Abstract
Objectives
The objective of this study was to evaluate the diagnostic accuracy of infrared thermography in cats with acute pelvic paralysis to differentiate feline aortic thromboembolism (FATE) from non-ischaemic conditions.
Methods
Thermographic images were prospectively obtained at admission from cats presented for acute bilateral pelvic paralysis. Based on the final diagnosis, cats were divided into a FATE and a control group (ischaemic and non-ischaemic related pelvic paralysis, respectively). The maximum (Tmax), minimum (Tmin) and average (Tav) temperatures were determined for each of the four limbs within a hand-drawn region of interest on the dorsal limb extremity. Temperature differences between the forelimb (non-affected) and hindlimb (affected) with the highest temperature (ΔT), with the lowest temperature (δT) and from the right and left side (RightΔT and LeftΔT, respectively) were calculated.
Results
The FATE and control groups included 10 and six cats, respectively. In the FATE group, right hindlimb mean Tmax (23.6°C ± 1.9), left hindlimb mean Tmax (23.6°C ± 2.2) and mean Tav (22.7°C ± 2.2) were significantly lower than in the control group (26.6°C ± 3.5 [P = 0.042]; 26.6°C ± 2.4°C [P = 0.024] and 25.7°C ± 2.0 [P = 0.020], respectively). ΔT, δT, RightΔT and LeftΔT were significantly higher in the FATE group than in the control group. A cut-off value of 2.4°C for RightΔTmax and LeftΔTmax allowed discrimination between the FATE and control groups with a sensitivity of 80% and 90%, respectively, a specificity of 100% for both, a positive predictive value of 100% for both, and a negative predictive value of 75% and 86%, respectively.
Conclusions and relevance
A minimal difference of 2.4°C between ipsilateral affected and non-affected limbs has an excellent specificity and high sensitivity for FATE diagnosis. Infrared thermography seems to be a promising, useful, easy, non-invasive and rapid method for detecting aortic thromboembolism in cats, particularly in emergency situations.
Introduction
Feline aortic thromboembolism (FATE) is a devastating condition in cats. 1 FATE is clinically manifested by an acute onset of unilateral or bilateral hindlimb paralysis, weak or absent femoral pulses, pain, pale or cyanotic footpads and nail-beds, and cold extremities of the affected limbs. 2 The clinical diagnosis is usually easy, but this condition could be mistaken with pelvic trauma or other causes of paralysis. The final diagnosis relies on ultrasound visualisation of the thrombus in the distal aorta, but is highly operator-dependent. 3
Consistent with the pathogenesis of FATE, differences in clinical or biochemical parameters between affected and non-affected limbs are of great interest. For example, glucose and lactate concentration differences between central and affected limbs have been studied,4,5 but other studies including other biochemical and physical parameters, such as toe temperature, are lacking.
Thermography is a painless, non-invasive, non-ionising diagnostic imaging technique recording the cutaneous thermal patterns generated by the emission of surface heat. 6 This technique has been used in dogs for the diagnosis of femoral arterial thrombosis, 7 mammary tumour, 8 thoracolumbar disc disease 9 and cranial cruciate ligament rupture. 10 In humans, thermography is an accurate imaging technique for diagnosis of arterial thromboembolism in infants and deep venous thrombosis in adults.11,12 In cats, thermography has been used for pain assessment and various diseases.13,14 However, thermography has never been assessed in FATE.
The purpose of this study was to evaluate the diagnostic accuracy of infrared thermography as a new diagnostic tool for FATE. Our hypothesis was that FATE results in diminished temperature of the affected limbs vs non-affected limbs, and could be detected by infrared thermography.
Materials and methods
Ethical statement
Our institution ethics committee approved the study (number 1713). Owner consent was obtained prior to cat enrolment.
Cats
All enrolled cats were admitted to the intensive care unit (SIAMU, VetAgro Sup) between June 2015 and April 2017. We included cats of any age, sex or breed presented for acute hindlimb paralysis. Cats were excluded from the study if external injuries were present, or if cutaneous temperature was modified by external factors (wet hair, urine, alcohol, etc).
Cats were separated into two groups based on the final diagnosis: FATE group and control group. In the FATE group, cats were diagnosed with bilateral distal aortic thromboembolism after direct Doppler ultrasonographic visualisation of vessel obstruction by a thrombus. Cats enrolled in the control group were presented for bilateral acute-onset of pelvic paralysis not related to ischaemic process. The final diagnosis in the control group was obtained by radiographs, CT, MRI or cerebrospinal fluid analysis based on the main clinical hypothesis.
Thermographic imaging
A thermal camera, with a resolution of 80 × 60 pixels (Flir C2; FLIR System) was used for the imaging. The display chosen was a high rainbow colour palette. Thermographic images were obtained from each cat immediately after admission. The cats were placed in lateral recumbency on a sliding sheet. Three views were taken: one of the entire cat with four limbs (four-limb view [Figure 1]), one of the two forelimbs and one of the two hindlimbs (two-limb view [Figure 2]).
Figure 1.

Four-limb view with high rainbow colour palette scale of a cat with aortic thromboembolism. Note the colour difference between forelimbs (non-affected) and hindlimbs (affected)
Figure 2.
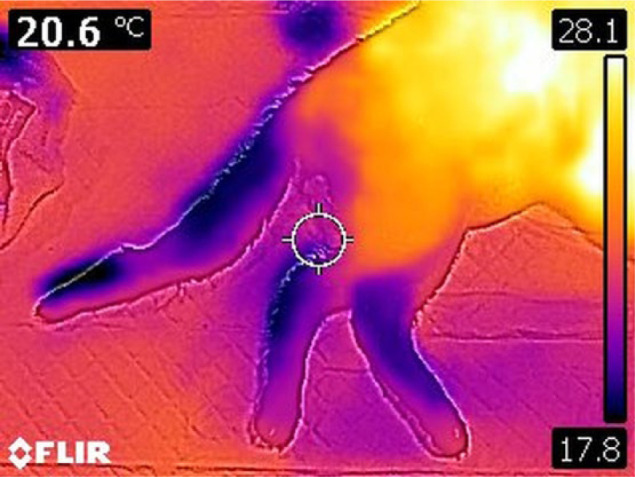
Two-limb view with high rainbow colour palette scale of a cat with aortic thromboembolism
The freeware FLIR Tools 2.1 (FLIR, 2014) was applied to interpret the thermographic images.
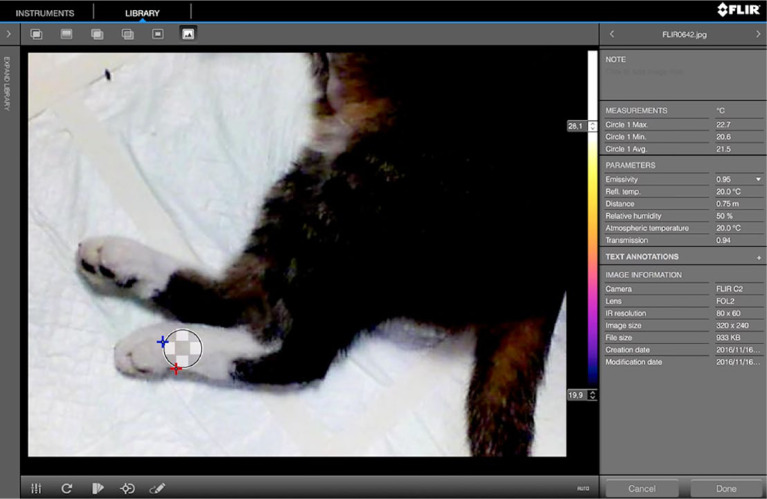
One veterinarian (CPN) trained in thermographic imaging evaluated the images. The veterinarian performing the evaluation was blinded to the cats’ history or signalment during the evaluation process, and non-rainbow-coloured images were used for analysis (Figure 3). For each limb, a circular region of interest (ROI) on the dorsal limb extremity was drawn on the two-limb view (Figure 3), and the maximum (Tmax), minimum (Tmin) and average (Tav) temperatures were determined. When the two-limb view did not allow good determination of the ROI, the four-limb view was used. All data were entered into an electronic spreadsheet (Excel 2013; Microsoft).
Figure 3.
Region of interest (ROI) on the two-limb view and determination of the maximal (circle 1 max), minimal (circle 1 min) and average (circle 1 avg) temperature of the ROI (Tmax, Tmin and Tav, respectively)
For each cat, temperature differences between Tmax, Tmin and Tav of forelimb (non-affected) and hindlimb (affected) were calculated between: (1) ΔT – the forelimb with the highest temperature and the hindlimb with the highest temperature (ΔTmax, ΔTmin, ΔTav); (2) δT – the forelimb with the lowest temperature and the hindlimb with the lowest temperature (δTmax, δTmin, δTav); (3) RightΔT – the right forelimb and the right hindlimb (RightΔTmax, RightΔTmin, RightΔTav); (4) LeftΔT – the left forelimb and the left hindlimb (LeftΔTmax, LeftΔTmin, LeftΔTav).
Statistical analysis
Data analysis was performed using the JMP 13.1 statistical software package (SAS Institute). All descriptive data are presented as the median (range) and expressed as the percentage of total cats. Normality was assessed using the Shapiro–Wilk test. As all variables had a normal distribution, they are expressed as mean ± SD. Comparisons between FATE and control groups were evaluated by the two-tailed unpaired Student’s t-test. A partition model was used to find the most accurate limb temperature difference for differentiation between FATE and control groups and to define the cut-off value. Sensitivity, specificity, and positive and negative predictive values (PPV and NPV, respectively) were calculated with standard methods based on contingency tables. A P value <0.05 was considered statistically significant.
Results
Case selection
The FATE group included 10 cats. Eight (80%) were domestic shorthairs, and there was one Bengal and one Devon Rex. Median age was 7 years (range 2–12 years), and median weight was 4.7 kg (range 2.8–6.0 kg). Eight cats (80%) were male; two (20%) were female. All cats were neutered. Underlying diseases leading to FATE were cardiomyopathy for eight cats and neoplasia for two cats. Seven cats (70%) did not survive to discharge.
The control group included six cats. Five (83%) were domestic shorthairs and the remaining one (17%) was a Bengal. Median age was 3 years (range 0.3–6.0 years), and median weight was 4.5 kg (range 1.4–5.5 kg). Five cats (83%) were male and one (17%) was female. Four (68%) cats were neutered. Underlying diseases leading to hindlimb paralysis were pelvic fracture (n = 3; 50%), vertebral fracture (n = 2; 33%) and infectious meningomyelitis (n = 1; 17%). Four cats (67%) did not survive to discharge.
Median age and weight were not significantly different between groups.
Thermographic results
Temperatures of each limb are summarised in Table 1. In the FATE group, mean Tmax (23.6 ± 1.9°C) of the right hindlimb, mean Tmax (23.6 ± 2.2°C) and mean Tav (22.7 ± 2.2°C) of the left hindlimb were significantly lower than in the control group (26.6 ± 3.5°C [P = 0.042]; 26.6 ± 2.4°C [P = 0.024]; and 25.7 ± 2.0°C [P = 0.020]; respectively) (Table 1).
Table 1.
Temperature of each limb in feline aortic thromboembolism (FATE) and control groups
| FATE group (n = 10) | Control group (n = 6) | P value | ||
|---|---|---|---|---|
| Right forelimb | Tmax | 27.3 ± 2.5 | 27.1 ± 3.2 | 0.889 |
| Tmin | 24.8 ± 2.3 | 23.2 ± 1.9 | 0.189 | |
| Tav | 25.9 ± 2.4 | 25.5 ± 2.4 | 0.737 | |
| Left forelimb | Tmax | 26.9 ± 2.6 | 26.1 ± 2.8 | 0.563 |
| Tmin | 24.4 ± 3.0 | 23.4 ± 1.9 | 0.427 | |
| Tav | 25.8 ± 2.9 | 24.8 ± 2.2 | 0.503 | |
| Right hindlimb | Tmax | 23.6 ± 1.9 | 26.6 ± 3.5 | 0.042* |
| Tmin | 22.0 ± 2.2 | 23.8 ± 2.8 | 0.169 | |
| Tav | 22.9 ± 1.9 | 25.3 ± 3.2 | 0.07 | |
| Left hindlimb | Tmax | 23.6 ± 2.2 | 26.6 ± 2.4 | 0.024* |
| Tmin | 22.1 ± 2.4 | 24.4 ± 1.6 | 0.052 | |
| Tav | 22.7 ± 2.2 | 25.7 ± 2.0 | 0.020* |
Data are mean ± SD of the maximum (Tmax), minimum (Tmin) and average (Tav) of each limb temperature in each region of interest
P <0.05 between FATE group and control group
ΔT and δT were significantly higher in the FATE group than in the control group for all temperatures (Tmax, Tmin and Tav) (Table 2).
Table 2.
Temperature differences between forelimbs and hindlimbs
| FATE group (n = 10) | Control group (n = 6) | P value | |
|---|---|---|---|
| ΔTmax | 3.6 ± 1.2 | −0.1 ± 1.8 | 0.0002* |
| ΔTmin | 2.6 ± 2.0 | −1.3 ± 1.8 | 0.0014* |
| ΔTav | 3.2 ± 1.6 | −0.6 ± 1.6 | 0.0004* |
| δTmax | 3.2 ± 1.1 | −0.2 ± 2.0 | 0.0018* |
| δTmin | 2.6 ± 1.5 | −0.1 ± 3.1 | 0.0339* |
| δTav | 3.0 ± 1.4 | −0.1 ± 2.4 | 0.0075* |
| RightΔTmax | 3.7 ± 1.3 | 0.5 ± 1.8 | 0.0011* |
| RightΔTmin | 2.8 ± 0.7 | −0.6 ± 3.3 | 0.0124* |
| RightΔTav | 3.1 ± 1.5 | 0.1 ± 2.4 | 0.0098* |
| LeftΔTmax | 3.3 ± 1.1 | −0.6 ± 2.2 | 0.0003* |
| LeftΔTmin | 2.3 ± 1.9 | −1.1 ± 2.4 | 0.0063* |
| LeftΔTav | 3.0 ± 1.4 | −0.8 ± 2.2 | 0.0008* |
Data are mean ± SD
P <0.05 between feline aortic thromboembolism (FATE) group and control group
Tmax = maximum temperature in the region of interest (ROI); Tmin = minimum temperature in the ROI; Tav = average temperature in the ROI; ΔT = temperature difference between the forelimb with the highest temperature and the hindlimb with the highest temperature; δT = temperature difference between the forelimb with the lowest temperature and the hindlimb with the lowest temperature; RightΔT = temperature difference between the right forelimb and the right hindlimb for Tmax (RightΔTmax), Tmin (RightΔTmin) and Tav (RightΔTav); LeftΔT = temperature difference between the left forelimb and the left hindlimb for Tmax (LeftΔTmax), Tmin (LeftΔTmin) and Tav (LeftΔTav)
When investigating the ipsilateral limbs, RightΔT and LeftΔT were significantly higher in the FATE group than in the control group for all temperatures (Table 2).
Between all studied limb temperature differences, the most accurate to discriminate between cats in the FATE and control groups was LeftΔTmax with a cut-off value of 2.4°C (sensitivity 90%, specificity 100%, PPV 100%, NPV 86%). For RightΔTmax, the best cut-off value to discriminate cats between the FATE and control groups was also 2.4°C (sensitivity 80%, specificity 100%, PPV 100%, NPV 75%).
Discussion
The main finding of our study is that a difference of at least 2.4°C between ipsilateral non-affected and affected limbs has an excellent specificity and high sensitivity for the diagnosis of FATE. Our study is the first to prospectively describe the use of infrared thermography in FATE, and this technique appears to be a simple, non-invasive and reliable method to diagnose FATE. Cold extremities are part of the major clinical signs of FATE and clinical comparison of the affected and non-affected limb temperature is important. However, a 2.4°C difference could be difficult to detect clinically. Thermography, by allowing a precise skin temperature measurement, can detect a decrease in limb temperature in the early course of the disease. Early diagnosis is essential for initiation of the appropriate treatment that could improve the prognosis. 2
When comparing right and left hindlimb temperature between groups, mean Tmax was significantly lower in the FATE group than in the control group, suggesting the usefulness of direct assessment of hindlimb temperature. However, as systemic alterations associated with the underlying condition leading to pelvic paralysis may affect limbs temperature (ie, hypovolaemia in trauma), analysing temperature from the paralysed limbs only may not be sufficient, and comparison of affected and non-affected limb temperature could be more accurate.
While a decrease of skin temperature assessed by infrared thermography has already been described after arterial thromboembolism in dogs, 7 our study is the first to report its use in cats. The maximum temperature of the dorsal limb extremity was the most reliable data, as the Tmax of the hindlimbs was significantly higher in the control group (approximately 26.6°C) than in the FATE group (approximately 23.3°C) on both sides. As a thermal camera can give an instantaneous temperature reading on one defined spot, the maximal temperature of the distal extremity of the limb could be easily assessed.
The admission of an acute paralysed cat, sometimes dyspnoeic (because of congestive heart failure in FATE or pulmonary trauma), could be very stressful in the emergency setting. Distinction between ischaemic or non-ischaemic causes of the paralysis usually rely on palpation of femoral pulses. However, palpation of the femoral pulse could be challenging in a painful or hypovolaemic cat. Thanks to the lack of sedation or physical restrain needed for imaging, infrared thermography has potential use as a screening test for triage of acute paralysed cats.
Final diagnosis of FATE was made by direct observation of the thrombus in the distal aorta in our study, used as the gold standard. Several imaging procedures have been described, such as abdominal ultrasonography, CT or MRI angiography,3,15 but these are technically challenging, time consuming, cost-prohibiting or require anaesthesia. Bedside tests have been developed to aid in the diagnosis of FATE. In one study, a difference in glucose concentration of 30 mg/dl between a systemic and locally affected limb blood sample has a sensitivity of 100% and a specificity of 90% in diagnosing FATE. 4 Blood glucose measurement is easy and widely available, but obtaining blood samples from affected limbs might be challenging because of the absence of blood supply. Thermography shows a similar accuracy in detecting FATE as blood glucose concentration measurement, but it is totally painless and non-invasive for the cat, and very easy to realise and to interpret. 13 Based on our experience, direct visual analysis of the thermal images was usually sufficient to suspect FATE, as distal extremities of pelvic limbs were a different colour to forelimbs (Figure 1). In cats with non-ischaemic pelvic paralysis, this colour asymmetry was not observed (Figure 4).
Figure 4.
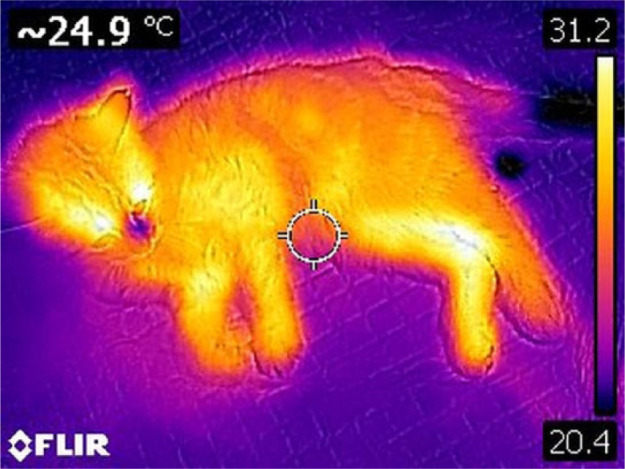
Four-limb view with high rainbow colour palette scale of a cat with pelvic fracture. Note the absence of colour difference between forelimbs (non-affected) and hindlimbs (affected)
This study has several limitations. First, the size of the study groups was rather limited, but was sufficient to show a statistical difference between groups. However, the results need to be applied to a larger population of cats for further verification. Second, this study was not designed to investigate the size or localisation of the thrombus, or the prognosis of FATE. Infrared thermography was used by Kim et al 7 for monitoring local arterial thrombolysis in a dog. Serial monitoring of limb temperature after thrombolysis may be valuable if an increase in limb temperature could be detected earlier than clinically, and if a correlation with functional recovery exists. Third, time between the start of limb paralysis and presentation was not constant, and could have influenced the results. If highly suspected, we recommend repeating the thermographic measurement within the first hour of admission. The last limitation is the availability of an infrared camera and potentially the cost. However, new small devices are now easily available.
Conclusions
The main finding of our study is that a minimum difference of 2.4°C between ipsilateral affected and non-affected limbs has an excellent specificity and high sensitivity for the diagnosis of FATE. Even if these results have to be confirmed in a larger population of cats, infrared thermography appears to be a promising, useful, easy, non-invasive and instant method for detecting aortic thromboembolism in cats, particularly in emergency practice.
Acknowledgments
The authors would like to thank Dr Alexandra Nectoux and Dr Amandine Violé for their help with the acquisition of the thermal images.
Footnotes
Accepted: 22 August 2017
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Borgeat K, Wright J, Garrod O, et al. Arterial thromboembolism in 250 cats in general practice: 2004–2012. J Vet Intern Med 2014; 28: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Luis Fuentes V. Arterial thromboembolism: risks, realities and a rational first-line approach. J Feline Med Surg 2012; 14: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams TP, Shaw S, Porter A, et al. Aortic thrombosis in dogs. J Vet Emerg Crit Care (San Antonio) 2017; 27: 9–22. [DOI] [PubMed] [Google Scholar]
- 4. Klainbart S, Kelmer E, Vidmayer B, et al. Peripheral and central venous blood glucose concentrations in dogs and cats with acute arterial thromboembolism. J Vet Intern Med 2014; 28: 1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMichael M, Rozansky EA, Rush JE. Low blood glucose levels as a marker of arterial thromboembolism in dogs and cats [abstract]. J Vet Emerg Crit Care (San Antonio) 1998; 8: 249–264. [Google Scholar]
- 6. Loughin CA, Marino DJ. Evaluation of thermographic imaging of the limbs of healthy dogs. Am J Vet Res 2007; 68: 1064–1069. [DOI] [PubMed] [Google Scholar]
- 7. Kim JH, Park HM. Unilateral femoral arterial thrombosis in a dog with malignant mammary gland tumor: clinical and thermographic findings, and successful treatment with local intra-arterial administration of streptokinase. J Vet Med Sci 2012; 74: 657–661. [DOI] [PubMed] [Google Scholar]
- 8. Pavelski M, Silva DM, Leite NC, et al. Infrared thermography in dogs with mammary tumors and healthy dogs. J Vet Intern Med 2015; 29: 1578–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grossbard BP, Loughin CA, Marino DJ, et al. Medical infrared imaging (thermography) of type I thoracolumbar disk disease in chondrodystrophic dogs. Vet Surg 2014; 43: 869–876. [DOI] [PubMed] [Google Scholar]
- 10. Infernuso T, Loughin CA, Marino DJ, et al. Thermal imaging of normal and cranial cruciate ligament-deficient stifles in dogs. Vet Surg 2010; 39: 410–417. [DOI] [PubMed] [Google Scholar]
- 11. Saxena AK, Willital GH. Infrared thermography: experience from a decade of pediatric imaging. Eur J Pediatr 2008; 167: 757–764. [DOI] [PubMed] [Google Scholar]
- 12. Deng F, Tang Q, Zeng G, et al. Effectiveness of digital infrared thermal imaging in detecting lower extremity deep venous thrombosis. Med Phys 2015; 42: 2242–2248. [DOI] [PubMed] [Google Scholar]
- 13. Vainionpaa MH, Raekallio MR, Junnila JJ, et al. A comparison of thermographic imaging, physical examination and modified questionnaire as an instrument to assess painful conditions in cats. J Feline Med Surg 2013; 15: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Redaelli V, Tanzi B, Luzi F, et al. Use of thermographic imaging in clinical diagnosis of small animal: preliminary notes. Ann Ist Super Sanita 2014; 50: 14014–14016. [DOI] [PubMed] [Google Scholar]
- 15. Sharpley J, Thode H, Sestina L, et al. Distal abdominal aortic thrombosis diagnosed by three-dimensional contrast-enhanced magnetic resonance angiography. Vet Radiol Ultrasound 2009; 50: 370–375. [DOI] [PubMed] [Google Scholar]