Abstract
Objectives
The objective of this study was to evaluate the cardiovascular effects of low-dose atipamezole administered intravenously to isoflurane-anesthetized cats receiving dexmedetomidine. We hypothesized that atipamezole would increase heart rate (HR) and reduce arterial blood pressure in isoflurane-anesthetized cats receiving dexmedetomidine.
Methods
Six healthy adult domestic shorthair cats were anesthetized with isoflurane and instrumented for direct arterial pressures and cardiac output (CO) measurements. The cats received a target-controlled infusion of dexmedetomidine (target plasma concentration 10 ng/ml) for 30 mins before administration of atipamezole. Two sequential doses of atipamezole (15 and 30 μg/kg IV) were administered at least 20 mins apart, during dexmedetomidine administration. The effects of dexmedetomidine and each dose of atipamezole on HR, mean arterial blood pressure (MAP), CO and systemic vascular resistance (SVR) were documented.
Results
Dexmedetomidine reduced the HR by 22%, increased MAP by 78% (both P ⩽0.01), decreased CO by 48% and increased SVR by 58% (both P ⩽0.0003). Administration of atipamezole 15 and 30 μg/kg intravenously increased HR by 8% (P = 0.006) and 4% (P = 0.1), respectively. MAP decreased by 39% and 47%, respectively (both P ⩽0.004). Atipamezole 30 μg/kg returned CO and SVR to baseline values.
Conclusions and relevance
Low doses of atipamezole (15 and 30 μg/kg) administered intravenously to anesthetized cats decreased arterial blood pressure with only marginal increases in HR. Atipamezole 30 μg/kg restored CO and SVR to baseline values before dexmedetomidine administration.
Introduction
The alpha (α)2-agonist dexmedetomidine is a widely used sedative, often administered to cats prior to general anesthesia. Dexmedetomidine is approved for use as a preanesthetic agent in the USA, European Union, Australia and New Zealand, and has gained wide acceptance for this purpose owing to its sedative, analgesic and anesthetic-sparing effects.1,2 Dexmedetomidine, however, also produces substantial bradycardia (and other bradyarrhythmias), increased systemic vascular resistance (SVR) and a reduced cardiac output (CO) in cats. 3
Dexmedetomidine-induced bradycardia may persist throughout the perianesthetic period, and owing to its high incidence and severity, its prevention or treatment with anticholinergic agents such as atropine or glycopyrrolate, was proposed initially. Co-administration of anticholinergics and α2-agonists, however, exacerbates arterial hypertension and results in the appearance of ventricular tachycardia and pulsus alternans.4–6 Moreover, no evidence of improvement in cardiac function was demonstrated when glycopyrrolate was used to treat xylazine-induced bradycardia in cats or dogs.7,8 For these reasons, treatment with anticholinergic agents might be detrimental in hypertensive animals, and is currently discouraged by several authors.8,9
An alternate approach for treating dexmedetomidine-induced bradycardia in cats under general anesthesia, is to administer low doses of the competitive α2-adrenoceptor antagonist, atipamezole. In non-anesthetized cats receiving dexmedetomidine, atipamezole readily reverses sedation and restores the heart rate (HR). 1 Administration of atipamezole, however, may also reduce arterial blood pressure via α2-adrenoceptor blockade. 10 This effect might be augmented when atipamezole is administered concurrently with inhalant anesthetics, owing to their additional vasodilatory effects.
In this study, we evaluated the cardiovascular effects of low-dose atipamezole administered to isoflurane-anesthetized cats receiving dexmedetomidine. We hypothesized that intravenous atipamezole would increase HR and reduce arterial blood pressure in isoflurane-anesthetized cats receiving dexmedetomidine.
Materials and methods
This work was approved by the local Institutional Animal Care and Use Committee. Six healthy adult domestic shorthair cats (two intact females, two spayed females and two neutered males) (Liberty Research), aged between 1 and 2 years and weighing 2.6–4.7 kg were enrolled. Cats were deprived of solid food overnight prior to anesthesia. Each cat was anesthetized with isoflurane (5%) in an induction chamber. After loss of consciousness, the larynx was desensitized with lidocaine (2%, 0.1 ml sprayed) and a cuffed orotracheal tube (4–4.5 mm ID) was placed. General anesthesia was maintained with isoflurane (end-tidal 1.4–1.5%) in oxygen (2 l/min). The lungs were mechanically ventilated to maintain the end-tidal CO2 tension between 35 and 50 mmHg (Ohmeda Modulus SE; GE Healthcare). A catheter was placed in a cephalic vein and atracurium (0.2 mg/kg initial dose, 0.1 mg/kg subsequent doses) was administered intravenously (IV) as needed, to prevent spontaneous ventilation or sudden movements during arterial catheterization and data collection. Cats were positioned in dorsal recumbency. Monitoring under general anesthesia included electrocardiography, pulse oximetry, capnography, direct arterial blood pressure, inspired and expired isoflurane concentration and esophageal temperature (Cardell Touch; Midmark), which was maintained between 36ºC and 38°C by the use of a forced warm air blanket. A 22 G catheter was placed percutaneously in either a caudal (tail) or femoral artery, and the blood pressure waveform was recorded continuously for subsequent analysis (LabChart Pro, v8; ADInstruments).
The CO, SVR, total end-diastolic volume index (TEDVI), which represents the volume of blood contained in the heart at end-diastole (preload), and total ejection fraction (TEF), were calculated with the transpulmonary ultrasound dilution technique (COstatus; Transonic Systems). 11 Briefly; an 18 G, 1 ¾ inch catheter was placed percutaneously in a jugular vein and a 1 ml/kg bolus of normal saline (37°C) was injected into the jugular catheter. In one cat a catheter could not be placed in a jugular vein, and hence a femoral vein catheter (20 G, 3 1/16 inches) was used for CO measurements.
The injection of a bolus of normal saline produces a decrease in ultrasound velocity; this transient decrease in ultrasound velocity was measured in the systemic arterial circulation. For this purpose, blood was withdrawn from the arterial catheter at a rate of 6–12 ml/min, and the ultrasound velocity measured as blood circulated through an ultrasound sensor. The withdrawn blood was then returned at the venous catheter through a temporary arteriovenous extracorporeal loop; hence, there was no net blood loss. CO was calculated automatically using the Stewart-Hamilton method; likewise, the TEDVI was calculated from the cord of the indicator-dilution transient, and the TEF was calculated from the ratio between the stroke volume and the TEDV. 11
Data collection
After instrumentation was completed, a 30 min period was allowed for stabilization before data collection began. At the end of the stabilization period, baseline HR, systolic arterial pressure (SAP), mean arterial pressure (MAP), diastolic arterial pressure (DAP) and pulse pressure (SAP − DAP; PP) were recorded. For each variable the average of 10 consecutive beats was used.
Once baseline values were obtained, a target-controlled infusion (TCI) of dexmedetomidine (Dexdomitor; Zoetis) was started. The infusion of dexmedetomidine (Medfusion 3500; Smiths Medical ASD) was guided by computer software (CCIP V2.5; Department of Anesthesia and Intensive Care, The Chinese University of Hong Kong) for a target plasma concentration of 10 ng/ml. We utilized parameters previously published from a three-compartment model following administration of dexmedetomidine 20 μg/kg, where the y-intercepts for extrapolated lines for fast and slow distribution, and elimination portions of the curves were 67.2 ng/ml, 34.7 ng/ml and 9.3 ng/ml, respectively, and the slopes associated with fast and slow distributions and eliminations were 0.508/min, 0.056/min and 0.012/min, respectively. 12 This TCI was continued throughout all subsequent observations. A 30 min period during TCI was allowed before collecting more data. During dexmedetomidine administration, changes in systemic pressure were observed until a new, stable value was reached. We considered arterial pressure to be stable when MAP varied by <5 mmHg over a 5 min period. Using the averaging procedure described above, the HR and arterial pressures were recorded again to assess the effects of dexmedetomidine. Subsequently, two intravenous doses of atipamezole (Antisedan; Zoetis) (15 and 30 μg/kg, administered sequentially) were then evaluated during dexmedetomidine TCI. Each dose was diluted to 1 ml normal saline and injected manually over 10 s. The HR and arterial pressures were measured immediately prior to the administration of each dose, and at maximal effect, when MAP reached a nadir following atipamezole injection. The second dose of atipamezole was administered at least 20 mins after the first dose, and only when blood pressure was stable again (MAP varied by <5 mmHg during a 5 min period). Cardiac output and associated variables were measured at baseline (during isoflurane anesthesia), during dexmedetomidine TCI and when blood pressure was at its lowest after the second dose of atipamezole; that is, they were not measured between the two atipamezole doses. Figure 1 illustrates the timeline of the experiment.
Figure 1.
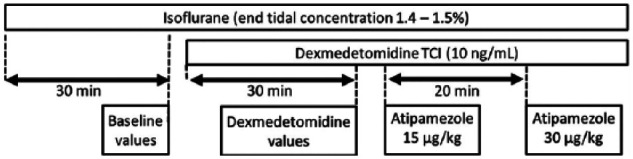
Timeline of events. Heart rate and blood pressures were measured for 10 consecutive beats and averaged at the end of the baseline period, after 30 mins of dexmedetomidine target-controlled infusion (TCI; when mean arterial pressure was stable for at least 5 mins), and at maximal effect after injection of atipamezole 15 and 30 µg/kg intravenously (IV). In addition, cardiac output and other associated variables were also measured at baseline, during dexmedetomidine TCI, and at maximal effect of atipamezole 30 µg/kg IV
Data collection ceased when the effects of the second dose of atipamezole had subsided. Cats received ondansetron 0.2 mg/kg and meloxicam 0.1 mg/kg IV. Atropine (0.02 mg/kg) and edrophonium (0.5 mg/kg) were administered IV to reverse neuromuscular block. Isoflurane administration was discontinued and atipamezole (equivalent to five-fold of the total dose of dexmedetomidine infused) was administered intramuscularly (IM) to hasten recovery from general anesthesia.
Statistical analysis
Normal distribution of the results was confirmed with the Shapiro–Wilk test. To assess the effects of dexmedetomidine and atipamezole on HR and blood pressure, HR and blood pressure values were separately compared for each intervention with paired t-tests: between baseline and after reaching a stable MAP after dexmedetomidine TCI, and between values recorded prior to each atipamezole dose and values obtained at the nadir MAP after atipamezole was administered. The onset time to peak effect, and the duration of effect for each dose of atipamezole on MAP are also reported and compared with paired t-tests. Onset time was defined as the interval (in seconds) between atipamezole injection and maximal decrease in blood pressure, and duration was defined as the interval (in mins) between injection of atipamezole and recovery of MAP (ie, when a plateau after the nadir in MAP was reached so that variation was <5 mmHg in 5 mins).
The CO and the hemodynamic variables derived from the ultrasound dilution method (at baseline, after reaching a stable MAP during dexmedetomidine TCI and at the MAP nadir after atipamezole 30 µg/kg) were compared with ANOVA for repeated measures and Tukey’s post-hoc tests. Differences were considered significant when P <0.05. Values are summarized as mean ± SD or as the percentage change from preceding values.
Results
All cats recovered without complications from general anesthesia. Dexmedetomidine infusion produced a 22% reduction in HR and a 78% increase in MAP (Figure 2). When 15 µg/kg atipamezole was administered, HR increased by 8% and MAP decreased by 39%. After 30 µg/kg atipamezole, the increase in HR was 4% and MAP decreased by 47% (Figure 2). In one cat, blood pressure decreased by ⩾50% after the second dose of atipamezole (SAP of 65 mmHg and MAP of 52 mmHg). Administration of 15 and 30 µg/kg atipamezole had onset times of 79 ± 23 and 70 ± 11 s, respectively. The decrease in blood pressure lasted for 7.3 ± 2 and 8.5 ± 1 min, respectively. Differences in neither the onset nor duration were statistically significant between the two doses of atipamezole (all P >0.2).
Figure 2.
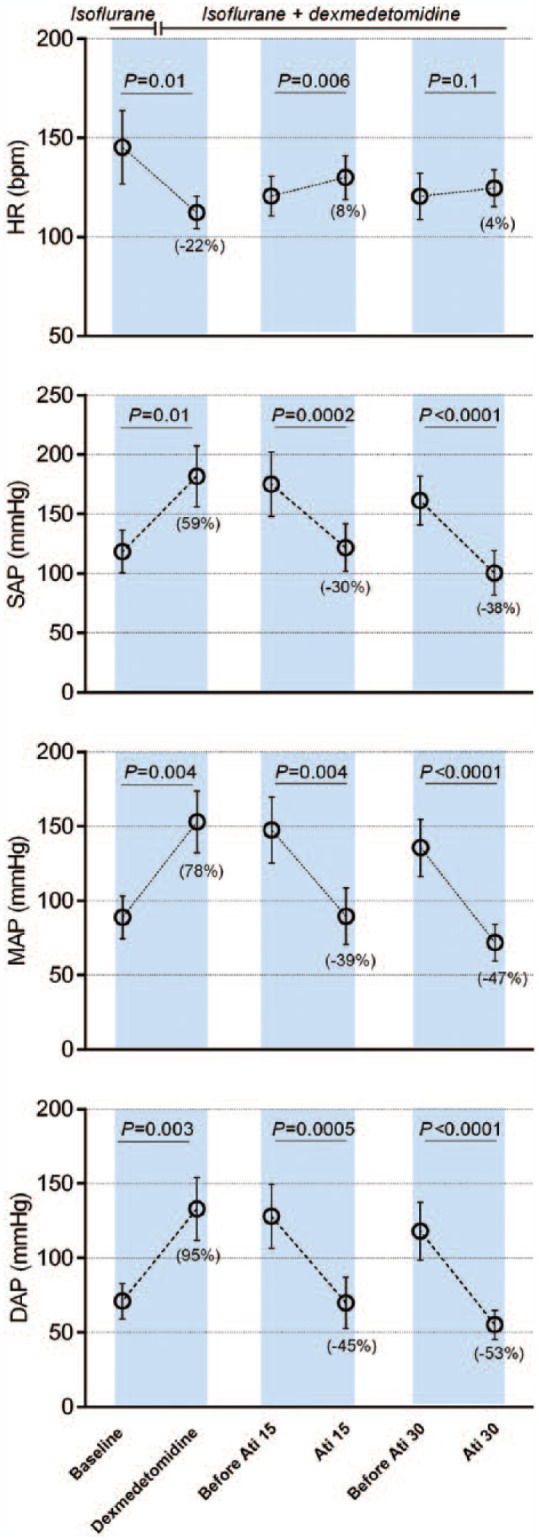
Mean ± SD heart rate (HR), systolic (SAP), mean (MAP) and diastolic (DAP) arterial blood pressures measured at baseline, during dexmedetomidine infusion, and before and after intravenous atipamezole (Ati) 15 and 30 µg/kg in isoflurane-anesthetized cats (n = 6). The percentage change for each value is indicated in brackets. bpm = beats per min
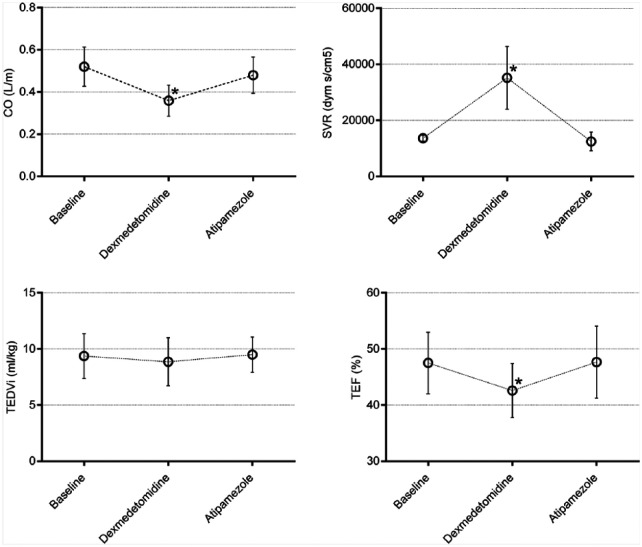
Dexmedetomidine infusion reduced the CO by 48% and TEF by 12%, whereas SVR increased by 58% (Figure 2). Atipamezole 30 µg/kg returned CO, SVR and TEF towards values prior to dexmedetomidine administration (Figure 3). Neither dexmedetomidine infusion nor co-administration of atipamezole with dexmedetomidine changed TEDVI (Figure 3).
Figure 3.
Mean ± SD cardiac output (CO), systemic vascular resistance (SVR), total end-diastolic volume index (TEDVI) and total ejection fraction (TEF), measured at baseline, during dexmedetomidine infusion, and after intravenous atipamezole 30 µg/kg in isoflurane-anesthetized cats (n = 6). *Significant difference
Significant changes in PP were not observed at the aforementioned observation times. However, inspection of individual systemic blood pressure traces revealed that a transient decrease in PP, coincident with the increase in MAP, was observed consistently soon after dexmedetomidine TCI was initiated (Figure 4). This decrease in PP resolved shortly thereafter (Figure 4). When atipamezole was administered, a transient increase in PP occurred consistently and coincident with a decrease in blood pressure (Figure 4).
Figure 4.
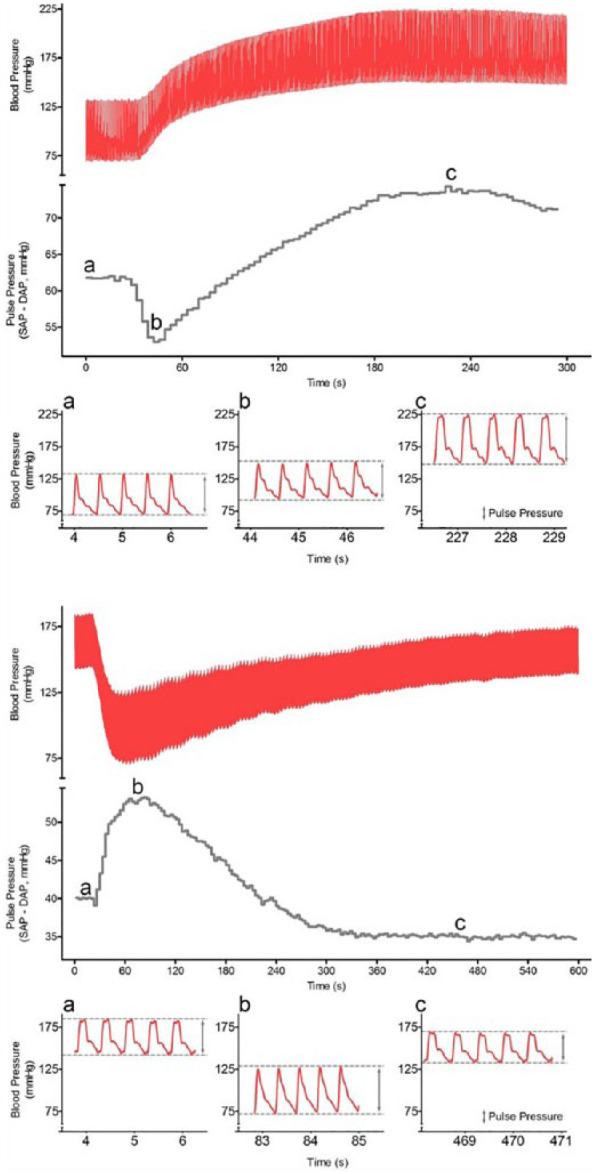
Top and bottom panels: arterial blood pressure trace (red) and pulse pressure (PP; systolic arterial pressure [SAP] – diastolic arterial pressure [DAP]) in one cat, at the beginning of dexmedetomidine infusion (top panel) and during administration of intravenous atipamezole 30 µg/kg. Note that the changes in arterial pressures during dexmedetomidine or atipamezole administration are accompanied by a short and temporary change in PP in the opposite direction. The absolute blood pressure values of the PP trace can be observed in inserts a, b and c
Discussion
This investigation found that intravenous administration of low doses of atipamezole in isoflurane-anesthetized cats receiving dexmedetomidine reduced arterial blood pressure, but only marginally increased HR. At 30 µg/kg, atipamezole restored CO and SVR to pre-dexmedetomidine values. The magnitude of the decrease in arterial blood pressure, however, raises the concern of potential complications with this treatment.
Bradycardia and arterial hypertension are likely the most frequently observed side effects when dexmedetomidine is administered to cats. The practice of using anticholinergic agents to prevent and treat dexmedetomidine-induced bradycardia has been discouraged because it produces severe hypertension, ventricular dysrhythmias, increases myocardial work and does not improve hemodynamic function.4,5,7,9 Hence, despite a lack of licensing for this use, veterinarians, including the authors, might be tempted to use atipamezole to treat bradycardia and hypertension. To this end, small intravenous doses of atipamezole might be administered in an attempt to elevate the HR while avoiding complete reversal of the sedative and analgesic effects, as it might result from the administration of larger doses. We hypothesized that administration of small doses of atipamezole could increase HR and reduce blood pressure. Our results show that administration of atipamezole 15 µg/kg IV resulted in an increase in HR of 8%. While that increase was statistically significant, the clinical value is questionable. Doubling the dose of atipamezole produced an even smaller (not significant) effect in HR, whereas the decrease in MAP was 45%. The decrease in blood pressure was substantial for all cats; however, severe arterial hypotension (MAP <60 mmHg) was only observed in one animal after the second, higher dose of atipamezole was injected. The fact that severe arterial hypotension was not observed more frequently does not necessarily mean that treatment with atipamezole at these doses would be safe under all circumstances, or that our results should be extrapolated to a clinical scenario without reservations. It should be noted that we administered atipamezole to cats during a state of substantial arterial hypertension; the MAP at the time of atipamezole administration was ~150 mmHg and then decreased by ~40%. We cannot comment on the potential effects of atipamezole if administered to cats with lower arterial pressure than that of our cats. If this magnitude of decrease in blood pressure occurs when atipamezole is administered to cats with lower blood pressures, as could easily happen if blood pressure is not monitored, clinically important arterial hypotension could ensue. Second, administration of dexmedetomidine was not interrupted during treatment with atipamezole. The effects of atipamezole in cats that have previously received a single dose of dexmedetomidine (eg, as a preanesthetic sedative) might differ from the ones reported here, as the plasma concentration of the dexmedetomidine will decay over time.
Dexmedetomidine-induced bradycardia has been attributed to a dual mechanism: reflexive bradycardia secondary to increased blood pressure (baroreflex), and a central effect mediated via activation of presynaptic α2-adrenoceptors resulting in a decreased sympathetic outflow. In our study, low doses of atipamezole reduced the blood pressure to values similar to baseline, however, only partially increased the HR. It is possible that the central component of dexmedetomidine-induced bradycardia was not fully antagonized by these doses of atipamezole. It is also possible that isoflurane might have contributed to the apparent lack of effect of atipamezole in increasing the cats’ HR in this study.
The doses of atipamezole we evaluated (15 and 30 μg/kg IV) are a fraction of those previously used to antagonize dexmedetomidine sedation in cats (200 μg/kg IM) 1 and in dogs (100–300 μg/kg IM). 13 We did not evaluate higher doses of atipamezole, and hence we cannot speculate whether increasing the dose of atipamezole may result in more efficient treatment of dexmedetomidine-induced bradycardia or more severe hypotension. Moreover, no apparent differences were observed when the dose was doubled, from 15 to 30 μg/kg. Our data do not allow us to conclude whether a dose-dependent effect could occur with larger doses, or whether atipamezole is unable to antagonize dexmedetomidine-induced bradycardia when administered to anesthetized cats, at any dose.
Inspection of our results also suggest that the changes in blood pressure were of greatest magnitude for DAP; the increase in DAP observed during dexmedetomidine infusion, and the decreases observed after treatment with atipamezole, appear to be greater than those observed for SAP and MAP (Figure 1). In addition, we consistently observed transient changes in PP after infusion of dexmedetomidine was started, and after each dose of atipamezole was administered. Shortly after dexmedetomidine infusion was started the PP decreased because DAP increased more than SAP, and after atipamezole was administered, PP increased because DAP decreased more than SAP (Figure 4). These observations suggest that the effect of both agents on DAP occurred first. The suggestion that DAP is affected first is likely the result of direct changes in vasomotor activity in the systemic circulation.
Consistent with observations in other species, infusion of dexmedetomidine reduced CO and increased SVR. The TEF was also significantly decreased during dexmedetomidine infusion, despite TEDVI, an indicator of cardiac preload, being stable. The decrease in TEF is likely secondary to the substantial increase SVR observed, although our data do not allow us to rule out a negative inotropic effect of dexmedetomidine. Administration of atipamezole 30 µg/kg returned the CO and SVR to baseline. Because HR remained constant, the increase in cardiac output after atipamezole must be entirely (or almost entirely) due to increased stroke volume. This observation, coupled with the contemporary decrease in SVR and an unchanged TEDVI, supports the contention that the changes in CO were largely secondary to changes in afterload, not bradycardia.
There are limitations to this study. A TCI of dexmedetomidine was chosen in preference to a bolus, so that the effects of atipamezole could be evaluated under what were presumably steady-state conditions. Dexmedetomidine concentration in plasma was not measured and hence we cannot comment on how stable the plasma concentration of dexmedetomidine remained throughout the experiment, especially as pharmacokinetic values were obtained from awake cats. However, HR and direct arterial pressures remained stable during the infusion of dexmedetomidine. Utilizing a TCI differs from usual clinical anesthetic practice; however, it allowed us to observe the cardiovascular effects of atipamezole without the variability caused by the changing plasma concentration inherent after a single dose of dexmedetomidine. Hence, the effect of atipamezole administration on cats that had received an intramuscular dose of dexmedetomidine prior to anesthesia might differ from our results. Isoflurane administration was not adjusted during dexmedetomidine administration, but rather was maintained constant. While dexmedetomidine has documented anesthetic-sparing effects, we elected to keep isoflurane stable during data collection to better isolate the effects of dexmedetomidine and isoflurane. The doses of atipamezole were administered sequentially, and although stability in HR and blood were achieved between doses, we cannot quantify if any residual effects from the first dose affected the second one. Such residual effect would affect the results of a dose-finding study, which was not the purpose of this investigation. These factors should be considered before extrapolating results of this experimental project to a clinical scenario. Lastly, the monitor used to determine CO and other related variables has not yet, to our knowledge, been validated in cats. However, this monitor has been extensively evaluated in vitro, 11 and in several species, including human children,14,15 rats, 16 piglets 17 and dogs. 18 As the location of the tip of the jugular catheter was not confirmed radiologically we did not include central venous pressure (CVP) during calculations of SVR. However, the contribution of CVP to SVR is minimal and hence unlikely to introduce a substantial error.
Conclusions
Low doses of atipamezole (15 and 30 μg/kg) administered IV to anesthetized cats decreased arterial blood pressure with only marginal increases in HR. Atipamezole 30 μg/kg restored CO and SVR to pre-dexmedetomidine baseline values.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was funded by the Feline Health Center, College of Veterinary Medicine, Cornell University.
Accepted: 29 June 2017
References
- 1. Granholm M, McKusick BC, Westerholm FC, et al. Evaluation of the clinical efficacy and safety of dexmedetomidine or medetomidine in cats and their reversal with atipamezole. Vet Anaesth Analg 2006; 33: 214–223. [DOI] [PubMed] [Google Scholar]
- 2. McSweeney PM, Martin DD, Ramsey DS, et al. Clinical efficacy and safety of dexmedetomidine used as a preanesthetic prior to general anesthesia in cats. J Am Vet Med Assoc 2012; 240: 404–412. [DOI] [PubMed] [Google Scholar]
- 3. Lamont LA, Bulmer BJ, Grimm KA, et al. Cardiopulmonary evaluation of the use of medetomidine hydrochloride in cats. Am J Vet Res 2001; 62: 1745–1749. [DOI] [PubMed] [Google Scholar]
- 4. Alibhai HI, Clarke KW, Lee YH, et al. Cardiopulmonary effects of combinations of medetomidine hydrochloride and atropine sulphate in dogs. Vet Rec 1996; 138: 11–13. [DOI] [PubMed] [Google Scholar]
- 5. Ko JC, Fox SM, Mandsager RE. Effects of preemptive atropine administration on incidence of medetomidine-induced bradycardia in dogs. J Am Vet Med Assoc 2001; 218: 52–58. [DOI] [PubMed] [Google Scholar]
- 6. Monteiro ER, Campagnol D, Parrilha LR, et al. Evaluation of cardiorespiratory effects of combinations of dexmedetomidine and atropine in cats. J Feline Med Surg 2009; 11: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dunkle N, Moise NS, Scarlett-Kranz J, et al. Cardiac performance in cats after administration of xylazine or xylazine and glycopyrrolate: echocardiographic evaluations. Am J Vet Res 1986; 47: 2212–2216. [PubMed] [Google Scholar]
- 8. Congdon JM, Marquez M, Niyom S, et al. Evaluation of the sedative and cardiovascular effects of intramuscular administration of dexmedetomidine with and without concurrent atropine administration in dogs. J Am Vet Med Assoc 2011; 239: 81–89. [DOI] [PubMed] [Google Scholar]
- 9. Sinclair MD, O’Grady MR, Kerr CL, et al. The echocardiographic effects of romifidine in dogs with and without prior or concurrent administration of glycopyrrolate. Vet Anaesth Analg 2003; 30: 211–219. [DOI] [PubMed] [Google Scholar]
- 10. Vainio O. Reversal of medetomidine-induced cardiovascular and respiratory changes with atipamezole in dogs. Vet Rec 1990; 127: 447–450. [PubMed] [Google Scholar]
- 11. Krivitski NM, Kislukhin VV, Thuramalla NV. Theory and in vitro validation of a new extracorporeal arteriovenous loop approach for hemodynamic assessment in pediatric and neonatal intensive care unit patients. Pediatr Crit Care Med 2008; 9: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pypendop BH, Ilkiw JE. Pharmacokinetics of dexmedetomidine after intravenous administration of a bolus to cats. Am J Vet Res 2014; 75: 441–445. [DOI] [PubMed] [Google Scholar]
- 13. Zoetis. Antisedan (Atipamezole hydrochloride). 2014.
- 14. Lindberg L, Johansson S, Perez-de-Sa V. Validation of an ultrasound dilution technology for cardiac output measurement and shunt detection in infants and children. Pediatr Crit Care Med 2014; 15: 139–147. [DOI] [PubMed] [Google Scholar]
- 15. Boehne M, Baustert M, Paetzel V, et al. Determination of cardiac output by ultrasound dilution technique in infants and children: a validation study against direct Fick principle. Br J Anaesth 2014; 112: 469–476. [DOI] [PubMed] [Google Scholar]
- 16. Veal N, Moal F, Wang J, et al. New method of cardiac output measurement using ultrasound velocity dilution in rats. J Appl Physiol 2001; 91: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 17. Darling E, Thuramalla N, Searles B. Validation of cardiac output measurement by ultrasound dilution technique with pulmonary artery thermodilution in a pediatric animal model. Pediatr Cardiol 2011; 32: 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shih A, Giguere S, Vigani A, et al. Determination of cardiac output by ultrasound velocity dilution in normovolemia and hypovolemia in dogs. Vet Anaesth Analg 2011; 38: 279–285. [DOI] [PubMed] [Google Scholar]