria for assigning gait and respiratory scores in cats affected by tick paralysis
| Gait score 1 Mild weakness 2 Can stand but not walk 3 Cannot stand but can right itself and maintain sternal recumbency 4 Unable to right itself, cannot maintain sternal recumbency Respiratory score A Normal B Mild: increased respiratory rate and effort C Moderate: any respiratory distress or dyspnoea, restrictive breathing pattern, coughing, gagging or retching D Severe: severe dyspnoea, cyanosis, progressive reduction in respiratory rate, open-mouth breathing |
Breeds were grouped into domestic shorthair (DSH), domestic not-shorthair (including domestic mediumhair and domestic longhair), purebred shorthair, purebred not-shorthair, purebred shorthair cross, purebred not-shorthair cross. Each breed was categorised as brachycephalic or not; brachycephalic breeds were British Shorthair, British Longhair, British Blue, Exotic Shorthair, Himalayan, Persian, Scottish Fold and Chinchilla. Each breed was also categorised based on coat length as either short or not short (where not short consisted of mediumhair and longhair breeds).
Numbers of adult ticks, as well as craters, found by the owner, the referring veterinarian prior to presentation and at initial presentation at AES were pooled by location. Each crater was counted as one adult tick. Numbers of nymphs were also analysed by location. Numbers of additional ticks (adult ticks and nymphs separately) found during entire body coat clip were also identified. Before coat clipping, cats were either heavily sedated with a variety of combinations of drugs or lightly anaesthetised with alfaxalone (Alfaxan; Jurox) and had an endotracheal tube placed for the duration of the clipping. Locations of ticks were categorised into the following body regions: head, neck, shoulder, thorax, abdomen, flank, anus, lumbar, forelimb(s), hindlimb(s) and tail.
Treatments, complications and outcomes
Treatments
Cases were divided into two subsets: (a) cats that did not receive TAS at either the referring veterinary clinic prior to presentation to AES or at AES (ie, cases where TAS was either not recommended or owners declined all treatment); and (b) cats that received TAS at either the referring veterinary clinic prior to presentation to AES and/or at AES (ie, the veterinarian had advised the use of TAS and the owner had agreed with that decision).
Treatment details extracted included oxygen requirements (subsequent to initial triage and stabilisation), TAS details – including route of administration (intravenous [IV] or intraperitoneal [IP]) – and details of treatments prior to referral to AES. TAS batches and brands varied over the study period, and had not been recorded. Whether cases required intubation and/or mechanical ventilation, and durations of each, were also identified.
Treatment protocol
A computerised tick paralysis template was used at all four clinics, which prompted for history, triage, physical findings, standardised treatment protocol and complications to be documented for every case. This protocol evolved over the duration of study as new knowledge emerged. Treating veterinarians were still able to make individual case-based decisions on treatment options, including preferred sedation, anaesthesia and premedication choices.
The treatment protocol for cases admitted to hospital was as follows:
Triage and physical examination performed at presentation, including initial gait and respiratory score and a tick search (by hand).
Oxygen and sedation. Sedation was given to all patients on presentation, subcutaneously (SC) or intramuscularly (IM) unless an intravenous (IV) cannula was in place from the referring clinic in which case sedatives were given IV. Oxygen was either provided by ‘fly by’ (oxygen supplementation provided via a mask or tubing held in front of the animal’s face) or within an oxygen cage, depending on initial respiratory status. This was continued if the cat was hypoxaemic, hypercapnic or was perceived to have increased work of breathing. Sedation was continued throughout hospitalisation with a continuous rate infusion (CRI) of butorphanol (Torbugesic; Zoetis) or additional boluses of the treating veterinarian’s preferred drug combination.
IV cannula placed once the cat was sedated.
TAS administration. Premedication protocols evolved throughout the study period. Many different combinations of the following drugs were used: prednisolone sodium succinate (Solu-Delta-Cortef; Zoetis), dexamethasone (Dexapent; Troy Laboratories), adrenaline (Adrenaline; Aspen Pharmacare), chlorpheniramine (Histamil; Troy Laboratories) and atropine (Atrosite; Troy Laboratories). Towards the end of the study period no premedication was used. A dose of 10 ml TAS per cat was recommended in the protocol, which was diluted to 20 ml with 0.9% sodium chloride and administered IV via a syringe driver over 1 h with constant monitoring of all vitals. Treating veterinarians could deviate from the protocol if they wanted to give a lower or higher dose; therefore, TAS doses (ml/kg) varied over the study period. Some owners declined the recommended treatment; instead, cases were given TAS intraperitoneally (IP) and discharged home for owners to monitor.
TAS adverse reaction. If an adverse reaction (any change in cardiorespiratory parameters) was noted, the TAS infusion was stopped. Further treatment depended on clinical signs and severity of adverse reaction and involved different combinations of adrenaline (bolus or CRI), atropine, chlorpheniramine, fluid bolus, supplemental oxygen and endotracheal intubation with intermittent positive pressure ventilation, as required.
All cases received IV fluids (Hartmann’s, 0.9% sodium chloride or Plasma-Lyte 148 [Baxter]).
All cases received nursing care. This involved positioning and repositioning in sternal recumbency, eye care, bladder care and active warming, as required (utilising various combinations of warm bedding, cage heat, hot water bottles or forced air-warming blanket). All supportive treatments were performed with a risk–benefit analysis. If any of the above treatments caused stress, a more hands-off approach was taken.
Coat clipping. This was offered as standard of care but could be declined by owners for cost or aesthetic reasons, or was not performed owing to time constraints for cases admitted close to closing time, or delayed if the cat was critical. All cases received heavy sedation or light anaesthesia using alfaxalone and an endotracheal tube was placed for the duration of the clipping. Some cases received fipronil (Frontline; Merial) spray with or without clipping. However, this was not consistently recorded.
Mechanical ventilation. Standard guidelines for recommendation of mechanical ventilation were used across all clinics. Hypoxaemia (SpO2 <90% or PaO2 <60 mmHg; arterial samples were rarely performed owing to stress), hypercapnia (PvCO2 >60 mmHg or ETCO2 >60 mmHg), unsustainable breathing effort (a subjective category as determined by the treating veterinarian), respiratory arrest or a combination of the above. Some cases were recommended to receive mechanical ventilation but this was declined owing to costs.
Discharge from hospital. Cases were classified as recovered from tick paralysis when they had minimal or no signs of paralysis and had eaten food and drunk water safely. Some cats would not eat in hospital. Cases discharged home early, against veterinary advice, all had treatment waiver forms signed and were assessed to be still showing varying degrees of clinical signs of tick paralysis. Cases that were critical and not stable for transport were offered referral to VSS. All other cases were either discharged home (if recovered) or transferred back to the referring clinic when AES closed.
Complications
Complications including cardiac arrest, respiratory arrest and laryngeal paralysis, and changes on any thoracic radiographs were recorded. Adverse reactions to TAS administration were recorded. Owing to the retrospective design of this study, exact classification of all reactions was not possible, and so TAS reaction cases were pooled.
Outcomes
Total duration (h) of hospitalisation at AES was recorded. Based on the notes in each case record, each case was categorised based on its outcome. Categories were: (a) euthanased or died (without euthanasia) at AES; (b) discharged home having recovered from tick paralysis; (c) discharged home early against veterinary advice; (d) discharged back to referring veterinary practice for ongoing care; or (e) transferred to VSS.
Regarding cases discharged (categories b–e), if AES records indicated that at a later date the cat had re-presented >5 days later, it was classified as having survived the previous episode of tick paralysis. Cases that were discharged home having recovered from tick paralysis were not followed-up but were assumed to have survived. Of the remaining cases, those that had gait and respiratory signs at discharge (equivalent to scores >2 and/or B, respectively) (Table 1) were followed-up with the owner, referring veterinary practice or VSS. Those with lower scores or no gait and respiratory signs were not followed-up but instead assumed to have survived.
Deaths were further categorised into: (a) those without euthanasia, (b) cost-based euthanasia (financial constraints documented in the clinical record) or (c) prognosis-based euthanasia (had treatment continued, the case was highly likely to have died due to continued clinical deterioration, despite a high level of supportive care). Cases that suffered cardiopulmonary arrest, received cardiopulmonary resuscitation, had a return of spontaneous circulation (but no spontaneous breathing) and were then euthanased, were classified as deaths without euthanasia (rather than having been euthanased). As it was not possible to obtain the necessary information to categorise the reason for euthanasia at the referring veterinary practices, these cases were not included in mortality outcome analyses. All deaths occurred within 5 days of presentation to AES.
Statistical analysis
Data were analysed using Stata (version 14; StataCorp). The individual instance of tick paralysis (ie, each case) was the unit of analysis. For analyses of the binary variable, mortality by day 5, cases were excluded if they ended in cost-based euthanasia or euthanasia at the referring veterinary practices after discharge from AES, or if mortality status could not be determined from records or follow-up. Associations between potential risk factors and mortality by day 5 were assessed using logistic regression, with owner fitted as a random effect. Models were fitted using Stata’s -xtlogit- command. Likelihood ratio test P values were used to assess the overall significance of each exposure variable. Alternatively, where no cases died in one exposure group, exact logistic regression was used, fitted using Stata’s -exlogistic- command. These models did not fully account for clustering of cases within cats within owners. Multi-level models would have better accounted for all three hierarchical levels, but results from the simpler models used would have been very similar to those from multi-level models, as few owners had more than one study cat and few cats contributed more than one study case.
Based on univariable results, distributions of values and consideration of likely causal pathways, seven variables were selected for multivariable modelling: initial gait score, initial respiratory score, rectal body temperature at presentation, route of administration of TAS (IV or IP), and whether or not the cat was coat clipped, experienced an adverse reaction to TAS administration and/or required mechanical ventilation. All were initially fitted simultaneously and a final multivariable model containing five of these variables was selected after using a backwards elimination approach based on overall likelihood ratio test P values for each variable. The discriminatory ability and goodness of fit of this multivariable model were assessed using predicted probabilities from the fixed part of the model with the random effect set to zero. Because Wald P values and confidence intervals (CIs) can be conservative (ie, unduly large and wide, respectively) when data are sparse, likelihood-based P values and CIs were reported for the final multivariable model, with values calculated with profile likelihood models fitted using Stata’s -pllf- command.
Results
Number of cases
A total of 2213 cases were identified using the search strategy. Of these, three cases had no ticks or tick craters located, 131 had no clinical signs of tick paralysis recorded and concurrent trauma was recorded in two cases. Thus, these 136 cases did not meet our case definition and were excluded. The 2077 cases that met the inclusion criteria occurred in 2025 cats; 1976 cats presented with tick paralysis on a single occasion during the study period, 46 cases presented on two occasions and three cats presented with tick paralysis three times.
Outcome and crude mortality incidences
Mortality status after presentation to AES was obtained for 1742/2077 cases (84%); of these, 54/1742 (3%) died by day 5 (27 died at AES, three were euthanased due to poor prognosis, 11 died at home after discharge against veterinary advice and 13 died at referring clinics after discharge from AES). For the remaining 335 cases (16%), 5 day mortality was not determined as: (1) follow-up information could not be obtained (n = 116); (2) the case ended in a cost-based euthanasia (n = 210); or (3) the cat was euthanased after being discharged back to the referring practice (n = 9). Cases euthanased owing to costs included those at presentation (n = 160/210 [76%]) and those euthanased during treatment (n = 50/210 [24%]). Times from presentation to AES to death for the 54 cases that died by day 5 ranged from 2–108 h (median 15.5 h).
Potential risk factors for mortality by day 5 were assessed in the 1410 cases that: (1) received TAS; (2) received mechanical ventilation (when recommended); and (3) had not been discharged home early against veterinary advice. Based on these three criteria, this group was broadly considered to have received recommended treatment. Of these cases, 28/1410 (2%) died by day 5.
Seasonal distribution
Although cases occurred all year, numbers steadily increased after April (autumn) and then declined over December to February (summer; Figure 1), with a peak seen in spring (October). The majority of cases occurred in spring (n = 1365/2077 [66%]), with fewer cases in winter (n = 487 [23%]), summer (n = 177 [9%]) and autumn (n = 48 [2%]). Odds of mortality by day 5 did not vary significantly by season (P = 0.921), calendar month (P = 0.985) or financial year (July 2008 to June 2009, July 2009 to June 2010, etc) (P = 0.705). From the locally weighted regression plot of mortality, regressed on date of presentation, percentages of cases that died by day 5 were generally slightly lower later in the study period (July 2013 onwards).
Figure 1.
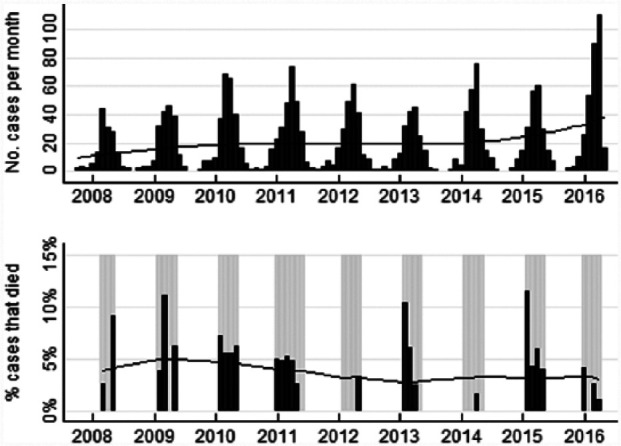
Numbers of cases, and percentages of cases that died on or before day 5 after presentation to the Animal Emergency Service, by month and year. Total number of cases = 2077; percentages of cases that died are shown only for months with at least 20 cases for which mortality outcome was known (grey-shaded months). Years are centred on mid-winter (July; shown as vertical marks). Lines are locally weighted regression (lowess) lines. For percentages of cases that died, the lowess line used all months where results are shown but disregarded the sizes of the denominators in those months
Demographics and clinical presentations
Clinic location, sex, breed, brachycephalic conformation, age, coat length and body weight
The majority of cases were seen at the Underwood (n = 1064/2077 [51%]) and Gold Coast (n = 693/2077 [33%]) clinics. The majority of cases were neutered male (n = 949/2069 [46%]) and female (n = 805/2069 [39%]). DSH was the most common breed (n = 1223/2075 [59%]); the majority had short coat length (n = 1441/2075 [69%]) and were non-brachycephalic breeds (n = 1972/2075 [95%]) (Table 2). Ages varied substantially (median 48 months; range 1 month to 22 years; interquartile range [IQR] 24–96 months). Body weights were recorded for 812/2077 cases (39%); these varied substantially (median 4.6 kg; range 1.5–10.6 kg; IQR 4.0–5.4 kg). Odds of mortality (by day 5) did not differ significantly by clinic location, sex, breed, brachycephalic conformation, age, coat length and body weight (univariable analyses, P ⩾0.183; Table 2).
Table 2.
Univariable associations between cat demographics and mortality by day 5 after presentation to the Animal Emergency Service for cats with tick paralysis
| Variable and categories | All cases |
Analysis of death by day 5 |
||||
|---|---|---|---|---|---|---|
| % (n) | No. of cases receiving recommended treatment | % (n) died by day 5 | OR | 95% CI | P value* | |
| Clinic | 0.183 | |||||
| Gold Coast | 33 (693) | 473 | 2.3 (11) | Ref. group | ||
| Noosa | 2 (44) | 29 | 6.9 (2) | 3.1 | 0.7–14.7 | 0.153 |
| Sunshine Coast | 13 (276) | 179 | 0.6 (1) | 0.2 | 0.0–1.8 | 0.168 |
| Underwood | 51 (1064) | 729 | 1.9 (14) | 0.8 | 0.4–1.8 | 0.631 |
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Sex | 0.273 | |||||
| Female entire | 5 (105) | 52 | 1.9 (1) | Ref. group | ||
| Female neutered | 39 (805) | 553 | 1.3 (7) | 0.7 | 0.1–5.4 | 0.694 |
| Male entire | 10 (210) | 127 | 3.9 (5) | 2.1 | 0.2–18.3 | 0.506 |
| Male neutered | 46 (949) | 674 | 2.2 (15) | 1.2 | 0.2–9.0 | 0.886 |
| Not recorded | (8) | 4 | 0.0 (0) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Breed † | 0.501 | |||||
| DNSH | 15 (304) | 197 | 1.0 (2) | Ref. group | ||
| DSH | 59 (1223) | 812 | 2.0 (16) | 2.0 | 0.4–8.6 | 0.372 |
| PNSH | 11 (235) | 175 | 2.3 (4) | 2.3 | 0.4–12.6 | 0.345 |
| PNSHX | 5 (95) | 62 | 4.8 (3) | 5.0 | 0.8–30.4 | 0.084 |
| PSH | 9 (189) | 143 | 2.1 (3) | }1.8 | 0.3–11.1 | 0.512 |
| PSHX | 1 (29) | 20 | 0.0 (0) | |||
| Not recorded | (2) | 1 | 0.0 (0) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Brachycephalic | 0.219 | |||||
| No | 95 (1972) | 1333 | 1.9 (25) | Ref. group | ||
| Yes | 5 (103) | 76 | 3.9 (3) | 2.2 | 0.6–7.3 | 0.219 |
| Not recorded | (2) | 1 | 0.0 (0) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Age (months) † | 0.241 | |||||
| <12 | 8 (169) | 108 | 5.6 (6) | Ref. group | ||
| 12–23 | 15 (299) | 195 | 1.5 (3) | }0.3 | 0.1–0.9 | 0.036 |
| 24–35 | 13 (262) | 162 | 1.9 (3) | |||
| 36–47 | 10 (199) | 141 | 2.8 (4) | }0.4 | 0.1–1.3 | 0.125 |
| 48–59 | 7 (138) | 83 | 1.2 (1) | |||
| 60–71 | 7 (136) | 100 | 2.0 (2) | }0.4 | 0.1–1.3 | 0.122 |
| 72–83 | 6 (124) | 92 | 2.2 (2) | |||
| 84–95 | 6 (111) | 80 | 1.3 (1) | }0.1 | 0.0–0.9 | 0.037 |
| 96–107 | 6 (121) | 86 | 0.0 (0) | |||
| 108–119 | 4 (71) | 53 | 1.9 (1) | }0.5 | 0.1–2.0 | 0.304 |
| 120–131 | 4 (78) | 57 | 3.5 (2) | |||
| 132–143 | 3 (66) | 55 | 0.0 (0) | }0.2 | 0.1–1.0 | 0.053 |
| ⩾144 | 11 (216) | 152 | 2.0 (3) | |||
| Not recorded | (87) | 46 | 0.0 (0) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Coat length | 0.877 | |||||
| Not short (medium and long) | 31 (634) | 434 | 2.1 (9) | Ref. group | ||
| Short | 69 (1441) | 975 | 1.9 (19) | 0.9 | 0.4–2.1 | 0.877 |
| Not recorded | (2) | 1 | 0.0 (0) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Body weight (kg) | 0.572 | |||||
| 1.5–<4.0 | 25 (199) | 148 | 1.4 (2) | Ref. group | ||
| 4.0–<5.0 | 36 (291) | 223 | 1.3 (3) | 1.0 | 0.2–6.1 | 0.996 |
| 5.0–<6.0 | 23 (189) | 152 | 0.7 (1) | 0.5 | 0.0–5.4 | 0.554 |
| ⩾6.0 | 16 (133) | 104 | 2.9 (3) | 2.2 | 0.4–13.4 | 0.401 |
| Not recorded | (1265) | 783 | 2.4 (19) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
Values in bold are likelihood ratio test overall P values for the variable; values not in bold are Wald P values for the particular category relative to the reference group (Ref. group)
Some adjoining categories were pooled for analyses as indicated by odds ratio (OR) positions
CI = confidence interval; DNSH = domestic not-shorthair; domestic mediumhair and domestic longhair; DSH = domestic shorthair; PNSH = purebred not-shorthair; PNSHX = purebred not-shorthair cross; PSH = purebred shorthair; PSHX = purebred shorthair cross
Numbers of ticks, locations and use of coat clipping
Total numbers of adult ticks and nymphs could be calculated for 1929 cases. The majority of cases (1628 [84%] had only one adult tick detected and only 53 (3%) had one or more nymphs detected. Total numbers ranged from 0–47 (adults) and 0–21 (nymphs). Ten cases had only nymphs detected (range 4–12). The median numbers of adult ticks on short- and longhair cats were both 1. Using univariable analysis, odds of mortality did not differ significantly between cases with ⩾2 adult ticks and those with 0 or 1 (P = 0.608) nor between cases with ⩾1 nymph and those with none (P = 0.410; Table 3).
Table 3.
Univariable associations between clinical presentation and mortality by day 5 after presentation to the Animal Emergency Service for cats with tick paralysis
| Variable and categories* | All cases |
Analysis of death by day 5 |
||||
|---|---|---|---|---|---|---|
| % (n) | No. of cases receiving recommended treatment | % (n) died by day 5 | OR | 95% CI | P value † | |
| Initial gait score | <0.001 | |||||
| 1 | 40 (840) | 618 | 0.3 (2) | Ref. group | ||
| 2 | 27 (562) | 423 | 1.4 (6) | 4.4 | 0.9–22.1 | 0.069 |
| 3 | 26 (546) | 329 | 4.0 (13) | }17.7 | 4.1–76.0 | <0.001 |
| 4 | 6 (128) | 40 | 17.5 (7) | |||
| Not recorded | (1) | |||||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Initial respiratory score | <0.001 | |||||
| A | 22 (463) | 333 | 0.3 (1) | Ref. group | ||
| B | 56 (1165) | 839 | 1.5 (13) | 5.2 | 0.7–40.1 | 0.112 |
| C | 18 (375) | 223 | 5.4 (12) | }20.8 | 2.7–159.0 | 0.004 |
| D | 4 (73) | 15 | 13.3 (2) | |||
| Not recorded | (1) | |||||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Body temperature at presentation (°C) | 0.001 | |||||
| <35 | 3 (51) | 24 | 20.8 (5) | 23.6 | 6.3–88.6 | <0.001 |
| 35–<36 | 6 (99) | 57 | 5.3 (3) | 5.0 | 1.2–21.5 | 0.031 |
| 36–<37 | 14 (236) | 164 | 4.9 (8) | 4.6 | 1.5–14.3 | 0.008 |
| 37–<38 | 38 (646) | 454 | 1.1 (5) | Ref. group | ||
| 38–<39 | 37 (628) | 460 | 0.2 (1) | }0.2 | 0.0–1.6 | 0.120 |
| ⩾39 | 3 (46) | 36 | 0.0 (0) | |||
| Not recorded | (371) | 215 | 2.8 (6) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Total no. of adult ticks | 0.608 | |||||
| 0 | 1 (10) | 9 | 0.0 (0) | }Ref. group | ||
| 1 | 84 (1628) | 1091 | 2.0 (22) | |||
| 2 | 10 (191) | 130 | 0.8 (1) | }0.7 | 0.2–2.5 | 0.608 |
| 3 | 2 (45) | 38 | 2.6 (1) | |||
| ⩾4 | 3 (55) | 37 | 2.7 (1) | |||
| Not recorded | (148) | 105 | 2.9 (3) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Total no. of nymphs | 0.410 | |||||
| 0 | 97 (1876) | 1263 | 2.0 (25) | Ref. group | ||
| ⩾1 | 3 (53) | 42 | 0.0 (0) | 0.8 | 0.0–3.9 | 0.438 |
| Not recorded | (148) | 105 | 2.9 (3) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
Some adjoining categories were pooled for analyses as indicated by odds ratio (OR) positions
Values in bold are likelihood ratio test overall P values for the variable; values not in bold are Wald P values for the particular category relative to the reference group (Ref. group)
CI = confidence interval
Of the 872 cases that were clipped, numbers for additional adult ticks and nymphs were available for 865 cases. Of these, adult ticks were detected during coat clipping for 55 (6%) and the number of additional adult ticks detected varied from 1–6. Nymphs were detected during coat clipping for 31 cases (4%), and the number of additional nymphs detected varied from 1 to an estimated 200. A total of 80 cases (9%) had additional adult ticks and/or nymphs detected associated with clipping.
Locations of ticks found during coat clipping had not all been recorded, so locations were assessed primarily for numbers of adult ticks and nymphs detected up to and including initial presentation at AES (n = 1745). The most common locations of ticks (adult ticks and nymphs combined) were the neck (n = 844 [48%]), head (n = 441 [25%]) and shoulder (n = 300 [17%]). Percentages of cases with more than one tick were also highest at these locations (Figure 2). Collectively, 90% of ticks were found from the shoulder forward.
Figure 2.
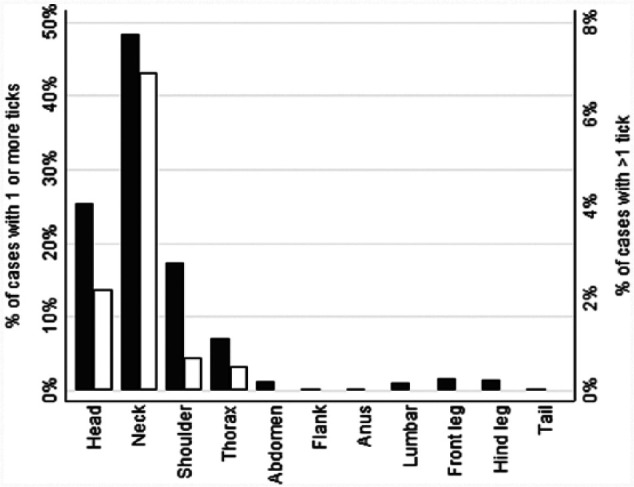
Locations of ticks found at initial presentation for cats with tick paralysis; black bars indicate percentages of the 1745 cases with ticks that had one or more ticks (adult and nymphs combined) at that location; white bars indicate percentages of cases that had more than one tick (adult and nymphs combined) at that location
Coat clipping was performed in 872/2011 cases (43%). Cats that were clipped had a 10/817 (1%) mortality incidence vs 18/535 cases (3%) where the cat was not clipped (univariable analysis, P = 0.010 [Table 4]; final multivariable model P = 0.020 [Table 5]).
Table 4.
Univariable associations between treatment variables and mortality by day 5 after presentation to the Animal Emergency Service (AES) for cats with tick paralysis
| Variable and categories | All cases |
Analysis of death by day 5 |
||||
|---|---|---|---|---|---|---|
| % (n) | No. of cases receiving recommended treatment | % (n) died by day 5 | OR | 95% CI | P value* | |
| TAS route † | 0.321 | |||||
| IP | 9 (148) | 21 | 4.8 (1) | Ref. group | ||
| IV | 91 (1544) | 1323 | 1.7 (23) | 0.4 | 0.0–2.7 | 0.321 |
| Not included‡ | (385) | 66 | 6.1 (4) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| TAS reaction † | 0.001 | |||||
| No | 77 (1574) | 1251 | 1.4 (18) | Ref. group | ||
| Yes | 8 (161) | 133 | 6.0 (8) | 4.4 | 1.9–10.3 | 0.001 |
| Not recorded § | (38) | 25 | 4.0 (1) | |||
| TAS not administered | 15 (304) | 1 | 100.0 (1) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Coat clip performed | 0.010 | |||||
| No | 57 (1139) | 535 | 3.4 (18) | Ref. group | ||
| Yes | 43 (872) | 817 | 1.2 (10) | 0.4 | 0.2–0.8 | 0.010 |
| Not recorded | (66) | 58 | 0.0 (0) | |||
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
| Mechanically ventilated | <0.001 | |||||
| No | 99 (2052) | 1388 | 1.7 (24) | Ref. group | ||
| Yes | 1 (25) | 22 | 18.2 (4) | 12.6 | 4.0–40.1 | <0.001 |
| Total | 100 (2077) | 1410 | 2.0 (28) | |||
Values in bold are likelihood ratio test overall P values for the variable; values not in bold are Wald P values for the particular category relative to the reference group (Ref. group)
For cases that received tick antitoxin serum (TAS) at either the referring clinic prior to presentation to the AES and/or at AES
>1 dose of TAS at either referring clinic prior to presentation to the AES and/or at AES, or route not known, or route was other than intravenous (IV) or intraperitoneal (IP)
TAS was administered but the occurrence of a TAS reaction or not was not specified
OR = odds ratio; CI = confidence interval
Table 5.
Adjusted odds ratios (ORs) for mortality by day 5 after presentation at the Animal Emergency Service from 1127 cases of tick paralysis in cats that were treated with tick antitoxin serum (TAS)
| Variable | Adjusted OR* | 95% CI* | P value † |
|---|---|---|---|
| Coat clip | 0.020 | ||
| No | Ref. group | ||
| Yes | 0.3 | 0.1–0.9 | 0.030 |
| Initial gait score | 0.047 | ||
| 1 | Ref. group | ||
| 2 | 1.8 | 0.3–13.7 | 0.536 |
| 3 or 4 | 5.4 | 1.2–47.0 | 0.044 |
| Mechanically ventilated | 0.017 | ||
| No | Ref. group | ||
| Yes | 7.0 | 1.4–119.0 | 0.021 |
| TAS reaction | 0.002 | ||
| No | Ref. group | ||
| Yes | 5.3 | 1.7–90.8 | 0.003 |
| Body temperature at presentation (°C) | <0.001 | ||
| <35 | 10.4 | 2.2–155.5 | 0.004 |
| 35–<36 | 1.3 | 0.1–7.0 | 0.797 |
| 36–<37 | 2.4 | 0.7–10.6 | 0.178 |
| 37–<38 | Ref. group | ||
| ⩾38 | 0.2 | 0.0–1.1 | 0.115 |
ORs and 95% confidence intervals (CIs) from profile likelihood models adjusted for the other four variables
Values in bold are the usual likelihood ratio test overall P values for the variable; values not in bold are P values for the particular category relative to the reference group (Ref. group) from the profile likelihood models
Gait and respiratory scores
Initial gait and respiratory scores were both significantly associated with mortality (P <0.001) on univariable analyses (Table 3). From the final multivariable model, cats with advanced gait scores (3 or 4) had an increased risk of mortality (odds ratio [OR] 5.4; 95% confidence interval [CI] 1.2–47.0 [P = 0.044]; Table 5). Within gait scores 2 and 3, mortality was only slightly higher for cases with respiratory score C rather than A or B. Among cases with gait scores of 4, there was no evidence that the respiratory scores gave further predictive information about mortality, as cases with respiratory scores of B, C and D all had a similar risk of mortality (17–20%) (Table 6). In a bivariable model with gait and respiratory scores fitted simultaneously, the overall P values for the associations between gait and respiratory scores, and mortality (adjusted for each other) were <0.001 and 0.064, respectively. When fitted with all five variables included in the final multivariable model, the overall P value for respiratory score was 0.686. The median tick number was the same (1) for each category of gait and respiratory scores.
Table 6.
Incidences of mortality by day 5 after presentation to the Animal Emergency Service by initial gait and respiratory scores
| Initial respiratory score | Initial gait score |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total | |
| A | 0 (1/256) | 0 (0/58) | 0 (0/19) | 0 (1/333) | |
| B | 0 (1/331) | 1 (4/305) | 3 (6/193) | 20 (2/10) | 2 (13/839) |
| C | 0 (0/30) | 3 (2/60) | 6 (7/115) | 17 (3/18) | 5 (12/223) |
| D | 0 (0/1) | 0 (0/2) | 17 (2/12) | 13 (2/15) | |
| Total | 0 (2/618) | 1 (6/423) | 4 (13/329) | 18 (7/40) | 2 (28/1410) |
Data are % (n)
Rectal body temperature
Rectal body temperatures at presentation ranged from 30.9–40.9°C (median 37.7°C). There were 51 cases with body temperatures <35°C on presentation; the majority (43/51 [84%]) of these had gait scores of 3 or 4. From univariable and multivariable analyses, cases with body temperatures <35°C on presentation were significantly more likely to die than those that were 37ºC to <38°C (P <0.001 [Table 3] and P = 0.004 [Table 5], respectively). The case with the lowest rectal body temperature of 30.9°C survived, requiring mechanical ventilation and intensive care.
Treatments, complications and durations of hospitalisation
TAS administration
There were 1742 cases where mortality status at 5 days was known. These consisted of: (1) 52 that did not receive TAS, of which four (8%) died by day 5 (two had mild clinical signs and were admitted for monitoring only, and for 50 the owner declined all treatment and the cat was taken home against advice); and (2) 1655 that received TAS, of which 49 (3%) died by day 5. TAS was given at the referring clinic prior to presentation to the AES in 98 cases (6%), at the AES in 1537 cases (93%) or at both in 18 cases (1%). A further 35 cases did not meet the criteria for either subset as the owner declined treatment at the AES and the cat received TAS at the referring clinic after discharge from the AES.
The effects of receiving TAS were estimated by comparing the mortality risk between the 52 cases that did not receive TAS with the 1410 cases that received TAS (after excluding cases discharged home early against veterinary advice [n = 228], cases recommended to receive mechanical ventilation but did not receive it [n = 16] and one case where both applied). The risk of death was markedly higher in those that did not receive TAS (OR for death 4.1 [P = 0.011]; Table 6). Generally, cases not treated with TAS had lower initial gait and respiratory scores, and were less likely to have low body temperatures at presentation. Thus, after adjusting separately for each of these three confounders, the estimated effect of not receiving TAS was larger (OR 8.2–13.3; Table 7).
Table 7.
Associations between receiving tick antitoxin serum (TAS) and mortality by day 5 after presentation to the Animal Emergency Service in cats with tick paralysis unadjusted and adjusted for confounding by initial gait score, initial respiratory score and rectal temperature
| Received TAS | No. of cases | % (n) died by day 5 | OR adjusted for | OR | 95% CI | P value* |
|---|---|---|---|---|---|---|
| Yes | 1410 | 2.0 (28) | Ref. group | |||
| No | 52 | 7.7 (4) | No adjustments | 4.1 | 1.4–12.2 | 0.011 |
| Initial gait score | 8.2 | 2.4–27.6 | 0.001 | |||
| Initial respiratory score | 10.6 | 3.2–34.9 | <0.001 | |||
| Body temperature at presentation | 13.3 | 3.6–49.7 | <0.001 |
Wald P values for ‘No’ relative to the reference group (‘Yes’); all likelihood ratio test P values were <0.001
OR = odds ratio; CI = confidence interval; Ref. group = reference group
Mechanical ventilation
Mechanical ventilation was recommended for 131/2077 cases (6%) and specifically for 39 of the 1655 cases (2%) that received TAS and for which mortality status was known. Of these 39 cases: (a) mechanical ventilation was recommended but not implemented for 17 cases (owing to owners declining) – of these cases, 15 (88%) died by day 5; (b) mechanical ventilation was recommended and implemented in 22 cases – four (18%) died (OR relative to group [a] 0.03; 95% CI 0.00–0.18; P <0.001); and (c) mechanical ventilation was neither recommended nor implemented in 1616 instances and 30/1616 (2%) died (OR relative to group [a] 0.00; 95% CI 0.00–0.01; P <0.001).
Intubation, oxygen supplementation and temporary tracheostomy placement
Of the cases that were admitted for hospitalisation to the AES (1771/2077 [85%]), data on the use of prolonged oxygen supplementation was available for 1707 cases – prolonged oxygen therapy was administered to 194 (11%). Endotracheal intubation without mechanical ventilation was performed for 30/1771 cases (2%); duration ranged from 2–40 h. Laryngeal paralysis was clearly documented in 24 of these cases. A further seven cases were initially intubated but developed severe hypoventilation and required mechanical ventilation. Mortality status was known for six cases; of which four survived, one was euthanased owing to cost and one had mechanical ventilation discontinued owing to cost and died at the referring clinic. Mechanical ventilation was recommended after endotracheal intubation but not implemented for three cases (all three died by day 5). Temporary tracheostomies were placed for two cases and both survived.
Laryngeal paralysis (with and without requiring endotracheal intubation) was documented in a total of 64/1771 cases (4%). Of these 64, 60 had received TAS and their TAS reaction status was known; 19/60 (32%) had a TAS reaction.
Complications
TAS reactions occurred in 161/1735 cases (9%) treated with TAS at either the referring clinic prior to presentation to the AES, at the AES or both. Of those cases that had a TAS reaction, 8/133 (6%) died by day 5 vs 18/1251 (1%) that did not have a TAS reaction (cases that received recommended treatment and mortality status was known; univariable analysis, P <0.001 [Table 4]; multivariable analysis, P = 0.002 [Table 5]). Spontaneous cardiopulmonary arrest was documented in 55 cases, seven of which had not received TAS. Of the remaining 48 cases, TAS reaction status was known for 47. TAS reactions occurred in 13/47 (28%). Of these, 11/13 (85%) suffered cardiopulmonary arrest during TAS administration or within 1 h of finishing TAS. Two cases survived to day 5. Respiratory arrest (without cardiac arrest) occurred in 11 cases, all of which had received TAS. TAS reaction status was known for eight cases; 3/8 cases (38%) had respiratory arrest during TAS administration or within 1 h of finishing TAS.
Thoracic radiographs were taken for 18 cases; 16 of which had an alveolar lung pattern. All cases had received TAS; 13 had TAS reaction status known, and a TAS reaction occurred in 9/13 cases (69%).
Durations of hospitalisation and discharge status
Of the 2077 cases, 1771 were admitted to hospital; their median duration of hospitalisation at the AES was 12 h (IQR 8–24; range 1–360). Of the 542 cases that recovered and were discharged, 541 had been hospitalised, with a median time of 24 h (IQR 18–40). Of 342 cases discharged against veterinary advice, 266 were hospitalised for a median of 4 h (IQR 1–6). Of 950 cases that were discharged to the referring clinic, 881 were hospitalised (median 12 h; IQR 10–14). Of the 242 cases that died or were euthanased at the AES, 82 were hospitalised for a median of 8 h (IQR 4–12).
Considering cases that received TAS, the risk of dying varied markedly according to discharge status; of those discharged against veterinary advice, 7/229 (3%) died vs 10/853 (1%) discharged back to referring clinics for ongoing care (OR 2.7; 95% CI 0.9–7.1; P = 0.063).
Multivariable model of risk factors for mortality
Seven variables were selected for multivariable modelling, five of which were retained in the final model (Table 5): the case’s initial gait score, rectal body temperature at presentation at the AES, whether or not the case experienced a reaction to TAS, was coat clipped and received mechanical ventilation. With all seven variables fitted simultaneously, respiratory score had the highest P value and did not contribute significantly to the model (P = 0.546) and so was removed. With the remaining six variables fitted simultaneously, route of administration of TAS (IV or IP) also did not contribute significantly (P = 0.437) and was therefore removed. Each of the remaining five variables was significantly associated with mortality (Table 5). Cases with gait scores of 3 or 4 at, or shortly after, presentation, and those with body temperatures <35°C at presentation were more likely to die, as were those that experienced an adverse reaction to TAS and those that required mechanical ventilation. Coat clipping was associated with a reduced risk of death. Estimated ORs for these five effects were large but imprecise. The final model used 1127/1410 eligible cases (80%); the remaining cases could not be included owing to missing the status of coat clip (n = 58), reaction to TAS (n = 26) and/or body temperature (n = 215). Of these 1127 cases, 1.9% (n = 21) died by day 5 vs 2% (n = 28) of all 1410 cases.
These five risk factors were collectively highly discriminatory between cases that died and those that survived. The area under the receiver operating characteristics curve was 0.97 (95% CI 0.94–0.99), indicating outstanding discrimination. 17 Using the predicted probability that the case died of 0.0043 as the cut-off point, 86% and 87% of cases that died and survived, respectively, were correctly predicted by the model as dying and surviving. The model had moderately good fit, with no deaths in the lowest six predicted probability groups where the expected number of deaths was also zero. However, in higher predicted probability groups, the numbers of deaths exceeded that expected, indicating that among cases with higher probabilities, some were exposed to additional determinants of death over and above the five risk factors included in the model, and/or that some of the five risk factors interacted synergistically in predisposing to death.
Discussion
The prognosis for feline tick paralysis cases is very good with recommended treatment, but subsets of affected cats are at markedly increased risk of death. Analyses identified eight risk factors for mortality. TAS reduces the risk of mortality, but the occurrence of a TAS reaction increases mortality risk. Cats for which mechanical ventilation is recommended (but not implemented) are at an increased risk of death, and there is some evidence that cats discharged home early against veterinary advice are at an increased risk of death relative to those discharged back to the referring veterinary clinic for ongoing care. Among cats treated as recommended (ie, treated with TAS, mechanically ventilated when indicated and not discharged home early against veterinary advice), those with profound weakness (gait scores of 3 or 4) and with rectal temperatures <35°C are more likely to die, as are those cats that experience an adverse reaction to TAS or require mechanical ventilation. Cases that were coat clipped were associated with a reduced risk of death. These last five risk factors (Table 5) were collectively highly discriminatory between cases that died and those that survived, suggesting that they collectively account for most of the pathophysiological mechanisms that lead to death in cats with tick paralysis.
Mortality
The 5 day mortality risk for cases that received recommended treatment in this cohort was 2%. If cases that were discharged home against veterinary advice or were recommended mechanical ventilation but did not receive it were included, the mortality risk was 3%. These are higher than <1% (n = 1/158), which was previously reported. 15 In the present study, the study population consisted of a combination of referred cases requiring 24 h care, as well as out-of-hours primary case accessions. Collectively, these cases may have been more severely affected. Reported mortality incidences for dogs presenting to general practice with tick paralysis are 5% and 6.9%.18,19 These may be higher than in cats because of the reported complications (pulmonary oedema and bronchopneumonia) in dogs. 10 Mortality risk for affected dogs presenting to an emergency 24 h facility is yet to be determined.
When calculating the mortality risks of 2% and 3% above, we excluded cases where follow-up information could not be obtained, where the case ended in a cost-based euthanasia, or where the cat was euthanased after being discharged back to the referring practice. Many of these excluded cases had low gait and respiratory scores and body temperatures at presentation >35°C, and hence had good prognoses. Even with quite extreme assumptions about the risk of mortality in these excluded cases, had those euthanasias not been performed, the mortality risk for all cases would still have been quite low.
TAS administration and reactions
TAS administration in this cohort of cases improved outcome and reduced the risk of death, even though those cases that had a TAS reaction were at higher risk of mortality.
Adverse reactions to TAS resulting in cardiopulmonary arrest were documented. Thus, administration of TAS should be considered with a risk vs benefit approach. For example, risks may exceed benefits in cats with mild, non-progressive clinical signs, particularly for those at high risk of experiencing a TAS reaction (eg, cats with previous sensitisation to TAS).15,20,21
Initial gait and respiratory scores
Advanced gait and respiratory scores were predictive of mortality in univariable analyses. When respiratory score was correlated with gait score or with all five variables included in the final multivariable model, it did not significantly improve prediction of mortality. Risk of mortality among cats with advanced gait scores of 4 (profound weakness) were similar regardless of the initial respiratory score. This is a very different situation from canine tick paralysis where a respiratory visual analogue scale is more predictive of mortality than the gait score. 19 Dogs with tick paralysis frequently develop aspiration pneumonia or pulmonary oedema, 10 which leads to an altered breathing pattern and dyspnoea, even in patients with low gait scores. In cats with high gait scores (3 or 4), close monitoring of respiratory function and recognition of hypoventilation is important so that positive pressure ventilation can be implemented if respiratory failure occurs.
Rectal body temperature at presentation
Hypothermia on presentation was a risk factor for mortality. Of the cats that were hypothermic, 84% had a gait score of 3 or 4 and were profoundly weak. This may be explained by the following mechanisms: (1) cats with more advanced paralysis lose the ability to thermoregulate because they are unable to shiver and muscle movement to generate heat is not possible;22,23 and (2) exposure to low ambient temperatures or cold weather may contribute to hypothermia prior to presentation as cats are unable to move to warmer areas. The case with the lowest rectal body temperature of 30.9°C survived, requiring mechanical ventilation and intensive care, emphasising that, despite a guarded prognosis, cats with tick paralysis and severe hypothermia can survive.
Coat clipping and tick numbers
Cats that had full body clipping had a reduced risk of death, probably as additional ticks were found. The total number of ticks found at presentation was not correlated with mortality. Historically, the risks vs benefits of clipping have been debated. In this cohort of cats, benefits outweighed risks; it is hypothesised that this was owing to missed tick(s) and prolonged attachment increasing mortality among those cases not clipped. This result should be interpreted with caution as confounding factors may have contributed to this association. Cats that had their coats clipped also received additional treatment, including either heavy sedation or light anaesthesia and endotracheal intubation for the duration of the clip. Prospective studies are needed to assess the effects of various approaches for detecting or killing all ticks. The frequency of use of acaricides or both preventative treatment and treatment within hospital for study cases had not been recorded consistently; it was therefore not possible to describe these.
Mechanical ventilation
Where mechanical ventilation was recommended but not implemented, cats were much more likely to die. In addition, generally, cases that required mechanical ventilation had a higher mortality. The proportion of cases where mechanical ventilation was recommended was higher than previously reported anecdotally.15,24 The current study most likely included more severely affected cases. In addition, these previous studies were based on knowledge from >10 years ago, and the ability to successfully ventilate domestic pets has improved. Equipment, facilities and trained staff are more readily available for treatment of severe cases. Close monitoring of respiratory function, recognition of hypoventilation and early intervention have been shown to improve outcomes in cases with respiratory failure.25,26
Multivariable model
The five risk factors in the multivariable model were collectively highly discriminatory between cases that died and those that survived (Table 5), and the model had a moderately good fit. In addition, it is possible that some of the five risk factors in that model interact synergistically in causing death. For example, the adverse effects of hypothermia may be exacerbated if the cat has an adverse reaction to TAS administration or requires mechanical ventilation. In addition, the combination of high gait score and hypothermia may be worse than expected based on each considered separately. We had too few deaths to meaningfully assess these hypothesised interactions; further larger prospective studies would be required for this purpose.
Study limitations
The current study was retrospective, precluding prospective data collection and, instead, limiting our data to that recorded in the course of veterinary practice. Cases were selected from multiple clinics from different geographical areas, and had been treated by multiple veterinarians, which increased the external validity of the study results. The consistency and detail of histories were variable. The computer system, however, was the same across all four clinics and had not changed over the 9 year study period. Medical records were overall of a high quality, and a computerised tick template at all four clinics had ensured more thorough data recording. Individual assessment and treatment decisions varied between veterinarians at both AES and referring clinics, including suitability for time of discharge. In addition, throughout the 9 year study period, improved knowledge regarding tick paralysis and treatment influenced treatment and clinic protocols. TAS batches and brands would have varied greatly over the study period but had not been recorded so we could not compare any effects by batch and brand. Case follow-up was difficult for some of the earlier cases due to disconnected phone numbers and reduced access to referring clinic histories. Selection factors included increased severity of cases requiring transfer for 24 h care. However, this gave a true representation of the severity of cases seen by after-hours emergency hospitals. Future studies could utilise presentation disease severity scores to standardise for this bias.
Conclusions
Cats affected by tick paralysis caused by I holocyclus have a low mortality risk with recommended treatment. Risk factors for mortality are high gait scores or severe respiratory signs and hypothermia. As these can be identified on presentation, they can inform prognosis and guide treatment and supportive care. TAS administration reduces overall mortality risk; however, those cases that have a reaction to TAS have a higher mortality risk. Thus, the risks vs benefits of TAS administration should be considered for each case. TAS may be contraindicated in cases with mild, non-progressive clinical signs, particularly those at high risk of experiencing a TAS reaction (eg, cats with previous sensitisation to TAS). Coat clipping is associated with a reduced mortality risk. Respiratory failure may occur, as well as other severe complications. However, with close monitoring and early intervention with mechanical ventilation, it is possible to markedly reduce the risk of death. Further prospective clinical trials are needed to evaluate all aspects of treatment in cats with tick paralysis and to further understand the pathophysiology and mechanism of TAS reactions.
Acknowledgments
We thank all staff at the Animal Emergency Service, Veterinary Specialist Services, referring veterinary clinics and clients who provided support and information for case follow-up. In addition, we would like to thank Dr Steven Epstein for his help with the study design and structure of the paper and Dr Anne Fawcett for her continued support and guidance through all stages of scientific writing.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 30 August 2017
References
- 1. Clunies-Ross I. Tick paralysis: a fatal disease of dogs and other animals in eastern Australia. J Counc Sci Ind Res 1935; 8: 8–13. [Google Scholar]
- 2. Stone BF, Aylward JH. Tick toxicosis and the causal toxins: tick paralysis. In: Gopalakhrishnakone P, Tan C. (eds). Progress in venom and toxin research. Singapore: National University of Singapore Press, 1987, pp 594–682. [Google Scholar]
- 3. Padula A. Tick paralysis of animals in Australia. In: Gopalakrishnakone P, Faiz S, Gnanathasan C. (eds). Clinical toxinology: clinical toxinology. Dordrecht: Springer Netherlands, 2016, pp 1–20. [Google Scholar]
- 4. Schull DN, Litster AL, Atwell RB. Tick toxicity in cats caused by Ixodes species in Australia: a review of published literature. J Feline Med Surg 2007; 9: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atwell RB. Laryngeal paresis caused by I holocyclus. Aust Vet Pract 2002; 32: 44. [Google Scholar]
- 6. Cooper B, Cooper H, Ilkiw, et al. Tick paralysis. Proceedings No. 30 Refresher Course in Neurology; August 9–13 1976; Sydney, Australia. Sydney: The Post Graudate Committee in Veterinary Science, The University of Sydney, 1976, pp 57–61. [Google Scholar]
- 7. Strakosh M. Tick paralysis in cats. In: Control and therapy. Sydney: University of Sydney, CVE, 1988, p 1218. [Google Scholar]
- 8. Campbell FE. The cardiovascular effects of the toxin(s) of the Australian paralysis tick, Ixodes holocyclus. MS thesis, University of Queensland, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Campbell FE, Atwell RB, Fenning A, et al. Cardiovascular effects of the toxin(s) of the Australian paralysis tick, Ixodes holocyclus, in the rat. Toxicon 2004; 43: 743–750. [DOI] [PubMed] [Google Scholar]
- 10. Webster RA, Mackie JT, Haskins SC. Histopathological changes in the lungs from dogs with tick paralysis: 25 cases (2010–2012). Aust Vet J 2013; 91: 306–311. [DOI] [PubMed] [Google Scholar]
- 11. Webster RA, Briscoe K, Campbell FE, et al. Round table discussion – part 2. In: Control and therapy. Perspective No. 93. Sydney: University of Sydney, CVE, 2012, pp 31–48. [Google Scholar]
- 12. Ilkiw JE, Turner DM, Howlett CR. Infestation in the dog by the paralysis tick Ixodes holocyclus. 1. Clinical and histological findings. Aust Vet J 1987; 64: 137–139. [DOI] [PubMed] [Google Scholar]
- 13. Westwood MN, Emery DL, Dhand NK. Clinical presentation and treatment of tick paralysis in dogs and cats in Sydney (2001–2010). Aust Vet J 2013; 91: 491–498. [DOI] [PubMed] [Google Scholar]
- 14. Eppleston KR, Kelman M, Ward MP. Distribution, seasonality and risk factors for tick paralysis in Australian dogs and cats. Vet Parasitol 2013; 196: 460–468. [DOI] [PubMed] [Google Scholar]
- 15. Musca F, Gunew M. Tick paralysis in cats – a retrospective review. In: Control and therapy. Perspective No. 40. Sydney: University of Sydney, CVE, 2004, pp 1–7. [Google Scholar]
- 16. Atwell RB, Campbell FE. Reactions to tick antitoxin serum and the role of atropine in treatment of dogs and cats with tick paralysis caused by Ixodes holocyclus: a pilot survey. Aust Vet J 2001; 79: 394–397. [DOI] [PubMed] [Google Scholar]
- 17. Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. New York: John Wiley and Sons, 2000. [Google Scholar]
- 18. Atwell RB, Campbell FE, Evans EA. Prospective survey of tick paralysis in dogs. Aust Vet J 2001; 79: 412–418. [DOI] [PubMed] [Google Scholar]
- 19. Atwell RB, Hannan P, Conacher C, et al. The clinical outcomes in a large cohort of natural general practice cases of canine tick (Ixodes holocyclus) toxicity from different geographic areas in the same season. Publically Listed 2008. [Google Scholar]
- 20. Bovens C, Gruffydd-Jones T. Xenotransfusion with canine blood in the feline species: review of the literature. J Feline Med Surg 2013; 15: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. FitzGerald M. Ixodes holocyclus poisoning. Proceedings of University of Sydney Post Graduate Foundation in Veterinary Science 318; Clinical Toxicology; November 11–14 1998; Sydney, Australia. Sydney: Post Graduate Foundation in Veterinary Science, The University of Sydney, 1998, pp 203–220. [Google Scholar]
- 22. Fearnley AF. The effect of ingestation with the Australian paralysis tick, Ixodes holocyclus, on thermoregulation and respiration in the dog. MS Thesis, University of Queensland, 2007. [Google Scholar]
- 23. Brodeur A, Wright A, Cortes Y. Hypothermia and targeted temperature management in cats and dogs. J Vet Emerg Crit Care 2017; 27: 151–163. [DOI] [PubMed] [Google Scholar]
- 24. Malik R. (ed). Tick paralysis in the cat. Proceedings of University of Sydney Post Graduate Foundation in Veterinary Science 318; Clinical Toxicology; November 11–14 1998; Sydney, Australia. Sydney: Post Graduate Foundation in Veterinary Science, The University of Sydney, 1998, pp 147–148. [Google Scholar]
- 25. Webster RA, Mills PC, Morton JM. Indications, durations and outcomes of mechanical ventilation in dogs and cats with tick paralysis caused by Ixodes holocyclus: 61 cases (2008–2011). Aust Vet J 2013; 91: 233–239. [DOI] [PubMed] [Google Scholar]
- 26. Trigg NL, Leister EM. Outcomes of mechanical ventilation in 302 dogs and cats in Australia (2005–2013). Aust Vet Pract 2014; 44: 698–703. [Google Scholar]


