Abstract
A spayed 14-year-old female domestic shorthair cat presented with a squamous cell carcinoma of the nasal planum and was treated with intralesional chemotherapy. During nasal infiltrations with cisplatin mixed with the cat’s own serum, a new carcinomatous lesion developed at the medial canthus of the right eye, which was also treated using intralesional chemotherapy. Two months after the treatment course, the cat developed a new mass at the site of the eyelid chemotherapy, which was diagnosed as a soft tissue sarcoma. At the owner’s request, the tumour was marginally excised, but it recurred after 10 months. No lung or lymph node metastases were evident at the time of euthanasia. The histotype of the tumour, the coincidence with injections and the histological description make the hypothesis of an injection-site sarcoma likely. To the authors’ knowledge, this is the first case of an injection-site sarcoma at the site of a cisplatin injection.
Case Report
A spayed 14-year-old female domestic shorthair cat presented with a clinically suspected squamous cell carcinoma (SCC) of the nasal planum. A complete work-up, including a clinical examination of the lesion and the regional lymph nodes (that were normal in size, shape and consistency), thoracic radiographs, a serum biochemical profile, feline immunodeficiency virus (FIV) and feline leukaemia virus (FeLV) tests, a complete blood cell count and a wedge biopsy of the lesion was performed. The histology report described an unencapsulated and poorly demarcated epithelial neoplasia composed of basal cells and mature keratinocytes arranged in clusters infiltrating the dermal tissue. Neoplastic cells with abundant eosinophilic cytoplasm and paracentral nuclei with one amphophilic nucleolus were present. Multifocal foci of central keratinisation within concentric layers of abnormal squamous cells were visible. A high mitotic index (5–6/HPF) and an abundant neutrophilic lymphocytic and plasmacellular inflammatory infiltrate were present. A diagnosis of SCC was formulated and intralesional administration of cisplatin (Cisplatino; Teva) (1.5 mg/cm3 of lesion mixed with an equal volume of the cat’s own blood serum) was performed because the tumour was too large for surgical excision and the owner declined radiation. Cisplatin was administered under injectable anaesthesia once a week for 4 weeks and then every other week for an additional four doses. During the treatment course a new SCC lesion was detected and cytologically confirmed on the medial canthus of the right eye. The new lesion was also infiltrated with cisplatin/serum. Following treatment, both tumours were in complete clinical remission and only a nasal planum deformity due to tumour erosion was evident. Two months after the completion of the intralesional chemotherapy course, a mass developed at the site of the eyelid infiltration, where it grew to a diameter of 1.5 cm within a few weeks. Differential diagnoses included a SCC recurrence, a granulomatous process and other tumour types. A fine needle aspirate was performed and the mass was subsequently diagnosed as a soft tissue sarcoma. Surgical excision was recommended, but the owner was reluctant; therefore, doxorubicin (Doxorubicina; Sandoz) (1 mg/kg body weight) was administered intravenously twice, with a 3-week interval between doses. The lesion partially necrotised and reduced in size, but renal failure developed and doxorubicin administration was stopped. At this point, the owner was still reluctant for us to perform surgery on the cat. After 3 months the tumour began growing again and surgical excision was finally agreed to by the owner. The nasal SCC was still under control at that time, although a crusty lesion was evident (Figure 1). The clinical condition of the cat was determined to be satisfactory after a blood work-up; therefore, surgery was performed after radiographs of the chest had been performed in three standard views, the regional lymph nodes had been palpated and evidence of metastasis was ruled out. The excision involved the lesion and a small amount of macroscopically normal tissue around the gross tumour burden. This excision was not a wide excision, as indicated for feline injection-site sarcoma (FISS), but both the localisation of the mass and the owner’s reluctance to enucleate the eye did not allow for a wider resection. The surgical site was closed with a transposition skin flap 1 and an Elizabethan collar was worn by the cat until complete healing occurred (in approximately 20 days without major complications) (Figure 2).
Figure 1.
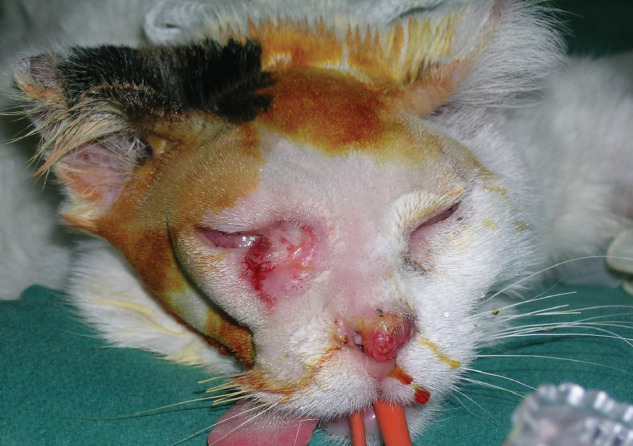
Pre-operative image of the FISS. An adequately wide surgical excision of the FISS would have led to the removal of the majority of both lids, the enucleation of the eye and reconstruction by a caudal auricular axial pattern flap or a superficial temporal axial pattern flap. The nasal planum is still erythematous, but no erosions, which were observed before treatment, are evident. The nasal deformity is a result of both the tumour erosive behaviour and the drug infiltration
Figure 2.
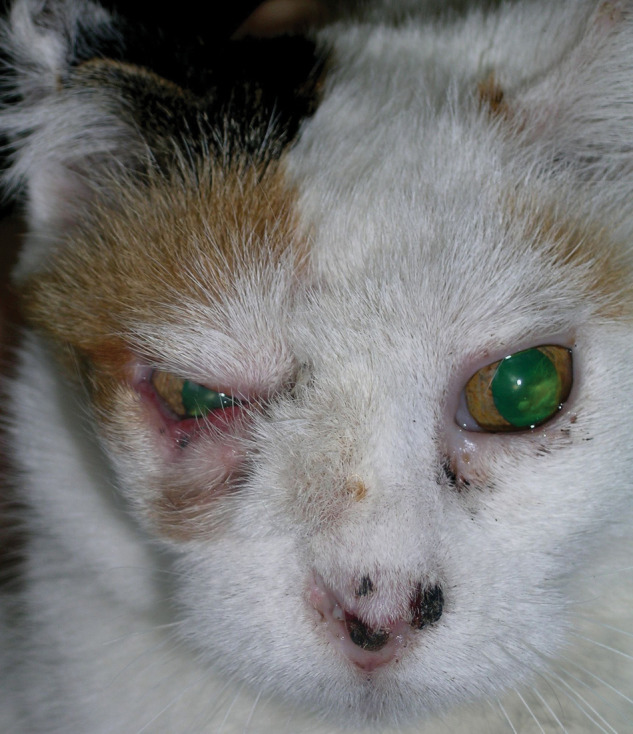
Clinical characteristics of the cat 1.5 months after surgery. Although the hair regrew near the eye, the cat did not seem to have any ocular problems. The crusty lesions of the nasal planum remained similar to the ones visible in the photograph for the rest of the cat’s life
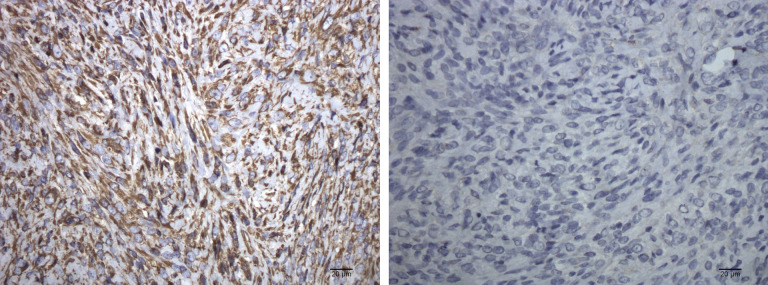
A histological examination, performed according to the criteria of the World Health Organization (WHO) Classification for Tumours of Domestic Animals, 2 revealed a partially pseudoencapsulated tumour extending to the cut borders. The tumour comprised a monomorphic population of spindle cells immersed in an abundant hyaline matrix. The cells were characterised by indistinct borders, abundant cytoplasm and one paracentral nucleus with 1–2 prominent nucleoli, and a low mitotic index. A small central necrotic area and peripheral multifocal inflammatory infiltrates were present. Moderate and diffuse chronic inflammation was also visible in the perinodular dermis. The final diagnosis of a well-differentiated fibrosarcoma was confirmed with immunohistochemical staining for vimentin (monoclonal mouse anti-vimentin, diluted 1:50, DakoCytomation) and cytokeratin (monoclonal mouse anti-human cytokeratin clone AE1-AE3, ready to use, DakoCytomation) (Figure 3a, b). No further treatment was proposed.
Figure 3.
Immunohistochemical results. (a) Neoplastic cells are characterised by diffuse and strong cytoplasmic immunolabelling for vimentin (40× streptavidin-biotin-peroxidase method; Mayer’s haematoxylin counterstaining). (b) Negativity for cytokeratin (40× streptavidin-biotin-peroxidase method; Mayer’s haematoxylin counterstaining)
Examinations of the nasal and peri-ocular areas were planned every month for the first 3 months, and every 3 months thereafter, and radiographs of the thorax were planned after 6 months. The FISS recurred locally 10 months after surgery. The owner declined any further therapy and the cat was euthanased a few days after the last examination. Necropsy was not allowed; therefore, no information is available regarding the presence of metastasis, with the exclusion of the lungs and regional lymph nodes, which were clean on radiographs and at the clinical examination, respectively.
Injection-site sarcomas have been described in cats since 1991. 3 Although an abnormal inflammatory response to vaccines and/or their adjuvant was initially thought to be primarily responsible for their development, further studies have suggested that any substance injected subcutaneously or intramuscularly in predisposed cats, as well as any foreign body, can induce tumour formation. 4 Sarcoma formation has been described at the site of long-acting antibiotic injections, 5 lufenuron, 6 non-absorbable sutures 7 and meloxicam. 8 Microchip implantation was also recently mentioned as a possible cause of sarcoma formation.9,10 Because the pathogenesis of FISS is related to an abnormal inflammatory process, the main concern in this complex of slowly developing, quickly progressive tumours (many histotypes have been described 4 ) is local recurrence, because it is difficult to adequately define their microscopic extent. For this reason, wide surgical excision, including 3–5 cm of macroscopically healthy tissue around the gross tumour and two fascial planes deep, is the standard of care; 11 a combination of the surgery with adjuvant or neoadjuvant irradiation of the surgical field can improve the result. 12 Pre-operative computed tomography enhances the likelihood of the complete excision of the mass. Despite such an invasive treatment, the 2-year recurrence rate is approximately 14–40%.11,13 Chemotherapy may be indicated when radiation is not available and the surgical excision is incomplete. 14 The metastatic rate, mainly to the lungs, varies between 0% and 28%. 13
Intralesional chemotherapy is one of the options for the treatment of SCCs that form on the feline nasal planum. 15 The drugs usually employed to treat these SCCs are bleomycin, cisplatin and carboplatin. Many additional substances have been added to the formulation to improve the local persistence of the drug, such as sesame oil, the cat’s own serum and vasoactive substances.16,17 This route of administration can either be used alone or in combination with radiation therapy, 18 and better results are obtained when in situ or superficial tumours are treated. Electrochemotherapy can be used as an alternative. 19 The lack of an electroporator device and the unwillingness of the owner to change clinic made this choice unavailable in this case. At the time of FISS occurrence and for the entire follow-up of the case described here, SCC nasal recurrence did not occur. An incisional biopsy of the crust on the nose would have ruled out any doubt regarding the nature of this lesion.
Because the cat was repeatedly photographed because of the nasal SCC, any pre-existing ocular lesion would have been noted; moreover, the first cytological diagnosis of the primary eyelid lesion identified a SCC. Interestingly, a FISS did not develop on the nasal planum, in which a greater number of injections was administered. Although the development of the tumour was rapid (2 months) compared with the long latency period often observed for FISS, 13 this hypothesis is the most likely as a result of the unusual site for the development of a sarcoma and the coincidence with the chemotherapy injections when considered with both the morphological and immunohistochemical characterisation of the tumour. The cat’s serum was not considered causative of tumour formation: autologous serum should be well tolerated. Cisplatin, however, is considered to be irritating to the tissues therefore, it is plausible that its presence, made longer-lasting by the addition of the serum, may have caused an abnormal inflammatory response.
Doxorubicin was suggested as palliation in the hope of partial tumour regression to at least make surgery easier, but renal failure became a problem before completion of the chemotherapeutic protocol.
In this cat, a better oncological result could have been obtained if a wider surgical excision, including a larger part of both of the eyelids, and the enucleation of the eye was performed, or if radiotherapy would have followed the surgical procedure.
To our knowledge, this is the first case of FISS development at the site of a chemotherapeutic drug injection.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
The authors do not have any potential conflicts of interest to declare.
Accepted: 1 May 2012
References
- 1. Schmidt K, Bertani C, Martano M, et al. Reconstruction of the lower eyelid by third eyelid lateral advancement and local transposition cutaneous flap after ‘en bloc’ removal of feline squamous cell carcinoma: report of 5 cases. Vet Surgery 2005; 34: 78–82. [DOI] [PubMed] [Google Scholar]
- 2. Goldschmidt MH, Dunstan RW, Stannard AA, et al. Histological classification of epithelial and melanocytic tumors of the skin of domestic animals. Washington DC: WHO International Histological Classification of Tumors of Domestic Animals, 1998. [Google Scholar]
- 3. Hendrick MJ, Goldschmidt M. Do injection site reactions induce fibrosarcomas in cats? J Am Vet Med Assoc 1991; 199: 68. [PubMed] [Google Scholar]
- 4. Vaccine-Associated Feline Sarcoma Task Force. The current understanding and management of vaccine-associated sarcomas in cats. J Am Vet Med Assoc 2005; 226: 1821–1842. [DOI] [PubMed] [Google Scholar]
- 5. Kass PH, Spangler WR, Hendrick MJ, et al. Multicenter case-control study of risk factors associated with development of vaccine-associated sarcomas in cats. J Am Vet Med Assoc 2003; 223: 1283–1292. [DOI] [PubMed] [Google Scholar]
- 6. Esplin DG, Mcgill LD. Fibrosarcoma at the site of lufenuron injection in a cat. Vet Cancer Soc Newsletter 1999; 23: 8–9. [Google Scholar]
- 7. Buracco P, Martano M, Morello E, Ratto A. Vaccine-associated-like fibrosarcoma at the site of a deep nonadsorbable suture in a cat. Vet J 2002; 163: 105–107. [DOI] [PubMed] [Google Scholar]
- 8. Munday JS, Banyay K, Aberdein D, French AF. Development of an injection site sarcoma shortly after meloxicam injection in an unvaccinated cat. J Feline Med Surg 2011; 13: 988–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carminato A, Vascellari M, Marchiori W, et al. Microchip-associated fibrosarcoma in a cat. Vet Dermatol 2011; 22: 565–569. [DOI] [PubMed] [Google Scholar]
- 10. Daly MK, Saba CF, Crichik SS, et al. Fibrosarcoma adjacent to the site of microchip implantation in a cat. J Feline Med Surg 2008; 10: 202–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Phelps HA, Kuntz CA, Milner RJ, et al. Radical excision with five-centimeter margins for treatment of feline injection-site sarcomas: 91 cases (1998–2002). J Am Vet Med Assoc 2011; 239: 97–106. [DOI] [PubMed] [Google Scholar]
- 12. Eckstein C, Guscetti F, Roos M, et al. A retrospective analysis of radiation therapy for the treatment of feline vaccine-associated sarcoma. Vet Compar Oncol 2009; 7: 54–68. [DOI] [PubMed] [Google Scholar]
- 13. Martano M, Morello E, Buracco P. Feline injection-site sarcoma: past, present and perspectives. Vet J 2011; 188: 136–141. [DOI] [PubMed] [Google Scholar]
- 14. Poirier VJ, Thamm DH, Kurzman ID, et al. Liposome-encapsulated doxorubicin (Doxil) and doxorubicin in the treatment of vaccine-associated sarcoma in cats. J Vet Intern Med 2002; 16: 726–731. [PubMed] [Google Scholar]
- 15. Thomson M. Squamous cell carcinoma of the nasal planum in cats and dogs. Clin Tech Small Anim Pract 2007; 22: 42–45. [DOI] [PubMed] [Google Scholar]
- 16. Kitchell BK, Orenberg EK, Brown DM, et al. Intralesional sustained-release chemotherapy with therapeutic implants for treatment of canine sun-induced squamous cell carcinoma. Eur J Cancer 1995; 31A: 2093–2098. [DOI] [PubMed] [Google Scholar]
- 17. Théon AP, VanVechten MK, Madewell BR. Intratumoral administration of carboplatin for treatment of squamous cell carcinomas of the nasal plane in cats. Am J Vet Res 1996; 57: 205–210. [PubMed] [Google Scholar]
- 18. deVos JP, Burm AGD, Focker BP. Results from the treatment of advanced stage squamous cell carcinoma of the nasal planum in cats, using a combination of intralesional carboplatin and superficial radiotherapy: a pilot study. Vet Compar Oncol 2004; 2: 75–81. [DOI] [PubMed] [Google Scholar]
- 19. Spugnini E, Vincenzi B, Citro G, et al. Electrochemotherapy for the treatment of squamous cell carcinoma in cats: a preliminary report. Vet J 2009; 179: 117–120. [DOI] [PubMed] [Google Scholar]