Abstract
Objectives
Non-surgical contraceptive management of free-roaming cat populations is a global goal for public health and humane reasons. The objectives of this study were to measure the duration of contraception following a single intramuscular injection of a gonadotropin-releasing hormone-based vaccine (GonaCon) and to confirm its safe use in female cats living in colony conditions.
Methods
GonaCon (0.5 ml/cat) was administered intramuscularly to 20 intact female cats (queens), and saline was administered to 10 queens serving as sham-treated controls. Beginning in late February, 4 months after injection, all cats were housed with fertile male cats in a simulated colony environment. Time to pregnancy, fetal counts and vaccine-elicited injection-site reactions were evaluated.
Results
All control cats (n = 10/10) and 60% (n = 12/20) of vaccinated cats became pregnant within 4 months of the introduction of males. Two additional vaccinates became pregnant (70%; n = 14/20) within 1 year of treatment. Average fetal counts were significantly lower in vaccinated cats than in control cats. Vaccinates had a significantly longer (P = 0.0120) median time to conception (212 days) compared with controls (127.5 days). Injection-site reactions ranging from swelling to transient granulomatous masses were observed in 45% (n = 9/20) of vaccinated cats.
Conclusions and relevance
A single dose of GonaCon provided contraception lasting for a minimum of 1 year in 30% (n = 6/20) of treated cats. The level of contraception induced by this GonaCon dose and vaccine lot was not sufficiently effective to be recommended for use in free-roaming cats.
Introduction
Control of free-roaming cat (FRC) populations is desired by multiple stakeholders to improve the welfare of cats, other species and society. While surgical sterilization serves as the cornerstone of humane FRC management, long-acting non-surgical contraceptive methods are being explored as potential cost-effective adjuncts to surgery. GonaCon, a gonadotropin-releasing hormone (GnRH) vaccine developed by the US Department of Agriculture, Animal and Plant Health Inspection Service (USDA APHIS), Wildlife Services’ National Wildlife Research Center (NWRC), is one such approach. GonaCon Immunocontraceptive Vaccine and GonaCon-Equine are registered by the US Environmental Protection Agency (EPA) for contraception of adult female white-tailed deer, and wild horses and burros, respectively. GonaCon has undergone several formulation changes since its initial development by the NWRC, and the name has been loosely applied in the literature to different formulations. All GonaCon formulations have included a carrier protein presenting multiple copies of a GnRH peptide, emulsified in a mineral oil-based mycobacterium-containing adjuvant.
Levy et al were the first to demonstrate safety and efficacy of a previous formulation of the GnRH vaccine in the laboratory using commercial, vendor-acquired, specific-pathogen-free female cats. 1 Fifteen adult queens were injected intramuscularly with 0.5 ml of this GnRH vaccine, and five control cats were injected with a sham vaccine. In a 5 year breeding trial starting 120 days post-vaccination, vaccinated cats had a significantly longer median time to conception (39.7 months) compared with sham-treated cats (4.4 months). Granulomatous injection-site masses appeared 2 years after vaccination in 33% (n = 5/15) of treated cats. Stochastic modeling suggested that this median duration of infertility could position an injectable contraceptive to rival permanent sterilization of cats for short-term population reduction in the first 5 years of use, though it would be less effective over the longer term of 10 years. 2
Owing to changes in vaccine formulation following the Levy et al study, 1 plus consideration of the potential for injection-site reactions, a short-term safety study using the current formulation of GonaCon was conducted in six adult spayed female purpose-bred cats at the Cincinnati Zoo’s Center for Conservation and Research of Endangered Wildlife. 3 GnRH antibodies developed within 30 days in all vaccinates and persisted throughout the 4 month study. Although transient injection-site reactions were observed in 66% (n = 4/6) of cats, the levels of safety and immunogenicity demonstrated were deemed adequate for further evaluation.
The purpose of the current study was to determine the duration of immunocontraception induced by vaccination with 0.5 ml GonaCon in a diverse group of queens living under conditions that closely resembled a FRC population. It was hypothesized that GonaCon administration would result in prolonged and safe contraception of female cats under simulated FRC colony conditions. The design of the current study, when compared with the Levy et al study in female laboratory cats, 1 modified both the source of animals and their housing conditions. Specific study objectives were to measure duration of contraception following a single intramuscular injection of GonaCon and to confirm the clinical safety of a single injection of GonaCon.
Materials and methods
Animals and environment
Forty-five cats (38 females, seven males) were screened for potential participation in the study. All were domestic short- or medium-hair cats, with estimated ages ranging from 4 months to 3 years (females) and 1–3 years (males) at the time of screening. Forty cats (33 females, seven males) were acquired from four animal control agencies in four non-adjacent Illinois counties; efforts were made to select cats at high risk of euthanasia. Four cats were acquired from private individuals who posted cats for rehoming on a classified advertisement website, and one from a feline rescue. Six sister pairs were enrolled; beyond those sibling relationships, it is expected that the study population was genetically diverse owing to geographic distribution of cat sources. Cats that were screened but not enrolled in the study were surgically sterilized and adopted into private homes.
The study was performed at Clowder Concepts, a USDA-registered Class R research facility, and all procedures were approved by the facility’s Institutional Animal Care and Use Committee. The facility was located in a rural community outside of Urbana-Champaign, IL, USA, and was licensed and inspected by the Illinois Department of Agriculture as an animal shelter. Cats were group housed in a 1400-square-foot room with a loft and ample opportunities to perch, climb and hide. The room was maintained at ambient temperatures between 20ºC and 27°C. Cats had access during daylight hours to an outdoor fenced enclosure (one-third of an acre) with grasses, shrubs and trees. Light provided inside the facility was adjusted to reflect the natural photoperiod. Cats were fed a commercial dry cat food with access to fresh water ad libitum; canned food and treats were provided for enrichment.
Intake and screening procedures
Female cats
Prior to entering the facility, each female cat underwent a physical examination to rule out pregnancy, clinical signs of illness and behaviors incompatible with group housing or low-stress handling. Preventive healthcare was provided as described in Table 1. Cats were visually assessed twice daily for general wellbeing and received monthly physical examinations and monthly treatment with imidacloprid plus moxidectin (Advantage Multi; Bayer) for the duration of the study.
Table 1.
Cat intake and conditioning procedures
| Complete physical examination (repeated within 3–4 weeks) Scanned for presence of microchip Wood’s lamp examination for ringworm Vaccination against feline viral rhinotracheitis, calicivirus, panleukopenia/feline leukemia virus (FeLV) (repeated within 3–4 weeks) Treatment with imidacloprid/moxidectin and praziquantel Complete blood count and serum chemistry panel FeLV antigen and feline immunodeficiency virus antibody test* Abdominal ultrasonographic examination for general health and pregnancy detection (repeated within 3–4 weeks) Implantation of RFID microchip Rabies vaccination |
Cats were individually housed until this test was performed and yielded a negative result. All females were retested on study day 0, which was at least 30 days after the initial test
RFID = radio-frequency identification
Male cats
Male cats were transferred to the facility a minimum of 4 months after the females and underwent the procedures described in Table 1, except for ultrasound examinations. Additionally, all males were assessed for fertility. Semen was collected under sedation via urethral catheterization or electroejaculation.4,5 Immediately after collection, semen was subjected to a standardized semen analysis. 6
Study design
Thirty female cats were selected based on health and behavior. To randomly assign treatment groups, cats were listed from youngest to oldest based on estimated age. A randomization table was generated assigning each female cat to receive either GonaCon or sterile saline (control group), starting with cat 1, in groups of three (with two GonaCon and one control in each group). This randomization resulted in 20 GonaCon-treated cats and 10 control cats. With the exception of the veterinarian who administered the injections, the study statistician and the study monitor, all other study personnel were blinded to each cat’s treatment assignment until the cat was diagnosed as pregnant. Five male cats were selected for use in the study based on health, behavior and fertility.
Design and funding allowed for up to a 5 year study duration, based on cat safety and vaccine effectiveness. Lack of demonstration of vaccine effectiveness in the first year led to the decision to terminate the study 1 year post-vaccination. All cats were surgically sterilized and adopted to private homes at the end of the study.
Vaccination
‘GonaCon’ refers to a formulation identical to the GonaCon Immunocontraceptive Vaccine (EPA registration 56228-40) and GonaCon-Equine (EPA registration 56228-41) products but applied in a smaller dose for this unregistered, experimental use in domestic cats. GonaCon was provided by the USDA/NWRC, Fort Collins, CO, USA. Pre-filled syringe doses were shipped in a cooler with ice packs to the test facility and kept under refrigerated conditions until administration. The vaccine was kept at a refrigerated temperature from manufacture until use, as documented by chain of custody forms. In order to mimic field conditions, cats were not sedated and the injection site was not prepared prior to injection of 0.5 ml GonaCon or sterile saline into the right quadriceps muscle group using an 18 G needle. The day of vaccination was designated study day 0.
Safety observations
Injection sites were palpated daily for 1 week following treatment, then weekly for 1 month and then at least monthly. Cats were observed daily, and any adverse events were recorded on adverse event forms. Baseline serum chemistry and hematology analysis was performed prior to vaccination on each cat, and repeated on day 34.
Breeding trial
The breeding trial began with the first male cat introduced 115 days after treatment. This date corresponded with a period of increasing day length (late February) and the time of year typically associated with the beginning of increased feline estrous activity. A rotation of five breeding males mitigated effects of inter-cat behavioral incompatibility on breeding success. All estrous behaviors and breeding attempts that occurred in the presence of project personnel were recorded. Cats were not under 24 h observation, so it is likely that some estrous and breeding behaviors went unobserved and unrecorded.
Pregnancy diagnosis and ovariohysterectomy
A veterinarian performed monthly ultrasonographic examinations of each queen, beginning 1 month after initiation of the breeding trial. Cats identified as pregnant were ovariohysterectomized between 0 and 8 days after pregnancy was confirmed, and always prior to an estimated day 45 of gestation.
Time to conception was defined as the interval between day 0 and conception, which was estimated by measuring fetal crown–rump length. The length of each fetus was measured with calipers, and the median litter fetal crown–rump length value was calculated for each litter. Fetal age was estimated as per published guidelines. 7 If the median crown–rump length was <58 mm, the date of conception was arbitrarily designated as 30 days prior to the spay surgery. Fetuses were visually evaluated for normal development, but necropsies were not performed.
Statistical analyses
Differences in the median time to conception between the vaccinated and control groups were determined by the Kaplan–Meier survival analysis log rank test. Mean ± SEM and median were calculated for litter size. Means and medians were compared using the Student’s t-test and Wilcoxon rank-sum test, respectively.
Results
Breeding trial
Time to pregnancy is shown in Figure 1. Six control cats (60%) and eight GonaCon-treated cats (40%) displayed behavioral signs of estrus and became pregnant within the first month of the breeding trial. By the fourth month of the breeding trial all control cats (n = 10) had become pregnant, as had 12 (60%) of the GonaCon-treated cats. At 1 year post-treatment, two additional queens had conceived and therefore 14 GonaCon-treated cats (70%) had become pregnant. The GonaCon-vaccinated group had a longer median time to conception following treatment (212 days) than controls (127.5 days; P = 0.01; Figure 1). Litter size (mean ± SD) was significantly smaller in the GonaCon-vaccinated group (3.9 ± 1.7) compared with the control group (5.6 ± 1.8; P = 0.04) (Table 2). There was one unviable (likely resorbed) fetus detected in a vaccinate litter and one in a control litter.
Figure 1.
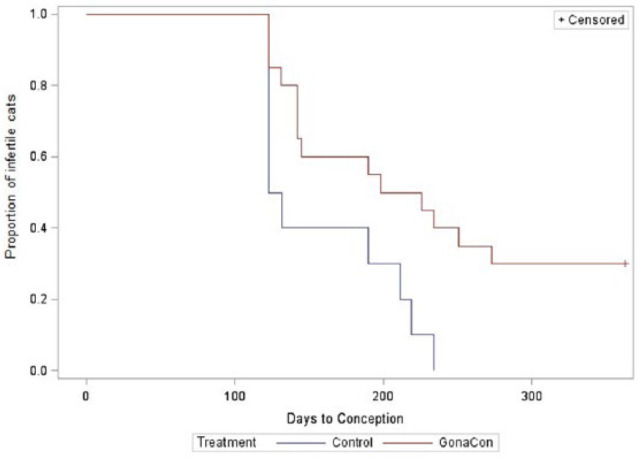
Fertility control in cats. Vaccinated cats (n = 20) received an intramuscular (IM) injection of GonaCon on study day 0. Control cats (n = 10) received an IM injection of saline. A breeding trial commenced on study day 115 and terminated on day 363. Vaccinated cats had longer median time to conception (212 days) than control cats (127.5 days; P = 0.0120). Six vaccinated cats remained infertile for the duration of the breeding trial
Table 2.
Time to conception, injection-site reaction incidence and litter size in individual control cats (n = 10) and GonaCon-treated cats (n = 20)
| Treatment | Cat | Conception (day) | Injection-site reaction | Fetuses at spay |
|---|---|---|---|---|
| Control | 1 | 123 | No | 8 |
| Control | 10 | 123 | No | 6 |
| Control | 13 | 123 | No | 7 |
| Control | 20 | 123 | No | 6 |
| Control | 32 | 123 | No | 7 |
| Control | 19 | 132 | No | 7 |
| Control | 7 | 190 | No | 4 |
| Control | 17 | 211 | No | 4 |
| Control | 24 | 219 | No | 5 |
| Control | 23 | 234 | No | 2 + 1* |
| GonaCon | 9 | 123 | Yes | 4 |
| GonaCon | 31 | 123 | Yes | 4 |
| GonaCon | 28 | 123 | No | 1 |
| GonaCon | 15 | 131 | Yes | 1 |
| GonaCon | 8 | 142 | Yes | 5 |
| GonaCon | 11 | 142 | No | 5 |
| GonaCon | 37 | 142 | No | 2 + 1* |
| GonaCon | 5 | 145 | No | 4 |
| GonaCon | 14 | 190 | No | 6 |
| GonaCon | 22 | 198 | No | 7 |
| GonaCon | 3 | 226 | Yes | 3 |
| GonaCon | 30 | 234 | No | 4 |
| GonaCon | 18 | 251 | Yes | 4 |
| GonaCon | 25 | 273 | No | 5 |
| GonaCon | 6 | NA | No | NA |
| GonaCon | 12 | NA | Yes | NA |
| GonaCon | 21 | NA | No | NA |
| GonaCon | 27 | NA | No | NA |
| GonaCon | 36 | NA | Yes | NA |
| GonaCon | 38 | NA | Yes | NA |
Six cats remained infertile for the duration of the 1 year study
Resorbed fetus
NA = not applicable
Estrous behaviors
Based on observations during working hours (typically 72 h per week and excluding overnight periods), estrous behaviors were never observed in 4/6 vaccinated cats that did not become pregnant. The other two vaccinates exhibited estrous behaviors, even before the breeding trial began, and then engaged in breeding multiple times over the course of the study but did not conceive. The last vaccinate that conceived (cat 25 on day 273) displayed persistent estrous behaviors and accepted the males repeatedly for 5 months prior to conception.
Safety
There were a variety of adverse events that occurred during the trial that would be expected in a colony of 30 co-housed female cats, plus a rotation of male cats. However, there was no pattern to indicate that any adverse events were related to treatment group, with the exception of injection-site reactions.
Three vaccinates (cats 9, 21, 36) were observed limping on the right hindlimb beginning 3–7 h after treatment, which resolved within 24 h. No other acute adverse reactions related to vaccination were observed.
Nearly half of the treated cats (n = 9/20) had a delayed (beginning at least 1 month after treatment) injection-site reaction in the region in which GonaCon was administered. Seven of these cats developed soft tissue masses near the site of injection at variable time points, and mass size fluctuated over variable durations. The other two cats developed swelling with no palpable mass.
Reactions for six cats with delayed reactions resolved within 2–3 months post-injection, whereas reactions for three cats were detected during or after month 7 and persisted through their adoption and exit from the study. Among these nine cats with delayed injection-site reactions, none demonstrated prolonged pain or difficulty ambulating, and there was no evidence of ulceration or other external irritation. A summary of injection-site reactions is presented in Table 3.
Table 3.
Injection-site reaction (swelling or mass) scores in cats vaccinated intramuscularly with GonaCon
| Cat | Days 0–29 |
30–59 | 60–89 | 90–119 | 120–149 | 150–179 | 180–209 | 210–239 | 240–269 | 270–299 | 300–329 | 330–359 | 360–389 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | – | – | – | – | – | – | – | – | – | – | + | A | + |
| 8 | – | – | – | – | – | – | – | ++++++ | ++++ | + | + | A | A |
| 9 | – | ++++ | +++ | – | – | – | – | – | – | – | – | – | – |
| 12 | – | ++ | ± | – | – | – | – | – | – | – | – | – | – |
| 15 | – | +++++ | – | – | – | – | – | – | – | – | – | – | – |
| 18 | – | – | – | – | – | – | – | +++++ | +++ | +++ | A | A | ++ |
| 31 | – | ± | – | – | – | – | – | – | – | – | – | – | – |
| 36 | – | ++++++ | +++++ | – | – | – | – | – | – | – | – | – | – |
| 38 | – | ± | – | – | – | – | – | – | – | – | – | – | – |
Days are numbered relative to GonaCon injection. Longest dimension of mass: + = <1 cm diameter; ++ = 1–1.9 cm; +++ = 2–2.9 cm; ++++ = 3–3.9 cm; +++++ = 4–4.9 cm; ++++++ = ⩾5 cm; ± = inflammation without palpable mass; A = cat in adoptive home, not assessed
Wedge biopsies were performed under general anesthesia on two of the later-onset masses (cats 8 and 18). Histological evaluation indicated the lesions were granulomatous to pyogranulomatous panniculitis with inter-lesional acid-fast bacteria. Aerobic, anaerobic and mycobacterial cultures performed on tissue from cat 18 were negative; tissue from cat 8 was not cultured. Mycobacteria PCR was negative for cat 8 (formalin-fixed tissue) and cat 18 (fresh tissue).
Two of the cats with masses did not become pregnant, and five became pregnant between days 123 and 251. Of the two cats that developed swelling in their hindlimb without palpable masses, one did not become pregnant and the other conceived at day 123. No injection-site reactions were observed in control cats. Tables 2 and 3 detail injection-site reactions, pregnancy and fetal numbers.
Discussion
This study evaluated the duration of contraception and safety of a single GonaCon injection in adult female cats in a simulated free-roaming indoor–outdoor environment. Conditions were as close to a FRC colony as feasible while ensuring a closed population, the ability to closely and reliably monitor vaccine response and individual cat health, and the ability to provide veterinary care as needed. Although vaccinated cats demonstrated significantly longer time to conception and a reduced number of fetuses compared with controls, the study found that a single dose of GonaCon provided contraception lasting for a minimum of 1 year in only 30% (n = 6/20) of treated cats. In a previous study, 1 a GnRH-based vaccine (a previous formulation of GonaCon) induced fertility control in 100% of treated laboratory cats with a duration of contraception that varied from 5 months to >5 years, with a median of nearly 40 months.
The poor contraceptive efficacy of this vaccine vs prior studies in both female and male cats was not anticipated.1,8,9 Three possibilities have been considered.
One possible explanation could be variability in the vaccine itself. Although the formulation used in the current study was the same as in the GonaCon safety confirmation study, during which a strong humoral immune response was measured in all treated cats, 3 the two studies used different batches of vaccine. A retrospective consideration of standard manufacturing quality-control measures did not offer an explanation for differences between the two batches, maintenance of appropriate cold-chain handling was well documented and the vaccine used in this study did not exceed its shelf life. A direct comparative analysis between vaccines used in safety confirmation and current studies was not performed because both batches had expired at the time of inquiry.
A second potential explanation relates to individual variability. As with any vaccine, duration of effect is expected to vary somewhat among individuals. 10 Even when breed, age, source and sex were kept consistent within previous studies using GnRH-based vaccines,1,8,9 individual cats responded with a variable GnRH antibody production. As increased individual variability was an essential part of the current study design, an inconsistent response to vaccination within this population was plausible.
It is noteworthy that individual variability was also observed in the mating responses of control cats in this study. Mating and conception was more broadly distributed in the current study than in the Levy et al study, 1 in which all of the sham-treated cats (n = 5) became pregnant 7–28 days after the introduction of the male. In the current study, 6/10 control cats conceived in a similarly rapid timeframe, whereas the remaining four control cats conceived later, 75–119 days after initiation of the breeding trial.
A third potential explanation stems from plausible differences between laboratory and free-roaming cat populations, including environmental conditions. A consideration of previous GnRH-based immunocontraceptive studies in female white-tailed deer reveals a lesser vaccine efficacy in free-roaming populations of wild deer than in penned,11,12 captive-bred/born populations that received regular veterinary care, housing and a consistent diet. 13 However, results in deer present a difficult analogy because, while history was not known prior to entering the research facility, cats in this study were normalized to a common plane of health, nutrition and environment prior to study initiation, and they received regular veterinary care throughout the study. This precludes equating cats in this study with the free-roaming wild deer population.
With regard to injection-site reactions, previous studies of cats treated with GonaCon formulations exhibited reactions similar to those seen in this study.1,3 It is possible that transient reactions in this study went undetected after the frequency of palpations changed from weekly to monthly. It is also possible that late-onset reactions could have developed after cats were removed from the study and adopted into homes. These findings are consistent with the aforementioned safety confirmation study, in which 4/6 cats developed soft tissue masses at the site of injection; three of these developed within a month after injection and one developed 8 months post-injection while in the adoptive home.1,3
Vaccine components that extend duration of response are believed to contribute to injection-site reactions. 14 Although the unpredictable timing, type and severity of the observed reactions could be perceived as a concern for field use of this vaccine, none of the reactions observed in the Levy et al laboratory cats, 1 the safety confirmation study and this field study appeared to cause more than transient pain or were considered life threatening. 3
The goal of testing a temporary contraceptive in this study was to provide a useful tool in the field to manage free-roaming cats, as an alternative to surgical sterilization or as an interim tool prior to the availability of a permanent method of non-surgical fertility control. Simulation modeling using the GonaCon contraceptive results achieved by Levy et al, 1 and using a negative long-term population growth rate as a generic criterion for population management success, required treating (or retreating) approximately 50% of the fertile cats in the population, every 6 months, on a sustained basis. This was equivalent to a long-term cumulative infertility rate of 75% of cats. Although it was not explicitly modeled, we suspect that employing contraception and surgery sequentially could be logistically advantageous. More specifically, high initial rates of fertility suppression could be most quickly achieved by contracepting a large number of cats, with surgery used over a longer subsequent time period to permanently sterilize contracepted cats before they revert to fertility. 2 Additional research is being conducted to better characterize the relationship between contraceptive efficacy and population reduction, including a comparison of the economic requirements of alternative management approaches (J Boone, 2017, personal communication). This could provide helpful guidance in designing non-permanent contraception for cats.
Conclusions
A 0.5 ml dose of the GonaCon vaccine formulated and approved for use in white-tailed deer and wild equid species did not provide contraception for a sufficient proportion of female cats housed under colony conditions during this year-long study to justify continuation of the study or deployment of this immunocontraceptive for the control of FRC populations.
Acknowledgments
We thank Doug Eckery of the USDA APHIS National Wildlife Research Center for providing GonaCon, and Doug Eckery and Lowell Miller for assisting with interpretation of results. We thank the University of Illinois staff, interns and volunteers who provided exceptional care for the cats, and the adopters who provided homes.
Footnotes
Accepted: 17 January 2018
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: We thank the Morris Animal Foundation and the John T and Jane A Wiederhold Foundation for funding this study.
References
- 1. Levy JK, Friary JA, Miller LA, et al. Long-term fertility control in female cats with GonaCon, a GnRH immunocontraceptive. Theriogenology 2011; 76: 1517–1525. [DOI] [PubMed] [Google Scholar]
- 2. Miller PS, Boone JD, Briggs JR, et al. Simulating free-roaming cat population management options in open demographic environments. PLoS One 2014; 9: e113553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vansandt LM, Kutzler MA, Fischer AE, et al. Safety and effectiveness of a single and repeat intramuscular injection of a GnRH vaccine (GonaCon) in adult female domestic cats. Reprod Domest Anim 2016; 51: 1–6. [DOI] [PubMed] [Google Scholar]
- 4. Zambelli D, Prati F, Cunto M, et al. Quality and in vitro fertilizing ability of cryopreserved cat spermatozoa obtained by urethral catheterization after medetomidine administration. Theriogenology 2008; 69: 485–490. [DOI] [PubMed] [Google Scholar]
- 5. Howard JG, Brown JL, Bush M, et al. Teratospermic and normospermic domestic cats: ejaculate traits, pituitary-gonadal hormones, and improvement of spermatozoa motility and morphology after swim up processing. J Androl 1990; 11: 204–215. [PubMed] [Google Scholar]
- 6. Howard JG. Semen collection and analysis in carnivores. In: Fowler ME. (ed). Zoo and wild animal medicine III. Philadelphia, PA: WB Saunders, 1993, pp 390–399. [Google Scholar]
- 7. Johnston SD, Root Kustritz MV, Olson PNS. Canine and feline theriogenology. Philadelphia, PA: WB Saunders, 2011, p 419. [Google Scholar]
- 8. Levy JK. Contraceptive vaccines for the humane control of community cat populations. Am J Reprod Immunol 2011; 66: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levy JK, Miller LA, Crawford C, et al. GnRH immunocontraception of male cats. Theriogenology 2004; 62: 1116–1130. [DOI] [PubMed] [Google Scholar]
- 10. Benka VAW, Levy JK. Vaccines for feline contraception. J Feline Med Surg 2015; 17: 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gionfriddo JP, Eisemann JD, Sullivan KJ, et al. Field test of a single-injection gonadotrophin-releasing hormone immunocontraceptive vaccine in female white-tailed deer. Wildlife Res 2009; 36: 177–184. [Google Scholar]
- 12. Gionfriddo JP, DeNicola AJ, Miller LA, et al. Efficacy of GnRH immunocontraception of wild white-tailed deer in New Jersey. Wildlife Soc Bull 2011; 35: 142–148. [Google Scholar]
- 13. Miller LA, Gionfriddo JP, Fagerstone KA, et al. The single-shot GnRH immunocontraceptive vaccine (GonaCon) in white-tailed deer: comparison of several GnRH preparations. Am J Reprod Immunol 2008; 60: 214–223. [DOI] [PubMed] [Google Scholar]
- 14. Miller L, Fagerstone K, Kemp J, et al. Immune mechanisms and characterization of injection site reactions involved in the multi-year contraceptive effect of the GonaCon vaccine. Proceedings of the 23rd Vertebrate Pest Conference; 2008 March 17–20; San Diego, CA. Davis, CA: University of California, Davis, 2008, pp 224–249. [Google Scholar]


