Abstract
The aims of this study were to investigate the prevalence of hypocobalaminaemia in UK cats presented for referral investigation of gastrointestinal signs and to ascertain whether the duration of clinical signs or severity of disease (based on WSAVA Gastrointestinal Standardization histopathological grading) related to cobalamin concentration. The study population comprised 39 cats, of which 11 (28.2%) had hypocobalaminaemia. Eight of these cats were diagnosed with a single cause of gastrointestinal signs: intestinal inflammation (five); alimentary lymphoma (two); and cholangitis (one). Two or more concurrent diseases were diagnosed in the three remaining cases. Alimentary lymphoma and the most severe grade of histological intestinal inflammation were associated most commonly with concurrent hypocobalaminaemia, but there was no statistically significant correlation between serum cobalamin concentrations and histopathological score or duration of clinical signs.
Introduction
Cobalamin (vitamin B12) is a water-soluble vitamin with an important role in various intracellular biochemical pathways. Cobalamin deficiency has been recognised in cats and has significant metabolic and clinical consequences, including methylmalonic acidaemia, which is associated with inappetence and lethargy, and may complicate primary gastrointestinal (GI) disease.1,2
The absorption of cobalamin from the GI tract is a complex, receptor-mediated process. In the stomach, cobalamin is released from dietary proteins and binds to a non-specific binding protein of salivary and gastric origin called haptocorrin (or R-protein). In the duodenum, haptocorrin is degraded by pancreatic proteases and free cobalamin is transferred to intrinsic factor (IF). IF is produced solely by the pancreas in cats 3 and has a high affinity for cobalamin at a neutral pH. Cobalamin-IF complexes pass through the small intestine until they bind to specific receptors (IF-cobalamin receptors or cubilin) in the ileum. Following receptor-mediated endocytosis by ileal enterocytes, cobalamin is released from IF and enters the portal circulation where it is transferred to a group of serum carrier proteins (the transcobalamins) which mediate the uptake of cobalamin by target cells. 4
Enterohepatic recycling of cobalamin occurs in cats, with cobalamin being secreted into bile bound to hepatic haptocorrin. 5 Cats have a lesser capacity to store cobalamin than humans and lack the binding protein transcobalamin 1. Consequently, cats can rapidly lose cobalamin and, in the presence of severe malabsorption, may become cobalamin depleted within a month. 6
The complexity of the cobalamin absorption and storage pathways means that a problem at any stage can lead to hypocobalaminaemia. Dietary deficiency is rare and, consequently, hypocobalaminaemia may result from four mechanisms: reduced IF availability [eg, in exocrine pancreatic insufficiency (EPI), chronic pancreatitis or extra-hepatic biliary disease]; intestinal bacterial competition (eg, in dysbiosis secondary to EPI, reduced gastric acid production or disturbed mucosal immune system function); ileal mucosal disease resulting in down regulation of IF-cobalamin receptors (eg, owing to exposure of toxic bile acids or inflammatory mediators); or failure of cubilin expression owing to genetic defects, as described in humans and specific canine, but not feline, breeds.7–10
Recent studies describing the prevalence of hypocobalaminaemia in cats in the UK have shown markedly different results. 1 Ibarrola et al 11 reported hypocobalaminaemia to be uncommon; however, a second study by Reed et al 12 found hypocobalaminaemia in 16.5% of cats with GI disease. The two studies used different cobalamin assays and different reference intervals. However, even the prevalence in the UK reported by Reed et al was still markedly lower than that reported from the USA, where 61% of cats with confirmed GI disease had concurrent hypocobalaminaemia. 13
The first aim of this study was to evaluate the prevalence of hypocobalaminaemia, using a single methodology, in UK cats presented for referral investigation of suspected GI disease. The second aim was to test the hypotheses that there is an association between the degree of hypocobalaminaemia and the severity of histopathological changes in small intestinal biopsy samples, and the duration of the clinical signs.
Materials and methods
The medical records of the Feline Centre at the University of Bristol were searched for cases presented for investigation of GI signs between 2006 and 2010. Inclusion criteria were cats that had undergone intestinal biopsy and concurrent measurement of serum cobalamin. Cats that had knowingly received parenteral cobalamin were excluded.
Review of records
For each case, information was recorded regarding signalment, clinical presentation, duration of clinical signs, retroviral status, haematological abnormalities, serum biochemical abnormalities, serum cobalamin and folate concentrations, feline pancreatic lipase immunoreactivity (fPLI), feline trypsin-like immunoreactivity (fTLI) and thyroxine, urinalysis, faecal analysis and abdominal imaging (radiographic and ultrasonographic findings).
Serum cobalamin determination
Serum samples were stored at -20°C until shipping on dry ice to the GI Laboratory, Texas, TX, USA for measurement by an automated chemiluminescence competitive binding immunoassay system (DPC Immulite 1000 Vitamin B12 Assay with heat denaturation). The reference interval for feline serum cobalamin concentrations was 290–1500 ng/l.
Histopathological assessment
The histopathological diagnosis for all tissue samples (GI, hepatic and pancreatic) was recorded. All sections of duodenal biopsy samples and other tissue samples were reviewed by a single board-certified pathologist (MJD) who was blinded to the original diagnosis. Cases of alimentary lymphoma were identified and those samples that were histologically normal or inflamed were reviewed according to the WSAVA Gastrointestinal Standardization Group template. 14 Each of the 10 histological changes considered individually was scored: normal = 0, mild = 1, moderate = 2, marked = 3, with a maximal possible cumulative score of 30 for very severe inflammation. Three of the morphological features used in the WSAVA template (villus stunting, epithelial injury and crypt distension) were also assessed for samples of alimentary lymphoma. Each category was assigned a score of 0–3 to give a maximum possible cumulative score of 9. Five cats had ileal biopsy samples obtained at exploratory laparotomy. These samples were also reviewed according to the WSAVA template, as in the study by Casamian-Sorrosal et al. 15
Statistical analysis
Using a commercial software package (PASW statistics version 18 for Microsoft Windows), univariate linear regression models assessed the relationship of serum cobalamin concentration to histopathological scores and duration of clinical signs. To perform linear regression a conservative value of 140 ng/l was assigned to cases with cobalamin measured below the assay limit of the detection (150 ng/l). Regression analysis was performed and a P value calculated.
The cats were also subdivided into two groups (inflammation and lymphoma) on the basis of the histopathological diagnosis; the distribution and median of the histopathology scores and duration of clinical signs for these groups were compared by a Mann-Whitney test. For all analyses, a P value <0.05 was considered significant.
Results
Forty-one cats fulfilled the inclusion criteria: 17 were domestic shorthair (DSH), two were domestic longhair (DLH) and 22 were purebreeds (including Siamese, British Shorthair, Persian, Birman, Burmese, Burmilla, Tonkinese, Russian Blue, Maine Coon and Singapura). The age range was 7 months to 18 years (median 7 years). There were 20 female cats (three entire) and 21 male cats (three entire).
Prevalence of hypocobalaminaemia
Serum cobalamin concentrations ranged from <150 ng/l to 8320 ng/l. One cat had a cobalamin concentration reported as >4000 ng/l, but insufficient serum was available to dilute and measure the concentration accurately. Another cat was reported to have a serum cobalamin concentration of 8320 ng/l. These last two results were suggestive of prior parenteral supplementation and so despite clear historical evidence of such treatment, these cats were excluded from further analysis.
A median value of 496 ng/l was documented for the remaining 39 cats. Hypocobalaminaemia (<290 ng/l) was found in 11 (28.2%) of the 39 cats (Table 1). In the hypocobalaminaemic cats, five had intestinal inflammation (all <150 ng/l); two had alimentary lymphoblastic lymphoma (both <150 ng/l); one had alimentary small lymphocytic lymphoma and chronic neutrophilic cholangitis (200 ng/l); one cat had intestinal inflammation, pancreatitis and diabetes mellitus (<150 ng/l); one cat had chronic neutrophilic cholangitis and severe intestinal inflammation (<150 ng/l); and one cat had only chronic neutrophilic cholangitis (224 ng/l).
Table 1.
Clinical features of 11 cats with hypocobalaminaemia
| Case number | Age | Sex | Breed | B12 (290–1500 ng/l) | Duration signs (mths) | Duodenal WSAVA score (max 30) | Duodenal morphological score (max 9) | Intestinal disease | Pancreatic disease fPLI (2–7 µg/l) TLI (35–130 µg/l) | Hepatic disease | Treatment and outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 yrs | MN | DSH | <150 ng/l | 2 | 3 | 1 | Neutrophilic enteritis | Not measured | No hepatic disease detected | Signs controlled with fenbendazole, diet, pot. amoxicillin for 4 wks. Died 1 year later, unrelated to GI disease |
| 2 | 8 yrs | FN | DLH | <150 ng/l | 1 | 3 | 1 | Lymphoplasmacytic enteritis | Pancreatitis fPLI 8.1 µg/l Diabetes mellitus |
No hepatic disease detected | Signs controlled with diet, fenbendazole and buprenorphine. Died 2 yrs later diabetic ketoacidosis |
| 3 | 4 yrs | FN | Burmese | <150 ng/l | 4 | 12 | 4 | Mixed inflammatory enteritis (lymphocytes, plasma cells, neutrophils, macrophages) | fPLI 1.5 µg/l | No hepatic disease detected | Signs controlled with dietary management alone in the long term, with metronidazole initially for 4 wks |
| 4 | 9 mths | M | Siamese | <150 ng/l | 5 | 1 | 0 | Neutrophilic enteritis | fPLI 6 µg/l | No hepatic disease detected | Signs controlled with ranitidine and sucralfate for 4 wks, diet for 6 mths. No treatment 12 mths later |
| 5 | 5 yrs | MN | Persian | <150 ng/l | 6 | 10 | 7 | Ulcerative enteritis (lymphocytic plasmacytic) and villous atrophy | Not measured | Chronic neutrophilic cholangitis, portal fibrosis, bile duct proliferation | Signs controlled with metronidazole, prednisolone and diet for 12 mths then lost to follow-up |
| 6 | 7 mths | FN | Siamese | <150 ng/l | 6 | 2 | 1 | Lymphoplasmacytic enteritis | TLI 136 µg/l | No hepatic disease detected | Signs controlled with fenbendazole, diet for 2 mths, then prednisolone and metronidazole for 2 yrs |
| 7 | 8 yrs | MN | BSH | <150 ng/l | 12 | 1 | 0 | Neutrophilic enteritis | TLI 44 µg/l | No hepatic disease detected | Poor response to diet, prednisolone, metronidazole for 4 wks, chlorambucil for 2 wks then euthanasia |
| 8 | 11 yrs | MN | DSH | <150 ng/l | 1.5 | Not applicable | 2 | Lymphoblastic lymphoma | Not measured | No hepatic disease detected | Euthanasia |
| 9 | 13 yrs | MN | DSH | 200 ng/l | 3 | Not applicable | 7 | Small lymphocytic lymphoma | Not measured | Chronic neutrophilic cholangitis, portal fibrosis, bile duct proliferation | Euthanasia |
| 10 | 3 yrs | M | DSH | <150 ng/l | 7 | Not applicable | 8 | Lymphoblastic lymphoma | Not measured | No hepatic disease detected | Euthanasia |
| 11 | 7 yrs | FN | Singapura | 224 ng/l | 0.5 | 0 | 0 | No enteritis detected | fPLI 2.6µg/l | Chronic neutrophilic cholangitis | Pot. amoxicillin, enrofloxacin for 6 wks. No signs 18 mths later |
fPLI = feline pancreatic lipase immunoreactivity, TLI = trypsin-like immunoreactivity, MN = male neutered, DSH = domestic shorthair, pot. amoxicillin = potentiated amoxicillin, FN = female neutered, DHL = domestic longhair, M = male, BSH = British Shorthair
Twenty-eight cats were normocobalaminaemic. Twenty-one cats had intestinal inflammation alone; one of these cats was positive for feline immunodeficiency virus and Isospora species infection; two cats had alimentary lymphoblastic lymphoma; one cat had intestinal inflammation and mild hepatic fibrosis; one cat had intestinal inflammation and pancreatitis; one cat had intestinal inflammation and chronic neutrophilic cholangitis with marked fibrosis; one cat had intestinal inflammation and a biliary cystadenoma; and one cat had intestinal inflammation, chronic neutrophilic cholangitis and pancreatitis.
Histopathological diagnosis
Duodenal biopsies were obtained endoscopically in 32 cats and surgically in seven cats. Of the 39 cats, 33 were diagnosed with intestinal inflammation and five with alimentary lymphoma. One cat was diagnosed with chronic neutrophilic cholangitis alone based on histopathological examination of hepatic, pancreatic and duodenal biopsy samples; however, ileal samples were not collected. Seven of the 33 cats with intestinal inflammation and one of the cats with alimentary lymphoma had either concurrent hepatobiliary and/or pancreatic disease (Table 1).
Histopathological scores and cobalamin levels
Of the 33 cats with intestinal inflammation, the cumulative duodenal histopathological scores ranged from 0 to 12 (median = 3) out of a possible 30. There was no correlation between the severity of inflammation, based on histopathological score, and cobalamin concentration (r = -0.18, r2 = 0.03, P = 0.30) (Figure 1).
Figure 1.
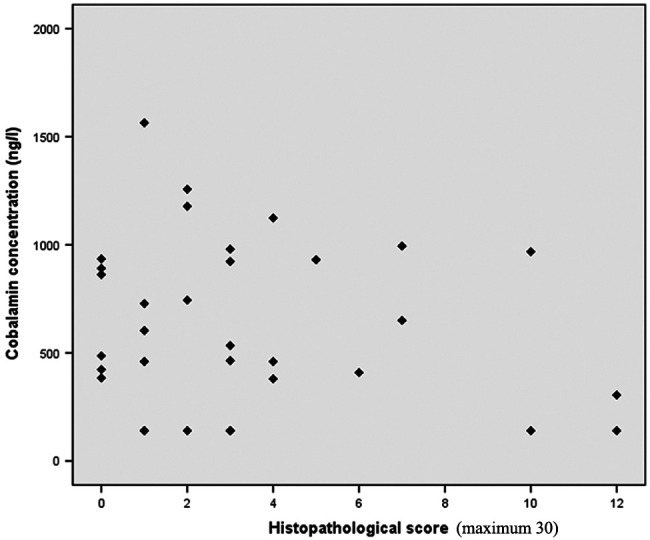
Correlation between histopathological score and serum cobalamin concentration in 33 cats with intestinal inflammation
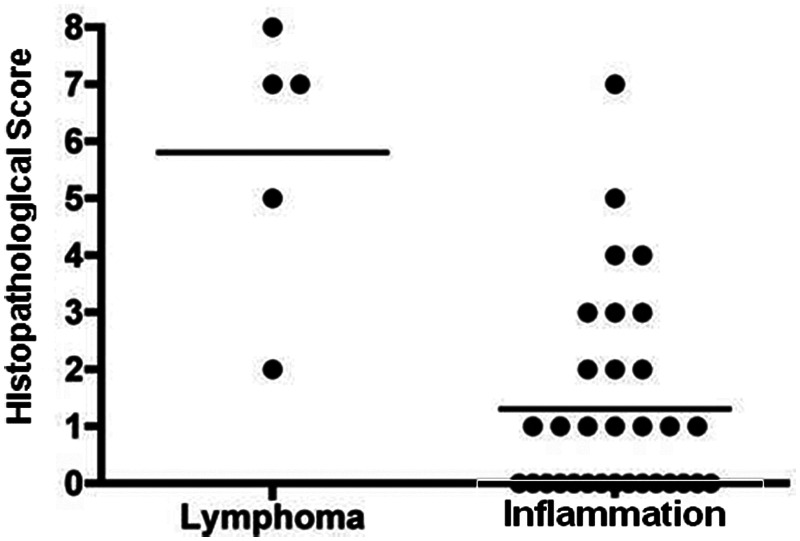
For the five cats with alimentary lymphoma, the cumulative morphological histopathological scores ranged from 2 to 8 (median = 7) out of a possible 9. The number of cats with lymphoma was too small to assess for statistical correlations.
The three morphological characteristics used to assess the microscopical changes in intestinal morphology in lymphoma were also applied to the 33 cats with intestinal inflammation. Using this assessment, the morphological scores ranged from 0 to 7 (median =1). If the samples from the inflammatory and lymphoma populations were compared, there was a significantly higher score in the median morphological histopathological score in those cats with lymphoma (P = 0.001) (Figure 2).
Figure 2.
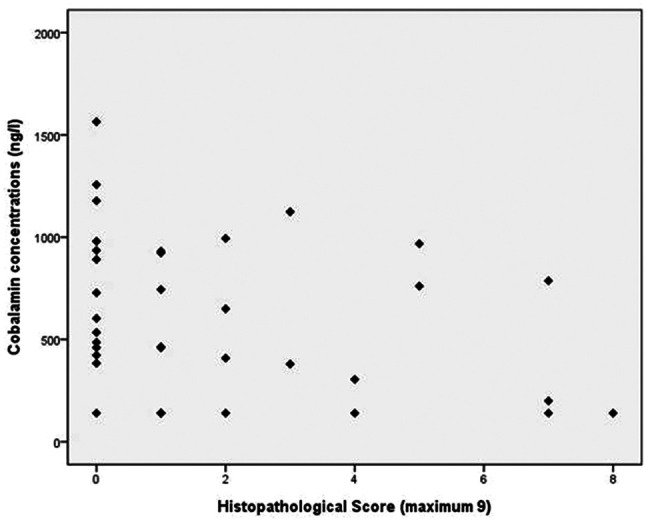
Comparison of morphological histopathological score between cats with intestinal inflammation (n = 33) and lymphoma (n = 5)
The morphological histopathological scores from cats with both intestinal inflammation and lymphoma were then grouped together but there was still no significant correlation with cobalamin concentration (r = −0.30, r2 = 0.09, P = 0.07) (Figure 3).
Figure 3.
Correlation between morphological histopathological score and cobalamin concentration in cats with intestinal inflammation (n = 33) and cats with lymphoma (n = 5)
Five cats had ileal biopsies collected during exploratory laparotomy. These were reviewed in order to compare the findings with those from duodenal samples. Of these five cats, one had alimentary small lymphocytic lymphoma diagnosed on examination of both duodenal and ileal samples. The severity of histopathological change, based on morphological score, was comparable (7 out of a maximum possible score of 9 for the duodenum and 4 out of a possible maximum score of 9 for the ileum). Two cats had an absence of ileal inflammation (histopathological score = 0), minimal inflammation in their duodenal biopsies (histopathological scores of 0 and 3) and normal cobalamin concentrations. The two remaining cats had minimal ileal inflammation (histopathological score = 1) but marked duodenal changes (histopathological scores 10 and 12). One of these cats was also hypocobalaminaemic but the presence of concurrent chronic neutrophilic cholangitis may explain the altered cobalamin concentration in the absence of ileal inflammation.
Duration of clinical signs and cobalamin levels
The duration of clinical signs for the 33 cats with intestinal inflammation ranged from 0.1 to 12 months (median 4 months). There was no correlation between serum cobalamin concentrations and duration of clinical signs (r = 0.26, r2 = 0.07, P = 0.13) (Figure 4).
Figure 4.
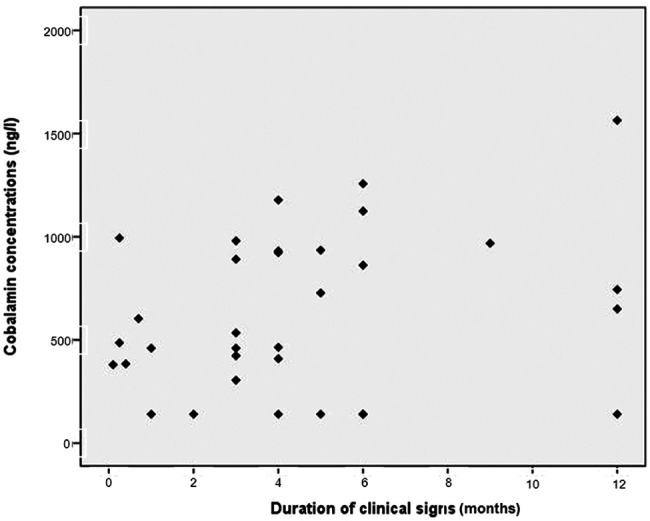
Correlation between duration of clinical signs and cobalamin concentration in 33 cats with intestinal inflammation
The duration of clinical signs for the five cats with alimentary lymphoma ranged from 0.5 to 7 months (median 1.5 months). No clear association between the duration of clinical signs and cobalamin concentrations could be determined given the small number of cases.
The duration of clinical signs in the two groups of cats were compared. There was no statistical difference in the distribution of the duration of clinical signs (P = 0.294).
Cats with intestinal inflammation and lymphoma were then assessed together and the correlation of serum cobalamin with duration of clinical signs analysed. This was not significant (r = 0.32 and r2 = 0.10, P = 0.05) (Figure 5).
Figure 5.
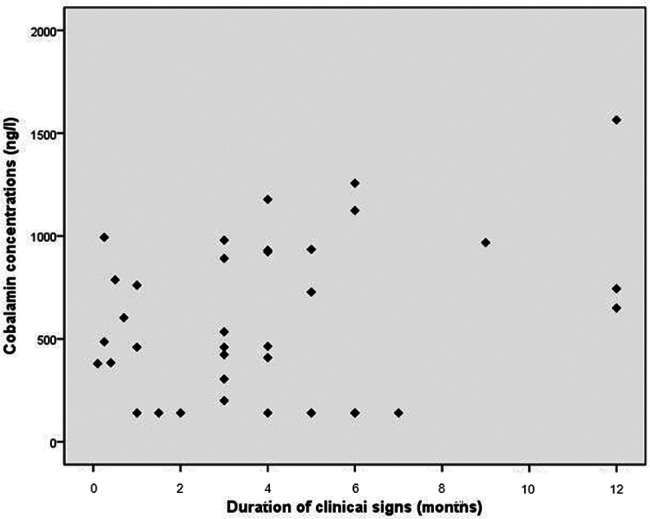
Correlation between duration of clinical signs and cobalamin concentration in cats with intestinal inflammation (n = 33) and lymphoma (n = 5)
Discussion
The tendency for cats to develop intestinal disease, pancreatic disease and biliary disease, often concurrently, makes them susceptible to developing hypocobalaminaemia. Simpson et al 13 demonstrated hypocobalaminaemia in cats with disease in several organ systems but was unable to attribute the hypocobalaminaemia to a specific disease entity. With chronic enteropathies there is presumed reduced absorption of IF–cobalamin complexes in the distal small intestine.
Cats have a more rapid turnover of cobalamin compared to humans. The biological t1/2 of cobalamin in people is approximately 1 year and signs of deficiency do not develop for several years after the onset of cobalamin malabsorption. The turnover of cobalamin in cats is more rapid, with an estimated t1/2 of 11–14 days in four healthy cats and an estimated half-life of 4.5 days and 5.5 days in two cats with intestinal inflammation and pancreatitis, respectively. 13 This is the basis for the current recommendation for weekly supplementation. Given the reduced ability of cats to store cobalamin, reserves of cobalamin could become depleted within 1 month in cases of severe malabsorption. Therefore, the aim of the present study was to look for a relationship between the serum cobalamin concentration and the severity of intestinal pathology or with the duration of clinical signs. The association between body condition score or body weight and cobalamin concentrations has been investigated previously. 12 Increased serum fPLI concentrations have also been shown to be negatively associated with cobalamin concentrations. 16 However, there have been no previous studies evaluating the severity of intestinal disease, based on a histopathological assessment, or the chronicity of disease and cobalamin concentrations.
Two UK studies of the prevalence of feline hypocobalaminaemia have reported contrasting results. Ibarrola et al found few cases, with only 1/682 samples submitted over a 3-year period recording a result below the reference interval. 11 The second study documented 16.5% of cats with GI disease as having low serum cobalamin concentrations. 12 In the present study, 28.2% (11/39 cats) with GI signs were shown to have low serum cobalamin concentrations using a single methodology to determine the cobalamin concentration. 1 Previous studies have either used a radioimmunoassay or a combination of methodologies. Low serum cobalamin concentrations do not necessarily directly reflect cellular cobalamin deficiency and true cellular deficiency of cobalamin is best assessed by measuring the serum concentration of methylmalonic acid (MMA). However, a previous study demonstrated that, with the methodology used in this study, a serum cobalamin concentration of ≤160 ng/l predicted the presence of significantly elevated serum MMA concentration (ie, cellular cobalamin deficiency) with a sensitivity of 74% and specificity of 80%. 1 The prevalence of hypocobalaminaemia (28.2%) in this study is still lower than the 61% (49/80) documented in the USA. 13 The same assay was used and so the age of the feline population studied, dietary factors and genetic variation may be explanations for the difference. Age has been shown to be inversely correlated with serum cobalamin concentrations in healthy cats; however, this correlation is hard to interpret in a sick cat population.17,18 The possibility of breed variation in serum cobalamin concentrations was suggested by Ibarrola et al who found significantly higher concentrations in Birman and Maine Coon cats. 11 Like Reed et al, there was also an over-representation of purebred cats in the present study (53.6%). This may reflect a willingness of purebred cat owners to pursue investigation or a breed susceptibility to GI disease. Hyperthyroidism has been associated with low serum cobalamin concentrations 19 but none of the cats in the present study was hyperthyroid. The possibility of occult hyperthyroidism cannot be excluded.
In this group of 39 cats, hypocobalaminaemia was found in those with intestinal inflammation or lymphoma, with or without concurrent pancreaticobiliary disease, and, on one occasion, in the absence of intestinal pathology on duodenal biopsy.
A variety of types of inflammation were noted, with lymphoplasmacytic enteritis being the most common diagnosis. Intestinal inflammation can only be termed idiopathic inflammatory bowel disease (IBD) when known underlying causes of inflammation, such as dietary allergies, are excluded. We, therefore, deliberately chose not to classify these cases as having idiopathic IBD. Hypocobalaminaemia might arise from the primary disease process leading to inflammation or from the inflammatory change itself.
The duodenal histological changes were scored using the WSAVA Gastrointestinal Standardization Group template and a numerical value was determined to enable statistical comparison. However, it must be recognised that the template is not a weighted scoring system and it is not yet clear whether all histopathological features are of equal importance in the diagnosis of intestinal inflammation. Selected morphological features of the scoring system were also adapted for use in scoring these changes in alimentary lymphoma. Although the template was devised to grade intestinal inflammation some of the architectural changes in the intestinal mucosa are similar in lymphoma and inflammation, although cellular changes in the mucosa are not. No association was found between the cobalamin concentrations and severity of histopathological change. This may reflect the use of duodenal biopsies to assign a score reflecting the severity of mucosal changes. Casamian-Sorrosal et al 15 suggested the correlation in histopathological changes between biopsy samples of duodenum and ileum in dogs was poor. A recent study comparing endoscopic duodenal and ileal biopsy samples in cats 20 documented intestinal inflammation in both duodenal and ileal biopsy samples in 19/33 cats (58%) but poor agreement in cases of small cell lymphoma, with a positive diagnosis in only 3/18 cats (17%). Of the five cats that had ileal biopsy samples collected, the duodenal and ileal histopathological scores were comparable in two cats with intestinal inflammation and in one cat with lymphoma. Two cats had discordant histopathological scores but the overall numbers were too small to allow statistical analysis.
In cats with alimentary lymphoma, the severity of histopathological change was significantly greater, but this did not appear to correlate with cobalamin concentrations, with only 3/5 cats documented as hypocobalaminaemic. The correlation of either histopathological change or duration of disease with serum cobalamin concentrations may be confounded in lymphoma: the marked severity of histopathological change would be anticipated to cause hypocobalaminaemia in almost all cases of lymphoma, but the rapid progression of disease, particularly in large cell lymphoma, may mean that insufficient time has elapsed for the cobalamin store to be depleted by the time of diagnosis. It is interesting to note that the two cats with a short clinical history (<4 weeks) had normal serum cobalamin concentrations and the three cats with a history of 6 weeks or more were all hypocobalaminaemic. One cat diagnosed with lymphoblastic alimentary lymphoma had a surprisingly long clinical history and may represent a case of lymphocytic lymphoma that developed blast transformation or of intestinal inflammation that later transformed to alimentary lymphoma.
Interestingly, there were two cats with cholangitis and hypocobalaminaemia in the present study. One of these cats had concurrent alimentary lymphoma that confounded interpretation, but the other cat had no other histopathological evidence of concurrent disease (enteropathy or pancreatitis). This cat also had an acute clinical presentation (2 weeks). It is not entirely clear in cases of cholangitis why hypocobalaminaemia develops, as a lack of enterohepatic recycling should limit exhaustion of cobalamin stores. The presence of undiagnosed concurrent ileal disease cannot be excluded in this patient given only duodenal biopsy samples were obtained, but cases of cholangitis may be worthy of further study.
Measurement of serum MMA concentrations is not readily available in the UK at present and so the serum concentration of cobalamin must be used as a guide to the likelihood of cellular deficiency. There is no direct proof that the chemiluminescent assay gave clinically relevant results, as serum MMA concentrations were not measured because of available serum sample size. However, we used a laboratory whose methodology has previously been validated against the presence or absence of MMA.
Cobalamin absorption occurs in the distal small intestine and therefore ileal biopsy would be the ideal way to investigate the presence of significant GI disease influencing cobalamin absorption. Only duodenal biopsies were obtained in most cases as it is usually assumed that infiltrative disease is diffuse and the changes in the distal small intestine are reflected in duodenal biopsies. Only five cats had ileal biopsies and these were collected at exploratory laparotomy when samples from liver, pancreas or lymph node were obtained concurrently to detect the presence of comorbid disease. The technicalities of lower GI endoscopy (patient preparation with laxative and enemas, longer duration of general anaesthesia and the technical difficulties in passing through the ileocaecocolic junction) meant this was not performed routinely.
Finally, there was no control group of cats in our study as under UK Home Office regulations we were unable to obtain biopsies for histopathological analysis and grading from clinically normal animals.
Hypocobalaminaemia was found in 11 (28.2%) of the 39 cats in the present study and associated most commonly with alimentary lymphoma and the most severe grade of histological intestinal inflammation. There was no statistically significant correlation between serum cobalamin concentrations and histopathological score or duration of clinical signs but further studies with larger numbers of cats might help establish if a causal relationship exists.
Acknowledgments
We would like to acknowledge the staff at the GI Laboratory and Steve Dodkin from Bristol University for their technical support; Dr Peter Graham (NorthWest Laboratories) for helpful advice, Drs Séverine Tasker and Jane Murray (University of Bristol) for help with statistical analysis; the clinicians and support staff of the Feline Centre at Langford Veterinary Services for managing clinical cases. We also acknowledge the referring veterinary surgeons for referring the cats that participated in this study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
JMS and JSS are members of the GI Laboratory at Texas A&M University, which offers a commercial serum cobalamin assay.
Accepted: 13 April 2012
References
- 1. Ruaux CG, Steiner JM, Williams DA. Relationships between low serum cobalamin concentrations and methylmalonic acidaemia in cats. J Vet Intern Med 2009; 23: 472–445. [DOI] [PubMed] [Google Scholar]
- 2. Ruaux CG, Steiner JM, Williams DA. Early biochemical and clinical responses to cobalamin supplementation in cats with signs of gastrointestinal disease and severe hypocobalaminaemia. J Vet Intern Med 2005; 19: 155–160. [DOI] [PubMed] [Google Scholar]
- 3. Fyfe JC. Feline intrinsic factor (IF) is pancreatic in origin and mediates ileal cobalamin absorption. J Vet Intern Med 1993; 7: 133. [Google Scholar]
- 4. Hornbuckle EH, Simpson KW, Tennant BC. Gastrointestinal function. In: Kaneko JJ, Harvey JW, Bruss ML. (eds). Clinical biochemistry of domestic animals. 6th ed. Missouri: Elsevier, 2008, pp 432–433. [Google Scholar]
- 5. Ruaux CG. Cobalamin and gastrointestinal disease. J Vet Intern Med 2002; 16: 318–389. [DOI] [PubMed] [Google Scholar]
- 6. Hall EJ, German AJ. Diseases of the small intestine. In: Ettinger SJ, Feldman EC. (eds), Textbook of veterinary internal medicine. 7th ed. St Louis: Saunders Elsevier, 2010, pp 1526–1572. [Google Scholar]
- 7. Fyfe JC, Giger U, Hall CA, et al. Inherited selective intestinal cobalamin malabsorption and cobalamin deficiency in dogs. Pediatr Res 1991; 1: 24–31. [DOI] [PubMed] [Google Scholar]
- 8. Morgan LW, McConnell J. Cobalamin deficiency associated with erythroblastic anemia and methyl malonic aciduria in a border collie. J Am Anim Hosp Assoc 1999; 5: 392–395. [DOI] [PubMed] [Google Scholar]
- 9. Fordyce HH, Callan MB, Giger U. Persistent cobalamin deficiency causing failure to thrive in a juvenile Beagle. J Small Anim Pract 2000; 9: 407–410. [DOI] [PubMed] [Google Scholar]
- 10. Battersby I A, Giger U, Hall EJ. Hyperammonaemic encephalopathy secondary to selective cobalamin deficiency in a juvenile Border collie. J Small Anim Pract 2005; 7: 339–344. [DOI] [PubMed] [Google Scholar]
- 11. Ibarrola P, Blackwood L, Graham PA, et al. Hypocobalaminaemia is uncommon in cats in the United Kingdom. J Feline Med Surg 2005; 6: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reed N, Gunn-Moore D, Simpson K. Cobalamin, folate and inorganic phosphate abnormalities in ill cats. J Feline Med Surg 2007; 4: 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simpson KW, Fyfe J, Cornetta A, et al. Subnormal concentrations of serum cobalamin (vitamin B12) in cats with gastrointestinal disease. J Vet Intern Med 2001; 15: 26–32. [DOI] [PubMed] [Google Scholar]
- 14. Day MJ, Bilzert T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008; 138: S1–S40. [DOI] [PubMed] [Google Scholar]
- 15. Casamian-Sorrosal D, Willard MD, Murray JK, et al. Comparison of histopathological findings in biopsies from the duodenum and ileum of dogs with enteropathy. J Vet Intern Med 2010; 24: 80–83. [DOI] [PubMed] [Google Scholar]
- 16. Bailey S, Benigni L, Eastwood J, et al. Comparisons between cats with normal and increased fPLI concentrations in cats diagnosed with inflammatory bowel disease. J Small Anim Pract 2010; 9: 484–489. [DOI] [PubMed] [Google Scholar]
- 17. Barron PM, Mackie JT, Evans NA, Langer N. Serum cobalamin concentrations in healthy cats and cats with non-alimentary illness in Australia. Aust Vet J 2009; 7: 280–283. [DOI] [PubMed] [Google Scholar]
- 18. Parnell NK, Moore GE, Suchodolski JS, Steiner JM. Influence of age on serum cobalamin and folate concentrations in healthy cats. J Vet Intern Med 2008; 22: 687–824. [Google Scholar]
- 19. Steiner JM, Peterson MA, Ruaux CG, et al. Serum cobalamin and folate concentrations in cats with hyperthyroidism. J Vet Intern Med 2005; 19: 386–488. [Google Scholar]
- 20. Scott KD, Zoran DL, Mansell J, et al. Utility of endoscopic biopsies of the duodenum and ileum for diagnosis of inflammatory bowel disease and small cell lymphoma in cats. J Vet Intern Med 2011; 25: 1253–1257. [DOI] [PubMed] [Google Scholar]


