Abstract
Twenty healthy cats (group 1) with clinically normal ears, 15 cats with systemic disease (group 2) and 15 allergic cats (group 3) were included in a prospective study. The experimental unit was the ear. A clinical score was established for each ear canal after otoscopic examination. Microbial population was assessed on cytological examination of smears performed with the cotton-tipped applicator smear technique. Fungal population was significantly more prominent in allergic cats (P <0.001) and in diseased cats compared with healthy cats (P <0.02). Bacterial population was significantly higher in allergic cats than in healthy cats (P <0.001) and cats suffering from systemic disease (P <0.001). Bacterial overgrowth was also higher in cats with systemic disease than healthy cats. In cats from group 2, only fungal overgrowth was associated with otitis severity. In group 3, only bacterial overgrowth was associated with otitis severity.
Short Communication
The external ear canal of cats hosts microorganisms, mostly yeasts and bacteria, which are considered to be normal constituents of feline ear microflora.1–4 Changes in the auricular microclimate and immunological dysfunctions can induce overgrowth of infectious elements and predispose the animal to develop infectious otitis.2,5 Systemic and cutaneous disorders can influence skin microclimate and modify the physiological balance, facilitating the multiplication of infectious agents.5,6 No data were available regarding ear microbiota in cats. The aim of the study was to compare the microflora composition and the influence of aural clinical stage in healthy cats, allergic cats and cats with a systemic disease. Our hypothesis was that systemic disease or allergic dermatitis could influence the auricular microclimate and then modify the normal flora.
Cats were recruited at the Small Animal Hospital of the Toulouse Veterinary School. Group 1 comprised healthy cats with normal ears visiting the preventive medicine service. Group 2 comprised cats suffering from a systemic disease and admitted to the internal medicine service. Group 3 comprised cats diagnosed with allergic dermatitis. Diagnosis of allergic dermatitis was based on clinical signs compatible with face and neck pruritus, self-induced alopecia, miliary dermatitis or eosinophilic granuloma complex after parasitic, bacterial and fungal infections had been ruled out. Cats that had received antibiotics or antifungal therapy (topical or systemic) during the 3 weeks prior to the visit were excluded. Healthy cats with abnormal auricular examination and cats suffering from a systemic disease with skin disease were also excluded. The experimental unit was the ear. Bilateral otoscopic examination was performed for each patient. Examination of the pinnae and ear canals was performed with a hand-held otoscope. The investigator assessed pinnal lesions, pain, pruritus, presence of otopedal reflex, erythema, canal stenosis, amount of cerumen and the tympanic membrane. Based on unpublished preliminary studies, scores were given for each of these parameters on a 0–2 severity scale, except for otopedal reflex (0 or 1); a clinical score ranging from 0 to 15 was calculated. With a score of 0 or 1, the ear was considered as normal (otitis-free). With a score between 2 and 5, otitis was considered as mild. With a score ranging from 6 to 9, the otitis was moderate; and with a score between 10 and 15, the otitis was severe. Aural microflora was evaluated by cytological examination. Ear swabs were taken from the horizontal ear canal and smeared on microscope slides. Slides were briefly heat-fixed before dipping into a commercial staining solution (Kit RAL-555; RAL Diagnostics) and subsequently examined microscopically under a 100× oil immersion lens. Infectious agents were recorded on 10 successive high-power fields. Quantitative reference intervals established by Ginel et al 4 were adapted: mean numbers were used to differentiate between absence of organisms (yeasts: 0–0.2; bacteria: 0–0.4), few infectious elements (yeasts: 0.3–1.0; bacteria: 0.5–1.5) or numerous elements (yeasts: >1; bacteria: >1.5). The operator was not blinded to the clinical status (healthy, allergic or systemic disease), but was blinded to the aural score. Data were entered into an Excel (Microsoft) spreadsheet and statistical tests were performed in SYSTAT (v. 13). To determine the significance of tested parameters, χ2 test, Student’s t-test and one-way analysis of variance (ANOVA) were used. Significance was set at 0.05.
Fifty cats (26 males, 24 females), that is, 100 ears, were included. Group 1 comprised 20 (11 males, nine females) healthy cats. Group 2 comprised 15 (eight males, seven females) diseased cats: five suffered from feline urinary syndrome, one had a chronic upper respiratory tract infection, three suffered from liver or pancreatic disease, three suffered from chronic kidney failure and one cat had an autoimmune disease. Group 3 was composed of 15 (seven males, eight females) allergic cats. Four were food-allergic, five were flea-allergic, six had non-flea-/non-food-induced hypersensitivity dermatitis (NFNFIHD). Gender distribution was homogeneous in this population. Ten animals were younger than 1 year of age, 32 were aged from 1 to 7 years and eight cats were older than 7 years. Age distribution was heterogeneous between groups; healthy cats were significantly younger than cats from the other groups (P <0.05), whereas there was no difference between group 2 cats and group 3 cats.
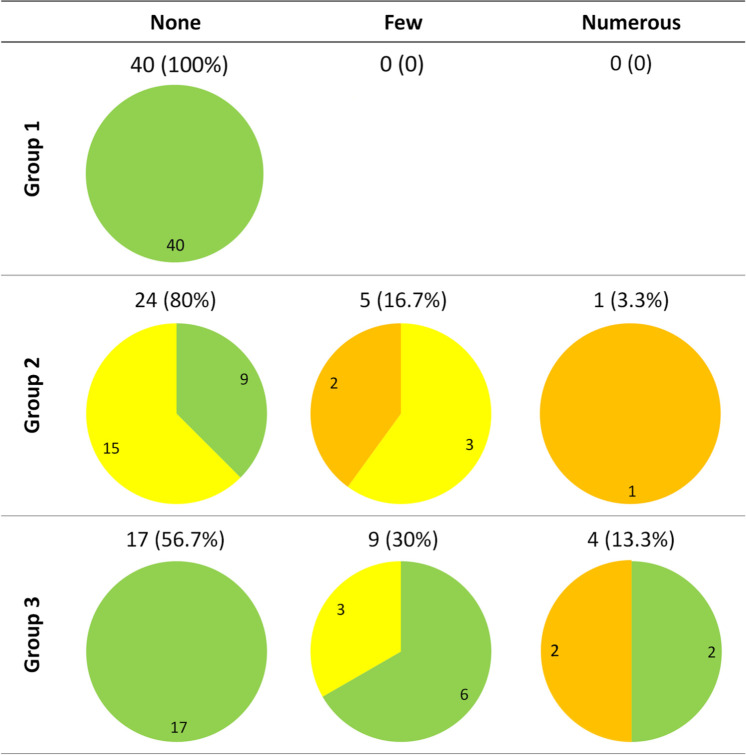
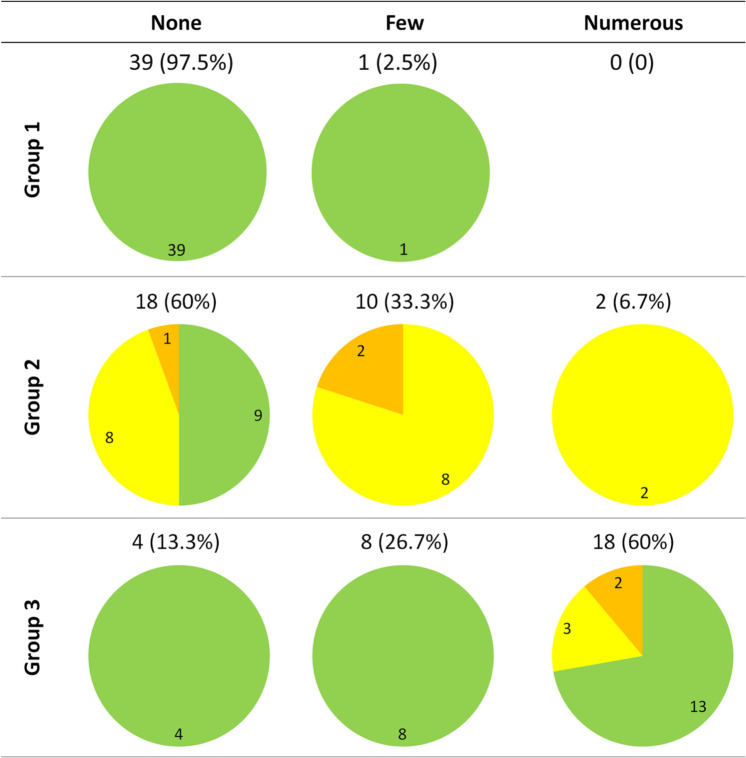
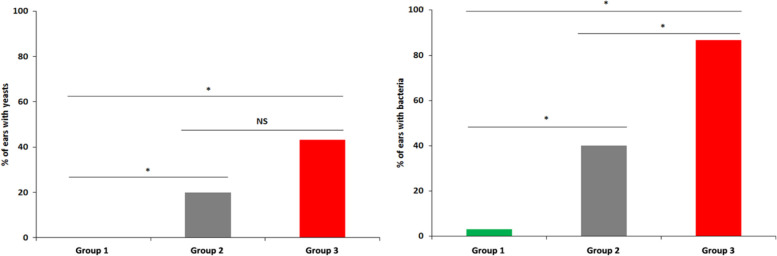
In all three groups of cats, only Malassezia yeasts and coccoid bacteria were detected. In cats from group 1, no yeasts were detected (Figure 1). Bacteria were isolated from a single ear (2.5%) and proliferation was mild (Figure 2). All ears were clinically normal, as required in the study design (Figures 1 and 2). In cats from group 2, yeasts were observed in six canals (20%), low yeast numbers were observed in five ears, three of which had mild otitis and two that had moderate otitis; a moderate otitis was detected in the single canal with numerous yeasts (Figure 1). Bacteria were detected in 12 ears (40%); few bacteria were present in 10 canals, of which eight presented with mild otitis and two with moderate otitis. Moderate otitis was observed in the ears harbouring numerous bacteria (Figure 2). In cats from group 3, yeasts were present in 13 ears (43.3%); nine ears had few yeasts and only 3/9 presented with mild otitis. Of the four canals with numerous yeasts, two had a moderate otitis (Figure 1). Bacteria were observed in 26 ears (86.7%); they were few in eight cases (26.7%) and numerous in 18 (60%). Five ears presenting numerous bacteria showed mild or moderate signs of otitis (Figure 2). Microbial overgrowth was compared between the 3 groups with a χ2 test and a Student’s t-test, regardless of the clinical status (Figure 3). Fungal population was significantly more prominent in allergic cats (P <0.001) and in diseased cats compared with healthy cats (P <0.001). No difference was found between cats from group 2 and group 3. Bacterial population was significantly higher in allergic cats than in healthy cats (P <0.001) and cats suffering from systemic disease (P <0.001). This overgrowth was also more prominent in group 2 than in group 1 (P <0.001). An ANOVA was used to determine a possible association between microbial overgrowth and clinical status. In cats from group 2, only fungal overgrowth was associated with otitis severity. In group 3, only bacterial overgrowth was associated with otitis severity.
Figure 1.
Yeast overgrowth in the ear canals of healthy cats (group 1), cats with a systemic disease (group 2) and allergic cats (group 3). Normal ear canals are in green, canals with mild otitis are in yellow and canals with moderate otitis are in orange
Figure 2.
Bacterial overgrowth in ear canals of healthy cats (group 1), cats with a systemic disease (group 2) and allergic cats (group 3). Normal ear canals are in green, canals with mild otitis are in yellow and canals with moderate otitis are in orange
Figure 3.
Yeast (left) or bacterial (right) overgrowth in ear canals of healthy cats (group 1), cats with a systemic disease (group 2) and allergic cats (group 3). NS = not significant, P = 0.05 *Difference between the two groups
In the present study, based on cytology, Malassezia yeasts were not observed in the ears of healthy cats. The rare data available in the literature report the presence of yeasts in 23–83% of healthy cats.7–9 The large difference could be explained by the use of cultures to detect microbial overgrowth,7,9 but Tater et al 8 also used cytology and detected Malassezia species in 83% of cases. Alternatively, the difference might be due to population selection, possibly influenced by climate or age.
Coccoid bacteria were detected in only 2.5% of the ears of healthy cats. Tater et al 8 reported the presence of bacteria in 71% of cases with a similar technique; Patel et al 10 cultured Staphylococcus felis in 45% of cases. In this study, Malassezia species yeasts were more prominent in diseased cats than in healthy cats. This is in accordance with literature, where Malassezia overgrowth on the skin is often associated with severe systemic diseases, particularly neoplasia. 11 Moreover, Sierra et al 12 demonstrated that a greater diversity of fungal elements was isolated from the external ear canals of retrovirus-infected cats (feline leukaemia virus [FeLV], feline immunodeficiency virus [FIV]); Malassezia species were more commonly detected from these cats than in non-infected cats. 12 Of the 15 systemically ill cats included in the study, only one was FeLV- and FIV-positive, and – according to inclusion criteria – none of the cats had skin lesions. Malassezia yeasts were also more often observed in the ear canals of allergic cats than in ear canals of healthy cats. This could be similar to findings in atopic dogs, which are prone to develop yeast overgrowth. 6 Ordeix et al 13 suggested a similar situation in feline on skin, but no data were available regarding ear overgrowth in allergic cats. Few signs of otitis were observed in this group compared with the other groups. As opposed to atopic dogs, otitis is not commonly observed in allergic cats; hence, otitis is not considered as a major diagnostic criterion of NFNFIHD. 14 However, 86% of ears presented excessive numbers of coccoid bacteria; they were considered as numerous in 60% of ears. Only 5/26 ear canals presenting with bacterial overgrowth showed slight or moderate signs of otitis. Similar features occur in atopic dogs where colonisation of the ear canal by Staphylococcus pseudintermedius is commonly reported; in a prospective study, it was higher than in healthy dogs and concerned 90% of dogs. 15 In cats, physiopathology of atopy is currently too poorly understood to explain these data. Furthermore, inclusion criteria comprised all types of allergy and not only NFNFIHD. Consequently, when there is evidence of microorganisms on aural cytology in the absence of clinical signs, the practitioner should consider investigating an underlying disease, possibly not yet clinically apparent.
Conclusions
Although pathogenicity of yeasts and bacteria remains unclear, Malassezia and bacterial overgrowths may be early signs of systemic and allergic diseases, respectively.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this short communication.
Accepted: 7 January 2014
References
- 1. Shokri H, Khosravi A, Rad M, et al. Occurrence of Malassezia species in Persian and domestic short hair cats with and without otitis externa. J Vet Med Sci 2010; 72: 293–296. [DOI] [PubMed] [Google Scholar]
- 2. Coutinho SD, Fedullo JD, Correa SH. Isolation of Malassezia spp. from cerumen of wild felids. Med Mycol 2006; 44: 383–387. [DOI] [PubMed] [Google Scholar]
- 3. Cox HU, Hoskins JD, Newman SS, et al. Distribution of staphylococcal species on clinically healthy cats. Am J Vet Res 1985; 46: 1824–1828. [PubMed] [Google Scholar]
- 4. Ginel PJ, Lucena R, Rodriguez JC, et al. A semiquantitative cytological evaluation of normal and pathological samples from the external ear canal of dogs and cats. Vet Dermatol 2002; 13: 151–156. [DOI] [PubMed] [Google Scholar]
- 5. Scott DW, Miller WH, Jr, Griffin CE. Fungal skin diseases. Muller & Kirk’s small animal dermatology. 6th ed. Philadelphia: WB Saunders, 2001, pp 336–422. [Google Scholar]
- 6. Chen TA, Hill PB. The biology of Malassezia organisms and their ability to induce immune responses and skin disease. Vet Dermatol 2005; 16: 4–26. [DOI] [PubMed] [Google Scholar]
- 7. Nardoni S, Mancianti F, Rum A, et al. Isolation of Malassezia species from healthy cats and cats with otitis. J Feline Med Surg 2005; 7: 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tater KC, Scott DW, Miller WH, Jr, et al. The cytology of the external ear canal in the normal dog and cat. J Vet Med A Physiol Pathol Clin Med 2003; 50: 370–374. [DOI] [PubMed] [Google Scholar]
- 9. Uchida Y, Nakade T, Kitazawa K. Clinico-microbiological study of the normal and otitic external ear canals in dogs and cats. Nihon Juigaku Zasshi 1990; 52: 415–417. [DOI] [PubMed] [Google Scholar]
- 10. Patel A, Lloyd DH, Lamport AI. Prevalence of feline staphylococci with special reference to Staphylococcus felis among domestic and feral cats in the south-east of England. In: Thoday KL, Foil CS, Bond R. (eds). Advances in veterinary dermatology. Vol 4. Oxford: Blackwell Science, 2002, pp 85–91. [Google Scholar]
- 11. Mauldin EA, Morris DO, Goldschmidt MH. Retrospective study: the presence of Malassezia in feline skin biopsies. A clinicopathological study. Vet Dermatol 2002; 13: 7–13. [DOI] [PubMed] [Google Scholar]
- 12. Sierra P, Guillot J, Jacob H, et al. Fungal flora on cutaneous and mucosal surfaces of cats infected with feline immunodeficiency virus or feline leukemia virus. Am J Vet Res 2000; 61: 158–161. [DOI] [PubMed] [Google Scholar]
- 13. Ordeix L, Galeotti F, Scarampella F, et al. Malassezia spp. overgrowth in allergic cats. Vet Dermatol 2007; 18: 316–323. [DOI] [PubMed] [Google Scholar]
- 14. Favrot C, Steffan J, Seewald W, et al. Establishment of diagnostic criteria for feline nonflea-induced hypersensitivity dermatitis. Vet Dermatol 2012; 23: 45–50, e11. [DOI] [PubMed] [Google Scholar]
- 15. Fazakerley J, Nuttall T, Sales D, et al. Staphylococcal colonization of mucosal and lesional skin sites in atopic and healthy dogs. Vet Dermatol 2009; 20: 179–184. [DOI] [PubMed] [Google Scholar]