Abstract
A 2-month-old kitten exhibited simultaneously an imperforate anus, hypospadias, rectourethral fistula and genital dysgenesis (penis restricted to the glans, absence of prepuce and bifid scrotum). Surgical correction consisted of separation of the urinary and digestive tracts, perineal urethrostomy and connection of the rectum to the newly made anal opening. Pathological examination of the testes, conventionally removed at 9 months of age, showed no mature spermatozoa and underdevelopment of germ and Leydig cells. In humans, the absence of an anal opening in association with abnormal sexual development defines the urorectal septum malformation sequence. Here, we describe the first case of this syndrome in a kitten with a normal male karyotype (38,XY) and a normal coding sequence for the SRY gene. Both the rectourethral fistula and observed genital abnormalities might have been induced by a disturbance in the hedgehog signalling pathway. However, although four polymorphic sites were identified by DHH gene sequencing, none cosegregated with the malformation.
Case Report
Various disorders of sex development (DSD), although rare, have been described in cats, including true hermaphrodism1,2 and pseudohermaphrodism.3–6 In all the feline DSD cases described in the literature, abnormalities were restricted to genital tract development, while other systems, such as the digestive tract, were normal. Conversely, normal genital differentiation has been observed in the case of anorectal abnormalities.7,8 In humans, abnormal sexual development and the absence of perineal openings are observed concurrently, and termed the urorectal septum malformation (URSM) sequence. 9 The presence of a single opening, draining a common cloaca, along with an absent (imperforate) anus, characterises a partial URSM (PURSM) sequence. 10 In human DSD nomenclature, URSM is classified as ‘syndromic associations of male genital development (eg, cloacal anomalies, cryptorchidism, etc)’. 11 This report describes, for the first time, a case of PURSM in a kitten that simultaneously displayed DSD (38,XY; SRY-positive) and a single perineal opening, common to the digestive and genitourinary tracts.
This 2-month-old Persian, presumed to be a female, was referred at the end of the weaning period for an imperforate anus. At the normal place of the anal opening, only a dimple was present. A single perineal orifice was draining both urine and faeces (Figure 1). Genital abnormalities described in association with PURSM in human males include hypospadias, phallic hypoplasia, penoscrotal transposition and bilateral undescended testes. 10 This kitten presented a large hypospadias and a penis restricted to the glans. The testes were palpable outside the inguinal ring at 2 months, but not in a definitive scrotal position. In other reports of kittens with rectogenital fistulae, no genital abnormalities have been described,3–5 and the associated non-genital abnormalities, found exclusively in females, were mainly partial tail agenesis and, less frequently, umbilical hernia, cleft palate, sacrocaudal dysgenesis and hydrocephalus. 8 None of the large number of abnormalities associated with URSM in humans (vertebral, limb, cardiac, tracheoesophageal defects) was identified in this kitten. In particular, ultrasound examination did not reveal any of the renal abnormalities (agenesis, dysplasia, hydronephrosis) observed in up to 82% of affected infants.9,10 Cytogenetic evaluation of in vitro-cultured lymphocytes revealed a normal, male chromosome complement (38,XY) (Figure 1 in the Supplementary material). The presence of the SRY gene was confirmed by polymerase chain reaction (1022 base pairs), with primers designed on the basis of the feline SRY gene sequence (Figure 2 in the Supplementary material). The earlier reported polymorphism (389G>C) 2 was not identified in this study, but two deletions in the 3’-flanking region of the SRY gene distinguished the SRY sequence of our kitten from the reference and control cat (Figure 3 in the Supplementary material).
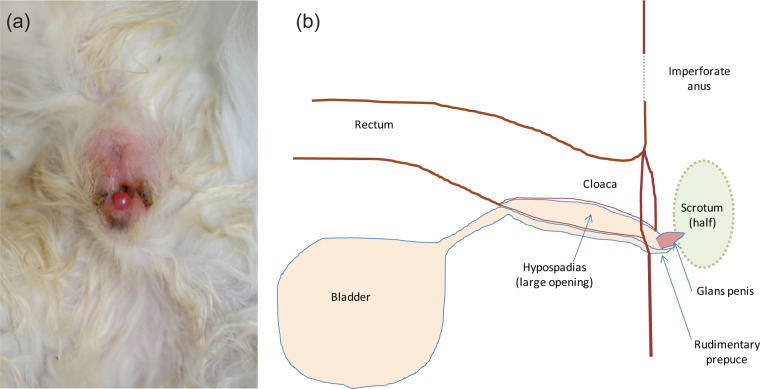
Figure 1.
(a) Anogenital region of the kitten (in ventral recumbency) at 2 months of age. (b) Diagrammatic representation in a sagittal view. The imperforate anus is visible as a dimple. The urethra, dilated in its pelvic part, forms dorsally a hypospadias connecting with the rectum. A single-opening draining, a cloaca common to the digestive and urinary tracts, is apparent at a distance similar to the anogenital distance in females, between two scrotal pouches (testes were not in a definitive scrotal position at 2 months). The glans penis was congested and only a rudimentary prepuce was present
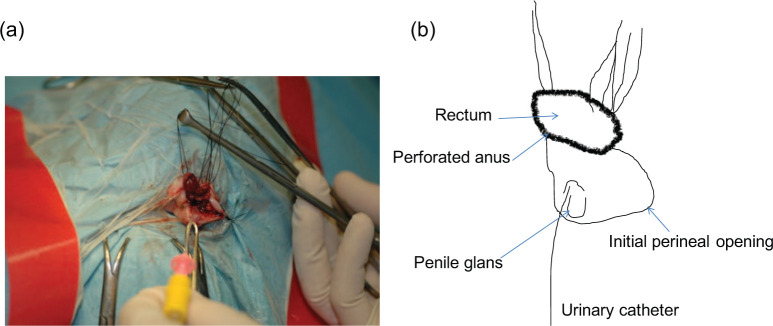
The radiographic appearance and size of the colon were unremarkable, suggesting normal colonic motility. A liquid diet was maintained until surgery, which consisted of perineal urethrostomy, reopening of the anus and connection of the rectum. After premedication (acepromazine, 0.05 mg/kg, IV; Calmivet, Vétoquinol), general anaesthesia was induced with propofol (6 mg/kg IV; Rapinovet, MSD) and maintained with isoflurane(Forene; Abbvie) in oxygen. Analgesics (0.1 mg/kg morphine sulfate IV; morphine chlorhydrate, Aguettant) and antibiotics (amoxicillin, 20 mg/kg, IV; Clamoxyl, Pfizer Santé Animale) were administered. The kitten was placed on a heating pad in ventral recumbency with its tail fixed over its back. The perineum was clipped and prepared for aseptic surgery. A midline dorsal incision of the perineal orifice, ending at the dorsal limit of the dimple, was performed. The external anal sphincter had been identified previously on the dorsal part of the incision. The rectourethral fistula connected the dorsal wall of a dilated urethra and the ventral portion of the terminal rectum. After fistula dissection, an incision was made to separate the urethra from the rectum. The rectal opening was carefully mobilised through the external anal sphincter (Figure 2) and sutured to the surrounding subcutaneous tissues with a simple continuous pattern of 4–0 polydioxanone sutures (PDS; Ethicon) and, finally, to the skin with simple interrupted sutures of 4–0 polyamide (Ethilon; Ethicon). 12 The urethral opening was sutured with 6–0 or 7–0 polydioxanone (PDS; Ethicon) simple interrupted sutures at the initial position of the perineal opening. The cutaneous incision was sutured with simple interrupted sutures of 4–0 polyamide (Ethilon; Ethicon).
Figure 2.
(a) Intraoperative view of the perineal region of the kitten (in ventral recumbency) during surgical reconstruction. (b) Schematic representation of the anatomical structures
One month after surgery, the kitten received a classical solid diet, and faecal elimination became normal after 2 months. Micturition and growth were normal. At the age of 9 months, the cat was of normal stature; a rudimentary prepuce was observed, just behind the penis glans protruding through the perineal urethrostomy orifice. Two testes were found in a bifid, but normally developed, scrotum (Figure 3). The time of testicular descent could not be documented. After conventional removal, the testes were found to be abnormally small, but the epididymis was present. Overall, pathological examination of several sections of both testes revealed seminiferous tubules of small diameter lined by a small number of germ cells layers. The paucity of germ cells may reflect an abnormality of spermatogenesis, either primary or secondary to possible delayed testes descent or to URSM. Sertoli cells and the early stages of spermatogenesis (until elongated spermatids) were present, but no mature spermatozoa were observed (Figure 4) and Leydig cells were rare. Five years later, the animal is still alive and healthy.
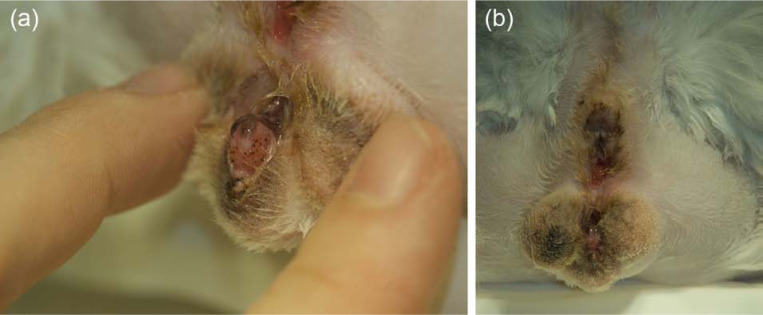
Figure 3.
(a) Anogenital region of the cat (in ventral recumbency) at 9 months of age. A rudimentary prepuce can be observed, just behind the glans penis. Two openings have been reconstructed: above, the anal opening connecting the rectum; below, the opening of the perineal urethrostomy. (b) Closer view of the glans penis protruding through the perineal urethrostomy opening
Figure 4.
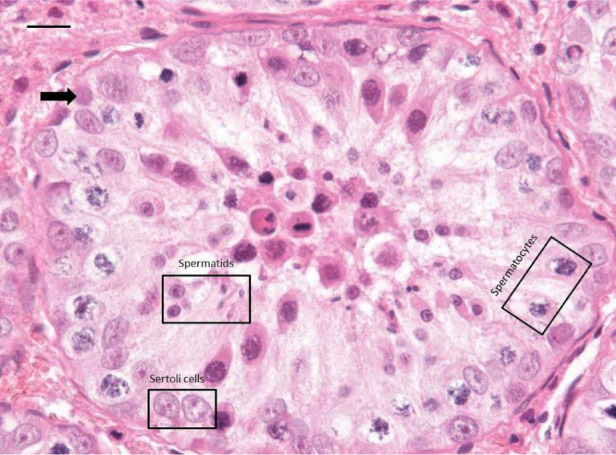
Histological view of a representative seminiferous tubule. Magnification ×400. The number of germ cell layers appears abnormally low (black arrow); Sertoli cells, spermatocytes and spermatids (all indicated in the corresponding boxes) are present, whereas mature spermatozoa are absent. Haematoxylin-eosin-safran stain. Bar = 15 µm
Both abnormal development of the perineal region and testicular disorders can result from a disturbance of the hedgehog signalling pathway. Sonic hedgehog (Shh) and other members of the hedgehog family (Gli2, Gli3) are known to be involved in epithelio-mesenchymal interaction, which is critical for urorectal septum formation and closure,13,14 whereas desert hedgehog (Dhh) is essential for Leydig cell development and linked to hypospadias.14,15 Indeed, knockout Dhh male mice are sterile owing to the absence of mature spermatozoa. 16 Comparison of the Dhh gene sequence in this kitten and in five control male cats revealed only four common polymorphic sites (Table 1 in the Supplementary Material) in exon 1 (deposited in GenBank: KF358988). None of them co-segregated with the malformation.
Conclusions
Newborn animals with discordant phenotypes regarding URSM within the same litters have been reported; for example, dizygous lambs 13 and human twins. 17 Here, the littermates of the affected kitten were normal. This makes it unlikely that URSM is of teratogenic origin. Indeed, genetic analyses of porcine lineages, in which the incidence of atresia ani with rectogenital fistulas is high, suggest an oligogenic or a polygenic determinism for URSM. 18
Supplemental Material
Karyotype of the studied kitten (38,XY). Chromosome preparations from in vitro lymphocyte culture. The chromosome set was assessed on 50 metaphase spreads after conventional Giemsa staining and following the international nomenclature for the cat karyotype
Karyotype of the studied kitten (38,XY). Chromosome preparations from in vitro lymphocyte culture. The chromosome set was assessed on 50 metaphase spreads after conventional Giemsa staining and following the international nomenclature for the cat karyotype
The SRY gene sequence of the case kitten (DSD cat), aligned with that of a control male cat and the reference sequence GenBank:DQ095188 (above). The START and STOP codons are underlined, while two deletions found in 3?-flanking region are indicated by arrows. Primers were designed on the basis of the feline SRY gene sequence (957bp; DQ095188): F: 5? aactttgctacccaccaacc and R: 5? caatctgcggaaaactggat. The PCR conditions were as follows: initial denaturation for 5 min. at 95?C; 34 cycles of: denaturation for 30 sec. at 95?C, primer annealing for 30 sec. at 58?C, elongation for 1 min at 72?C; final elongation for 10 min. at 72?C. Sequencing of the obtained amplicon revealed a normal coding sequence in the DSD cat when compared with the reference (GenBank: DQ095188) and control male cat sequences
Sequencing of the SRY gene fragment in 3?UTR with two identified deletions (g.*129C and g.*186C), behind the UGA stop codon (FJ829365)
Acknowledgments
We would like to thank Dr Henri Luengo for referring the case, Mrs Anne Calgaro from the cytogenetics laboratory at the National Veterinary School, Toulouse, France, for the initial karyotyping, and Dr Isabelle Raymond from the pathology unit at the National Veterinary School, Toulouse, France, for performing the histopathological examination of the testes.
Footnotes
Supplementary material: Figure 1: Karyotype of the studied kitten (38,XY).
Figure 2: The SRY gene sequence of the case kitten (DSD cat), aligned with that of a control male cat and the reference sequence GenBank:DQ095188
Figure 3: Sequencing of the SRY gene fragment in 3′UTR with two identified deletions (g.*129C and g.*186C), behind the UGA stop codon (FJ829365).
Table 1: Polymorphic sites identified in the Dhh gene.
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
Accepted: 10 March 2014
References
- 1. Felts JF, Randell MG, Greene RW, et al. Hermaphroditism in a cat. J Am Vet Med Assoc 1982; 181: 925–926. [PubMed] [Google Scholar]
- 2. Schlafer DH, Valentine B, Fahnestock G, et al. A case of SRY-positive 38,XY true hermaphroditism (XY sex reversal) in a cat. Vet Pathol 2011; 48: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hakala JE. Reproductive tract anomalies in 2 male cats. Mod Vet Pract 1984; 65: 629. [PubMed] [Google Scholar]
- 4. Meyers-Wallen VN, Wilson JD, Griffin JE, et al. Testicular feminization in a cat. J Am Vet Med Assoc 1989; 195:631–634. [PubMed] [Google Scholar]
- 5. Bredal WP, Thoresen SI, Kvellestad A, et al. Male pseudohermaphroditism in a cat. J Small Anim Pract 1997; 38: 21–24. [DOI] [PubMed] [Google Scholar]
- 6. Knighton E. Congenital adrenal hyperplasia secondary to 11beta-hydroxylase deficiency in a domestic cat. J Am Vet Med Assoc 2004; 225: 238–241. [DOI] [PubMed] [Google Scholar]
- 7. Van den Brock AHM, Else RW, Hunter MS, et al. Atresia ani and urethrorectal fistula in a kitten. J Small Anim Pract 1988; 29: 91–94. [Google Scholar]
- 8. Suess RP, Jr, Martin RA, Moon ML, et al. Rectovaginal fistula with atresia ani in three kittens. Cornell Vet 1992; 82: 141–153. [PubMed] [Google Scholar]
- 9. Escobar LF, Heiman M, Zimmer D, et al. Urorectal septum malformation sequence: prenatal progression, clinical report, and embryology review. Am J Med Genet 2007; A143: 2722–2726. [DOI] [PubMed] [Google Scholar]
- 10. Wheeler PG, Weaver DD. Partial urorectal septum malformation sequence: a report of 25 cases. Am J Med Genet 2001; 103: 99–105. [DOI] [PubMed] [Google Scholar]
- 11. Pasterski V, Prentice P, Hughes IA. Impact of the consensus statement and the new DSD classification system. Best Pract Res Clin Endocrinol Metab 2010; 24: 187–195. [DOI] [PubMed] [Google Scholar]
- 12. Mahler S, Williams G. Preservation of the fistula for reconstruction of the anal canal and the anus in atresia ani and rectovestibular fistula in 2 dogs. Vet Surg 2005; 34: 148–152. [DOI] [PubMed] [Google Scholar]
- 13. Jo Mauch T, Albertine KH. Urorectal septum malformation sequence: insights into pathogenesis. Anat Rec 2002; 268: 405–410. [DOI] [PubMed] [Google Scholar]
- 14. Franco HL, Yao HH. Sex and hedgehog: roles of genes in the hedgehog signaling pathway in mammalian sexual differentiation. Chromosome Res 2012; 20: 247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ostrer H. 46,XY disorder of sex development and 46,XY complete gonadal dysgenesis. In: Pagon RA, Adam MP, Bird TD, et al. (eds). GeneReviews. Seattle: University of Washington, 1993–2013. [Google Scholar]
- 16. Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol 1996; 6: 298–304. [DOI] [PubMed] [Google Scholar]
- 17. Achiron R, Frydman M, Lipitz S, et al. Urorectal septum malformation sequence: prenatal sonographic diagnosis in two sets of discordant twins. Ultrasound Obstet Gynecol 2000; 16: 571–574. [DOI] [PubMed] [Google Scholar]
- 18. Hori T, Giuffra E, Andersson L, et al. Mapping loci causing susceptibility to anal atresia in pigs, using a resource pedigree. J Pediatr Surg 2001; 36: 1370–1374. [DOI] [PubMed] [Google Scholar]
- 19. Ford CE, Pollock DL, Gustavsson I. Proceedings of the first international conference for the standardisation of banded karyotypes of domestic animals. Hereditas 1980; 92: 145–162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Karyotype of the studied kitten (38,XY). Chromosome preparations from in vitro lymphocyte culture. The chromosome set was assessed on 50 metaphase spreads after conventional Giemsa staining and following the international nomenclature for the cat karyotype
Karyotype of the studied kitten (38,XY). Chromosome preparations from in vitro lymphocyte culture. The chromosome set was assessed on 50 metaphase spreads after conventional Giemsa staining and following the international nomenclature for the cat karyotype
The SRY gene sequence of the case kitten (DSD cat), aligned with that of a control male cat and the reference sequence GenBank:DQ095188 (above). The START and STOP codons are underlined, while two deletions found in 3?-flanking region are indicated by arrows. Primers were designed on the basis of the feline SRY gene sequence (957bp; DQ095188): F: 5? aactttgctacccaccaacc and R: 5? caatctgcggaaaactggat. The PCR conditions were as follows: initial denaturation for 5 min. at 95?C; 34 cycles of: denaturation for 30 sec. at 95?C, primer annealing for 30 sec. at 58?C, elongation for 1 min at 72?C; final elongation for 10 min. at 72?C. Sequencing of the obtained amplicon revealed a normal coding sequence in the DSD cat when compared with the reference (GenBank: DQ095188) and control male cat sequences
Sequencing of the SRY gene fragment in 3?UTR with two identified deletions (g.*129C and g.*186C), behind the UGA stop codon (FJ829365)