Abstract
Objectives
The purpose of this study was to specify lymphoma subtypes according to the World Health Organization (WHO) classification in a group of cats and to investigate their potential prognostic value.
Methods
Records of cats from the University of Veterinary Medicine Vienna suffering from lymphoma were reviewed in this retrospective study. To diagnose various subtypes specified in the WHO classification, histopathological and immunohistochemical examinations, as well as clonality assays in some cases, were performed.
Results
Of the 30 cats included in this study and classified according to the WHO guidelines, peripheral T-cell lymphoma was the most prevalent lymphoma subtype (37% of cases; n = 11), followed by diffuse large B-cell (23%; n = 7), intestinal T-cell (10%; n = 3), T-cell-rich B-cell (10%; n = 3), large granular lymphocytic (7%; n = 2), anaplastic large T-cell (7%; n = 2), B-cell small lymphocytic (3%; n = 1) and T-cell angiotropic lymphoma (3%; n = 1). The median survival time (MST) was 5.4 months (range 6 days to 2.2 years), with two cats still alive after 1.7 and 2.0 years, respectively. Treating cats prior to chemotherapy with glucocorticoids did not worsen their prognosis. Adding to chemotherapy, radiotherapy or surgery did not improve the clinical outcome. We observed that patients with intestinal T-cell lymphoma lived significantly longer (MST 1.7 years) than those with a diffuse large B-cell (MST 4.5 months) or peripheral T-cell lymphoma (MST 6.1 months). Cats with T-cell-rich B-cell lymphoma survived significantly longer (MST 1.2 years) than those with a diffuse large B-cell lymphoma.
Conclusions and relevance
A detailed diagnosis of feline lymphoma can be obtained by allocating different subtypes according to the WHO classification. From the eight detected lymphoma subtypes, two, intestinal T-cell lymphoma and T-cell-rich B-cell lymphoma, showed promising survival times in cats.
Introduction
Lymphoma is the most frequent malignant tumour in cats. 1 In the majority of cases, fine-needle aspiration of the suspicious tissue with subsequent cytology confirms the diagnosis. Quite often this diagnosis is rendered adequate for starting an adjuvant therapy to treat ‘the lymphoma’. Valli et al stated more than a decade ago that a simple lymphoma diagnosis is not sufficient for veterinary oncologists to provide optimal tumour management. 2 Thus, to diagnose all possible lymphoma subtypes according to the established revised European–American Classification of Lymphoid Neoplasms/World Health Organization (REAL/WHO) classification of haematopoietic tumours in domestic animals, histopathological and immunohistochemical examinations are essential. 2 This veterinary classification system has been developed based on the REAL classification, which was established in 1994 in human medicine and then further developed into the WHO classification in 2001, with the goal of identifying well-defined lymphoid diseases within this complex matter. Knowledge of specific lymphoma subtypes in cats is still limited, especially in conjunction with outcome data. The purpose of this study was to determine the naturally occurring lymphoma subtypes according to the WHO classification in a group of Austrian cats. Furthermore, the determined subtypes and other patient parameters were analysed for their potential prognostic value.
Materials and methods
Patients
The inclusion criteria for the observed cats, which were examined at the University of Veterinary Medicine Vienna, were confirmation of the lymphoma by histopathology, subtyping according to the WHO classification, 2 and treatment with either chemotherapy (mostly with COP or VELCAP protocols), radiation therapy or both. Clinical data were collected using the computer information system of the University of Veterinary Medicine Vienna. Cases that met the inclusion criteria were recruited from 2002–2014. During the observation period, 272 feline lymphomas were detected by histopathology, 188 within necropsies and 84 in terms of diagnostic work-up. In more than half of diagnosed lymphoma cases no therapy was attempted; thus, 38 cats were primarily included as original histopathology reports and a treatment schedule existed. Reviewing the archived tissue for WHO classification, eight samples had to be excluded for not being a lymphoma (n = 2), evaluation not being possible owing to low tissue quality (n = 5) and one tissue sample being lost. All histopathological samples were archival tissues.
Alimentary lymphoma samples for WHO classification were collected by laparotomy (n = 9), by gastroduodenoscopy (n = 3), by autopsy (n = 1) and by ultrasound-guided core biopsy (n = 1). The number of collected samples varied between one and five per cat; in summary, samples were collected from the stomach (n = 4), the small intestines (n = 13), mesenterial lymph nodes (n = 8), liver (n = 3) and pancreas (n = 2).
Variables regarding age, weight, sex, glucocorticoid pretreatment, cytology results, anatomical location, mitotic rate, grade, immunophenotype of the tumour, lymphoma subtype according to the WHO classification, form of treatment and the overall survival time (OST) were recorded.
Histopathology and immunohistochemistry
From paraffin-embedded samples, 4 μm sections were stained with haematoxylin and eosin for microscopic analysis. Immunohistochemistry was performed on a LabVision Autostainer (Thermo Fisher Scientific). Slides were deparaffinised and pretreated with heat in a citrate buffer (pH 6) for 15 mins, for antigen unmasking. To decrease background staining, the slides were incubated in hydrogen peroxidase block (Thermo Fisher Scientific) for 5 mins and in Ultra V Block (Thermo Scientific) for 10 mins. A polyclonal rabbit antihuman antibody against CD3 (diluted 1:1000; Dako) and a monoclonal mouse antihuman antibody against CD79a (diluted 1:300; Dako) were used as pan-T-cell and pan-B-cell markers, respectively. The samples were incubated with the primary antibodies for 30 mins and, subsequently, with the secondary antibodies for 30 (biotinylated goat antirabbit; Thermo Fisher Scientific) and 15 mins (biotinylated goat antimouse; Thermo Fisher Scientific), respectively. The binding reaction was detected with streptavidin peroxidase (incubation for 20 mins; Thermo Fisher Scientific) and visualised with diaminobenzidine (Large Volume DAB Plus Substrate System for 5 mins; Thermo Fisher Scientific). The slides were counterstained with Mayer’s haematoxylin, dehydrated, put into Neo Clear and mounted in Neo-Mount (both from Merck). If a final diagnosis for B- or T-cell lymphoma was not possible by immunohistochemistry, clonality assays for T-cell receptor gamma and complete immunoglobulin heavy chain V-J gene rearrangements were performed.3,4
The mitotic rate was defined as the mean number of mitoses in 10 high-power fields (HPF); the grade was categorised according to Valli et al as low (0–5), medium (6–10) and high (>10 mitoses per HPF). 5 All histopathology samples were reviewed by one veterinary pathologist (AFB). If immunohistochemistry was inconclusive, showing either no or dual staining with CD79a and CD3,clonality assays were performed. Consensus between histopathology and cytology was defined when the cytologist was completely sure that a lymphoma was present, and this diagnosis was confirmed by histopathology.
Statistics
Survival analysis was conducted with the Kaplan–Meier method. The log rank test was used to assess the following parameters, which affected survival time: sex, glucocorticoid pretreatment, anatomical lymphoma form, immunohistochemistry, histopathological subtype, grading, additional radiation therapy and mass reduction surgery. To test the association between survival time and age, weight at diagnosis or the mitotic rate, the Spearman rank correlation was used. To assess a difference between glucocorticoid pretreated vs non-pretreated cats concerning the distribution of the variable lymphoma subtypes, a Pearson’s χ2 test was used. Statistical significance was set at P ⩽0.05. All statistical analyses were conducted with SPSS statistics software, version 19 (IBM).
Results
Cats
Thirty cats, which were examined at the University of Veterinary Medicine Vienna, met the inclusion criteria for this retrospective study. All cases were domestic shorthair cats, except for one Maine Coon, one Persian and one Norwegian Forest Cat, with a sex distribution of 53% females and 47% males. The mean age and weight at diagnosis was 9.3 years (range 2–15 years) and 3.8 kg (range 2.6–7.6 kg), respectively. Hypercalcaemia was not observed in our study group. Forty percent of the animals were pretreated with cortisone before initiating chemotherapy. The most prevalent anatomical form was alimentary lymphoma at 47% (n = 14), followed by 43% (n = 13) extranodal cases (17% [n = 5] cutaneous, 13% [n = 4] nasal, 10% [n = 3] laryngeal, and 3% [n = 1) ocular), 7% (n = 2) with a nodal and 3% (n = 1) with a mixed (nodal and renal) form.
In most cases, immunohistochemistry was sufficient for immunophenotyping the tumours. However, in four cases immunohistochemistry was inconclusive, showing a dual expression of CD3 and CD79a, which made clonality assays necessary to determine immunophenotype. Three cats were diagnosed with a T-cell and one with a B-cell lymphoma (see Table 1, cases 1, 15, 16, 30). Nearly two-thirds, namely 63% (n = 19), of the cats had a T-cell lymphoma, while only 37% (n = 11) suffered from B-cell lymphoma. According to the WHO classification, eight different lymphoma subtypes have been defined. Peripheral T-cell lymphoma (PTCL [37%; n = 11]) was the most common form, followed by diffuse large B-cell lymphoma (DLBCL [23%; n = 7]), intestinal T-cell lymphoma (ITCL [10%; n = 3]) and T-cell-rich B-cell lymphoma (TCRBCL [10%; n = 3]), T-cell large granular lymphocytic (T-LGL [7%; n = 2]), anaplastic large T-cell lymphoma (T-ALCL [7%; n = 2]), B-cell small lymphocytic lymphoma (B-SLL [3%; n = 1]) and T-cell angiotropic lymphoma (T-AL [3%; n = 1]; Table 1).
Table 1.
Classification, mitotic rate and survival times in cats treated for lymphoma
| Case | Anatomical classification | Mitoses/HPF | WHO classification | Treatment | OST (days) |
|---|---|---|---|---|---|
| 26 | Alimentary | 2.7 | PTCL | VELCAP | 681 |
| 22 | EN (laryngeal) | 3 | PTCL* | VELCAP, 6 × 4 Gy | 649 |
| 2 | EN (cutaneous) | 3.8 | PTCL* | COP + ELA, 5 × 4 Gy, 2 × 4 Gy | 259 |
| 7 | Alimentary | 2.4 | PTCL | sx, COP | 213 |
| 3 | Alimentary | 6.8 | PTCL | sx, prednisolone, 2 × 4 Gy | 201 |
| 1 | Nodal | 1.7 | PTCL | sx, COP + ELCyt | 183 |
| 17 | Alimentary | 7.7 | PTCL | sx, A | 163 |
| 4 | Alimentary | 8.2 | PTCL | sx, COP | 113 |
| 19 | EN (cutaneous) | 6 | PTCL | EL, 15 × 3.2 Gy | 56 |
| 9 | Alimentary | 2.5 | PTCL | COP + EL | 14 |
| 28 | Alimentary | 0.2 | PTCL* | VELCAP | 12 |
| 29 | Alimentary | 0 | DLBCL | COP + A | 355 |
| 11 | Nodal | 1.5 | DLBCL* | COP + ELACyt, 5 × 4, 2 × 4 Gy | 180 |
| 27 | EN (nasal) | 2 | DLBCL | VELCAP | 142 |
| 13 | EN (cutaneous) | 5.5 | DLBCL* | COP + A | 134 |
| 6 | EN (laryngeal) | 1 | DLBCL* | COP, 5 × 4 Gy | 66 |
| 23 | EN (nasal) | 0.9 | DLBCL* | VELCAP, 15 × 3.2 Gy | 63 |
| 14 | EN (nasal) | 3.4 | DLBCL* | COP + EL, 5 × 4 Gy, 3 × 4 Gy | 49 |
| 5 | M (nodal + renal) | 1.3 | TCRBCL | COP + EL | 746 † |
| 8 | Alimentary | 5.7 | TCRBCL | sx, COP + ACyt, 2 × 4 Gy, 2 × 4 Gy | 422 |
| 10 | EN (nasal) | 2.4 | TCRBCL | COP + A, 8 × 4 Gy | 152 |
| 20 | Alimentary | 0 | ITCL | sx, VELCAP | 796 |
| 30 | Alimentary | 0 | ITCL* | Chlorambucil + P | 625 † |
| 24 | Alimentary | 0 | ITCL* | VELCAP | 596 |
| 21 | Alimentary | 2.1 | T-LGL | sx, VELCAP | 455 |
| 25 | Alimentary | 8.3 | T-LGL | sx, VP | 19 |
| 16 | EN (cutaneous) | 2.3 | T-ALCL | sx, VELCAP | 190 |
| 18 | EN (cutaneous) | 3.2 | T-ALCL | sx, VELCAP | 36 |
| 12 | EN (ocular) | 5.2 | T-AL* | COP + EL, 3x3 Gy | 52 |
| 15 | EN (laryngeal) | 0 | B-SLL* | COP | 6 |
Pretreated with glucocorticoids
Still alive
EN = extranodal lymphoma; M = mixed lymphoma; HPF = high-power fields; WHO = World Health Organization; T = T-cell lymphoma; B = B-cell lymphoma; PTCL = peripheral T-cell lymphoma; DLBCL = diffuse large B-cell lymphoma; TCRBCL = T-cell-rich B-cell lymphoma; ITCL = intestinal T-cell lymphoma; LGL = large granular lymphocyte lymphoma; ALCL = anaplastic large cell lymphoma; AL = angiotropic lymphoma; SLL = small lymphocytic lymphoma; sx = cytoreductive surgery; V = vincristine; O = Oncovine; EL = Elspar (L-asparaginase); C = cyclophosphamide; A = Adriblastine (doxorubicin); P = prednisolone; Cyt = cytarabine; Gy = Gray; OST = overall survival time
Cytology of the tumours was available in 20/30 cases; 10% (n = 2) were non-diagnostic (one low-qualitysample and once it was not possible to make a precise diagnosis) and 40% (n = 8) showed no consensus with the histopathology report in the lymphoma diagnosis.
In the eight cases where cytology delivered a different diagnosis than histopathology, most commonly inflammatory reactions and in one case suspicion of an undifferentiated sarcoma were described. The samples involved both alimentary (n = 5) and extranodal (n = 3) cases and histopathology revealed PTCL (n = 4), DLBCL (n = 2), ITCL (n = 1) and ALCL (n = 1). Half of the samples (n = 10) demonstrated accordance in lymphoma diagnosis between cytology and histopathology. Immunohistochemistry was not performed on cytological smears.
Treatment, outcome and prognostic factors
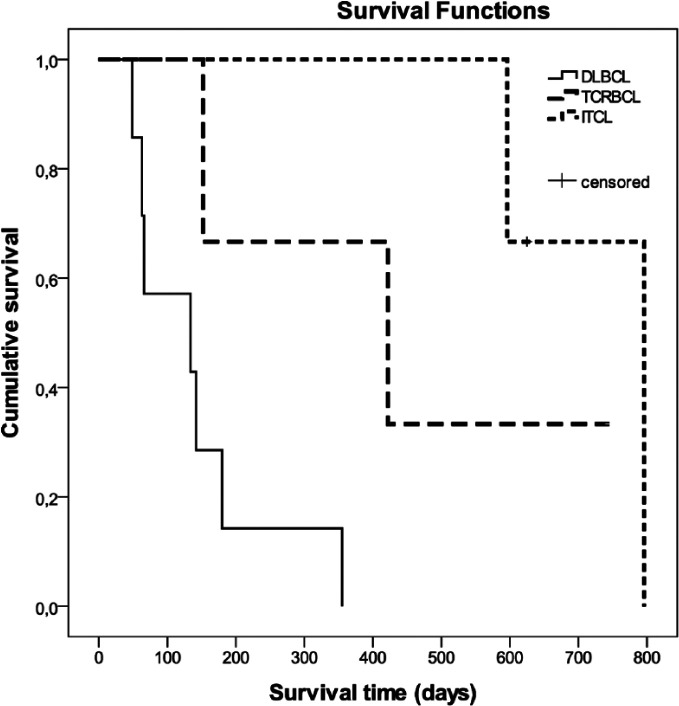
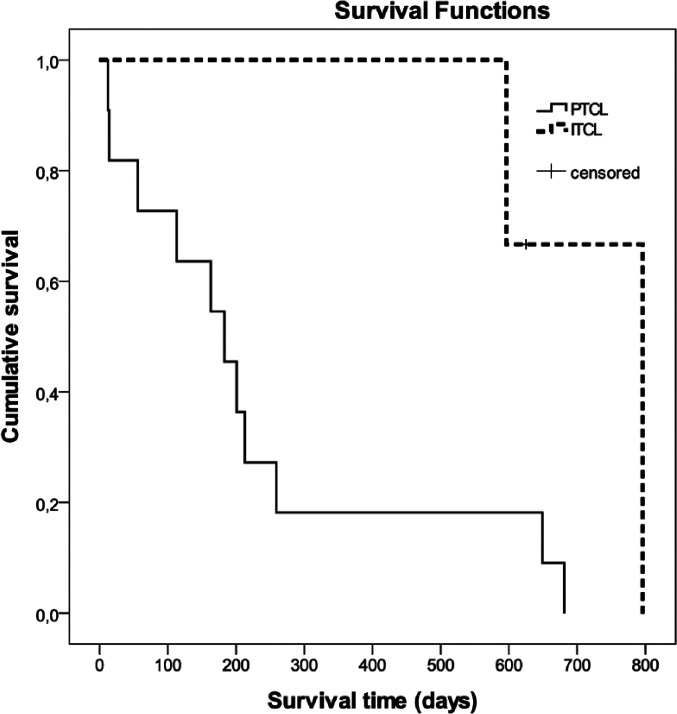
No correlation was observed between grading based on mitotic rate and outcome. Regarding WHO subtypes, cats suffering from TCRBCL (median survival time [MST] 1.2 years) or from ITCL (MST 1.7 years) had a significantly longer OST than patients with DLBCL (MST 4.5 months; P = 0.048 and 0.009, respectively) (Figure 1). Animals with PTCL (MST 6.1 months) had a significantly shorter MST than ITCL cats (MST 1.7 years; P = 0.039) (Figure 2). Survival times of the two cats with LGL lymphoma were 1.3 years and 19 days, respectively. The two cats with ALCL lived for 6.3 and 1.2 months, respectively; the cat with AL lived for 1.7 months and the cat with B-SLL survived for only 6 days. The cat with the longest survival time (2.2 years) had ITCL. The cats still alive after 2 and 1.7 years were diagnosed with TCRBCL and an ITCL, respectively. Nevertheless, long-term survivors (1.9 and 1.8 years, respectively) were also observed in cats with PTCL (Table 1).
Figure 1.
Kaplan–Meier survival curves showing survival of cats with different lymphoma subtypes according to the World Health Organization classification. Cats suffering from T-cell-rich B-cell lymphoma (TCRBCL) or from intestinal T-cell lymphoma (ITCL) had significantly longer overall survival times than cats with diffuse large B-cell lymphoma (DLBCL; P = 0.048 and 0.009, respectively)
Figure 2.
Kaplan–Meier survival curves showing survival of cats with different lymphoma subtypes according to the World Health Organization classification. Animals with intestinal T-cell lymphoma (ITCL) had a significantly longer survival time than cats with a peripheral T-cell lymphoma (PTCL; P = 0.039)
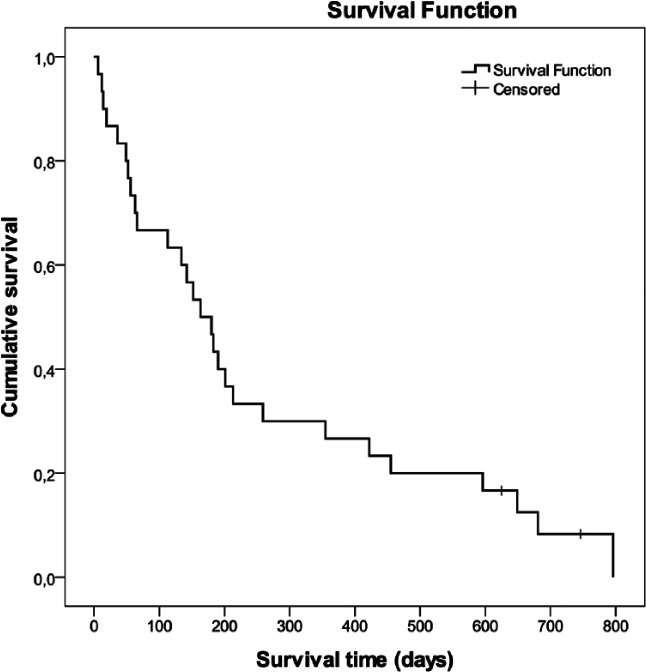
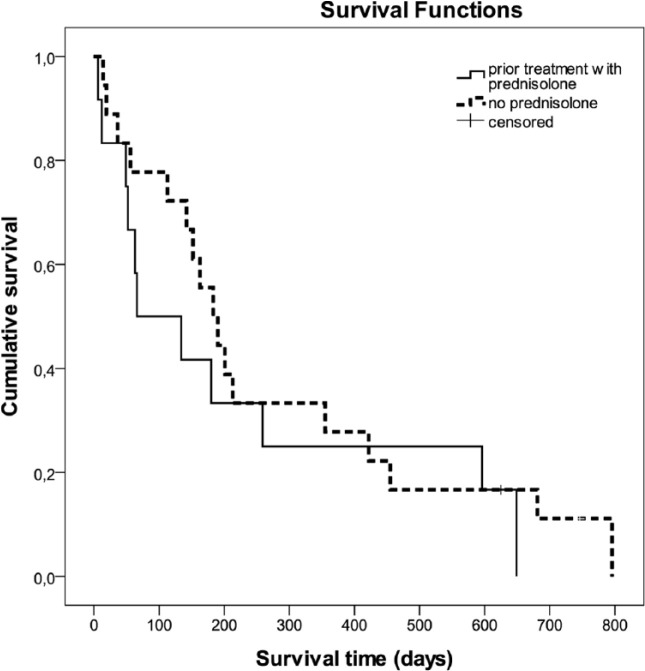
The median OST for the cats was 5.4 months (range 6 days to 2.2 years). Two cats with lymphoma were still alive after 1.7 and 2 years, respectively (Figure 3). There was no correlation between the survival time and age or weight at the time of the diagnosis. Animals that were pretreated with glucocorticoids were identified in Table 1. Six cats received prednisolone, two cats methylprednisolone and one dexamethasone; in three cats no information beside ‘glucocorticoid’ treatment could be gained. Duration of glucocorticoid treatment ranged from one-time application up to 1 month, dosages were either anti-inflammatory (n = 4) or immunosuppressive (n = 5). Pretreatment with glucocorticoids did not influence survival (Figure 4). No significant difference in the distribution of the various aggressive lymphoma subtypes between glucocorticoid pretreated vs non-pretreated cats could be detected (P = 0.095).
Figure 3.
A Kaplan–Meier survival curve showing the overall survival of 30 cats with lymphoma. The median survival time was 172 days (range 6–796 days)
Figure 4.
Kaplan–Meier survival curves showing survival of cats with or without prednisolone treatment before starting chemotherapy. There was no significant difference in survival times between the two groups (P = 0.438)
During surgery, intestinal resection, peripheral lymphadenectomy or removal of cutaneous lesions, with subsequent tumour mass reduction, was performed in 37% (n = 11) of the cases. All patients (with or without mass reduction) were treated with chemotherapy (mostly with COP or VELCAP protocols), except one cat, which was treated with radiation and prednisolone; 37% (n = 11) of the patients received a combination of chemotherapy and radiation therapy.
Cytoreductive surgery before chemotherapy or additional radiotherapy did not improve the outcome. Looking at the anatomical distribution, cats diagnosed with alimentary lymphoma survived significantly longer (7.1 months) than those diagnosed with the extranodal form (2.2 months; P = 0.027). There was no difference in survival between cats showing a B- or T-cell immunophenotype.
Discussion
At present, reports using the WHO classification to subdivide the feline lymphoma complex are still quite rare.6–9 One study applied the WHO classification for feline lymphoma in different anatomical sites similar to our study. 6 The most common subtype in the report from Vezzalli et al was ITCL (33% of cases; n = 16), 6 followed by PTCL at 23% (n = 11), which was the most frequent lymphoma type in our study (37%; n = 11). Diffuse DLBCL was the second most prevalent lymphoma subtype in our study at 23% (n = 7), followed by ITCL (10%; n = 3) and TCRBCL (10%; n = 3), the last of which was not observed by Vezzalli et al. 6 Some researchers solely analysed the alimentary form of lymphoma in cats, where the most common subtypes were ITCL (75%; n = 90), diffuse large B-cell immunoblastic type (33%; n = 16) and B-lymphoblastic lymphoma (30%; n = 8).7–9 Fifty percent (n = 7) of our cats with gastrointestinal lymphoma had PTCL. Pohlman et al found ITCL (31%; n = 15) to be the second most often diagnosed subtype, 7 whichis similar to the 21% (n = 3) observed in our study,but differs from other results, such as the incidence of DLBCL (14%; n = 17) and T-lymphoblastic lymphoma (19%; n = 5).8,9 With the exception of the study of Moore et al, 8 who examined 120 samples of lymphoma cats, the discussed papers were, as ours, predominantly smaller studies investigating 50, 48 and 27 cats, respectively.6,7,9 Thus, the described comparisons need to be considered critically, as investigations of larger groups of cats could yield different results.
Results could also differ owing to regional distinctions, as the studies evaluating feline lymphoma subtypes according to the WHO classifications originate from the USA,7,8 the UK, 9 Italy 6 and Austria (present study).
In summary, we found that the majority of cats had T-cell lymphomas, which is in accordance with previous studies,8,10 although some authors have reported a predominance of B-cell tumours in cats.7,9,11 There was no survival benefit in cats with a B- or T-cell immunophenotype, which has already been observed in other studies.11,12 In our study the small number of cats could have influenced the observed result. Additionally, we observed a survival benefit in ITCL as well as in TCRBCL, with one subtype being a T-cell the other one being a B-cell lymphoma; therefore, this advantage could be equalised when comparing survival data of cats with B-cell with T-cell lymphomas.
In this study, we wanted to know specifically if the various subtypes according to the WHO classification would have any prognostic value. We observed that cats with ITCL had a significantly longer MST (1.7 years) than cats with DLBCL (4.5 months) or PTCL (6.1 months). In the only other study evaluating the prognosis of cats diagnosed with different lymphoma subtypes of the gastrointestinal tract, a favourable outcome was observed in a small-cell, T-cell lymphoma group (equivalent to our ITCL subtype) compared with a large-cell, T-cell lymphoma group (equivalent to our PTCL subtype) with survival times of 2.3 years vs 1.5 months. 8 We also found that cats with TCRBCL had a significantly longer MST (1.2 years) than cats with DLBCL (4.5 months). The other diagnosed subtypes in our study had too few cats; therefore, statistical analysis was not possible. Feline LGL lymphoma has been described as an aggressive disease with a very poor prognosis, with MSTs <2 months.13–15 In our study, one of the two cats with T-cell LGL lymphoma also lived for a very short period (19 days); however, surprisingly, the other cat survived for 1.3 years. Indeed, more favourable clinical outcomes in individual cases were also observed by other researchers, including cats that were treated with a simple COP protocol, which survived for 9 months and 1.3 years, respectively. Additionally, one cat that was treated with only prednisone and cyclophosphamide therapy survived for 9.6 months and one cat that received masitinib therapy survived for 6 months.13,16 Angiotropic and anaplastic large T-cell lymphoma have rarely been described in cats,6,17 and the survival times of these lymphoma subtypes are unknown. Two cats with anaplastic large T-cell lymphoma of cutaneous origin and one cat with ocular angiotropic lymphoma in our study lived for 6.3 months, 1.2 months and 1.7 months, respectively.
Looking at the anatomical classification, alimentary lymphoma was the most frequent form in the current study, a finding that is consistent with many other studies.12,18–22 The median OST of the cats in this study was 5.4 months, whereas most survival analyses of cats predominantly with alimentary feline lymphoma had lower survival times of 2.1 months, 2.8 months, 3.2 months and 3.6 months, respectively.12,18–20 Milner et al described a slightly higher MST of 7 months. 23 In the past, the anatomical site mostly showed no prognostic influence on survival time.20,23–25 Recently, MST in cats with renal lymphoma vs all other forms was described to have significantly lower survival times (0.9 months vs 3.5 months) and cats with alimentary lymphoma lived significantly shorter (1.6 months) than cats with mediastinal (4.8 months) and nasal lymphoma (4.5 months).12,26 Cats diagnosed with alimentary lymphoma in our study survived significantly longer (7.1 months) than those with the extranodal form (2.2 months). Other studies found higher MSTs (8 months and 18.8 months) in cats with only or mostly extranodal forms.25,27 Cats with only alimentary lymphoma have been described with either low MSTs of 1.7 months, 2.8 months and 3.2 months, or longer survival times of 5.7 months and 9.3 months.10,28–31 In the current study, we had several cases with slowly progressive tumour types, such as intestinal T-cell lymphoma, which may have caused a slightly favourable outcome in the alimentary group as in studies investigating solely low-grade intestinal lymphomas, long survival times of 11 months, 14.9 months, 17 months and 23.5 months were observed.32–35
Before beginning chemotherapy, previous treatment with glucocorticoids is a concern in lymphoma patients because a resistance in neoplastic cells may diminish the effect of chemotherapeutics. 36 A poorer prognosis in feline lymphoma patients was not proven with glucocorticoid pretreatment in our study or in other reports.10,37 In a different report, a subgroup of lymphoma cats with complete remission had a significantly shorter MST after previous glococorticoid treatment (8 vs 18.8 months). When considering all cats, however, no statistical significance was observed (3.7 vs 5.9 months). 25 In our study, the rather low patient numbers with glucocorticoid treatment (n = 12) could be the reason for detecting no significant difference in survival times between the pretreated and the non-pretreated group; this could also apply in the studies of Zwahlen et al (n = 6) 10 and Fabrizio et al (n = 14), 37 contrary to the higher numbers in the study of Taylor et al (n = 37), 25 who achieved a significant difference in a subgroup of cats. Duration of prednisone treatment was not a significant predictor of survival in the cohort of Zwahlen et al; 10 dose and duration of corticosteroid treatment was not available in other studies.25,37 In most of the cases we could collect information about the drug, the dosage and the duration of glucocorticoid treatment; however, the pretreatment was very heterogeneous and may therefore also have influenced the outcome. One could also suppose that a different distribution of the various aggressive lymphoma subtypes, for example a higher number of less aggressive lymphoma subtypes in cats with glucocorticoid pretreatment, could influence our results. Nevertheless, statistical analysis showed no significant difference in the distribution of subtypes between the two cat groups.
Although the treatment of choice in cats suffering from lymphoma is still chemotherapy, radiation therapy has been applied for nasal, cutaneous and abdominal lymphoma.38–42 In addition to chemotherapy, 37% (n = 11) of our cats were also treated with radiotherapy; however, there was no survival benefit for these animals. One cat had gastrointestinal surgery, but the owners refused subsequent chemotherapy. This cat was diagnosed with PTCL and was then administered two abdominal radiation treatments on consecutive days, as well as with prednisolone. The cat had an excellent quality of life for the next 7 months and was euthanased after deteriorating. The survival time of this cat was the same as the median OST of 11 cats that were treated with two fractions of abdominal radiation after failing chemotherapy. 41 Presently, it is unknown which lymphoma subtypes would benefit most from radiation alone or as a combination therapy.
The therapeutic benefit of surgery in cats with alimentary lymphoma is still unknown, although it has been established that surgery is a safe diagnostic procedure, with the advantage of full-thickness samples.43,44 The cats in our study experienced no survival benefit after cytoreductive surgery for their alimentary lymphoma, which is in accordance with various other reports.10,12,28,30 After looking more closely at the literature, no statistical significance in survival was found when comparing four cats with alimentary lymphoma that were treated with chemotherapy and prior surgery with 24 cats treated only with chemotherapy; nevertheless, the median time to death was more than three times longer (4 months) in the small surgery group as opposed to the chemotherapy group (1.2 months). 28 In seven cases, only limited surgery was performed to obtain biopsy specimens without debulking. 10 The MST of 11 cats after mass resection of colonic lymphoma was 3.2 months with no significant difference to the very few cats (n = 4) that lived for 4.2 months after biopsy. 30 Cytoreductive surgery was conducted in a study by Collette et al; 12 however, only a small group of five cats were operated on vs 114 that had no surgery performed. Our study included only 11 cats that were treated with mass reduction surgery. It is possible that surgery does not benefit lymphoma cats, although the small number of cats may render the results statistically not significant.
It has been observed that individual cats can greatly benefit clinically from surgery. After surgical removal of a single mandibular lymph node, a cat had no recurrence after 4 years. 45 A histopathological subtype was not determined. In other reports, TCRBCL was diagnosed in masses in the submandibular and neck region. In three cats, the tumours did not recur after 6 months following a single removal, and two cats had three surgeries and survived for 1.5 years and 2.3 years.46,47 Only one cat with TCRBCL in our study had cytoreductive surgery for gastrointestinal lymphoma. The cat was treated with adjuvant chemotherapy and abdominal radiation therapy postsurgery and survived for 1.2 years. Another report described removal of a 4 cm ileocaecal mass with dirty margins, but the cat lived for another 5 years and died of causes other than lymphoma. The removed mass was diagnosed as mucosa-associated lymphoid tissue (MALT) lymphoma, which can stay localised in the lamina propria for quite a long period of time. It seems, therefore, that MALT lymphoma can be effectively treated with surgery alone. 48 Another cat with subtotal colectomy lived without any additional therapy for 3.7 years. 30 In addition to these singular cases, one recent study described surgical resection of discrete alimentary lymphoma in 20 cats, followed by chemotherapy. 44 For the time being, the MST of 1.1 years in their study is the highest reported in cats with intermediate- and high-grade alimentary lymphoma. 44 Thus, surgery as a treatment option should not be ruled out completely in specific cases, even if at the moment there is no scientific proof of a survival benefit postoperatively in lymphoma cats in general.
Limitations of this study are due to its retrospective nature. There was considerable heterogeneity in treatment schemes and a lack of uniform chemotherapy protocols. The limited sample size makes interpretation more difficult; therefore, prospective studies with larger patient cohorts are warranted.
Conclusions
With histopathology, immunohistochemistry and clonality assays, eight different lymphoma subtypes classified according to the WHO guidelines were identified in 30 cats. The most common lymphoma subtypes were PTCL and DLBCL; additionally, we identified subtype ITCL and TCRBCL, which both showed a significant survival advantage in cats. Using the WHO classification provides more detailed information on the disease than labelling the tumours as low-, intermediate- or high-grade lymphoma. However, in the majority of cases this scientifically valuable information does not change the line of treatment in practice yet. Thus, to optimise our treatment schedules in future, large scale studies will be essential to assess the benefit from surgery, radiation or chemotherapy on specific lymphoma subtypes classified according to the WHO classification.
Acknowledgments
We would like to thank Verena Greß and Siegfried Kosik very much for their support.
Footnotes
Accepted: 29 July 2016
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. MacVean DW, Monlux AW, Anderson PS, Jr, et al. Frequency of canine and feline tumors in a defined population. Vet Pathol 1978; 15: 700–715. [DOI] [PubMed] [Google Scholar]
- 2. Valli VE, Jacobs RM, Parodi AL, et al. Histological classification of hematopoietic tumors of domestic animals. 2nd series, Volume VIII. Washington, DC: Armed Forces Institute of Pathology in cooperation with the American Registry of Pathology and the World Health Organization Collaborating Center, 2002, pp 11–46. [Google Scholar]
- 3. Mochizuki H, Nakamura K, Sato H, et al. Multiplex PCR and Genescan analysis to detect immunoglobulin heavy chain gene rearrangement in feline B-cell neoplasms. Vet Immunol Immunopathol 2011; 143: 38–45. [DOI] [PubMed] [Google Scholar]
- 4. Mochizuki H, Nakamura K, Sato H, et al. GeneScan analysis to detect clonality of T-cell receptor γ gene rearrangement in feline lymphoid neoplasms. Vet Immunol Immunopathol 2012; 145: 402–409. [DOI] [PubMed] [Google Scholar]
- 5. Valli VE, San Myint M, Barthel A, et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet Pathol 2011; 48: 198–211. [DOI] [PubMed] [Google Scholar]
- 6. Vezzali E, Parodi AL, Marcato PS, et al. Histopathologic classification of 171 cases of canine and feline non-Hodgkin lymphoma according to the WHO. Vet Comp Oncol 2010; 8: 38–49. [DOI] [PubMed] [Google Scholar]
- 7. Pohlman LM, Higginbotham ML, Welles EG, et al. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet Pathol 2009; 46: 259–268. [DOI] [PubMed] [Google Scholar]
- 8. Moore PF, Rodriguez-Bertos A, Kass PH. Feline gastrointestinal lymphoma: mucosal architecture, immunophenotype, and molecular clonality. Vet Pathol 2012; 49: 658–668. [DOI] [PubMed] [Google Scholar]
- 9. Waly NE, Gruffydd-Jones TJ, Stokes CR, et al. Immunohistochemical diagnosis of alimentary lymphomas and severe intestinal inflammation in cats. J Comp Pathol 2005; 133: 253–260. [DOI] [PubMed] [Google Scholar]
- 10. Zwahlen CH, Lucroy MD, Kraegel SA, et al. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997). J Am Vet Med Assoc 1998; 213: 1144–1149. [PubMed] [Google Scholar]
- 11. Patterson-Kane JC, Kugler BP, Francis K. The possible prognostic significance of immunophenotype in feline alimentary lymphoma: a pilot study. J Comp Pathol 2004; 130: 220–222. [DOI] [PubMed] [Google Scholar]
- 12. Collette SA, Allstadt SD, Chon EM, et al. Treatment of feline intermediate- to high-grade lymphoma with a modified university of Wisconsin-Madison protocol: 119 cases (2004–2012). Vet Comp Oncol 2016; 14 Suppl 1: 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krick EL, Little L, Patel R, et al. Description of clinical and pathological findings, treatment and outcome of feline large granular lymphocyte lymphoma (1996–2004). Vet Comp Oncol 2008; 6: 102–110. [DOI] [PubMed] [Google Scholar]
- 14. Endo Y, Cho KW, Nishigaki K, et al. Clinicopathological and immunological characteristics of six cats with granular lymphocyte tumors. Comp Immunol Microbiol Infect Dis 1998; 21: 27–42. [DOI] [PubMed] [Google Scholar]
- 15. Roccabianca P, Vernau W, Caniatti M, et al. Feline large granular lymphocyte (LGL) lymphoma with secondary leukemia: primary intestinal origin with predominance of a CD3/CD8(alpha)(alpha) phenotype. Vet Pathol 2006; 43: 15–28. [DOI] [PubMed] [Google Scholar]
- 16. Sapierzyński R, Jankowska U, Jagielski D, et al. Large granular lymphoma in six cats. Pol J Vet Sci 2015; 18: 163–169. [DOI] [PubMed] [Google Scholar]
- 17. Roccabianca P, Avallone G, Rodriguez A, et al. Cutaneous lymphoma at injection sites: pathological, immunophenotypical, and molecular characterization in 17 cats. Vet Pathol 2016; 53: 823–832. [DOI] [PubMed] [Google Scholar]
- 18. Hadden AG, Cotter SM, Rand W, et al. Efficacy and toxicosis of VELCAP-C treatment of lymphoma in cats. J Vet Intern Med 2008; 22: 153–157. [DOI] [PubMed] [Google Scholar]
- 19. Kristal O, Lana SE, Ogilvie GK, et al. Single agent chemotherapy with doxorubicin for feline lymphoma: a retrospective study of 19 cases (1994–1997). J Vet Intern Med 2001; 15: 125–130. [DOI] [PubMed] [Google Scholar]
- 20. Waite AH, Jackson K, Gregor TP, et al. Lymphoma in cats treated with a weekly cyclophosphamide-, vincristine-, and prednisone-based protocol: 114 cases (1998–2008).J Am Vet Med Assoc 2013; 242: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 21. Vail DM, Moore AS, Ogilvie GK, et al. Feline lymphoma (145 cases): proliferation indices, cluster of differentiation 3 immunoreactivity, and their association with prognosis in 90 cats. J Vet Intern Med 1998; 12: 349–354. [DOI] [PubMed] [Google Scholar]
- 22. Louwerens M, London CA, Pedersen NC, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med 2005; 19: 329–335. [DOI] [PubMed] [Google Scholar]
- 23. Milner RJ, Peyton J, Cooke K, et al. Response rates and survival times for cats with lymphoma treated with the University of Wisconsin-Madison chemotherapy protocol: 38 cases (1996–2003). J Am Vet Med Assoc 2005; 227: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 24. Teske E, van Straten G, van Noort R, et al. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med 2002; 16: 179–186. [DOI] [PubMed] [Google Scholar]
- 25. Taylor SS, Goodfellow MR, Browne WJ, et al. Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. J Small Anim Pract 2009; 50: 584–592. [DOI] [PubMed] [Google Scholar]
- 26. Sato H, Fujino Y, Chino J, et al. Prognostic analyses on anatomical and morphological classification of feline lymphoma. J Vet Med Sci 2014; 76: 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simon D, Eberle N, Laacke-Singer L, et al. Combination chemotherapy in feline lymphoma: treatment outcome, tolerability, and duration in 23 cats. J Vet Intern Med 2008; 22: 394–400. [DOI] [PubMed] [Google Scholar]
- 28. Mahony OM, Moore AS, Cotter SM, et al. Alimentary lymphoma in cats: 28 cases (1988–1993). J Am Vet Med Assoc 1995; 207: 1593–1598. [PubMed] [Google Scholar]
- 29. Jeglum KA, Whereat A, Young K. Chemotherapy of lymphoma in 75 cats. J Am Vet Med Assoc 1987; 190: 174–178. [PubMed] [Google Scholar]
- 30. Slawienski MJ, Mauldin GE, Mauldin GN, et al. Malignant colonic neoplasia in cats: 46 cases (1990–1996). J Am Vet Med Assoc 1997; 211: 878–881. [PubMed] [Google Scholar]
- 31. Gustafson TL, Villamil A, Taylor BE, et al. A retrospective study of feline gastric lymphoma in 16 chemotherapy-treated cats. J Am Anim Hosp Assoc 2014; 50: 46–52. [DOI] [PubMed] [Google Scholar]
- 32. Carreras JK, Goldschmidt M, Lamb M, et al. Feline epitheliotropic intestinal malignant lymphoma: 10 cases (1997–2000). J Vet Intern Med 2003; 17: 326–331. [DOI] [PubMed] [Google Scholar]
- 33. Lingard AE, Briscoe K, Beatty JA, et al. Low-grade alimentary lymphoma: clinicopathological findings and response to treatment in 17 cases. J Feline Med Surg 2009; 11: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fondocaro JV, Richter KP, Carpenter JL. Feline gastrointestinal lymphoma: 67 cases (1988–1996). Eur J Comp Gastroenterol 1999; 4: 5–11. [Google Scholar]
- 35. Kiselow MA, Rassnick KM, McDonough SP, et al. Outcome of cats with low-grade lymphocytic lymphoma: 41 cases (1995–2005). J Am Vet Med Assoc 2008; 232: 405–410. [DOI] [PubMed] [Google Scholar]
- 36. Price GS, Page RL, Fischer BM, et al. Efficacy and toxicity of doxorubicin/cyclophosphamide maintenance therapy in dogs with multicentric lymphosarcoma. J Vet Intern Med 1991; 5: 259–262. [DOI] [PubMed] [Google Scholar]
- 37. Fabrizio F, Calam AE, Dobson JM, et al. Feline mediastinal lymphoma: a retrospective study of signalment, retroviral status, response to chemotherapy and prognostic indicators. J Feline Med Surg 2014; 16: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haney SM, Beaver L, Turrel J, et al. Survival analysis of 97 cats with nasal lymphoma: a multi-institutional retrospective study (1986–2006). J Vet Intern Med 2009; 23: 287–294. [DOI] [PubMed] [Google Scholar]
- 39. Sfiligoi G, Théon AP, Kent MS. Response of nineteen cats with nasal lymphoma to radiation therapy and chemotherapy. Vet Radiol Ultrasound 2007; 48: 388–393. [DOI] [PubMed] [Google Scholar]
- 40. Burr HD, Keating JH, Clifford CA, et al. Cutaneous lymphoma of the tarsus in cats: 23 cases (2000–2012). J Am Vet Med Assoc 2014; 244: 1429–1434. [DOI] [PubMed] [Google Scholar]
- 41. Parshley DL, Larue SM, Kitchell B, et al. Abdominal irradiation as a rescue therapy for feline gastrointestinal lymphoma: a retrospective study of 11 cats (2001–2008). J Feline Med Surg 2011; 13: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams LE, Pruitt AF, Thrall DE. Chemotherapy followed by abdominal cavity irradiation for feline lymphoblastic lymphoma. Vet Radiol Ultrasound 2010; 51: 681–687. [DOI] [PubMed] [Google Scholar]
- 43. Smith AL, Wilson AP, Hardie RJ, et al. Perioperativecomplications after full-thickness gastrointestinal surgery in cats with alimentary lymphoma. Vet Surg 2011; 40: 849–852. [DOI] [PubMed] [Google Scholar]
- 44. Gouldin ED, Mullin C, Morges M, et al. Feline discrete high-grade gastrointestinal lymphoma treated with surgical resection and adjuvant CHOP-based chemotherapy: retrospective study of 20 cases. Vet Comp Oncol 2017; 15: 328–335. [DOI] [PubMed] [Google Scholar]
- 45. Malik R, Gabor LJ, Foster SF, et al. Therapy for Australian cats with lymphosarcoma. Aust Vet J 2001; 79: 808–817. [DOI] [PubMed] [Google Scholar]
- 46. Day MJ, Kyaw-Tanner M, Silkstone MA, et al. T-cell-rich B-cell lymphoma in the cat. J Comp Pathol 1999; 120:155–167. [DOI] [PubMed] [Google Scholar]
- 47. Steele KE, Saunders GK, Coleman GD. T-cell-rich B-cell lymphoma in a cat. Vet Pathol 1997; 34: 47–49. [DOI] [PubMed] [Google Scholar]
- 48. Valli VE, Jacobs RM, Norris A, et al. The histologic classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest 2000; 12: 295–306. [DOI] [PubMed] [Google Scholar]