Abstract
This retrospective study evaluated the use of lomustine as a rescue agent for 39 cases of resistant feline lymphoma. Parameters assessed included lymphocyte cell size, number of previous chemotherapy drugs and number of previous chemotherapy protocols received, time from lymphoma diagnosis to initiation of lomustine therapy, body weight and anatomic location of lymphoma. Cell size, number of previous chemotherapy drugs, number of previous chemotherapy protocols and anatomic location were all significant prognostic factors for the progression-free interval. Twenty-one cats (54%) received more than one dose of lomustine. The overall median progression-free interval (MPFI) was 39 days (range 7—708 days). The MPFI for large versus small and intermediate cell lymphomas was 21 versus 169 days, respectively. The MPFI for gastrointestinal versus non-gastrointestinal lymphomas was 180 versus 25.5 days, respectively. Lomustine has an acceptable efficacy and safety for use as a rescue agent in feline lymphoma.
Introduction
Cyclohexylchlorethylnitrosourea, 1-(2-chloroethyl)-3- cyclohexcyl-1-nitrosurea (CCNU), also known as lomustine, is a nitrosourea monofunctional alkylating agent. Lomustine damages DNA and RNA and is partially cross resistant with other alkylating agents. 1 Owing to its high lipid solubility and relative lack of ionization at physiologic pH, it is one of the few chemotherapy agents known to cross the blood–brain barrier. 1 The cytochrome P450 system and hydroxylation are both thought to be involved in the metabolism of this drug and may play a part in the toxicity profile. 1 Lomustine has a delayed hematopoietic depression profile, particularly in human and feline patients which show a neutrophil nadir at 2–6 weeks post-administration. 1 It has been used to treat brain tumors, metastatic melanomas and lymphoma in humans.2–4
In dogs, lomustine has been used both as a single agent and in multi-drug protocols for the treatment of mast cell tumors, brain tumors, epitheliotrophic and non-epithetheliotrophic lymphomas.5–8 To date, there is limited information on the use of lomustine in cats. Rassnick et al 9 conducted a phase I study evaluating the effect of single dose lomustine in 25 tumor-bearing cats. Five out of 17 cats with lymphoma and one cat with a mast cell tumor demonstrated a measurable response. 9 Fan et al 10 evaluated administration (1–12 doses) and toxicity of lomustine in tumor-bearing cats. Five cats were found to have a partial response: two with lymphoma, two with fibrosarcoma and one with multiple myeloma. 10 Komori et al 11 evaluated lomustine in a feline case of cutaneous non-epitheliotropic lymphoma, which resolved within 3 months of starting treatment.
The purposes of this study were: (i) to evaluate the use of lomustine as a rescue agent for feline resistant lymphoma; (ii) to determine prognostic factors for progression-free interval; and (iii) to detail toxicities noted in the course of this study. The following parameters were evaluated: lymphocyte size, number of previous chemotherapy drugs received, number of previous chemotherapy protocols used, time from diagnosis to initiation of lomustine therapy, body weight and anatomic location of the lymphoma. To our knowledge, this is the first study evaluating lomustine as a rescue agent in cats with resistant lymphoma.
Materials and methods
The medical database of the Veterinary Cancer Group was searched for feline patients who received lomustine between 1 January 2007 and 30 June 2009. Inclusion criteria included histological or cytological confirmation of lymphoma, rescue agent lomustine therapy, an initial complete blood count (CBC) and follow-up data at least 1 week post-lomustine administration. Additional information obtained included age, sex, breed, weight, size of lymphocyte affected, feline leukemia virus (FeLV)/feline immunodeficiency virus (FIV) status (when available) and chemistry values (when available). Cases were categorized by cell size (large versus small and intermediate cell), number of previous chemotherapy drugs received (1–6 versus 7 or more), number of previous chemotherapeutic protocols (1–2 versus 3–4), time from lymphoma diagnosis to start of lomustine therapy and lymphoma location (gastrointestinal versus non-gastrointestinal). Cell size was obtained from the pathology reports and determined by the pathologist based on comparison of lymphocyte and red blood cell sizes. Lymphomas were not immunophenotyped. Final (binomial) categories were chosen based on clinical expertise and comparison with models developed using original categorization.
Cats were treated orally with a lomustine dose range of 5–15 mg (31.6–72.2 mg/m2), with a median dose of 10 mg/cat (44 mg/m2) every 3–4 weeks until disease progression was noted. Dose and dose intervals were based on the cat’s body weight, response to therapy, development of side effects [leukopenia, gastrointestinal (GI) upset] and owner’s compliance.
Evaluation of response and toxicity
Response to therapy was based on complete physical examinations, measurements of masses, laboratory data (CBC and serum chemistry panels) and clinical signs noted by owners between visits. Objective response to treatments was difficult to assess in many cases as imaging was not routinely performed. Therefore, we did not report clinical remission, partial remission, stable disease and progressive disease typical of many papers. Progression-free interval (PFI) was the main focus of this study and defined as the time from when an animal was placed on the lomustine protocol to subsequent progression of disease necessitating a protocol change or euthanasia. PFI was the endpoint of our study. Evaluation of hematologic, biochemical and clinical toxicity was conducted when data was available. Toxicity data was graded for alanine transaminase (ALT), creatinine, blood urea nitrogen (BUN) and platelets according to the VCOG-CTCAE scoring system. 12 The hematocrit (HCT), rather than packed cell volume (PCV) was recorded in the medical record and a modified VCOG-CTCAE score was used (Table 1). The total white blood count (WBC) rather than neutrophil counts were available, thus the VCOG-CTCAE scoring system was not applicable to these values. Different ranges were assigned to these values as listed in Table 2.
Table 1.
VCOG-CTCAE values used in this study
| Normal range a | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Platelets 103/µl | 200–500 | 100–<200 | 50–99 | 25–49 | <25 |
| BUN mg/dl | 14–36 | >36–54 | >54–72 | >72–108 | >108 |
| Creatinine mg/dl | 0.6–2.4 | >2.4–3.6 | >3.6–4.8 | >4.8–7.2 | >7.2 |
| ALT IU/l | 10–100 | >100–125 | >125–150 | >150–200 | >200 |
| HCT% b | 29–48 | 25–29 | 20–<25 | 15–<20 | <15 |
Normal ranges obtained for BUN, Creat, and ALT, from Antech Diagnostics Laboratory, Irvine, CA, USA
Modified from VCOG-CTCAE, because the HCT was documented in the medical records of our cases rather than the PCV used to determine grades of anemia in the VCOG article
Normal range obtained for HCT and PLT, from Heska HemaTrue Veterinary Hematology Analyzer, which is the in-house CBC machine used at both locations of the Veterinary Cancer Group where data was collected for this study
ALT = alanine transaminase, BUN = blood urea nitrogen, Creat = creatinine, HCT = hematocrit, PCV = packed cell volume, PLT = platelets
Table 2.
White blood cell (WBC) count grading scale
| Normal range a | Leukocytosis | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|---|
| WBC 103/µl | 3.5–16 | >16 | 2.5–<3.5 | 1.5–<2.5 | <1.5 |
Normal range obtained from Antech Diagnostic Laboratory, Irvine, CA, USA
Cox proportional hazards regression was used to assess the effect of various explanatory factors on risk of disease progression. Proportional hazards assumptions were tested and the following covariates were analyzed: cell size (large versus non-large), lymphoma location (GI versus non-GI), previous chemotherapy drugs, previous chemotherapy protocols, delay before starting CCNU and weight at the start of CCNU. Covariates were sequentially removed and tested for goodness of fit using χ2 difference tests (α = 0.05). Owing to the small number of observations at each level of the ordinal variables ‘previous drugs’ and ‘previous protocols’, and in the interest of simplifying the presentation and discussion of results, models using binomial (dichotomized) versions of these variables were tested in a similar manner. For example, after graphically examining PFI by the number of previous drugs we selected ≤6 and >6 as new categories and compared models using this categorization to the original data. Previous protocols were dichotomized in a similar way. Lymphoma cell size and disease location were specified as binomial in the a priori hypothesis as there were limited observations for several subcategories of these variables. Covariance was analyzed using stratified Cox proportional hazards models. Kaplan-Meier graphs were plotted for overall data and for each significant variable from the previous analysis. The three cats without cell size classification were excluded from statistical analysis. All statistical analyses were performed using R version 2.12. 13
Results
Thirty-nine cats were assessed during this study. The median time from diagnosis to the start of lomustine therapy was 158 days (range 28–2139 days). The median dose of lomustine was 10 mg and the median number of doses administered was two. Eighteen cats received a single treatment of lomustine, eight cats received 2–4 doses, nine cats received 5–9 doses and four cats received greater than 10 doses. Twenty-one cats (54%) received more than one dose of lomustine. The median progression free interval of all 39 cats in this study was 39 days (range 7–708 days). This number included six cases lost to follow-up, which were censored at their last known date of contact. Thirty-three cats (84.6%) were euthanased or died during this study period.
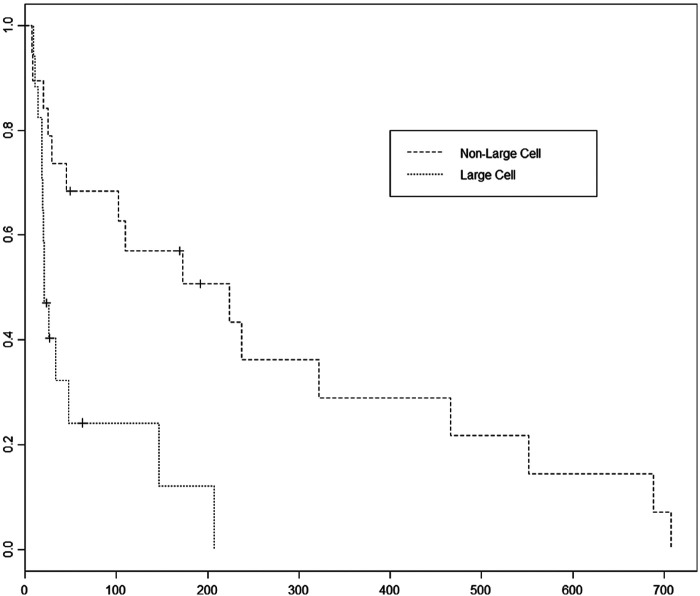
Thirty-nine cats met the inclusion criteria: 26 domestic shorthair (DSH), four domestic mediumhair (DMH), six domestic longhair (DLH) and one each of Russian Blue, American Shorthair and Oriental Shorthair. There were 19 castrated males, 18 spayed females, one intact male and one intact female. Median age was 10.6 years (range 5–17 years). Median weight was 3.8 kg (range 1.94–6.36 kg). The retroviral infection status was known for nine cats with one being FIV positive and one being FeLV positive. Diagnosis of lymphoma was obtained from biopsies in 28 cases and by cytology in the remaining 11 cases. Sixteen cats had small cell lymphoma, three cats had intermediate cell lymphoma, 17 had large cell lymphoma and three cats were not classified as to the lymphocyte cell size in the medical records. Cases with large cell lymphomas were 9.8 times as likely to have disease progression compared with those with non-large cell size lymphoma (P = 0.000491) (Figure 1). The median PFI (MPFI) for large versus non-large cell cases was 21 versus 169 days, respectively.
Figure 1.
Progression-free interval comparison between large cell lymphoma and non-large cell (small and intermediate) lymphoma cases. Large cell cases had a 9.8 times risk of disease progression compared to the small and intermediate cell cases (P = 0.00049). The median PFI of large cell cases was 21 days compared to 169 days for non-large cell cases
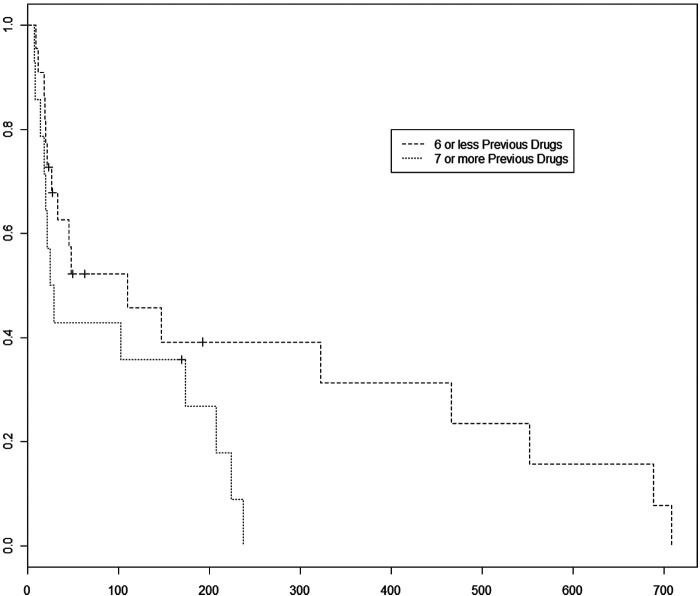
Cats received a median of six different chemotherapy drugs prior to initiation of lomustine therapy (range 1–9). Previous chemotherapy drugs included L-asparaginase, vincristine, cyclophosphamide, doxorubicin, methotrexate, mechlorethamine, vinblastine, procarbazine and chlorambucil. All cats had received some form of corticosteroids during their previous protocols and, as such, the effect of corticosteroids administered on PFI could not be assessed in this study. No cats had received corticosteroids as monotherapy prior to receiving lomustine. Thirty-six cats remained on corticosteroids during their lomustine therapy. Cats who received more than six chemotherapy agents prior to initiation of lomustine therapy were 3.8 times as likely to have disease progression during this study (P = 0.009015) (Figure 2). The MPFI for 1–6 versus ≥7 previous chemotherapy drugs was 46.5 versus 27 days, respectively.
Figure 2.
Progression-free interval comparison between patients receiving ≤6 previous chemotherapy drugs versus patients receiving ≥7 previous chemotherapy drugs. Cases that received more than six previous chemotherapy drugs had a 3.8 times increased risk of disease progression compared to cases receiving 1–6 previous chemotherapy drugs (P = 0.009). The median PFI for cases receiving 1–6 previous drugs was 46.5 days versus 27 days if they received more than six drugs
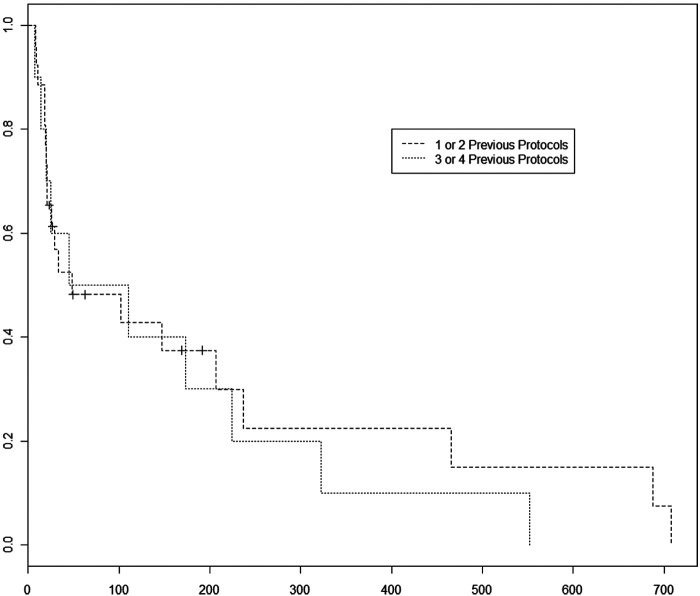
Cats in this study received an average of two previous chemotherapy protocols (range 1–4). Ten cats received one previous protocol, 18 cats received two previous protocols, eight cats received three previous protocols and three cats received four previous protocols. Cats that received three or four prior protocols were 3.6 times as likely to have disease progression during this study than cats receiving one or two prior protocols (P = 0.02) (Figure 3). The MPFI for 1–2 protocols versus 3–4 protocols was 31 versus 77.5 days, respectively.
Figure 3.
Progression-free interval comparison between patients receiving one or two previous protocols before beginning lomustine versus patients receiving three or four previous protocols before beginning lomustine. Cats that received 3–4 previous chemotherapy protocols were 3.6 times as likely to have disease progression compared to the cats that received 1–2 previous protocols (P = 0.02). The median PFI for cats that received 1–2 protocols was 31 days versus 77.5 days for cases receiving 3–4 previous protocols. However, if the cats survived past the median point, the cases with 1–2 previous protocols went on to stay on the protocol longer
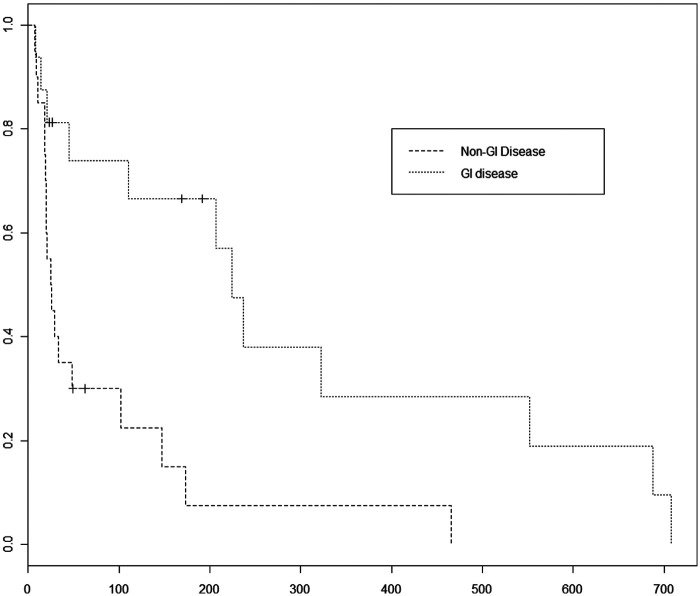
Seventeen cats had gastrointestinal lymphoma and nine cats had diffuse abdominal lymphoma. Eight cats were classified as having extra-nodal lymphoma, two cats had nodal lymphoma, two cats had lymphoma in multiple diverse sites and a single cat had nasal lymphoma. Nasal lymphoma was in its own category initially because, historically, cats have a more favorable response in this location than seen in other disease locations. However, owing to lack of numbers in each location, categories of GI versus non-GI locations were statistically evaluated. Cats with non-GI lymphoma were 4.7 times as likely to have progression of disease as cats with GI lymphoma (P = 0.001876) (Figure 4). The MPFI for GI versus non-GI locations were 180 versus 25.5 days, respectively. Stratified models resulted in inferior fits and provided no further significant differences.
Figure 4.
Progression-free interval comparison between patients with GI lymphoma versus patients with non-GI lymphoma. Cats with non-GI lymphomas had a 4.7 times increased risk of disease progression compared to cases with GI lymphoma (P = 0.0018). The median PFI for cats with GI lymphoma was 180 days versus 25.5 days for cats with non-GI lymphoma
Toxicity assessment was a tertiary endpoint of this study. Thirty non-hematological adverse events were noted. Gastrointestinal adverse events were most commonly reported, including nine vomiting, five diarrhea, two decreased appetites and two gagging/throat-clearing episodes. Other reported concerns included two with pleural effusion, two with fever and one of each of the following: retroperitoneal effusion, swollen face, cough and ocular ulceration. One cat had grade 2 vomiting and diarrhea which necessitated hospitalization for intravenous fluids. The cat recovered and went on to receive additional dosages of lomustine.
CBC parameters were evaluated for evidence of toxicity. All cats had baseline thrombocyte counts noted at their first dose of lomustine with a range of 19–626 103/µl. Eighteen cats had thrombocytopenia prior to the first dose of lomustine. Ten cats had a grade 1 thrombocytopenia, four had grade 2, two had grade 3 and two had grade 4. During the study there were a total of 62 episodes of thrombocytopenia noted with 50 episodes of grade 1, 10 episodes of grade 2 and two episodes (3% of total thrombocytopenic events) of grade 3. None required hospitalization (Table 1).
All cats had baseline WBC counts recorded at the first dose of lomustine administration with a range of 2.7–182 103/µl. Eleven cats had an initial leukocytosis while one cat developed a leukocytosis during the study. During the study, there were 27 total episodes of leukopenia with 14 episodes of grade 1 leukopenia, 10 episodes of grade 2 leukopenia and three episodes of grade 3 leukopenia (refer to Table 2 for modified WBC grades). One cat developed leukopenia after its first dose of lomustine and was euthanased owing to neurologic signs and suspicion of disease progression. Two other cats with grade 3 leukopenias (7% of total leukopenic cases) required hospitalization. One cat developed leukopenia 7 days after its fifth dose of lomustine and was lost to follow-up following discharge from the hospital. The other cat developed leukopenia a week after its third dose of lomustine, was hospitalized on antibiotics and went on to receive additional doses of lomustine in the future. This cat was not treated prophylactically with antibiotics when lomustine was administered in the future.
Pretreatment hematocrit values were documented for all cats and ranged from 12.6% to 38%. Twenty-one cases had an initial anemia noted with nine grade 1, 10 grade 2 and two grade 4. During the study, there were 29 total episodes of anemia noted with 23 episodes of grade 2 and six episodes of grade 3. Only one cat that was not previously anemic developed a grade 2 anemia while on this protocol. The rest of the noted episodes were all associated with previously anemic cats. None required hospitalization (Table 1).
Twenty-six cats had baseline ALT values obtained prior to the initial dose of lomustine administration (range 11–357 IU/l). Prior to lomustine, one cat had a grade 1 elevation, one had a grade 2 elevation and two had grade 4 elevations. During the study there were 23 reported episodes of elevated ALT values in seven patients. Three of these cats had previous ALT elevations noted (one cat with prior ALT elevation did not have follow-up ALT values). Two cats which did not have prior ALT elevations showed elevation while on lomustine and two cats which showed elevated ALT while on the study did not have baseline ALT values noted. Eight episodes of grade 1, seven episodes of grade 2, three episodes of grade 3 and five episodes of grade 4 elevations were recorded. Out of the total number of elevations noted, 21.7% of them were grade 4 (Table 1).
BUN and creatinine values were initially recorded for 12 and 15 cats, respectively. Three cats had grade 1 pretreatment elevations in their BUN. One cat had grade 1 elevation in both BUN and creatinine. One cat with resistant renal lymphoma had grade 4 BUN and grade 3 creatinine pre-treatment elevations. While on lomustine therapy, there were only seven BUN values recorded and four creatinine values. There were seven episodes of grade 1 elevation in BUN (all cases with recorded values) and one episode of grade 1 elevation in creatinine noted. Urinalyses were not available for comparison (Table 1).
Discussion
Twenty-one cats (54%) received more than one dose of lomustine. Although the overall MPFI after starting lomustine was only 39 days, there was a marked improvement in MPFIs for cats with small or intermediate cell lymphoma and cats with lymphoma of the GI tract. Cats with small or intermediate cell lymphoma had a PFI of 169 versus 21 days for cats with large cell lymphoma. Cats with GI lymphoma had a PFI of 180.5 days versus 25.5 days for cats with non-GI locations. Cats with non-large cell lymphoma with a GI location tended to have the longest MPFI (Table 3). The other two variables (previous number of chemotherapy drugs or chemotherapy protocols) did not have as marked a difference in their median PFIs but did provide statistically significant additional information explaining disease progression in this study. Future studies should evaluate the significance of these parameters prospectively, with larger sample sizes in each category. Blocking on one or more variables to ensure a balanced design may also be useful.
Table 3.
Lymphocyte cell size compared to GI versus non-GI lymphoma locations
| Median PFI (in days) for cats with non-GI location | Median PFI (in days) for cats with GI location | |
|---|---|---|
| Large cell lymphoma | 20.5 (n = 5) | 23 (n = 12) |
| Intermediate cell lymphoma | 101 (n = 1) | 322 (n = 2) |
| Small cell lymphoma | 37 (n = 10) | 208 (n = 6) |
PFI = progression-free interval
There is a paucity of literature evaluating lymphoma rescue protocols in our feline patients. Recently, two articles have been published looking at feline rescue protocols. Oberthaler et al 14 looked at doxorubicin-based chemotherapy for relapsing or refractory feline lymphoma. In that study, a 22% response rate was noted in 5/23 cats. Three out of the five responding cats received other drugs in addition to single agent doxorubicin as part of their rescue protocol. None of the responding cats had large cell lymphomas. 14 Our current study had a similar finding of non-large cell cases having a higher response rate and more durable remission durations to their lomustine rescue protocol. Another study by Parshley et al 15 looked at abdominal irradiation as a rescue therapy for feline GI lymphoma and found few, if any, acute effects and a response in 10/11 cats for a median survival of 214 days post-radiation. 15 In this study, the only significant factor affecting survival was body weight.
Thirty-six cats continued to receive a corticosteroid along with lomustine during this protocol, although all 39 had received corticosteroids during their previous protocol. As their disease had progressed while receiving previous chemotherapy with corticosteroids, the likelihood of the responses being attributed to the corticosteroids is decreased. Lomustine’s known list of toxicities include leukopenia, thrombocytopenia, hepatic and renal toxicities. 16 Hepatic failure and idiosyncratic acute renal failure have been documented in the dog.17,18 Pulmonary fibrosis is a less common side effect and has been documented in the cat. 19 In this study, the most commonly noted toxicities included: vomiting, diarrhea, thrombocytopenia, leukopenia and elevated ALT. It is difficult to determine whether the GI effects noted in this study were owing to lomustine or a result of the disease process itself, as most of the cats exhibiting these signs were not in a clinical remission at the time they were noted or had presented with these signs initially. Leukopenia was a prominent finding, with 27 episodes recorded. Two of these cases required hospitalization. While both cats recovered and were discharged from the hospital, these episodes highlight the importance of strict monitoring of the CBC while cats are on this particular drug, especially as the drug has a highly variable duration until neutrophil nadir, ranging between 1 and 6 weeks.
Lomustine has been documented to cause liver damage in humans and canines, whilst it has not been reported in cats to the our knowledge. 17 There were five grade 4 elevations of ALT during this study (21%). Two of these patients did not have baseline ALT measurement, whilst four of the patients had an elevation of their ALT while on the protocol. One cat’s ALT levels vacillated throughout the course of lomustine treatment. This cat eventually suffered liver failure after its twentieth dose of lomustine. A necropsy was not obtained to determine whether hepatic lymphoma was present, or to assess for histologic evidence of hepatotoxicity. With the knowledge of canine hepatic failure, it is imperative to elucidate if cats are at a similar risk. If so, additional studies investigating hepatoprotectants, such as S-adenosylmethionine, might be of benefit, as has been shown in canine patients. 20 Whilst idiosyncratic renal failure has been documented, we observed no cases of renal insufficiency in cats that were evaluated for this adverse effect. Only grade 1 toxicity was noted; however, few samples were assessed during the study and urinalysis were unavailable for a complete renal profile of these patients. Two cases of pleural effusion were noted in this study. The first case was diagnosed with congestive heart failure and therapy was discontinued. The second was diagnosed with mast cell disease and went on to receive additional doses of lomustine.
This study is limited by its retrospective nature. Because of this, it was neither possible to evaluate objective responses to treatment in all patients as multiple clinicians were involved in the clinical assessment of these patients, nor to obtain consistent toxicity data. It is also hard to ensure a balanced distribution of patients among the evaluated groups; hence, we applied binomial re-categorization to better distribute patients into clinically useful observation groups.
This is the first study to evaluate lomustine for resistant lymphoma in cats in a rescue setting. Results here suggest that cell size and anatomic location of lymphoma are significant prognostic factors affecting risk of disease progression in cats with relapsing lymphoma. This study clearly justifies a prospective trial looking at cats with small and intermediate cell lymphoma in various anatomic locations using lomustine as a rescue agent. This study also highlights the need for additional information adding to the toxicity profile of lomustine, use of lomustine in combination therapy, and the optimal dose and schedule for treatment of tumor-bearing cats.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 18 April 2012
References
- 1. Chabner BA, Longo DL. Cancer Chemotherapy and Biotherapy Principles and Practice. 4th edn. Philadelphia: Lippincott Williams and Wilkins, 2006. [Google Scholar]
- 2. Finlay JL, Boyett JM, Yates AJ, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine and prednisone with the eight drugs in 1 day regimen. J Clin Oncol 1995; 13: 112–123. [DOI] [PubMed] [Google Scholar]
- 3. Williams SD, Einhorn LH. Combination chemotherapy with doxorubicin and lomustine. Treatment of refractory Hodgkin’s disease. J Am Med Assoc 1977; 238: 1659–1661. [PubMed] [Google Scholar]
- 4. York RM, Foltz AT. Bleomycin, vincristine, lomustine, and DTIC chemotherapy for metastatic melanoma. Cancer 1998; 1: 61: 2183–2186. [DOI] [PubMed] [Google Scholar]
- 5. Williams LE, Rassnick KM, Power HT, et al. CCNU in the treatment of canine epitheliotropic lymphoma. J Vet Intern Med 2006; 20: 136–143. [DOI] [PubMed] [Google Scholar]
- 6. Risbon RE, de Lorimier LP, Skorupski K, et al. Response of canine cutaneous epitheliotropic lymphoma to lomustine (CCNU): A retrospective study of 46 cases (1999–2004). J Vet Intern Med 2006; 20: 1389–1397. [DOI] [PubMed] [Google Scholar]
- 7. Moore AS, London CA, Wood CA, et al. Lomustine (CCNU) for the treatment of resistant lymphoma in dogs. J Vet Intern Med 1999; 13: 395–398. [DOI] [PubMed] [Google Scholar]
- 8. Saba CF, Hafeman SD, Vail DM, Thamm DH. Combination chemotherapy with continuous L-asparaginase, lomustine and prednisone for relapsed canine lymphoma. J Vet Intern Med 2009; 23: 1058–1063. [DOI] [PubMed] [Google Scholar]
- 9. Rassnick KM, Gieger TL, Williams LE, et al. Phase I evaluation of CCNU (lomustine) in tumor-bearing cats. J Vet Intern Med 2001; 15: 196–199. [DOI] [PubMed] [Google Scholar]
- 10. Fan TM, Kitchell BE, Dhaliwal RS, et al. Hematological toxicity and therapeutic efficacy of lomustine in 20 tumor-bearing cats: critical assessment of a practical dosing regimen. J Am Anim Hosp Assoc 2002; 38: 357–363. [DOI] [PubMed] [Google Scholar]
- 11. Komori S, Nakamura S, Takahashi K, Masahiro T. Use of lomustine to treat cutaneous nonepitheliotropic lymphoma in a cat. J Am Vet Med Assoc 2005; 226: 237–239. [DOI] [PubMed] [Google Scholar]
- 12. Veterinary cooperative oncology group-common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1. Vet Comp Oncol. Epub ahead of print 20 July 2011. DOI: 10.1111/j.1476-5829.2011.00283.x 10.1111/j.1476-5829.2011.00283.x. [DOI] [PubMed] [Google Scholar]
- 13. R Development Core Team. R: A language and environment for statistical computing. http://www.R-project.org/ (2011).
- 14. Oberthaler KT, Mauldin E, McManus PM, et al. Rescue therapy with doxorubicin-based chemotherapy for relapsing or refractory feline lymphoma: a retrospective study of 23 cases. J Feline Med Surg 2009; 11: 259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parshley DL, LaRue SM, Kitchell B, et al. Abdominal irradiation as a rescue therapy for feline gastrointestinal lymphoma: a retrospective study of 11 cats (2001–2008). J Feline Med Surg 2011; 13: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore AS, Frimberger AE. Anticancer drugs and protocols: traditional drugs. In: Bonagura JD, Twedt DC. (eds). Kirk’s current veterinary therapy. Philadelphia, Saunders Elsevier, 2009, pp 309–310. [Google Scholar]
- 17. Pressler BM. Cancer and the kidney. In: Bonagura JD, Twedt DC. (eds). Kirk’s current veterinary therapy XIV. Philadelphia, Saunders Elsevier, 2009, p 930. [Google Scholar]
- 18. Hosoya K, Lord LK, Lara-Garcia A. Prevalence of elevated alanine transaminase activity in dogs treated with CCNU (Lomustine). Vet Comp Oncol 2009; 7: 244–255. [DOI] [PubMed] [Google Scholar]
- 19. Skorpupski KA, Durham AC, Duda L, Sorenmo KU. Pulmonary fibrosis after high cumulative dose nitrosourea chemotherapy in a cat. Vet Comp Oncol 2008; 6: 120–125. [DOI] [PubMed] [Google Scholar]
- 20. Skorupski KA, Hammond GM, Irish AM, et al. Prospective randomized clinical trial assessing the efficacy of denamarin for prevention of CCNU-induced hepatopathy in tumor-bearing dogs. J Vet Intern Med 2011; 25: 838–845. [DOI] [PubMed] [Google Scholar]