Abstract
Seizures are a common cause of neurologic disease, and phenobarbital (PB) is the most commonly used antiepileptic drug. Chronic oral dosing can be challenging for cat owners, leading to poor compliance. The purpose of this study was to determine if the transdermal administration of PB could achieve serum PB concentrations of between 15 and 45 μg/ml in healthy cats. Nineteen healthy cats were enrolled in three groups. Transdermal PB in pluronic lecithin organogel (PLO) was applied to the pinnae for 14 days at a dosage of 3 mg/kg q12h in group 1 (n = 6 cats) and 9 mg/kg q12h in group 2 (n = 7 cats). Transdermal PB in Lipoderm Activemax was similarly applied at 9 mg/kg q12h for 14 days in group 3 (n = 6 cats). Steady-state serum PB concentrations were measured at trough, and at 2, 4 and 6 h after the morning dose on day 15. In group 1, median concentrations ranged from 6.0–7.5 μg/ml throughout the day (observed range 0–11 μg/ml). Group 2 median concentrations were 26.0 μg/ml (observed range 18.0–37.0 μg/ml). For group 3, median concentrations ranged from 15.0–17.0 μg/ml throughout the day (range 5–29 μg/ml). Side effects were mild. One cat was withdrawn from group 2 owing to ataxia and sedation. These results show therapeutic serum PB concentrations can be achieved in cats following chronic transdermal administration of PB in PLO at a dosage of 9 mg/kg q12h. More individual variation was noted using Lipoderm Activemax. Transdermal administration may be an alternative for cats that are difficult to medicate orally.
Introduction
Seizures are a common cause of neurologic disease in cats, constituting 0.5–3.5% of all feline referrals to veterinary teaching hospitals.1–3 Phenobarbital (PB) is the most commonly recommended antiepileptic drug (AED) in cats with non-metabolic causes for seizures, and has been shown to control seizures in 93% of cats with a therapeutic serum PB concentration of between 15 and 45 μg/ml.4–9 Additionally, adverse biochemical, hematological or clinical effects were not observed during 21 days of treatment with PB in one feline study. 4
Chronic dosing of AEDs can be challenging for clients if a cat is resistant to taking oral medications. However, discontinuation of AEDs has been shown to result in a recurrence of seizures in 75% of cats. 10 Therefore, while the administration of AEDs is recommended for cats with seizures, chronic oral dosing may be impractical for some clients. Transdermal drug administration is an effective alternative to the oral route for some drugs in cats. 11 Therefore, we hypothesized that transdermal PB could reach therapeutic serum concentrations in cats. The purpose of this study was to determine whether transdermal PB in pluronic lecithin organogel (PLO) or Lipoderm Activemax (PCCA) vehicles would result in steady-state serum PB concentrations of between 15 and 45 μg/ml throughout a 12 h dosing interval in healthy cats.
Materials and methods
Study design
This study was designed as a prospective clinical trial. Nineteen healthy cats, owned by Veterinary Medical Teaching Hospital (VMTH) staff and students, were enrolled in the study. All owners provided written, informed consent, and the study was approved by the Institutional Animal Care and Use Committee at the University of Wisconsin–Madison. Cats were enrolled if they met the following inclusion criteria: (1) no history of seizures or other neurologic disease, (2) normal neurologic examination, (3) no current medication administration other than monthly heartworm disease prevention and/or flea prevention, (4) normal complete blood count and serum biochemistry, and a urine specific gravity >1.030, and (5) no concurrent participation in another clinical study. The trial was divided into three groups with six cats in each group.
For groups 1 and 2 of this study, PB (PB powder >99% purity; Sigma-Aldrich) was dissolved in propylene glycol or isopropyl alcohol 70%, and then custom formulated to 125 mg/ml in PLO (Polox Gel 20%, Poloxamer 407 [Fagron] and Lipoil [lecithen-isopropyl palmitate oil; Gallipot]) by a licensed VMTH pharmacist (MR). Owners were instructed to apply the PB to the cats’ pinnae at 3 mg/kg (group 1) or 9 mg/kg (group 2) q12h for days 1–14. The higher dose resulted in a larger volume administered, so each dose was split between both pinnae for group 2. For group 3, PB (PB powder; Sigma-Aldrich) was dissolved in propylene glycol or isopropyl alcohol 70%, and then custom formulated to 250 mg/ml in a proprietary compounding base (Professional Compounding Centers of America). Owners were instructed to apply the PB to the cats at 9 mg/kg q12h for days 1–14, alternating pinnae.
Each owner completed a daily log of side effects and dosing times. On day 15, serum PB (S-PB) concentrations were evaluated at trough, and 2, 4 and 6 h after a morning dose given at the VMTH. Serum samples were obtained by peripheral venepuncture. These time points were chosen to approximate peak concentrations for each cat based on a previous pharmacokinetic study. 5 As a precaution against possible withdrawal side effects, the cats were given the assigned dose q24h on days 15, 16 and 17; PB was then discontinued. Cats with unacceptable side effects were voluntarily withdrawn from the study at any point, at the discretion of the owners or principal investigators.
S-PB concentrations were assayed at a commercial laboratory (Marshfield Labs – Veterinary Services) using an immunoassay that is validated for cats. 5 The limit of quantitation of this assay was 5 μg/ml; therefore, samples <5 μg/ml were reported as <5 μg/ml, and medians were calculated using the detection limit divided by two (2.5 μg/ml). 12 All samples for each cat were run within the same batch. An interim data analysis was performed after the completion of group 1 in order to allow for a dose escalation, if necessary, to maintain serum drug concentrations between 15 and 45 μg/ml throughout the dosing interval in the majority of cats. The new dose was determined by the following equation:
Statistical evaluation
Data are reported for each group using median S-PB concentrations at each time point, as well as the full range of concentrations observed at steady state in each group. A successful outcome was defined as steady-state S-PB concentrations between 15 and 45 μg/ml at all time points in the majority of cats in a given group. The mean concentrations at steady state (Css) of S-PB were compared among the three groups using a Kruskal–Wallis test, followed by a Dunn’s multiple comparisons test (Prism 4.0, GraphPad Software).
Results
Group 1
All six cats were American domestic shorthair cats with a median age of 5 years (range 2–13 years). There were four male neutered cats and two female spayed cats. Median weight was 4.8 kg (range 3.4–7.6 kg).
In group 1, the median S-PB concentrations at trough and at 2, 4 and 6 h were 7.5 μg/ml (range 0–11.0 μg/ml), 6.5 μg/ml (range 0–10.0 μg/ml), 6.5 μg/ml (range 0–10.0 μg/ml) and 6.0 μg/ml (range 0–10 μg/ml), respectively. The Css was <15 μg/ml in all cats at this dosage. The volume of PB in PLO administered to cats in group 1 ranged from 0.08–0.18 ml per dose.
Mild adverse effects were reported in 4/6 cats in group 1. These effects included erythema of the inner surface of the pinnae (n = 1, days 6, 7 and 8), ear grooming (n = 1, day 9), mild increase in appetite (n = 1, days 2–9), mild increase in vomiting of trichobezoars (n = 1) and mild polydipsia (n = 1, day 4).
Group 2
Interim analysis performed on the data from group 1 cats predicted that a threefold higher dosage was needed to target the lower end of the therapeutic range at trough. Therefore, in group 2, six cats were enrolled at a dosage of 9 mg/kg q12h. One of the cats was withdrawn on day 3 owing to side effects considered intolerable by the owner, so the seventh cat was enrolled to maintain a total of six cats completing each study. Group 2 cats included four American domestic shorthairs, one American domestic longhair and one Abyssinian mixed breed. There were two male neutered cats and five female spayed cats. The median weight was 4.6 kg (range 4.0–6.1 kg) and the median age was 5 years (range 2–14 years).
Median S-PB concentrations at trough, and at 2, 4 and 6 h for the six cats that completed the second study were 26.0 μg/ml (range 20.0–37.0 μg/ml), 26.0 μg/ml (range 19.0–36.0 μg/ml), 25.5 μg/ml (range 18.0–36.0 μg/ml) and 25.5 μg/ml (range 18.0–36.0 μg/ml), respectively. The volume of PB in PLO administered to cats in group 2 ranged from 0.24–0.44 ml per dose.
Adverse effects were reported in 4/7 group 2 cats. The cat that was removed from the study after day 3 developed pelvic limb ataxia, polyphagia, sedation and inappropriate mentation. Adverse signs noted in the other three cats were mild: paraparesis (n = 2, day 11 for one and days 10–14 for another), pelvic limb ataxia (n = 1), lethargy (n = 1, day 3), vomiting a trichobezoar (n = 1, day 14) and ear grooming (n = 1, days 13 and 14).
Group 3
For group 3, six cats were administered PB in Lipoderm Activemax at 9 mg/kg q12h. There were five American domestic shorthair cats and one American domestic longhair; three were male neutered cats and three female spayed cats. The median weight was 4.7 kg (range 4.0–5.4 kg) and the median age was 2.5 years (range 1–6 years).
Median S-PB concentrations at trough, and at 2, 4 and 6 h appeared to be more variable than with the PLO vehicle, and were 17.0 μg/ml (range 5.0–25.0 μg/ml), 15.5 μg/ml (range 5.0–28.0 μg/ml), 15.0 μg/ml (range 5.0–26.0 μg/ml) and 15.5 μg/ml (range 6.0–29.0 μg/ml), respectively. The volume of PB in Lipoderm Activemax administered to cats in group 3 ranged from 0.14 to 0.19 ml per dose.
Adverse effects were reported in 5/6 group 3 cats. These effects included polyphagia (n = 1), polyuria/polydipsia (n = 1, days 3–10), pelvic limb ataxia (n = 2), mild lethargy (n = 2; days 8–14 and days 3–10) and increased ear grooming with medication crusting on the pinnae (n = 1, days 6–14).
There was a significant difference in mean Css for S-PB between groups 1 and 2 (P <0.01) (Figure 1). There was an apparent difference in the mean Css S-PB values between groups 2 and 3, but this did not reach significance (P = 0.06).
Figure 1.
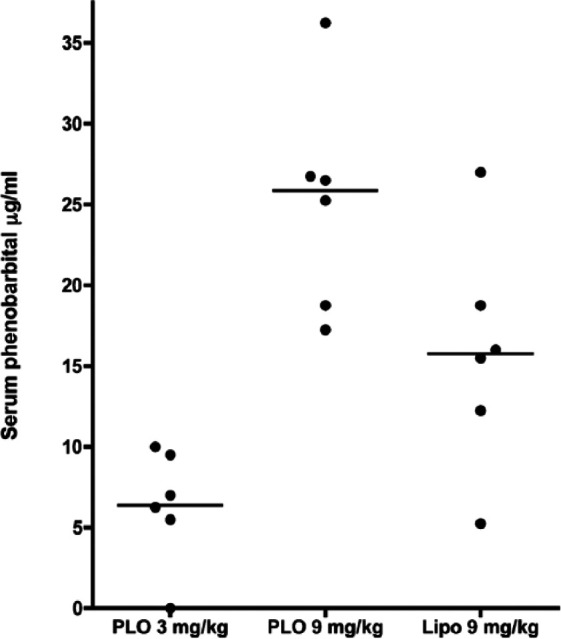
Mean concentration at steady state for serum phenobarbital (PB) levels between group 1 (PB in pluronic lecithin organogel [PLO] at 3 mg/kg q12h), group 2 (PB in PLO at 9 mg/kg q12h) and group 3 (PB in Lipoderm Activemax [Lipo] at 9 mg/kg q12h). Six cats were enrolled in each group
Discussion
The results of this study show that therapeutic S-PB concentrations can be achieved in cats following chronic transdermal administration of PB in PLO at a dosage of 9 mg/kg q12h, and in most cats at the same dosage in Lipoderm Activemax. Notably, S-PB concentrations were not achieved by the transdermal route at 3 mg/kg q12h, which is the standard PB dosage per os in cats.2,13 Interim analysis following the completion of group 1 predicted that a threefold higher dosage was needed to target the lower end of the therapeutic range at the trough level. Prior studies evaluating the bioavailability in cats have shown almost complete oral bioavailability. 5 We hypothesize that the difference in required dosage between per os and transdermal administration may be owing to lower transdermal vs oral bioavailability for PB in cats, although bioavailability relative to oral administration was not determined in this study. In addition, we used only partial sampling after 2 weeks rather than full pharmacokinetic analyses, as these were healthy client-owned cats. Therefore, it is possible that our sampling protocol could have missed a delayed serum peak concentration in some or all cats.
It is important to note that the range of therapeutic S-PB concentrations used for cats is extrapolated from the canine therapeutic range. The therapeutic range is determined from the minimally effective drug concentration and the maximum drug concentration observed without side effects in most patients. 14 As this is a population average, an individual animal may still show toxicity or lack of efficacy within this range. Therefore, there may be individual differences in clinical response, such as lack of efficacy or clinical toxicity, even within the therapeutic range in some epileptic cats. However, it has been shown that 93% of cats will achieve seizure control (defined as a reduction in seizures of ⩾50%) with a S-PB concentration of 15–45 μg/ml. 9
Overall, side effects were minor and transient in most cats given transdermal PB, and were limited to known side effects of oral PB, with the exception of erythema of the pinnae and increased ear grooming.6,15 While vomiting of trichobezoars was recorded as a potential adverse effect, there was no way of knowing if this was an effect of PB or coincidental. The cat that was removed from the study after day 3 owing to unacceptable side effects was not evaluated at a lower dosage, as might be done clinically in an epileptic cat. This cat could not be transported to the hospital on the day of disenrollment and S-PB concentrations were not determined, so it is unknown whether serum concentrations were above the therapeutic range.
Some cats were reported to occasionally groom their ears during the study. Oral ingestion of the transdermal product may have occurred during grooming in some cats, and may have contributed to the S-PB concentrations obtained in this study. While only one cat had signs of PB overdose, it is possible that excessive grooming of the higher 9 mg/kg dose could lead to sedation in some cats.
The main advantage of transdermal PB drug administration is to provide cat owners with an alternative to oral administration. Many cats become intolerant of oral administration, and transdermal application may be less stressful for some cats. The main limitation of the 9 mg/kg dose, as formulated in PLO (group 2), was the relatively large volume of medication required (range 0.24–0.44 ml/dose twice daily). This required a division of the amount between both pinnae, rather than using alternate pinnae for each dose. Formulations of PB in PLO at concentrations >125 mg/ml were attempted to minimize the volume administered, but solubility was poor at higher concentrations (unpublished data). Because of these larger volumes, an accumulation of PB in PLO on the pinnae is possible with chronic administration in a clinical setting, which could increase the risk of erythema or irritation to the cat. Indeed, most cats in group 2 presented on day 15 with encrusted debris on their pinnae, which was suspected to be the medication.
Formulation of PB in Lipoderm Activemax allowed for a smaller volume of administration with no apparent increase in the incidence of side effects compared with 9 mg/kg PB in PLO. However, there was more apparent individual variability in the mean S-PB concentrations in the cats receiving the drug in Lipoderm Activemax. One cat in group 3 had very low S-PB concentrations (range 5–6 μg/ml). This may have been due to poor compliance, poor transdermal bioavailability or rapid biotransformation of the PB. Additionally, differences in the volume of drug administered between dosing regimens could have also contributed to differences in the precision of dosing between groups. Given the possible trend towards lower S-PB concentrations with Lipoderm Activemax vs PLO, higher dosages of PB in Lipoderm Activemax might be necessary in some cats. The concentration of PB in Lipoderm Activemax could be adjusted up to 300 mg/ml as needed. Any cat receiving transdermal PB should have therapeutic S-PB levels monitored to assess individual response to the medication. The costs of the formulations of PB in PLO and Lipoderm Activemax at 9 mg/kg are approximately US$40–50 per month for 45 mg q12h for both formulations. PB in Lipoderm has the advantage of ease of administration and reduced crusting on the pinnae.
Conclusions
Transdermal PB in PLO or in Lipoderm Activemax at 9 mg/kg q12h provides S-PB concentrations between 15 and 45 µg/ml, with only minor side effects, in most cats. Individual cats given PB in Lipoderm Activemax may have lower S-PB concentrations, and S-PB concentrations should be monitored in any cats receiving transdermal PB. The increased solubility of Lipoderm Activemax may allow for more flexibility for increasing the dose above 9 mg/kg. Based on the results of this study, transdermal dosing may provide a therapeutic alternative to chronic oral administration of PB for cats. Any cat receiving transdermal PB should have therapeutic S-PB levels monitored to assess individual response to the medication. Future studies should evaluate this treatment route in cats with naturally occurring epilepsy.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This study was supported by the Department of Medical Sciences, University of Wisconsin–Madison, School of Veterinary Medicine, Madison, WI, USA.
Accepted: 4 July 2014
References
- 1. Schriefl S, Steinberg TA, Matiasek K, et al. Etiologic classification of seizures, signalment, clinical signs and outcomes in cats with seizure disorders: 91 cases (2000–2004). J Am Vet Med Assoc 2008; 233: 1591–1597. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz-Porsche D, Kaiser E. Feline epilepsy. Prob Vet Med 1989; 1: 628–649. [PubMed] [Google Scholar]
- 3. Pakozdy A, Leschnik M, Sarchahi AA, et al. Clinical comparison of primary versus secondary epilepsy in 125 cats. J Feline Med Surg 2010; 12: 910–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cochrane SM, Parent JM, Black WD, et al. Pharmacokinetics of phenobarbital in the cat following multiple oral administration. Can J Vet Res 1990; 54: 309–312. [PMC free article] [PubMed] [Google Scholar]
- 5. Cochrane SM, Black WD, Parent JM, et al. Pharmacokinetics of phenobarbital in the cat following intravenous and oral administration. Can J Vet Res 1990; 54: 132–138. [PMC free article] [PubMed] [Google Scholar]
- 6. Smith Bailey K, Dewey CW. The seizuring cat. Diagnostic work-up and therapy. J Feline Med Surg 2009; 11: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thomas WB. Idiopathic epilepsy in dogs and cats. Vet Clin North Am Small Anim Pract 2010; 40: 161–179. [DOI] [PubMed] [Google Scholar]
- 8. Kluger EK, Malik R, Govendir M. Veterinarians’ preferences for anticonvulsant drugs for treating seizure disorders in dogs and cats. Aust Vet J 2009; 87: 445–449. [DOI] [PubMed] [Google Scholar]
- 9. Finnerty K, Barnes Heller H, Mercier M, et al. Evaluation of therapeutic phenobarbital concentrations and application of a classification system for seizures in cats: 30 cases (2004–2013). J Am Vet Med Assoc 2014; 244: 195–199. [DOI] [PubMed] [Google Scholar]
- 10. Pakozdy A, Sarchahi AA, Leschnik M, et al. Treatment and long-term follow-up of cats with suspected primary epilepsy. J Feline Med Surg 2013; 15: 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sartor LL, Trepanier LA, Kroll MM, et al. Efficacy and safety of transdermal methimazole in the treatment of cats with hypertension. J Vet In Med 2004; 18: 651–655. [DOI] [PubMed] [Google Scholar]
- 12. LaFleur B, Lee W, Billihiemer D, et al. Statistical methods for assays with limits of detection: serum bile acid as a differentiator between patients with normal colons, adenomas and colorectal cancer. J Carcinog 2011; 10: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parent JML, Quesnel AD. Seizures in cats. Vet Clin North Am Small Anim Pract 1996; 26: 811–825. [PubMed] [Google Scholar]
- 14. Rowland M, Tozer TN. Therapeutic response and toxicity. In: Rowland M, Tozer TN. (eds). Clinical pharmacokinetics: concepts and applications. 4th ed. Philadelphia, PA: Williams and Wilkins, 1995, pp 57–61. [Google Scholar]
- 15. de Lahunta A, Glass E. Seizure disorders: narcolepsy. In: de Lahunta A, Glass E. (eds). Veterinary neuroanatomy and clinical neurology. 3rd ed. St Louis, MO; Saunders, 2009, p 466. [Google Scholar]


