Abstract
Objectives
Gastrointestinal (GI) perforation is a well described complication of GI lymphoma in people, commonly occurring within days of initiation of chemotherapy. There are no studies documenting the prevalence of GI perforation in cats with intermediate- or large-cell GI lymphoma or whether it is associated with induction of chemotherapy. The objectives of this study were to document the prevalence and timing of post-chemotherapy perforation in cats with discrete GI masses caused by intermediate- or large-cell lymphoma.
Methods
Cats with a diagnosis of intermediate- or large-cell lymphoma based on cytologic or histopathologic examination of a mass lesion of the GI tract and treated with chemotherapy were identified by searching the patient record database of three large specialty referral hospitals. Cats undergoing surgical resection of a GI mass prior to chemotherapy were excluded from the study. A clinical diagnosis of GI perforation was made using ultrasound findings and analysis of abdominal fluid.
Results
Twenty-three cats with intermediate- (n = 3) or large-cell (n = 20) lymphoma were included in the study. GI perforation was confirmed in 4/23 cats (17%), and occurred at 23, 56, 59 and 87 days after induction. There was no association between tumor size, the presence of hypoproteinemia or suppurative inflammation within the mass at the time of diagnosis and subsequent perforation. Post-hoc analysis revealed that the magnitude of weight loss within 15–28 days of diagnosis was greater in cats with perforation.
Conclusions and relevance
In this pilot study, we found that post-chemotherapy GI perforation in cats with intermediate- or large-cell GI lymphoma occurs. Acute perforation after induction of chemotherapy was not documented. Larger prospective studies are needed to determine risk factors associated with perforation and whether surgical excision would reduce the risk of subsequent GI perforation in these patients.
Introduction
Lymphoma is the most common neoplasm of the cat.1–3 The gastrointestinal (GI) tract is the most common anatomic location of lymphoma in cats, accounting for up to 72% of newly diagnosed cases.2,4 The National Cancer Institute Working Formulation classification scheme is used to classify feline GI lymphoma into high grade, intermediate grade or low grade based on cell morphology and mitotic rate.5,6 High-grade and low-grade lymphoma are commonly referred to as large-cell and small-cell lymphoma, respectively. 1
Intermediate- and large-cell GI lymphoma (ICGIL/LCGIL) are commonly described together owing to similarities in clinical presentation and response to treatment.1,7 I/LCGIL presents as a discrete intestinal mass or multifocal masses, and can involve mesenteric lymph nodes or other organs. 7 Owing to the characteristic microscopic appearance of intermediate- and large-cell (high-grade) lymphoma, fine-needle aspiration with cytologic examination is often utilized to obtain a diagnosis. 1
Cyclophosphamide, vincristine and prednisolone, with or without doxorubicin (COP or CHOP) is considered the chemotherapeutic protocol of choice for feline I/LCGIL,1,3 with complete remission rates reported between 32% and 38%.8,9 Additionally, a combination of lomustine and prednisolone can be used as a first-line therapy in cats with I/LCGIL when an oral chemotherapy option is preferred, with 22% of cats achieving complete remission. 10 Palliative therapy with prednisolone at a cytotoxic dose is sometimes prescribed if owners do not wish to pursue another chemotherapy protocol because of financial limitations or other concerns. The role of surgery in the treatment of cats with I/LCGIL characterized by a discrete mass is unclear. In certain cases, an exploratory laparotomy is clearly indicated prior to induction of chemotherapy, either to obtain biopsies for definitive diagnosis or in cases of GI perforation or obstruction. 11 In cases of I/LCGIL in which there is a discrete mass with no signs of obstruction or perforation, there is debate among veterinary clinicians regarding the utility of surgical excision of the primary mass prior to chemotherapy. Surgical excision of solitary masses prior to chemotherapy may be unnecessary or even detrimental. Because lymphoma is a systemic disease, elimination of neoplastic cells is not possible with surgery alone. In addition, there is a theoretical risk of delayed healing and dehiscence if there is pathology at the tumor margins. 11 Furthermore, chemotherapy is commonly delayed after surgery based on recommendations in people. 12
Conversely, it is possible that reduction of tumor burden prior to chemotherapy might improve remission rates. In addition, there is a theoretical risk that acute chemotherapy-associated apoptosis of neoplastic cells in a discrete GI mass could lead to GI perforation and result in peritonitis or tumor lysis syndrome due to the large tumor burden.11,13 Therefore, surgical resection might help prevent these complications. GI perforation is a well-described complication of GI lymphoma in people, commonly occurring within days of initiating chemotherapy.14–17 There are no studies published that document the prevalence of GI perforation in cats with I/LCGIL after chemotherapy is initiated, and the timing of perforation in relation to initiation of chemotherapy.
We hypothesized that larger tumors, tumors with evidence of necrosis or evidence of GI protein loss might be associated with an increased risk of perforation with chemotherapy. Histological studies have shown that GI tumors often start within the lamina propria and grow to considerable size before invading surrounding tissue. 6 Large masses may have compromised blood supply and necrotic areas within them. 6 Chemotherapy-induced tumor cell death within large tumors may therefore be more likely to lead to compromise of the GI wall. The presence of suppurative inflammation in tumor cytology obtained by fine-needle aspirate may indicate pre-existing areas of tissue damage and necrosis. Hypoalbuminemia and panhypoproteinemia are associated with GI compromise, 18 and hypoalbuminemia may impede healing, 19 thus potentially increasing risk of perforation in diseased bowel.
The objective of this pilot, retrospective study was to document the prevalence and timing of post-chemotherapy perforation in cats with discrete GI masses of I/LCGIL. We hypothesized that tumor size, suppurative inflammation within the mass at the time of diagnosis and presence of hypoproteinemia would be associated with perforation.
Materials and methods
Case selection
A retrospective review of medical records from the patient record database of three institutions was performed. Medical records of cases presenting between June 2006 and May 2012 were reviewed at the Veterinary Specialty Hospital San Diego and the Veterinary Specialty Hospital North County. Medical records of cases presenting between December 2007 and May 2015 were reviewed at the Angell Animal Medical Center. Records were identified using diagnostic codes utilized by the institution to include cats with GI lymphoma. Cats with a cytologic or histopathologic diagnosis of I/LCGIL and the appearance of a discrete GI mass lesion on abdominal ultrasound were included in the study. In addition, all cats were required to have been treated with either an injectable or oral chemotherapy protocol or palliative therapy with prednisolone. Any cat that underwent surgical resection of the mass prior to chemotherapy was excluded from the study.
Medical records review
Data collected for all cats at the time of initial diagnosis of I/LCGIL included age, sex, breed, weight, clinical signs, physical examination findings, ultrasound findings, complete blood cell count, and cytologic or histopathologic findings. If available, additional data collected at the time of initial diagnosis of I/LCGIL included serum biochemical analysis, retroviral status and pre-chemotherapy tumor size based on the largest single axis measurement on ultrasound. Initial treatments were recorded for all cats included in the study. Weights at recheck evaluations and follow-up abdominal ultrasound findings were documented if available. A clinical diagnosis of GI perforation was made if cytologic analysis of abdominal effusion revealed suppurative inflammation and presence of bacteria and at least one of the following ultrasound findings were present: free gas, hyperechoic mesentery or abdominal effusion. For cats diagnosed with perforation, remission status at the time of the recheck preceding perforation, remission status at the time when perforation was detected and weight at the time of perforation was recorded.
Variables
Variables examined to determine their association with the outcome of perforation or no perforation included tumor size, presence of hypoalbuminemia or hypoglobulinemia and presence of suppurative inflammation on cytology at the time of diagnosis. Post-hoc analysis of weight change at 15–28 days was performed after examining the data.
Statistics
Statistical analyses were performed using the commercially available MaxStat software. Normal distribution of the data were confirmed by means of a Shapiro–Wilk test. An unpaired t-test was used to compare continuous variables. Fisher’s exact test was used to compare categorical outcomes. The threshold for statistical significance was set at P = 0.05.
Results
Twenty-three cats met the inclusion criteria. There were 13 castrated males, nine spayed females and one intact female. The median age at diagnosis was 12.7 years (range 4.2–18 years). The median age at perforation was 10.4 years (range 4.3–12 years). Breeds included domestic shorthair (n = 12), domestic mediumhair (n = 4), domestic longhair (n = 3), Siamese cross (n = 2), Himalayan cross (n = 1) and Norwegian Forest Cat (n = 1) (Table 1). The most frequent presenting clinical signs at the time of initial diagnosis with I/LCGIL were complete or partial anorexia (18/23), vomiting (13/23), a subnormal body condition score (12/21) and lethargy (11/23).
Table 1.
Case description, examined variables, treatment, and presence of perforation for the study population
| Case number | Age at diagnosis (years) | Lymphoma grade | Tumor size (cm) | Tumor location | Suppurative inflammation on cytology | Albumin (g/dl)* | Globulin (g/dl) † | % change in body weight at 2–4 weeks | Treatment | Presence of perforation |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | High | 5.3 | SI | No | 3.4 | 3.3 | NA | PL | No |
| 2 | 16.5 | High | 4.3 | IC | No | 2.2 | 3.8 | –1.1 | CHOPLA | No |
| 3 | 6.2 | High | NA | G | No | 2.5 | 3.6 | –11.3 | CHOPLA | No |
| 4 | 16.8 | Intermediate | 4.0 | SI | No | 2.7 | 4.0 | NA | CHOPLA | No |
| 5 | 11 | High | 3.2 | G | No | 2.3 | 3.5 | –2.9 | CHOPA | No |
| 6 | 14.4 | High | 5.3 | SI | Yes | 2.6 | 4.2 | 9.1 | CHOPA | No |
| 7 | 11 | High | NA | G | No | NA | NA | –4.7 | CHOPLAM | No |
| 8 | 14 | High | 6.0 | G | No | 3.2 | 3.2 | NA | P | No |
| 9 | 14 | High | 4.0 | G | No | 2.0 | 3.9 | NA | PO | No |
| 10 | 15 | High | 1.5 | G | Yes | 1.9 | 3.2 | –1.2 | PCO | No |
| 11 | 14 | High | 2.0 | G | No | 2.3 | 3.1 | NA | PO | No |
| 12 | 14 | High | 2.7 | SI | No | 2.2 | 3.9 | 9.8 | CHOPA | No |
| 13 | 7 | High | 1.4 | G | No | 2.6 | 3.6 | 17.3 | PL | No |
| 14 | 10 | Intermediate | 5.2 | G | No | 3.0 | 3.0 | NA | PO | No |
| 15 | 16.6 | High | NA | G | No | 2.0 | 2.3 | 8.0 | COPAL | No |
| 16 | 7.6 | High | NA | G | No | 2.2 | 1.9 | NA | PO | No |
| 17 | 18 | High | NA | IC | Yes | 3.2 | 3.6 | NA | P | No |
| 18 | 12.7 | High | NA | SI | No | 3.5 | 5.0 | –1.5 | CHOP | No |
| 19 | 12.6 | High | NA | IC | No | 2.7 | 3.0 | –7.9 | P | No |
| 20 | 11.9 | High | 5.6 | SI | No | 3.1 | 2.5 | –6.5 | CHOPA | Yes |
| 21 | 9.4 | Intermediate | 4.9 | SI | No | 3.3 | 2.7 | 0 | CHOPL | Yes |
| 22 | 11 | High | 0.53 | SI | No | 2.3 | 2.9 | –18.8 | CHOPAM | Yes |
| 23 | 4.2 | High | 3.6 | G | No | 3.7 | 3.3 | –17.3 | CHOPA | Yes |
SI = small intestine; NA = not available; PL = prednisolone and lomustine; IC = ileocolic; CHOPLA = cyclophosphamide, doxorubicin, vincristine, prednisolone, lomustine and L-asparaginase; G = gastric; CHOPA = cyclophosphamide, doxorubicin, vincristine, prednisolone and L-asparaginase, L-asparaginase and mitoxantrone; CHOPLAM = cyclophosphamide, doxorubicin, vincristine, prednisolone, lomustine, L-asparaginase and mitoxantrone; P = prednisolone; PO = prednisolone and vincristine; PCO = prednisolone, cyclophosphamide and vincristine; COPAL = cyclophosphamide, vincristine, prednisolone, lomustine and L-asparaginase; CHOP =cyclophosphamide, doxorubicin, vincristine and prednisolone; CHOPL = cyclophosphamide, doxorubicin, vincristine, prednisolone and lomustine; CHOPAM = cyclophosphamide, doxorubicin, vincristine, prednisolone, L-asparaginase and mitoxantrone.
Reference interval 2.5–3.9 g/dl
Reference interval 2.6–4.9 g/dl
Abdominal ultrasound at the time of initial diagnosis of I/LCGIL revealed either a gastric mass (n = 11), a small intestinal mass (n = 9) or an ileocolic junction mass (n = 3). Twenty-one cats had a diagnosis obtained via fine-needle aspirate and cytology from the GI mass, one cat had diagnosis obtained via fine-needle aspirate and cytology from an enlarged mesenteric lymph node, and one cat had a diagnosis obtained based on endoscopic biopsy and histopathology. Twenty cats had a diagnosis of LCGIL and three cats had a diagnosis of ICGIL. A diagnosis of GI perforation was made in 4/23 cases (17%); three of these had a small intestinal mass and one had a gastric mass. All cats that experienced GI perforation had the initial diagnosis of lymphoma made by fine-needle aspirate of the GI mass.
Suppurative inflammation of the mass at the time of diagnosis was identified in 3/23 cases (13%), but was not observed in any cat that experienced GI perforation. Measurements of tumor size were available in 16 cats and, for these cases, the mean ± SD tumor size based on the largest single axis measurement obtained was 3.7 ± 1.6 cm for cats without perforation and 3.7 ± 2.2 cm for cats with perforation. Hypoalbuminemia (albumin ⩽2.5 g/dl) was recorded in 9/18 of cases without GI perforation and 1/4 cases with GI perforation. The mean ± SD albumin (reference interval [RI] 2.5–3.9 g/dl) was 2.6 ± 0.5 for cats without perforation and 3.1 ± 0.6 for cats with perforation (P = 0.08). Hypoglobulinemia (globulin ⩽2.5 g/dl) was recorded in 2/18 cases without GI perforation and 1/4 cases with GI perforation. The mean ± SD globulin (RI 2.6–4.9 g/dl) was 3.5 ± 0.7 for cats without perforation and 2.85 ± 0.3 for cats with perforation (P = 0.11). There was no association between tumor size, hypoalbuminemia or hypoglobulinemia and subsequent perforation. Ten cats had retroviral testing available. Two cats without GI perforation tested positive for feline immunodeficiency virus and all 10 cats tested negative for feline leukemia virus. Fifteen cats had body weight measurement available 2–4 weeks (range 15–28 days) after induction of chemotherapy available for review (4/4 cats with perforation and 11/19 cats without perforation). Change in body weight at 2–4 weeks after diagnosis was found to be significantly more decreased in cats with GI perforation (mean change in body weight −10.7% ± 8.9) compared with cats without evidence of GI perforation (mean change in body weight 1.24% ± 8.7; P = 0.037) (Figure 1).
Figure 1.
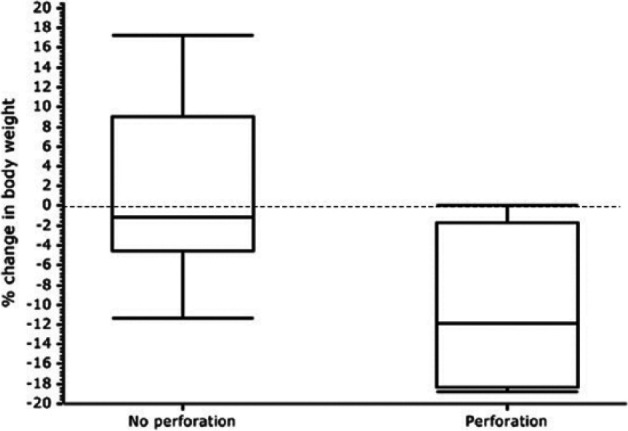
Box and whisker chart of percentage change in body weight at 15–28 days of cats without perforation (n = 19) vs cats with perforation (n = 4). Body weight at 15–28 days after initial diagnosis was found to be significantly decreased in cats that went on to experience gastrointestinal perforation (P = 0.037)
Eighteen cats were treated with a COP- or CHOP-based chemotherapy, as described in previously published treatment protocols.20,21 Three cats received prednisolone at a cytotoxic dose (1.2–1.9 mg/kg/day). Two cats were treated with oral lomustine (10 mg/cat or 34–43 mg/m2) and either subcutaneous methylprednisolone acetate (4.2 mg/kg every 14 days) or oral prednisolone (2.9 mg/kg/day). Eleven cats received L-asparaginase as an adjunct to treatment or a rescue therapy during their treatment. Two cats treated with a CHOP-based protocol received intravenous (IV) mitoxantrone (6 mg/m2) as a rescue therapy in addition to the standard protocol.
The four cats that experienced GI perforation received a COP- or CHOP-based chemotherapy protocol as first-line therapy. At the recheck evaluation preceding perforation, one cat was in partial remission and three cats had progressive disease (PD). Of the cats with PD, one received IV mitoxantrone and one received oral lomustine as a rescue therapy. All cats with perforation presented for emergency evaluation of acute onset of clinical signs, including lethargy (4/4), complete or partial anorexia (4/4), vomiting (3/4), dyspnea (2/4) and diarrhea (1/4). One cat (case 22, Table 1) received mitoxantrone at the time of emergency evaluation, 2 weeks after the preceding recheck had shown progressive disease, and developed GI perforation 3 days later while hospitalized for supportive care. The other three cats presented for emergency evaluation after developing acute onset clinical signs 1–19 days after the preceding recheck and were diagnosed with perforation shortly after presentation. Perforation occurred at 23, 56, 59 and 87 days after induction of chemotherapy and 1 day after receiving vincristine, 19 days after receiving lomustine, 3 days after receiving mitoxantrone and 3 days after receiving vincristine, respectively. All cats with GI perforation had lost weight at the time of perforation compared with the time of initial diagnosis (Table 2). Two cats were euthanized prior to exploratory laparotomy, one cat was euthanized intraoperatively after perforation of a gastric mass was confirmed via exploratory laparotomy and one cat was euthanized 3 days after jejunal resection and anastomosis of the perforated segment of intestine. The cat with a gastric perforation had initially been diagnosed with a gastric mass on abdominal ultrasound and the cat with a jejunal perforation had initially been diagnosed as having a small intestinal mass on abdominal ultrasound.
Table 2.
Timing of gastrointestinal (GI) perforation events
| Case number | Days from initiation of chemotherapy to GI perforation | Days from prior chemotherapy to GI perforation | Chemotherapy received prior to GI perforation | Tumor location | Remission status at evaluation preceding perforation | % change in body weight at time of perforation |
|---|---|---|---|---|---|---|
| 20 | 23 | 1 | O | SI | PR | –6.5 |
| 21 | 56 | 19 | L | SI | PD | –10.5 |
| 22 | 87 | 3 | M | SI | PD | –27.2 |
| 23 | 59 | 3 | O | G | PD | –22.9 |
O = vincristine; SI = small intestine; PR = partial remission; L = lomustine; PD = progressive disease; M = mitoxantrone; G = gastric
Discussion
This is the first report in the veterinary literature to describe the prevalence of GI perforation in cats with I/LCGIL and document the time from induction of chemotherapy to GI perforation. We found that, in cats with I/LCGIL, GI perforation occurred in 4/23 cats (17%). The median time to perforation after induction of chemotherapy was 57.5 days (range 23–87 days), and of the variables examined only the change in body weight at 2–4 weeks was found to be significantly associated with perforation.
Perforation is a well-documented complication in people treated with chemotherapy for GI lymphoma.14–17 In a recent large study of 1062 people with GI lymphoma, perforation was documented in 92 patients (8.6%) overall. Fifty-one percent of the perforations were diagnosed at presentation, while 49% occurred after chemotherapy. Twenty-five cases of post-chemotherapy perforation occurred during the first chemotherapeutic regimen. Thirty-two percent of perforations in this group occurred within the first 2 weeks after induction, 12% in weeks 3 and 4, and 56% beyond week 4. The mean time to perforation was 83 days, with a range of 2–298 days. Most patients received a CHOP-based protocol. 15 Twenty-five cases of perforation occurred during re-induction of chemotherapy after relapse or for resistant lymphoma. The median time to perforation in this group was 35 days after re-induction, with a range of 4–493 days. 15 Smaller studies of perforation in people with GI lymphoma have demonstrated perforation occurring 4–17 days after induction of chemotherapy.16,17
Whether perforation is due to chemotherapy-induced tumor lysis or tumor progression in people is not known. However, a recent study that histopathologically examined perforation sites in people with GI lymphoma showed that 73% were associated with tumor, whereas 27% had no viable tumor cells present. The authors speculated that the lack of tumor cells was consistent with chemotherapy-associated tumor destruction and/or inflammation as a cause for perforation in the latter group. 15
For the cats described in the present study, the shortest interval between induction of chemotherapy and perforation was 23 days. Because of the length of time between chemotherapy induction and the occurrence of perforation in our study, it seems unlikely that apoptosis and cell death associated with induction caused GI perforation in these cats. However, three cats in our study had GI perforation occur 1–3 days after the most recent chemotherapy treatment, and perforation occurred in two cats after the introduction of a novel chemotherapy drug as a rescue therapy. Given the temporal relationship between a recent or novel chemotherapy treatment and perforation, a combination of tumor cell death and progressive disease may have contributed to GI perforation.
The prevalence of perforation in cats in this study was similar to that in people with more aggressive GI lymphoma phenotypes and with masses in the intestines or colon, which are more associated with higher risk of perforation than less aggressive GI lymphoma tumor phenotypes or a gastric location. 22 Information regarding tumor phenotype was not available for the cats in this study so conclusions regarding associations of tumor phenotype and perforation risk cannot be made. However, it is interesting to note that perforations in 3/4 affected cats originated from intestinal masses, whereas perforation was due to a gastric mass in one cat. It has been suggested that greater thickness of the stomach wall may explain the reduced risk of perforation associated with gastric location of lymphoma in people. 22
Because hypoalbuminemia and hypoglobulinemia can signal GI compromise and hypoalbuminemia is a risk factor for impaired healing of the GI tract, 19 we evaluated whether there was an association between low serum albumin and low serum globulin and GI perforation. Although we found a large proportion of the cats in this study to be hypoalbuminemic, we did not find an association between hypoalbuminemia or hypoglobulinemia and GI perforation.
We also hypothesized that suppurative inflammation observed on cytologic examination of the mass would indicate inflammation which may lead to compromise of the intestinal wall after chemotherapy. No cats with documented suppurative inflammation developed perforation. Owing to small sample size and the potential of overlooking the presence of suppurative inflammation without histopathology, determining whether the presence of suppurative inflammation on cytologic examination is a risk factor for GI perforation requires further study.
In this study, the cats with GI perforation had significantly more weight loss 2–4 weeks after induction of chemotherapy than the cases without perforation. Weight loss ⩾5% 1 month after induction of chemotherapy was recently shown to be a poor prognostic factor in cats with LCGIL. 23 While seven cats in the group without perforation lost weight at 2–4 weeks, their changes in body weight were typically small, with only one cat of 11 losing ⩾5% body weight. In contrast, 3/4 cats with GI perforation lost ⩾5% of their body weight at 2–4 weeks after induction of chemotherapy. Three of the cats with GI perforation had progressive disease and one cat had a partial response to chemotherapy at the time of perforation. It is possible that weight loss in these cats was a biomarker of the incomplete response to chemotherapy and progression of disease. Whether loss of ⩾5% body weight at 2–4 weeks after induction of chemotherapy may help predict risk of subsequent perforation in cats with I/LCGIL is worthy of further investigation.
In this study, which excluded cats undergoing surgery at initial diagnosis, a gastric mass was detected in 48%, a small intestinal mass in 39% and an ileocolic mass in 13% of cats. In contrast, a recent study of 20 cats with high-grade GI lymphoma treated with surgical excision found that 75% had a small intestinal mass, 15% had a gastric mass and 10% had a colonic mass. 24 Given that small intestinal masses are more amenable to surgical excision, differences in case selection criteria may explain the higher prevalence of small intestinal masses in the latter study.
Whether surgical resection prior to chemotherapy improves outcomes in cats with localized disease and whether location and tumor grade affect outcomes has not been directly examined. However, in one retrospective study of 28 cats with GI lymphoma, nine cats had stage 2 disease. Three of these cats underwent surgical resection of a localized GI mass prior to chemotherapy. Median survival time (MST) in this group was 154 days vs 71 days for the other six cats with stage 2 GI lymphoma treated with chemotherapy alone. 9 Although the MST for cats undergoing surgical resection was longer, the difference was not statistically significant. Given the small sample size, it is possible that the study was underpowered to detect a difference if one existed. Interestingly, a recent retrospective study of 20 cats with a discrete mass of I/LCGIL that underwent surgery prior to chemotherapy reported an overall MST of 417 days and no GI perforation during the study period. 24 Although direct comparisons of separate studies cannot be made, it is interesting to note that the MST reported in this study is longer than a MST of 280 days reported for cats with GI lymphoma treated with CHOP-based chemotherapy protocols. 8 Another recent retrospective study evaluated the use of lomustine as a first-line chemotherapeutic agent in 32 cats with I/LCGIL and large granular lymphoma, and found a MST of 108 days and no significant difference in survival time of four cats that had surgical debulking prior to chemotherapy. 10
The decision to perform surgical resection before chemotherapy in people with GI lymphoma is dependent on multiple factors. 22 Surgical resection followed by chemotherapy has been associated with better outcomes in some studies of human patients with localized disease. 25 Histopathologic grade and location affect the risk of perforation, with gastric location and low grade being associated with lower risk and intestinal location, and high grade being associated with higher risk of perforation, respectively. 22 It has been suggested that risk of perforation may help guide clinical decision making in this context. 22
There are several limitations of this pilot study. Although the prevalence of perforation in cats with localized I/LGGIL treated with chemotherapy was determined, the prevalence in this cohort of patients may differ from other cohorts of cats with GI lymphoma as it does in people. 22 In addition, the sample size was small. Therefore, a lack of association of hypoalbuminemia, hypoglobulinemia, tumor size and the presence of suppurative inflammation may be because the study may have been insufficiently powered to detect a difference between cats that experienced a GI perforation and cats that did not. However, given the data reported here, prospective studies exploring factors that affect the prevalence and risk of perforation cats with I/LGGIL are warranted.
Another limitation was that the diagnosis of lymphoma in most cats was obtained via fine-needle aspirate. The lack of histopathology prior to treatment limits information regarding tumor invasiveness, the integrity of the remaining intestinal tissue and the ability to perform immunophenotyping. Furthermore, a combination of ultrasound and cytological findings were used to diagnose perforation, and while these ultrasound findings are frequently seen in cases of GI perforation, 26 it is possible that some cases of early or healed perforation were not detected, and that the prevalence was therefore underestimated. Necropsy was not available in any of the cases, and whether perforation occurred from tumor cell death due to chemotherapy or tumor progression could not be determined. Finally, given the retrospective nature of the study, chemotherapeutic protocols were not standardized between patients and institutions. Therefore, associations between chemotherapeutic agent and risk of perforation could not be explored.
Conclusions
In this pilot study, we found that post-chemotherapy GI perforation in cats with ICGIL or LCGIL occurred in 17% of patients, and that weight loss of >5% 2–4 weeks after induction was associated with perforation. Acute perforation after induction of chemotherapy was not documented. Based on these results, larger prospective clinical trials examining risk factors associated with perforation and whether surgical excision prior to chemotherapy may improve outcome in some cohorts of cats with GI lymphoma are warranted.
Acknowledgments
The authors would like to thank Virginia Sinnott-Stutzman for her assistance with statistical analysis.
Footnotes
Accepted: 6 July 2017
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This paper was funded by a research grant from the research office at Western University of Health Sciences College of Veterinary Medicine.
References
- 1. Gieger T. Alimentary lymphoma in cats and dogs. Vet Clin North Am Small Anim Pract 2011; 41: 419–432. [DOI] [PubMed] [Google Scholar]
- 2. Louwerens M, London CA, Pedersen NC, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med 2005; 19: 329–335. [DOI] [PubMed] [Google Scholar]
- 3. Vail DM. Feline lymphoma and leukemia. In: Withrow SJ, Vail DM. (eds). Withrow and MacEwen’s small animal clinical oncology. 4th ed. St Louis, MO: Saunders Elsevier, 2007, pp 733–756. [Google Scholar]
- 4. Waite AH, Jackson K, Gregor TP, et al. Lymphoma in cats treated with a weekly cyclophosphamide, vincristine, and prednisone based protocol: 114 cases (1998–2008). J Am Vet Med Assoc 2013; 8: 1104–1109. [DOI] [PubMed] [Google Scholar]
- 5. Pohlman LM, Higginbotham ML, Welles EG, et al. Immunophenotypic and histologic classification of 50 cases of feline gastrointestinal lymphoma. Vet Pathol 2009; 46: 259–268. [DOI] [PubMed] [Google Scholar]
- 6. Valli VE, Jacobs RM, Norris A, et al. The histological classification of 602 cases of feline lymphoproliferative disease using the National Cancer Institute working formulation. J Vet Diagn Invest 2000; 12: 295–306. [DOI] [PubMed] [Google Scholar]
- 7. Barrs V, Beatty J. Feline alimentary lymphoma: classification, risk factors, clinical signs and non-invasive diagnostics. J Feline Med Surg 2012; 14: 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zwahlen CH, Lucroy MD, Kraegel SA, et al. Results of chemotherapy for cats with alimentary malignant lymphoma: 21 cases (1993–1997). J Am Vet Med Assoc 1998; 213: 1144–1148. [PubMed] [Google Scholar]
- 9. Mahony OM, Moore AS, Cotter SM, et al. Alimentary lymphoma in cats: 28 cases (1988–1993). J Am Vet Med Assoc 1995; 207: 1593–1597. [PubMed] [Google Scholar]
- 10. Rau SE, Burgess KE. A retrospective evaluation of lomustine (CeeNU) in 32 treatment naïve cats with intermediate to large cell gastrointestinal lymphoma (2006–2013). Vet Comp Oncol 2017; 15: 1019–102. [DOI] [PubMed] [Google Scholar]
- 11. Richter KP. Feline gastrointestinal lymphoma. Vet Clin North Am Small Anim Pract 2003; 33: 1083–1098 [DOI] [PubMed] [Google Scholar]
- 12. Payne WG, Naidu DK, Wheeler CK, et al. Wound healing in patients with cancer. Eplasty 2008; 8: e9. [PMC free article] [PubMed] [Google Scholar]
- 13. Calia CM, Hohenhaus AE, Fox PR, et al. Acute tumor lysis syndrome in a cat with lymphoma. J Vet Intern Med 1996; 10: 409–11. [DOI] [PubMed] [Google Scholar]
- 14. Ara C, Coban S, Kayaalp C, et al. Spontaneous intestinal perforation due to non-Hodgkin’s lymphoma: evaluation of eight cases. Dig Dis Sci 2007; 52: 1752–1756. [DOI] [PubMed] [Google Scholar]
- 15. Vaidya R, Habermann TM, Donohue JH, et al. Bowel perforation in intestinal lymphoma: incidence and clinical features. Ann Oncol 2013; 24: 2439–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wada M, Onada M, Tokunaga A, et al. Spontaneous gastrointestinal perforation in patients with lymphoma receiving chemotherapy and steroids: report of 3 cases. J Nippon Med Sch 1999; 66: 37–40. [DOI] [PubMed] [Google Scholar]
- 17. Maisey N, Normal AN, Prior Y, et al. Chemotherapy for primary gastric lymphoma: does an in-patient observation prevent complications? Clin Oncol 2004; 16: 48–52. [DOI] [PubMed] [Google Scholar]
- 18. Peterson PB, Willard MD. Protein-losing enteropathies. Vet Clin Small Anim 2003; 33: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 19. Ralphs SC, Jessen CR, Lipowitz AJ. Risk factors for leakage following intestinal anastomosis in dogs and cats: 115 cases (1991–2000). J Am Vet Med Assoc 2003; 1: 73–77. [DOI] [PubMed] [Google Scholar]
- 20. Moore AS, Cotter SM, Frimberger AE, et al. A comparison of doxorubicin and COP for maintenance of remission in cats with lymphoma. J Vet Intern Med 1996; 10: 372–375. [DOI] [PubMed] [Google Scholar]
- 21. Rowan MJ, Peton J, Cooke K, et al. Response rates and survival times for cats with lymphoma treated with the University of Wisconsin-Madison chemotherapy protocol: 38 cases (1996–2003). J Am Vet Med Assoc 2005; 7: 1118–1122. [DOI] [PubMed] [Google Scholar]
- 22. Vaidya R, Witzig TE. Incidence of bowel perforation in gastrointestinal lymphomas by location and histology. Ann Oncol 2014; 25: 1249–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krick EL, Moore RH, Cohen RB, et al. Prognostic significance of weight changes during treatment of feline lymphoma. J Feline Med Surg 2011; 13: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gouldin ED, Mullin C, Morges M, et al. Feline discrete high-grade gastrointestinal lymphoma treated with surgical resection and adjuvant CHOP based chemotherapy: retrospective study of 20 cases. Vet Comp Oncol 2017; 15: 328–335. [DOI] [PubMed] [Google Scholar]
- 25. Talamonti MS, Dawes LG, Joehl RJ, et al. Gastrointestinal lymphoma. A case for primary surgical resection. Arch Surg 1990; 125: 972–976. [DOI] [PubMed] [Google Scholar]
- 26. Boysen SR, Tidwell AS, Penninck DG. Ultrasonographic findings in dogs and cats with gastrointestinal perforation. Vet Radiol Ultrasound 2003; 44: 556–564. [DOI] [PubMed] [Google Scholar]


