Abstract
Objectives
The objective of this study was to evaluate the analgesic effect and absorption of buprenorphine after buccal administration in cats with oral disease.
Methods
Six adult client-owned cats with chronic gingivostomatitis (weighing 5.1 ± 1.1 kg) were recruited for a randomised, prospective, blinded, saline-controlled, crossover study. Pain scores, dental examination, stomatitis score and buccal pH measurement were conducted on day 1 under sedation in all cats. On day 2, animals were randomised into two groups and administered one of the two treatments buccally (group A received buprenorphine 0.02 mg/kg and group B received 0.9% saline) and vice versa on day 3. Pain scores and food consumption were measured at 30, 90 and 360 mins after the administration of buprenorphine. Blood samples were taken at the same time and plasma buprenorphine concentration was measured by liquid chromatography–mass spectrometry. Data were statistically analysed as non-parametric and the level of significance was set as P <0.05.
Results
There were no major side effects after buprenorphine administration. Buccal pH values ranged between 8.5 and 9.1 and the stomatitis disease activity index between 10 and 22 (17.8 ± 4.5), with the scale ranging from 0–30. The maximum buprenorphine plasma concentration (14.8 ng/ml) was observed 30 mins after administration and there was low inter-individual variability. There was a significant difference between baseline pain scores compared with pain scores after buprenorphine (P <0.05), and between the saline and buprenorphine group at 30 mins (P = 0.04) and 90 mins (P = 0.04). There was also a significant effect of the stomatitis index on the pain score. Regarding the pharmacokinetic parameters, cats with stomatitis showed lower bioavailability and shorter absorption half-life after buccal administration of buprenorphine compared with normal cats in previous studies.
Conclusions and relevance
Buccal administration of buprenorphine in cats with gingivostomatitis produces an analgesic effect and low inter-individual variability in plasma concentration, and it can be incorporated in their multimodal analgesia plan.
Introduction
Pain management is the cornerstone of veterinary practice and constitutes not only a professional obligation, but also a way to enhance animals’ quality of life. In recent years, there has been increased interest in pain assessment and management in cats, which have been historically undertreated for pain compared with other species.1–3
Opioids play an important role in the multimodal approach to pain management in cats with buprenorphine being one of the drugs most widely used. 4 Buprenorphine, a highly lipophilic semi-synthetic partial agonist at mu opioid receptors, is considered a unique drug with complex pharmacology. 5 It is the most commonly used opioid in small animal practice in the UK, 1 and is also widely used in the vast majority of countries in continental Europe, Australia and South Africa.2,6 Common morphine and hydromorphone side effects such as nausea, vomiting and salivation are rarely seen after buprenorphine. 7 This advantage, along with its efficacy and long duration of action,8,9 justifies its popularity.
In feline patients, studies have proven that the buccal route of administration (OTM) of buprenorphine shows a bioavailability similar to the intravenous (IV) and intramuscular (IM) routes.10–12 According to Robertson et al, 10 the analgesia provided by buccal administration is comparable to that of alternative routes. However, among others, the study by Giordano et al 13 demonstrated an inferior analgesic effect of the buccal route vs the IV and IM routes after ovariectomy, and Santos et al 14 found a lower sedative effect after buccal administration of dexmedetomidine and buprenorphine than the IM route.
The systemic absorption of buprenorphine after buccal administration depends on the mucosal pH. Buprenorphine is a weak base (pKa 8.24) and therefore an alkaline environment, such as the cat’s oral cavity, which has a pH between 8 and 9, favours its unionised form and enhances its bioavailability by avoiding the first pass elimination.10,15
The blood sampling site also has an impact on the buprenorphine concentration–time profile. Following buccal administration in cats, venous blood sampling from a jugular site is not an acceptable substitute for arterial blood sampling, 16 as the perfusion of the oral mucosa drains from the same vein, resulting in overestimation of the drug’s systemic availability. This can explain the high bioavailability of buprenorphine (116%) found in previous studies following buccal administration when the external jugular was used for sampling. 10
Severe inflammation of the oral cavity, described using the term ‘gingivostomatitis’, 17 is a multifactorial disease often seen in feline patients and it can be a chronic, devastating and painful condition. The exact aetiology of the condition is unknown, with environmental factors, and bacterial and viral infection being most often implicated, 18 although neoplastic, autoimmune, developmental and congenital conditions can be recognised as co-factors as well. Clinical signs include oral pain, halitosis, dysphagia, anorexia and weight loss, whereas some cats are euthanased because of poor quality of life. 19 Treatment of gingivostomatitis is mainly symptomatic and involves antibiotics, corticosteroids, opioids, non-steroidal anti-inflammatory drugs (NSAIDs), laser thermoablation, ciclosporin, oral surgery and tonsillectomy. Plasmapheresis, human immunoglobin and feline interferon omega have also been used. 20 It is not known whether the presence of gingivostomatitis affects the salivary pH and thereby the absorption and the bioavailability of buprenorphine after buccal administration.
We designed a saline-controlled, crossover efficacy and pharmacokinetic study in cats with gingivostomatitis to assess whether the presence of oral inflammation in the oral cavity affected the rate of oral transmucosal absorption, the overall systemic uptake and the analgesic efficacy of buprenorphine. Our alternative hypothesis was that there would be a difference in analgesia between the buprenorphine and saline groups after buccal administration, with buprenorphine providing superior analgesia. The prevalence of feline gingivostomatitis in the UK is 0.7% but appears to be much higher (13.1%) in studies in the USA and Southern Europe. 18 Owing to the higher prevalence of oral diseases in Southern Europe we recruited patients at Aristotle University, Thessaloniki, Greece. 21
Materials and methods
The study was designed as a randomised, prospective, saline-controlled, blinded, crossover study (Figure 1). Ethics approval was granted by the Aristotle University of Thessaloniki, Greece, and written owner consent was obtained for this clinical trial.
Figure 1.
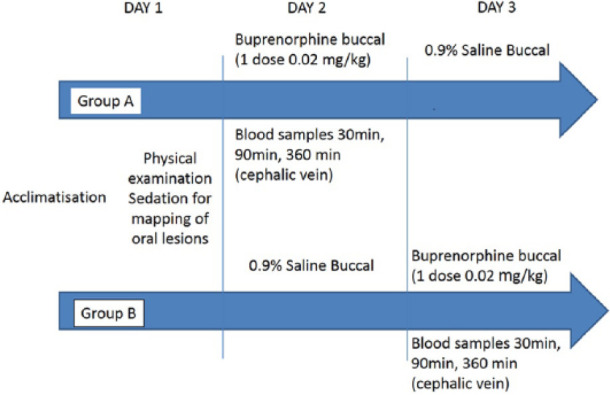
Crossover design of the study for buccal administration of buprenorphine 0.02 mg/kg or saline
Six client-owned adult cats, American Society of Anaesthesiologists physical status I or II, with evidence of oral inflammation were included in the study. No abnormal finding other than signs of gingivostomatitis was detected during physical examination. The cats had not received any opioids 5 days prior their arrival. Concurrent NSAIDs and/or antibiotics course were not exclusion criteria.
Allocation of the first treatment was randomised by the means of sealed envelopes containing the number of each cat. The first three chosen by a blinded investigator were assigned to group A and the rest to group B.
On day 1, physical examination was performed and baseline pain scores were recorded, according to a modified Botucatu pain scale (range 0–27, see Appendix 1 in the supplementary material). 22 All cats were subsequently sedated with 0.02 mg/kg medetomidine IM (Sedastart; Animalcare). During sedation, oral pH was measured with pH stripes (Simplex Health), oral lesions were staged and mapped using a dental examination form and stomatitis disease activity index (see Appendix 2 and Appendix 3 in the supplementary material). 23 An IV peripheral catheter (22 G, 25 mm [Jelco; Smiths Medical]) was placed in a cephalic vein to facilitate blood sampling and to decrease any additional discomfort for the patients. Sedation was reversed with 0.05 mg/kg of atipamesole IM (Sedastop; Animalcare). The catheters were flushed every 4 h with 2 ml heparinised saline to secure their patency and a light bandage was placed for protection.
On day 2, the cats from group A received 0.02 mg/kg buprenorphine OTM (‘BUP group’ [Buprecare; Animalcare]) and group B received an equal volume of 0.9% saline (‘SAL group’ [Vetivex1; Dechra Animal Products]) by the same route. Both treatments were administered with a 1 ml syringe (B Braun Medical) in the right cheek pouch by the principal investigator (TS), who was blinded to treatment allocation. Cats were assessed for the presence of hypersalivation, mydriasis, grooming activity and food consumption (yes/no) 30, 90 and 360 mins following the treatment administration. Pain assessments were performed by the same investigator at the same times using the same scale (modified Botucatu pain scale) as for baseline and for day 1.
Blood samples were collected by the assessor (MK), who was aware of treatment allocation, 30, 90 and 360 mins after buprenorphine buccal administration but not after saline administration. Following pain scoring, samples were taken from the cephalic catheter after 2 ml blood was aspirated to ensure a non-diluted blood sample. One millilitre of blood was collected in potassium EDTA blood tubes (Vetlab). The samples were centrifuged (Heraeus Centrifuge; Harz Simplex, GE) for 8 mins at 4039 g within 30 mins of collection. The plasma (0.5–0.7 ml) was separated and stored at −80ºC (Model 725; Thermo-Forma) in labelled Eppendorf tubes.
On day 3, the alternative treatment was administered, with group A receiving the 0.9% saline treatment and group B receiving 0.02 mg /kg buprenorphine buccally, and the same procedure as on day 2 was followed.
Plasma samples were shipped to the UK on dry ice and analysed by St George’s University, London. Plasma buprenorphine was measured using a validatedliquid chromatography–tandem mass spectrometry method (LC/MS/MS), 24 initially validated in people. The method was revalidated for feline plasma and met standards for sensitivity, linearity, precision, accuracy and stability generally accepted in bioanalytical chemistry. 25 The lower limit of quantification of the assay was 0.025 ng/ml.
Population pharmacokinetic modelling was performed with Phoenix NMLE, version 1.3 (Certara). Briefly, a two-compartmental model was built to be simultaneously fitted to the plasma buprenorphine concentration–time data from the present study (sparse sampling) and those from a previously published study performed in healthy cats administered the same dose of buprenorphine IV and by the buccal route (rich sampling). 26 Full description of the joint population PK model is provided in Appendix 4 (see the supplementary material). The goal of including external IV and buccal route data in the pharmacokinetic model was to leverage information (clearances and volumes of distribution assumed to be distributed similarly in stomatitis and healthy cats) and increase the degrees of freedom, as undertaken by Pelligand et al. 27 This allowed the fitting of the most likely plasma concentration–time curve in sparsely sampled cats and the estimation of bioavailability and absorption rate constant in the study with stomatitis cats.
Statistical analysis
A commercially available program was used for the statistical analysis (IBM SPSS Statistics 22). Data distribution was assessed for normality graphically and by the results of Kolmogorov–Smirnov statistic. Owing to violation of the assumption of normality, the Wilcoxon matched pairs signed rank test was used to compare pain scores obtained as baseline, after saline and after buprenorphine administration and at 30, 90 and 360 mins. The level of significance was set as P <0.05. Pharmacokinetic parameter distributions were compared between cats with gingivostomatitis and normal cats from a previous study, 26 using the Mann–Whitney U-test.
Correlation analysis was used to describe the strength and the direction of the linear relationship between variables. Spearman rank order correlation was used for non-parametric data testing of correlation between the stomatitis activity index score and both pH and pain scores. Food consumption (yes/no) was tested at each time point with a Fisher’s exact test.
Results
Six, client-owned, adult cats were included in this clinical study (four male neutered and two female neutered). Their age ranged from 7–10 years (mean 9.1 years) and their body weight ranged from 4–7 kg (mean 5.1 kg). Two of the cats were receiving antibiotics; one was also receiving meloxicam for stomatitis and the last dose was given 48 h before presentation.
No adverse effects were noted in this study except hypersalivation in two of the cats after the administration of buprenorphine; it resolved within minutes. All cats developed mydriasis within 5 mins of the administration of buprenorphine, except in one cat in which it could not be evaluated owing to bilateral enucleation. Mydriasis persisted for several hours after buprenorphine administration. Mydriasis does not correlate with analgesia or antinociception. 9
The oral pH values ranged from 8.5–9 and the stomatitis disease activity index ranged from 10–22 (mean 17.8 ± 4.5). Three of the cats had partial mouth extractions of the premolar and molar teeth and three had previously had full-mouth extractions; however, these were completed at least a year before presentation. The positive correlation between the variables of pH and stomatitis disease index and pH was not significant (P = 0.152).
Food consumption evaluation was part of the total pain scores. A small amount of wet and dry food was offered repeatedly at these time points. Overall, at 30 mins, all cats in the buprenorphine groups ate some wet food vs two in the saline groups (P = 0.061). At 90 mins, cats treated with buprenorphine had a significantly higher chance of eating than those treated with saline (six cats for buprenorphine vs one for saline; P = 0.0152). There was no difference at 360 mins (two cats for buprenorphine vs three cats for saline; P = 0.54). None of the cats started eating dry food at any time point.
Pain scores decreased significantly with buprenorphine and saline administration compared to baseline (P = <0.001) (Figure 2). When testing each time point, the pain scores for the BUP group were significantly lower than baseline at 30 mins (P = 0.0007) and 90 mins (P = 0.011) and were significantly lower than the SAL group at 30 mins (P = 0.04) and at 90 mins (P = 0.04) but not at 360 mins (P = 0.09). A linear mixed model also revealed a significant effect of the stomatitis index score on the pain score (P = 0.001).
Figure 2.
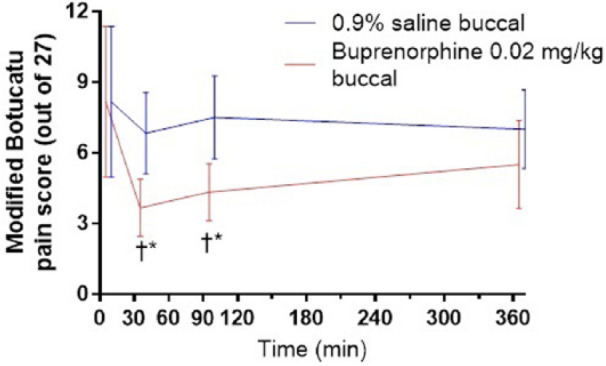
Modified Botucatu pain scores in six client-owned cats with gingivostomatitis after receiving saline and buprenorphine (0.02 mg/kg) buccally. †*P <0.05. Wilcoxon matched pairs signed rank test
The time of maximum buprenorphine plasma concentrations in cats with gingivostomatitis was at the 30 min blood sample, when concentrations ranged from 274–1621 ng/ml. One cat (a 10-year-old female neutered cat weighing 4.2 kg treated with clindamycin and meloxicam; dental score 18) had a very high plasma concentration (84,979 ng/ml). This data point was excluded from the analysis on the basis that such high plasma concentrations were not reached even in early 1 and 3 min samples after IV administration, 22 and was likely a result of contamination of the sample. The most likely buprenorphine plasma concentration–time plot for the cats with gingivostomatitis is shown in Figure 3. For all parameters listed below, the inter-individual variability is reported immediately following each estimate where appropriate. Pharmacokinetic parameter estimates for clearance, intercompartmental clearance, volume of distribution of the central and peripheral compartment displayed low inter-individual variability, even in a mixed group and were close to values previously reported (Table 1). 26
Figure 3.
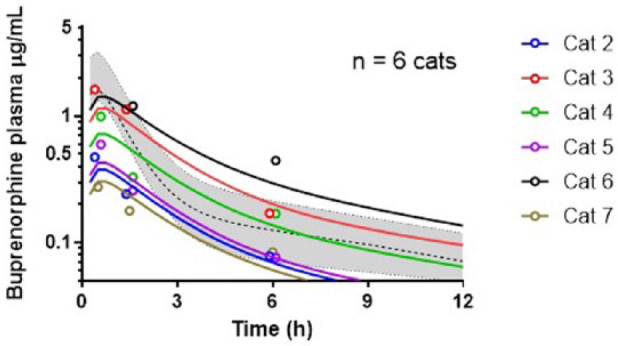
Plot of the buprenorphine concentration (μg/ml) in cats with gingivostomatitis and normal cats after receiving 0.02 mg/kg of buprenorphine buccally. For the cats with gingivostomatitis, coloured circles represent the individual measured plasma concentrations and coloured lines the most likely plasma concentration–time profile as informed by the pharmacokinetic model. For reference, the dashed line and grey area represent the median concentration and area between the minimum and maximum plasma concentration observed by Hedges et al26 after administration of the same dose of buprenorphine buccally in healthy cats
Table 1.
Pharmacokinetic parameters estimated to simulate population pharmacokinetic modelling of the data from the present study (n = 6 cats, buccal administration) and data from the study by Hedges et al (n = 6 cats, intravenous and buccal administration) 26
| Parameter | Estimate | Inter-individual variability (%) |
|---|---|---|
| Buprenorphine bioavailability OTM in gingivostomatitis cats (present study) | 19.5% | 65.7 |
| Buprenorphine bioavailability OTM in normal cats (Hedges et al 26 ) | 28.8% | 19.6 |
| Clearance (l/kg/h) | 1.26 | 1.1 |
| Volume of distribution central compartment (l/kg) | 0.65 | 0.9 |
| Inter-compartmental clearance (l/kg/h) | 1.19 | 2.3 |
| Volume of distribution peripheral compartment (l/kg) | 6.96 | 7.8 |
| Absorption rate constant OTM gingivostomatitis cats (l/h) | 0.573 | NE |
| Absorption half-life OTM gingivostomatitis cats (h) | 1.2 | |
| Absorption rate constant OTM normal cats (l/h) | 1.387 | NE |
| Absorption half-life OTM normal cats (h) | 0.49 |
OTM = buccal administration; NE = could not be estimated for individual
The pharmacokinetic parameters are presented in Table 1 and described in Appendix 4 (see supplementary material) (Figure 4).
Figure 4.
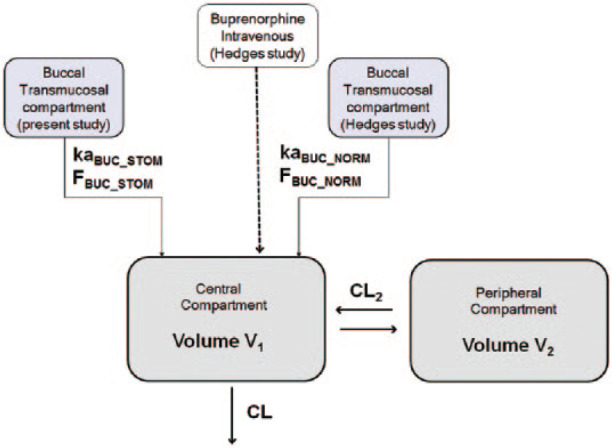
Population pharmacokinetic–pharmacodynamic modelling. A classic two-compartment model with first-order absorption was the starting point for compartmental modelling of the buccal route. CL = body clearance; CL2 = inter-compartmental clearance; V1 = central volume of distribution; V2 = peripheral volume of distribution; kaBUC_STOM = absorption rate constant in cats with stomatitis; kaBUC_NORM = absorption rate constant in normal cats; FBUC_STOM = bioavailability in cats with stomatitis; FBUC_NORM = bioavailability in normal cats
Discussion
During this study, no side effects were identified, except for hypersalivation in two cats. All cats, except the one that had bilateral enucleation, developed mydriasis.
There is a lack of evidence in the veterinary literature on whether oral inflammation affects buccal pH values. The values of buccal pH in our study ranged between 8.5 and 9.1 and were relatively lower than those in the study by Robertson et al (pH 9.0) 10 but higher than those in the study by Hedges et al (pH8.0). 26 A correlation between the buccal pH and the stomatitis disease activity index was not identified. An increase in pH is associated with increased salivation in humans owing to an increase of sodium and bicarbonate.28,29 In cats, stomatitis is often related with signs of hypersalivation. 17
Cats showed increased appetite at 30 and 90 mins after buprenorphine administration, which could have been due to additional analgesia or euphoria. An increase in food consumption is a rare manifestation of pain in cats. 30 None of the cats ate dry food, which could have been because of insufficient pain relief or owing to preference, as cats were simultaneously offered wet and dry food. The influence of a hospital environment should also be considered. Some cats remain unresponsive and passive in new environments or can be hyperactive.31,32 Increased food intake would be an important benefit, considering that compromised nutrition is one of the most important problems encountered with gingivostomatitis. 33
Pain scores following buprenorphine administration were lower than at baseline and following saline administration. This can be attributed to pain relief, as well as the euphoria produced by opioids. In addition, the local effect of buprenorphine needs to be considered since a study in humans found that buprenorphine decreased postoperative pain and increased the duration of analgesia when added to the inferior alveolar nerve block for dental surgery compared with IM administration. 34 The fact that the pain scores were lower after saline administration than at baseline could be attributed to acclimatisation to the new environment, as well familiarisation with the pain-scoring process and the evaluator. The effect of stomatitis index on pain score was expected, as cats with more severe stomatitis are expected to be more painful. Our alternative hypothesis that pain scores would be lower following buprenorphine than following saline was confirmed, as there was a significant difference at 30 and 90 mins. The plasma buprenorphine concentration at 360 mins may have been inadequate to provide analgesia. In any case, the results may suggest that the duration of effect of buprenorphine at the dose used may be shorter than previously reported.
The time of maximum plasma buprenorphine concentration was 30 mins after administration, and pharmacokinetic analysis showed low inter-individual variability with values close to those obtained by Hedges et al 26 in cats with normal oral mucosa. However, transmucosal drug absorption depends on many different factors, such as its concentration and mucosal contact time. 35 Buprenorphine was administered in the cheek pouch, but the degree of inflammation on the specific area could not be determined. Inflammation-induced vasodilation could have led to an earlier maximum concentration that we were unable to detect as our first blood sample was at 30 mins. In addition, cats might have swallowed or spitted a portion of the drug, as they were sensitive to handling of the head and did not tolerate their mouth being held closed after treatment. The formulation used in this study was a multidose vial (Buprecare; Animalcare) containing 0.135% chlorocresol as a preservative and it is possible that the preservative-free buprenorphine could be better tolerated, although there was no difference in pH among the formulations. 36 The multidose vials are commonly used in practice owing to cost-effectiveness and easy usage and storage.
In our study, the mean absorption half-life of buprenorphine was longer than found by the study of Hedges et al, 26 which included normal cats. However, there was no significant difference in bioavailability, although the present study may have been underpowered to detect a difference. The difference in absorption rate could be caused by the different formulations of buprenorphine that were used in the two studies, the actual modalities of administration or as an effect of the higher pH and the presence of gingivostomatitis.
The study had several limitations. The lack of a sensitive and validated pain scale for oral pain is a major limitation. The UNESP-Botucatu scale is the only pain-scoring system for cats with published data on reliability, validity and sensitivity, 30 and we modified it for oral pain using the oral cavity as the painful reference point and the head and neck area as the surrounding tissues. We omitted the blood pressure measurement because it can be stressful and unreliable when repeated at frequent intervals. The maximum point of our pain scale was 27 instead of 30 in the original scale. The small sample size is another limitation that could have affected our statistical analysis. Furthermore, the use of historical data for modelling constitutes another limitation, as it involves the use of data from another study obtained under different conditions and analysed using a different assay, despite the fact that the data were remodelled using the study population model. The fact that one of the cats was receiving meloxicam constitutes another limitation. However, the last dose was given 48 h before presentation and the baseline pain score of this cat was similar to the rest of the cats. In addition, there was no possibility that co-administration of NSAIDs interfered with the quantitative analysis of buprenorphine by LC/MS because of the high specificity of the method. Finally, the values of buccal pH were also obtained on day 1 after the administration of medetomidine, which could have also affected the value, so we were not aware of the actual pH value at the time of buprenorphine administration.
Conclusions
OTM administration of buprenorphine in cats with gingivostomatitis produces an analgesic effect and has low inter-individual variability regarding plasma concentration. Further studies are needed to elucidate the role of oral inflammation on buccal drug absorption in cats, as well as the potential benefit and appropriateness of opioids vs the current analgesia alternatives such as NSAIDs. Furthermore, considering that sublingual buprenorphine constitutes an effective treatment of chronic pain in humans and that subcutaneous buprenorphine prevented hyperalgesia in cats,37,38 studies on the long-term use of buprenorphine by the buccal route in cats with chronic gingivostomatitis and the evaluation of the potential benefits and side effects would be of clinical interest.
Supplemental Material
UNESP-Botucatu Multidimensional Composite Pain Scale for assessing postoperative pain in cats, modified to assess oral pain
Feline dental chart
Stomatitis disease activity index score
Population pharmacokinetic-pharmacodynamic modelling
Footnotes
Accepted: 26 July 2017
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplementary material: The following files are available.
Appendix 1: UNESP-Botucatu Multidimensional Composite Pain Scale for assessing postoperative pain in cats, modified to assess oral pain
Appendix 2: Feline dental chart
Appendix 3: Stomatitis disease activity index score
Appendix 4: Population pharmacokinetic pharmacodynamic modelling
References
- 1. Lascelles D, Capner C, Waterman-Pearson A. Current British veterinary attitudes to perioperative analgesia for cats and small mammals. Vet Rec 1999; 145: 601–604. [DOI] [PubMed] [Google Scholar]
- 2. Dohoo SE, Dohoo IR. Factors influencing the postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can Vet J 1996; 37: 552. [PMC free article] [PubMed] [Google Scholar]
- 3. Watson A, Nicholson A, Church D, et al. Use of anti-inflammatory and analgesic drugs in dogs and cats. Aust Vet J 1996; 74: 203–210. [DOI] [PubMed] [Google Scholar]
- 4. Bortolami E, Love EJ. Practical use of opioids in cats: a state-of-the-art, evidence-based review. J Feline Med Surg 2015; 17: 283–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol 2004; 2: 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joubert K. The use of analgesic drugs by South African veterinarians: continuing education. J South Afr Vet Assoc 2001; 72: 57–60. [DOI] [PubMed] [Google Scholar]
- 7. Robertson S, Taylor P, Sear J. Systemic uptake of buprenorphine by cats after oral mucosal administration. Vet Rec 2003; 152: 675–678. [DOI] [PubMed] [Google Scholar]
- 8. Taylor P, Robertson S, Dixon M, et al. Morphine, pethidine and buprenorphine disposition in the cat. J Vet Pharmacol Ther 2001; 24: 391–398. [DOI] [PubMed] [Google Scholar]
- 9. Steagall P, Monteiro-Steagall B, Taylor P. A review of the studies using buprenorphine in cats. J Vet Intern Med 2014; 28: 762–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robertson S, Lascelles B, Taylor P, et al. PK-PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration. J Vet Pharmacol Ther 2005; 28: 453–460. [DOI] [PubMed] [Google Scholar]
- 11. Abbo LA, Ko DJC, Maxwell LK, et al. Pharmacokinetics of buprenorphine following intravenous and oral transmucosal administration in dogs. Vet Ther 2008; 9: 83–93. [PubMed] [Google Scholar]
- 12. Ko JC, Freeman LJ, Barletta M, et al. Efficacy of oral transmucosal and intravenous administration of buprenorphine before surgery for postoperative analgesia in dogs undergoing ovariohysterectomy. J Am Vet Med Assoc 2011; 238: 318–328. [DOI] [PubMed] [Google Scholar]
- 13. Giordano T, Steagall PV, Ferreira TH, et al. Postoperative analgesic effects of intravenous, intramuscular, subcutaneous or oral transmucosal buprenorphine administered to cats undergoing ovariohysterectomy. Vet Anaesth Analg 2010; 37: 357–366. [DOI] [PubMed] [Google Scholar]
- 14. Santos LCP, Ludders JW, Erb HN, et al. Sedative and cardiorespiratory effects of dexmedetomidine and buprenorphine administered to cats via oral transmucosal or intramuscular routes. Vet Anaesth Analg 2010; 37: 417–424. [DOI] [PubMed] [Google Scholar]
- 15. Mendelson J, Upton RA, Everhart ET, et al. Bioavailability of sublingual buprenorphine. J Clin Pharmacol 1997; 37: 31–37. [DOI] [PubMed] [Google Scholar]
- 16. Hedges A, Pypendop BH, Shilo Y, et al. Impact of the blood sampling site on time–concentration drug profiles following intravenous or buccal drug administration. J Vet Pharmacol Ther 2014; 37: 145–150. [DOI] [PubMed] [Google Scholar]
- 17. Lyon KF. Gingivostomatitis. Vet Clin North Am Small Anim Pract 2005; 35: 891–911. [DOI] [PubMed] [Google Scholar]
- 18. Dolieslager SMJ, Lappin DF, Bennett D, et al. The influence of oral bacteria on tissue levels of Toll-like receptor and cytokine mRNAs in feline chronic gingivostomatitis and oral health. Vet Immunol Immunopathol 2013; 151: 263–274. [DOI] [PubMed] [Google Scholar]
- 19. Dowers KL, Hawley JR, Brewer MM, et al. Association of Bartonella species, feline calicivirus, and feline herpesvirus 1 infection with gingivostomatitis in cats. J Feline Med Surg 2010; 12: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leal R, Gil S, Mcgahie D, et al. The use of oral recombinant feline interferon-omega in naturally feline immunodeficiency virus infected cats: new insights into an alternative immunomodulation therapy. J Vet Intern Med 2014; 28: 729. [Google Scholar]
- 21. Healey KA, Dawson S, Burrow R, et al. Prevalence of feline chronic gingivo-stomatitis in first opinion veterinary practice. J Feline Med Surg 2007; 9: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brondani JT, Mama KR, Luna SP, et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res 2013; 9: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lommer MJ. Efficacy of cyclosporine for chronic, refractory stomatitis in cats: a randomized, placebo-controlled, double-blinded clinical study. J Vet Dent 2013; 30: 8–17. [DOI] [PubMed] [Google Scholar]
- 24. McKeown DA, Lee TD, Button J, et al. A sensitive and specific liquid chromatography–tandem mass spectrometry method for the analysis of buprenorphine and norbuprenorphine in human plasma. In: Monitoring TD. (ed). 10th International Congress of Therapeutic Drug Monitoring & Clinical Toxicology. Nice: Therapeutic Drug Monitoring, 2007, pp 460–551. [Google Scholar]
- 25. US Food and Drug Administration. FDA guidance for industry: bioanalytical method validation. Rockville, MD: US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, 2001. [Google Scholar]
- 26. Hedges A, Pypendop BH, Shilo-Benjamini Y, et al. Pharmacokinetics of buprenorphine following intravenous and buccal administration in cats, and effects on thermal threshold. J Vet Pharmacol Ther 2014; 37: 252–259. [DOI] [PubMed] [Google Scholar]
- 27. Pelligand L, Soubret A, King J, et al. Modeling of large pharmacokinetic data using nonlinear mixed-effects: a paradigm shift in veterinary pharmacology. A case study with robenacoxib in cats. CPT Pharmacometrics Syst Pharmacol 2016; 5: 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Preoteasa E, Tâncu A, Iosif L, et al. Salivary changes related to systemic diseases in the edentulous patients. J Med Life 2014; 7: 577. [PMC free article] [PubMed] [Google Scholar]
- 29. Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release 2011; 153: 106–116. [DOI] [PubMed] [Google Scholar]
- 30. Merola I, Mills DS. Systematic review of the behavioural assessment of pain in cats. J Feline Med Surg 2016; 18: 60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ellis SL. Environmental enrichment: practical strategies for improving feline welfare. J Feline Med Surg 2009; 11: 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodan I. Understanding feline behavior and application for appropriate handling and management. Top Comp Anim Med 2010; 25: 178–188. [DOI] [PubMed] [Google Scholar]
- 33. Southerden P. Review of feline oral disease 1. Periodontitis and chronic gingivostomatitis. In Pract 2010; 32: 2–7. [Google Scholar]
- 34. Chhabra N, Sharma P, Chhabra S, et al. Efficacy of buprenorphine added to 2% lignocaine plus adrenaline 1: 80,000 in providing postoperative analgesia after lower third molar surgery. Int J Oral Maxillofac Surg 2016; 45: 1644–1651. [DOI] [PubMed] [Google Scholar]
- 35. Madhav NS, Shakya AK, Shakya P, et al. Orotransmucosal drug delivery systems: a review. J Control Release 2009; 140: 2–11. [DOI] [PubMed] [Google Scholar]
- 36. Bortolami E, Slingsby L, Love E. Comparison of two formulations of buprenorphine in cats administered by the oral transmucosal route. Vet Anaesth Analg 2011; 38: 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther 2005; 12: 379–384. [DOI] [PubMed] [Google Scholar]
- 38. Taylor P, Steagall P, Dixon M, et al. Carprofen and buprenorphine prevent hyperalgesia in a model of inflammatory pain in cats. Res Vet Sci 2007; 83: 369–375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UNESP-Botucatu Multidimensional Composite Pain Scale for assessing postoperative pain in cats, modified to assess oral pain
Feline dental chart
Stomatitis disease activity index score
Population pharmacokinetic-pharmacodynamic modelling


